Abstract
The Corynebacterium glutamicum R genome contains a total of eight genes encoding proteins with sequence similarity to C4-dicarboxylate transporters identified from other bacteria. Three of the genes encode proteins within the dicarboxylate/amino acid:cation symporter (DAACS) family, another three encode proteins within the tripartite ATP-independent periplasmic transporter family, and two encode proteins within the divalent anion:Na+ symporter (DASS) family. We observed that a mutant strain deficient in one of these genes, designated dcsT, of the DASS family did not aerobically grow on the C4 dicarboxylates succinate, fumarate, and malate as the sole carbon sources. Mutant strains deficient in each of the other seven genes grew as well as the wild-type strain under the same conditions, although one of these genes is a homologue of dctA of the DAACS family, involved in aerobic growth on C4 dicarboxylates in various bacteria. The utilization of C4 dicarboxylates was markedly enhanced by overexpression of the dcsT gene. We confirmed that the uptake of [13C]labeled succinate observed for the wild-type cells was hardly detected in the dcsT-deficient mutant but was markedly enhanced in a dcsT-overexpressing strain. These results suggested that in C. glutamicum, the uptake of C4 dicarboxylates for aerobic growth was mainly mediated by the DASS transporter encoded by dcsT. The expression level of the dcsT gene transiently increased in the early exponential phase during growth on nutrient-rich medium. This expression was enhanced by the addition of succinate in the mid-exponential phase and was repressed by the addition of glucose in the early exponential phase.
C4-dicarboxylate intermediates in the tricarboxylic acid cycle, i.e., succinate, fumarate, and malate, are utilized by bacteria as carbon and/or energy sources. Various types of transporters are involved in the utilization of C4 dicarboxylates (20). The proteins of the DctA family, which is a subgroup of the dicarboxylate/amino acid:cation symporter (DAACS) family (5, 6), are well conserved in aerobes and facultative anaerobes and mediate the uptake of C4 dicarboxylates under aerobic conditions in various bacteria, i.e., Escherichia coli (7), Salmonella enterica serovar Typhimurium (2), Bacillus subtilis (1), and some species of Rhizobium (10, 36, 51, 55, 57). Other types of transporters have also been implicated in C4-dicarboxylate uptake, but the functional characterization has been limited. The tripartite ATP-independent periplasmic transporter (TRAP-T) is essential for aerobic growth on C4 dicarboxylates in the purple photosynthetic bacterium Rhodobacter capsulatus (11, 41). SdcS, which is a member of the divalent anion:Na+ symporter (DASS) family (5, 6), in Staphylococcus aureus was functionally characterized by being expressed in E. coli and by being reconstituted into proteoliposome, indicating that it functioned as a Na+/dicarboxylate symporter (13, 14).
Corynebacterium glutamicum, which is a nonpathogenic high-GC-content, gram-positive soil bacterium, is widely used for the industrial production of amino acids such as glutamate and lysine (23, 24). The entire genome sequence of C. glutamicum ATCC 13032 is helpful for the elucidation of various cellular functions of this microorganism important to industry (15, 21). We have developed a bioprocess for production of lactate, succinate, and ethanol by C. glutamicum R (16, 17, 26, 27). We performed transcriptome analyses during organic acid production (18) based on the genome sequence of C. glutamicum R (56). It is important to understand the mechanism of chemical transport across the cellular membrane for the development of bioprocesses using the microorganism. C. glutamicum has the ability to utilize C4 dicarboxylates (54). The uptake of succinate by C. glutamicum cells was characterized as a Na+-coupled mechanism (8). However, the relevant transporter remains unknown.
In this study, we searched for a transporter involved in the utilization of C4 dicarboxylates in C. glutamicum. In the genome sequence of C. glutamicum R, there are eight genes encoding proteins with some sequence similarity to the previously identified C4-dicarboxylate transporters classified into the DAACS, TRAP-T, or DASS family in other bacteria. Analyses of a gene-deficient mutant and a gene-overexpressing strain indicated that the utilization of succinate, fumarate, and malate for aerobic growth is mediated mainly by a member of the DASS family in C. glutamicum cells. Furthermore, we showed the growth-phase-dependent expression of the corresponding gene, designated dcsT, of which expression was positively affected by succinate and negatively affected by glucose.
MATERIALS AND METHODS
Bacterial strains.
C. glutamicum R (56) was used as a wild-type strain for our experiments. Mutant strains deficient in a C4-dicarboxylate transporter homologue (cgR_0299, cgR_1933, cgR_2199, cgR_2220, cgR_2306, cgR_2451, cgR_2497, and cgR_2914) were obtained from a single-gene-disruptant mutant library constructed by transposon-mediated mutagenesis (46).
Culture conditions.
For genetic manipulations, E. coli strains were grown at 37°C in Luria-Bertani medium (39). C. glutamicum strains were grown at 33°C in nutrient-rich A medium (17) with 4% glucose. Where appropriate, the culture medium was supplemented with 50 μg ml−1 of kanamycin and 50 μg ml−1 of chloramphenicol for E. coli. For C. glutamicum, the final concentrations of antibiotics were 50 μg ml−1 for kanamycin and 5 μg ml−1 for chloramphenicol.
For growth on organic acids as the sole carbon sources, C. glutamicum cell starter culture was grown aerobically until the late exponential phase in 10 ml A medium containing 4% glucose at 33°C in a 100-ml test tube. The cells were harvested by centrifugation at 4,000 × g for 10 min at 4°C. The cell pellet was subsequently washed twice with BT minimal medium (17). The washed cells were suspended with 100 ml BT medium containing 50 mM disodium succinate, sodium fumarate, or sodium malate and then aerobically cultured at 33°C in a 500-ml flask.
For growth on nutrient-rich A medium, C. glutamicum cell starter culture was grown aerobically in 10 ml A medium at 33°C in a 100-ml test tube overnight. A part of the culture was added to 100 ml A medium supplemented with disodium succinate or glucose at 50 mM, then aerobically cultured at 33°C in a 500-ml flask.
Uptake of [13C]succinate.
C. glutamicum cell starter culture was grown aerobically until the mid-exponential phase in 100 ml A medium with 4% glucose at 33°C in a 500-ml flask. The cells were harvested and washed twice with 50 mM Tris-HCl, pH 8.0, and then suspended with 80 ml of the same buffer at an optical density at 610 nm (OD610) of 3 in a 500-ml flask. After preincubation at 33°C for 5 min with shaking, the cell suspension was supplemented with 10 mM [1,4-13C]succinate and aerobically incubated with shaking at 33°C.
Analytical methods.
Cell growth was monitored by measuring the OD610 by using a spectrophotometer (DU640; Beckman Coulter, CA).
The cell culture was centrifuged at 10,000 × g for 10 min at 4°C, and the supernatants were analyzed for organic acids and glucose. Organic acids were quantified by high-performance liquid chromatography (8020 system; Tosoh, Tokyo, Japan) equipped with an electric conductivity detector and TSKgel OAPak column (Tosoh) operating at 40°C with a 0.75 mM H2SO4 mobile phase at a flow rate of 1.0 ml min−1. The concentration of glucose was measured by an enzyme electrode glucose sensor (BF-4; Oji Scientific Instruments, Hyogo, Japan).
Sample preparation for analysis of 13C-labeled metabolites.
Intracellular metabolites in C. glutamicum were extracted by modifying methods described previously (4, 44, 53). Cells were separated by vacuum filtration (PTFE membrane, 0.5-μm pore size, 47 mm diameter; Advantec, Tokyo, Japan) and washed four times with 5 ml NaCl solution (0.9%; 33°C). Subsequently, the filter was plunged into 1 ml of methanol (−20°C) for rapid deactivation of enzymes and simultaneously extraction of intracellular metabolites in C. glutamicum (53). An internal standard containing 5 μl of 0.5 g liter−1 ribitol was added to the methanol solution for quantitation of intracellular metabolites. The whole sampling procedure for methanol quenching and extraction was finished in less than 30 s. After incubation for 60 min at −20°C, the extract was obtained by a method described previously (44). The extract was derivatized by methoxyamine hydrochloride in pyridine and subsequently N-methyl-N-(trimethylsilyl)trifluoroacetamide (trimethylsilyl [TMS] derivatization) as previously described (35, 45). The derivatized sample was analyzed by gas chromatography-mass spectrometry (GC-MS).
GC-MS analysis.
GC-MS was carried out using a gas chromatograph equipped with a DB-5MS capillary column (30 m by 0.25 mm by 0.25 μm; J&W) that was directly connected to a mass spectrometer (QP-2010 Plus; Shimadzu, Kyoto, Japan). For the measurements of TMS derivatives, an initial oven temperature of 70°C was maintained for 5 min, then increased to 320°C at 10°C min−1, and maintained for 5 min. The total running time was 33 min. The other settings were the same as those for the measurements previously described (35). In this study, the ion fragment of [M-15] was measured by GC-MS (42). The concentration of m2 isotopomer originating from [1,4-13C] succinate was obtained from the ratio of the intensity of m2 of succinate-TMS derivatives to the sum of the intensities of all the [M-15] groups, multiplied by the succinate concentration.
DNA techniques.
Chromosomal DNA was isolated from C. glutamicum cells by using GenomicPrep cells and tissue DNA isolation kit (GE Healthcare, Buckinghamshire, England) according to the manufacturer's instructions but modified by using 10 mg ml−1 lysozyme at 37°C for 1 h. Plasmid DNA was isolated using the QIAprep Spin miniprep kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions but modified for extraction from C. glutamicum cells by using 10 mg ml−1 lysozyme at 37°C for 1 h. PCR was performed using DNA thermal cycler 480 (PerkinElmer, MA). After the reaction mixture was heated at 96°C for 3 min, the PCR proceeded under 30 cycles of 15 s at 96°C, 30 s at 58°C, and 1 min 30 s at 68°C by using DNA polymerase KOD Plus (Toyobo, Osaka, Japan).
C. glutamicum cells were transformed by electroporation as described previously (49). E. coli cells were transformed by the CaCl2 procedure (39).
DNA sequencing was performed by the dideoxy chain termination method, as described previously (40), with an ABI Prism 3100 genetic analyzer (Applied Biosystems, Foster City, CA) by using the BigDye Terminator version 3.1 cycle sequencing kit (Applied Biosystems). DNA sequence data were analyzed with the Genetyx program (Software Development, Tokyo, Japan). Sequences were aligned and phylogenetically analyzed by the program CLUSTAL W (47) using the neighbor-joining method (38). The phylogenetic tree was displayed using the program TREEVIEW (28).
Plasmid construction.
The dcsT-overexpressing strain was obtained as follows. The region for the DcsT open reading frame was amplified by PCR from the C. glutamicum R chromosomal DNA by using a set of primers, 5′-GGAATTCCATGAGCACACCTGACATTAAC-3′ and 5′-TCCCCCGGGTTAAAGCATGATGCCAAAGA-3′, with EcoRI and SmaI restriction sites, respectively. The EcoRI-SmaI fragment of the PCR-amplified product was inserted into the corresponding sites of E. coli-Corynebacterium shuttle vector pCRB1 (25), yielding pCRC300 for expression of dcsT under the control of lac promoter. This plasmid was introduced into C. glutamicum R by electroporation.
Quantitative reverse transcription-PCR (RT-PCR).
Total RNA was prepared from C. glutamicum cells by using the RNeasy minikit (Qiagen) according to the manufacturer's instructions. Single-stranded cDNA was synthesized from 0.2 μg of total RNA by using PrimeScript reverse transcriptase (Takara, Osaka, Japan) with hexadeoxyribonucleotide mixture as a primer in 20 μl reaction mixture, and then 2 μl of the cDNA mixture was added as a template in 18 μl of reaction mixture containing each primer (0.3 μM) and Power Sybr green PCR master mix (Applied Biosystems). After the reaction mixture was heated at 95°C for 10 min, PCRs proceeded via 40 cycles of 15 s at 95°C and 40 s at 60°C. The amount of amplified DNA was monitored by fluorescence at the end of each cycle by using the Applied Biosystems 7500 Fast real-time PCR system. Primers used for analyses of dcsT were 5′-TGCTGTCCTGGTGTTGTTCCT-3′ and 5′-ACCTGCGGTCAGTCCGATAC-3′. Primers for 16S rRNA were 5′-TCGATGCAACGCGAAGAAC-3′ and 5′-GAACCGACCACAAGGGAAAAC-3′. Specific amplification of the targeted DNA was confirmed by electrophoresis and sequencing of the PCR product. The relative abundances of the targeted mRNAs were quantified based on the cycle threshold value, which is defined as the number of cycles required in order to obtain a fluorescence signal above the background level. To standardize the results, the relative abundance of 16S rRNA was used as the internal standard.
RESULTS
Search for a single-gene-deficient mutant(s) incapable of growing on a C4-dicarboxylate-containing plate.
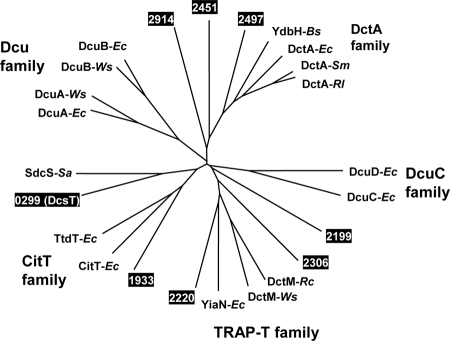
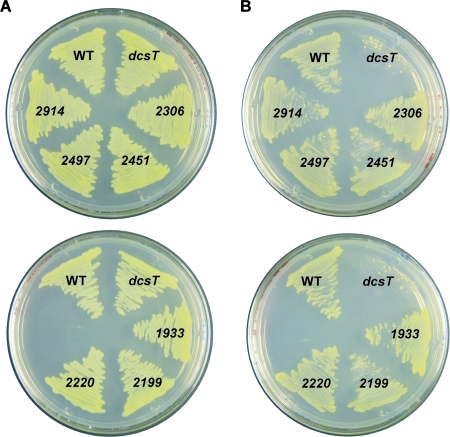
As summarized in Table 1, homology searches revealed that eight genes (cgR_0299, cgR_1933, cgR_2199, cgR_2220, cgR_2306, cgR_2451, cgR_2497, and cgR_2914) from the C. glutamicum R genome encode proteins exhibiting 20 to 50% amino acid sequence identity to the functionally characterized C4-dicarboxylate transporters classified into the DAACS family, the TRAP-T family, or the DASS family in other bacteria (5, 13, 20). Seven of the eight genes also exist in the genome of C. glutamicum ATCC 13032, but cgR_2306 is specific to strain R (Table 1). Figure 1 shows the results of a phylogenetic analysis of the bacterial C4-dicarboxylate transporter families. The protein CgR_2497 exhibited approximately 50% amino acid sequence identity to DctA family proteins involved in aerobic growth on C4 dicarboxylates in various bacteria. Although the other seven C. glutamicum proteins exhibited relatively low sequence similarity to previously identified transporters, they could be classified into three families based on the transporter classification system (Table 1) (5, 52). Mutant strains deficient in each of the eight transporter homologues were obtained from the mutant library previously constructed by transposon-based insertion of a selection marker (46). Figure 2 shows growth of these mutant strains on an agar plate with either glucose or succinate as the sole carbon source. The strain deficient in cgR_0299 (the gene was designated dcsT) barely grew on the succinate-containing plate, in contrast to the other mutant strains and the wild-type strain. All strains, including the dcsT strain, grew equally well on glucose-containing plates. Growth of all strains on fumarate- or malate-containing plates resembled that on the succinate-containing plate (data not shown).
TABLE 1.
C4-dicarboxylate transporter homologues in C. glutamicum
| C. glutamicum R protein family and protein | C. glutamicum ATCC 13032 homologuea |
|---|---|
| DAACS family | |
| CgR_2451 | NCgl2463 |
| CgR_2497 | NCgl2506 |
| CgR_2914 | NCgl2924 |
| DASS family | |
| CgR_0299 (DcsT) | NCgl0222 |
| CgR_1933 | NCgl1968 |
| TRAP-T family | |
| CgR_2199 | NCgl2236 |
| CgR_2220 | NCgl2256 |
| CgR_2306 |
The putative transporter proteins were classified according to the transporter classification system (52).
FIG. 1.
Unrooted phylogenetic tree showing the relationship between bacterial C4-dicarboxylate transporter families and the homologues of C. glutamicum. The proteins were DctA, DcuA, DcuB, DcuC, DcuD, YiaN, CitT, and TtdT from Escherichia coli (Ec) (7, 19, 34, 43, 58); YdbH from Bacillus subtilis (Bs) (1); DctA from Rhizobium leguminosarum (Rl) (36); DctA from Sinorhizobium meliloti (Sm) (10); DcuA, DcuB, and DctM from Wolinella succinogenes (Ws) (48); SdcS from Staphylococcus aureus (Sa) (13); DctM from Rhodobacter capsulatus (Rc) (11); and the putative transporter proteins in C. glutamicum R encoded by cgR_0299 (designated dcsT), cgR_1933, cgR_2199, cgR_2220, cgR_2306, cgR_2451, cgR_2497, and cgR_2914 (shown by their last four numbers). The amino acid sequences were aligned using the CLUSTAL W program (47), and the tree was constructed using the neighbor-joining method (38).
FIG. 2.
Growth of C. glutamicum cells on a plate with glucose or succinate as the sole carbon source. The C. glutamicum R wild-type strain (WT) and mutant strains deficient in a C4-dicarboxylate transporter gene homologue (cgR_0299 [designated dcsT], cgR_1933, cgR_2199, cgR_2220, cgR_2306, cgR_2451, cgR_2497, or cgR_2914 [shown by their last four numbers]) were streaked on BT minimal medium plates supplemented with glucose (A) or succinate (B) and incubated at 33°C for 1 day or 6 days, respectively. For controls, wild-type and dcsT-deficient strains were grown on all the plates.
Effects of disruption or overexpression of dcsT on C4-dicarboxylate utilization.
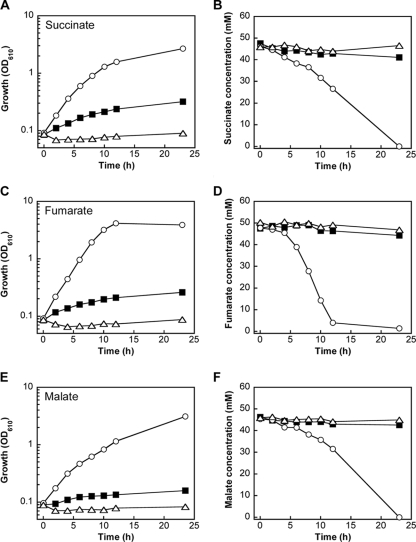
A plasmid carrying the dcsT gene under the control of lac promoter was introduced into C. glutamicum R wild-type strain. The dcsT-overexpressing strain was cultured in liquid medium using succinate as the sole carbon source, and its growth was compared to those of the dcsT-deficient strain and the wild-type strain carrying a vector plasmid without dcsT. The wild-type strain with the vector plasmid grew slowly during the 23-h culture, whereas for the deficient mutant, growth was completely suppressed by disruption of dcsT (Fig. 3A). On the other hand, the dcsT-overexpressing strain grew much better than the wild-type strain with the vector plasmid. The concentration of succinate in the medium decreased, accompanied by the growth of the dcsT-overexpressing strain, and succinate was fully consumed by the strain within 23 h (Fig. 3B). Similar results were observed when these strains were cultured in medium with fumarate or malate as the sole carbon source (Fig. 3C to F). The dcsT-overexpressing strain apparently utilized fumarate better and utilized malate less than succinate. These results indicated that utilization of the C4 dicarboxylates was markedly enhanced by overexpression of dcsT and was completely suppressed by disruption of this gene. The dcsT-deficient strain grew as well on plates with glucose (Fig. 2A), lactate, acetate, or citrate, as the wild type (data not shown). These results indicated that the putative transporter encoded by dcsT was specifically involved in the utilization of C4 dicarboxylates, i.e., succinate, fumarate, and malate.
FIG. 3.
Utilization of C4 dicarboxylates for aerobic growth of C. glutamicum cells. C. glutamicum R wild-type strain containing a control vector plasmid (black squares), the dcsT-deficient strain (white triangles), and the dcsT-overexpressing strain (white circles) were aerobically grown on BT minimal medium supplemented with succinate (A and B), fumarate (C and D), or malate (E and F). The OD610 (A, C, and E) were monitored. The time courses of changes in the concentrations of succinate (B), fumarate (D), and malate (F) in the medium are shown. Similar results were obtained from two independent experiments, and the mean values are shown.
The concentrations of C4 dicarboxylates in the culture medium changed little during growth of the wild type with the vector plasmid, probably because of low densities of the cells with little ability for C4-dicarboxylate utilization (Fig. 3B, D, and F). Actually, the concentration of succinate in the medium suspending the cells at a higher density, at an OD610 of 3, significantly decreased during 4-h incubation, while the decrease was not observed for the dcsT-deficient strain (data not shown). Under these conditions, uptake of succinate was analyzed using [13C]succinate. GC-MS analysis of intracellular succinate showed that [13C]succinate was taken up by the wild-type cells and accumulated in the cells within 5 min (Fig. 4). Expectedly, the uptake of succinate was not detected in the dcsT-deficient strain, while it was markedly stimulated by overexpression of dcsT. A decrease in the concentration of the labeled succinate after the rapid accumulation in the cells of dcsT-overexpressing strain implied that the succinate taken up was metabolized in the cells.
FIG. 4.
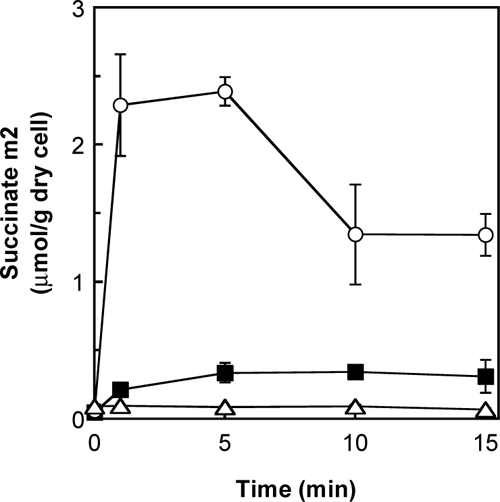
Succinate uptake analysis using C. glutamicum cell suspension. C. glutamicum cells were suspended at an OD610 of 3 in Tris-HCl buffer with [1,4-13C]succinate. The C. glutamicum R wild-type strain (black squares), the dcsT-deficient strain (white triangles), and the dcsT-overexpressing strain (white circles) were used. Intracellular succinate was analyzed by GC-MS, and the time course of changes in m2 isotopomer originating from the labeled succinate is shown. Mean values from three independent experiments are shown with standard errors (error bars).
Effects of succinate and glucose on the growth-phase-dependent expression of dcsT.
Expression of the dcsT gene in C. glutamicum cells cultured in nutrient-rich medium, which allowed the cells to grow well in the presence or absence of succinate, was examined by quantitative RT-PCR. When the cells in the stationary phase were transferred to the fresh medium without supplementation of additional carbon sources, the level of dcsT mRNA markedly increased in the early exponential growth phase and then rapidly decreased from the mid-exponential phase to the stationary phase (Fig. 5A). In the presence of succinate, which slightly stimulated the cell growth, a similar growth-phase-dependent pattern of expression was observed, but the decrease in the mRNA level from the early exponential phase to the mid-exponential phase was relatively repressed (Fig. 5B). On the other hand, in the presence of glucose, the mRNA level in the early exponential phase was markedly repressed compared with the level in the absence of glucose (Fig. 5C).
FIG. 5.
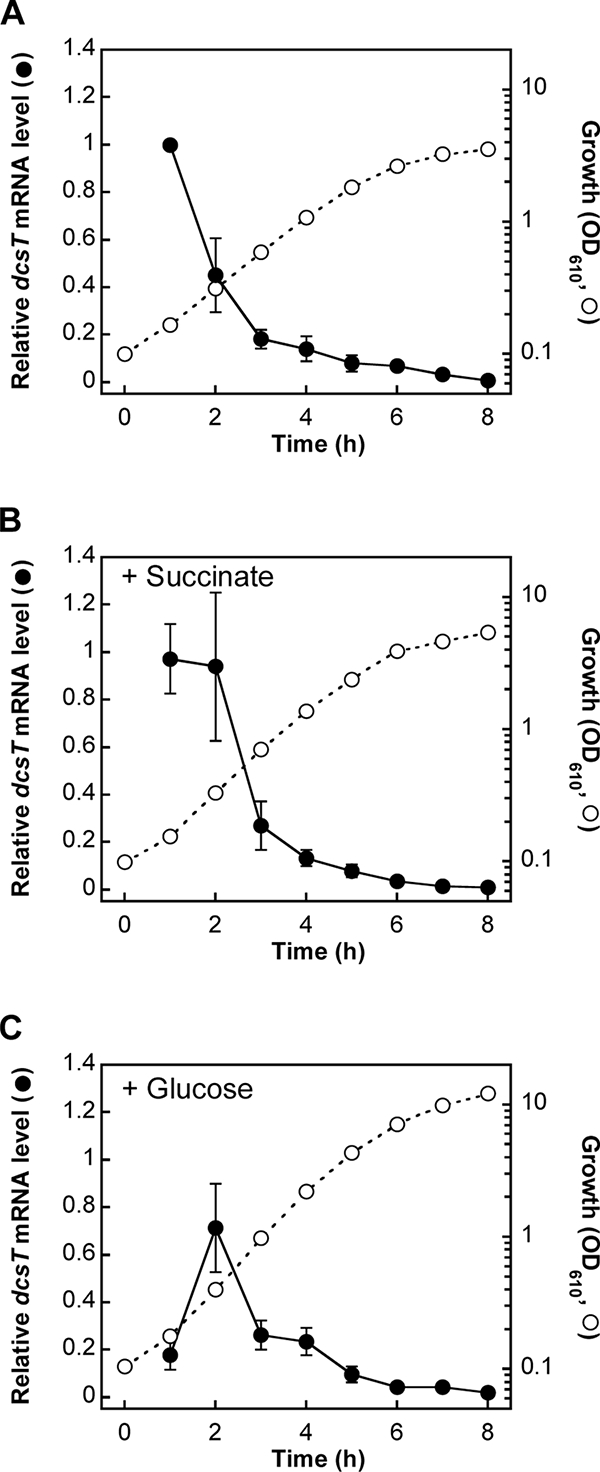
Effects of supplementation of succinate or glucose on dcsT expression during aerobic growth of C. glutamicum cells in nutrient-rich medium. C. glutamicum cells were aerobically grown on nutrient-rich A medium (A) or on the nutrient-rich medium supplemented with succinate (B) or with glucose (C). The levels of dcsT mRNA in the cells were determined by quantitative RT-PCR, and all the levels for the three different cultures are presented relative to the value for the 1-h culture on A medium without additional carbon sources. The OD610s are also shown. The values represent the means and standard errors (error bars) from four independent experiments.
DISCUSSION
In this study, we showed that disruption of dcsT, one of the eight C4-dicarboxylate transporter gene homologues in C. glutamicum, completely suppressed the aerobic growth on the C4 dicarboxylates succinate, fumarate, and malate as sole carbon sources. On the other hand, overexpression of this gene enhanced the utilization of these dicarboxylates markedly. The uptake of [13C]succinate observed for C. glutamicum cells was eliminated by disruption of the dcsT gene, while it was markedly stimulated by overexpression of dcsT. These results indicated that utilization of C4 dicarboxylates for growth was mediated mainly by the transporter encoded by dcsT. This gene was predicted to encode a protein of 528 amino acid residues and a molecular mass of 55.9 kDa. The DcsT protein showed significant similarity to Na+-coupled dicarboxylate transporters belonging to the DASS family (5, 6, 30, 31). Functionally characterized proteins within the DASS family transport organic di- and tricarboxylates as well as dicarboxylate amino acids, inorganic sulfate, and phosphate. These proteins are widespread among all three domains of life. However, within the DASS family, only a few prokaryotic proteins, SdcS (13, 14), CitT (34), and TtdT (22), have been functionally characterized. S. aureus SdcS was characterized as a Na+/dicarboxylate symporter (13, 14), while CitT is involved in citrate/succinate antiport for anaerobic growth by citrate fermentation (34). E. coli TtdT, homologous to CitT, is involved in the utilization of tartrate instead of citrate under anaerobic conditions (22). The observed similarity of C. glutamicum DcsT to SdcS (39% amino acid identity) was greater than that of DcsT to CitT (25%) or TtdT (22%). DcsT also shares approximately 30% identity to Na+/dicarboxylate symporters NaDC-1 and NaDC-3 in mammals (29, 32, 33, 50). It has been reported that uptake of succinate by C. glutamicum cells is dependent on Na+ (8). These findings may suggest that the DcsT protein functions in the uptake of C4 dicarboxylates coupled with Na+ in C. glutamicum. Uptake of C4 dicarboxylates by S. aureus SdcS in E. coli cells expressing the protein (13) and also in the partially purified protein reconstituted into proteoliposome prepared from E. coli cells was characterized (14). However, a physiological function of this transporter in S. aureus has not been reported. Our genetic analyses of dcsT in C. glutamicum revealed the involvement of the encoded DASS family protein in the utilization of C4 dicarboxylates for aerobic bacterial growth. Homology searches revealed that DcsT showed more similarity to proteins with unknown function predicted from genomic sequences of various bacterial species, Helicobacter pylori 26695 (HP_0214), Campylobacter fetus subsp. fetus 82-40 (CFF8240_0346), and Oceanobacillus iheyensis HTE831 (OB2540), than to S. aureus SdcS. Uptake systems for C4 dicarboxylates via this type of transporter may be widely distributed in bacteria.
It has been reported that uptake of C4 dicarboxylates under aerobic conditions is mediated by a member of DctA family in various bacteria, e.g., E. coli (7), B. subtilis (1), and some rhizobial species (10, 36, 37, 51, 55, 57). According to the transporter classification system based on transport mechanism and molecular phylogeny, DctA proteins are classified into the DAACS family. They show low similarity (about 20% amino acid identity) to C. glutamicum DcsT, which is classified into the DASS family. The uptake of C4 dicarboxylates by bacterial cells, mainly using the DctA protein, is dependent on H+ potential across the membrane (3, 12, 20), while the uptake of succinate is dependent on Na+ in C. glutamicum (8). It is consistent with the notion that a C4-dicarboxylate transporter for aerobic growth in C. glutamicum is different from the DctA family transporters. It should be noted that C. glutamicum has a gene (cgR_2497) encoding a protein grouped into the same cluster of the DctA family proteins with relatively high similarity (Fig. 1). However, a cgR_2497-deficient strain grew on succinate (Fig. 2), fumarate, and malate (data not shown) as well as the wild-type strain. It is noteworthy that the cgR_2497 gene retained in the dcsT-deficient strain did not complement utilization of C4 dicarboxylates for aerobic growth (Fig. 2 and Fig. 3). Therefore, the DctA-like cgR_2497 protein may be involved in the transport for the substrate(s) other than C4 dicarboxylates and may play a physiological role different from that associated with DcsT.
In this study, we showed that expression of the dcsT gene was dependent on the growth phase. When cells were cultured in nutrient-rich medium, dcsT expression was induced in the early exponential growth phase, while it was markedly repressed from the mid-exponential phase to the stationary phase. It is in contrast to expression of the E. coli dctA gene, whose expression was reported to be enhanced in the stationary phase, which was ascribed to the common feature of the genes subjected to cyclic AMP receptor protein-dependent catabolite repression (7). We observed that dcsT expression was enhanced in the presence of succinate in the mid-exponential phase, while the expression was repressed by glucose in the early exponential phase. However, the response to succinate appeared to be small, and this may correspond to the weak ability of C. glutamicum to utilize C4 dicarboxylates. In this context, it should be noted that the wild-type strain grew very slowly on C4 dicarboxylates as sole carbon sources, but overexpression of dcsT markedly enhanced the utilization of C4 dicarboxylates.
C. glutamicum excretes succinate in addition to lactate under conditions of oxygen deprivation (17). We observed that disruption of dcsT did not affect the succinate excretion under conditions of oxygen deprivation (data not shown), suggesting that transporters other than DcsT are involved in the different modes of C4-dicarboxylate transport for distinct physiological functions under different environmental conditions in C. glutamicum. In E. coli, DcuA, DcuB, and DcuC seem to be involved in the multiple modes of C4-dicarboxylate transport, i.e., uptake, exchange, and excretion, under anaerobic conditions, while uptake of C4 dicarboxylates for aerobic growth is mediated mainly by DctA (9, 20, 43, 58). However, homologues to DcuA, DcuB, and DcuC proteins were not found in the C. glutamicum genome, suggesting that a different type of transporter is involved in C4-dicarboxylate transport under conditions of oxygen deprivation in C. glutamicum. Identification of more transporters will be needed to understand the mechanism for the distinct modes of C4-dicarboxylate transport in this industrially important microorganism.
Acknowledgments
We thank Crispinus A. Omumasaba (RITE) for critical reading of the manuscript.
This work was financially supported in part by the New Energy and Industrial Technology Development Organization (NEDO), Japan.
Footnotes
Published ahead of print on 27 June 2008.
REFERENCES
- 1.Asai, K., S. H. Baik, Y. Kasahara, S. Moriya, and N. Ogasawara. 2000. Regulation of the transport system for C4-dicarboxylic acids in Bacillus subtilis. Microbiology 146:263-271. [DOI] [PubMed] [Google Scholar]
- 2.Baker, K. E., K. P. Ditullio, J. Neuhard, and R. A. Kelln. 1996. Utilization of orotate as a pyrimidine source by Salmonella typhimurium and Escherichia coli requires the dicarboxylate transport protein encoded by dctA. J. Bacteriol. 178:7099-7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bisschop, A., H. Doddema, and W. N. Konings. 1975. Dicarboxylic acid transport in membrane vesicles from Bacillus subtilis. J. Bacteriol. 124:613-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolten, C. J., P. Kiefer, F. Letisse, J. C. Portais, and C. Wittmann. 2007. Sampling for metabolome analysis of microorganisms. Anal. Chem. 79:3843-3849. [DOI] [PubMed] [Google Scholar]
- 5.Busch, W., and M. H. Saier, Jr. 2004. The IUBMB-endorsed transporter classification system. Mol. Biotechnol. 27:253-262. [DOI] [PubMed] [Google Scholar]
- 6.Busch, W., and M. H. Saier, Jr. 2002. The transporter classification (TC) system, 2002. Crit. Rev. Biochem. Mol. Biol. 37:287-337. [DOI] [PubMed] [Google Scholar]
- 7.Davies, S. J., P. Golby, D. Omrani, S. A. Broad, V. L. Harrington, J. R. Guest, D. J. Kelly, and S. C. Andrews. 1999. Inactivation and regulation of the aerobic C4-dicarboxylate transport (dctA) gene of Escherichia coli. J. Bacteriol. 181:5624-5635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ebbighausen, H., B. Weil, and R. Krämer. 1991. Na+-dependent succinate uptake in Corynebacterium glutamicum. FEMS Microbiol. Lett. 77:61-65. [DOI] [PubMed] [Google Scholar]
- 9.Engel, P., R. Krämer, and G. Unden. 1994. Transport of C4-dicarboxylates by anaerobically grown Escherichia coli. Energetics and mechanism of exchange, uptake and efflux. Eur. J. Biochem. 222:605-614. [DOI] [PubMed] [Google Scholar]
- 10.Engelke, T., D. Jording, D. Kapp, and A. Pühler. 1989. Identification and sequence analysis of the Rhizobium meliloti dctA gene encoding the C4-dicarboxylate carrier. J. Bacteriol. 171:5551-5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forward, J. A., M. C. Behrendt, N. R. Wyborn, R. Cross, and D. J. Kelly. 1997. TRAP transporters: a new family of periplasmic solute transport systems encoded by the dctPQM genes of Rhodobacter capsulatus and by homologs in diverse gram-negative bacteria. J. Bacteriol. 179:5482-5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gutowski, S. J., and H. Rosenberg. 1975. Succinate uptake and related proton movements in Escherichia coli K12. Biochem. J. 152:647-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall, J. A., and A. M. Pajor. 2005. Functional characterization of a Na+-coupled dicarboxylate carrier protein from Staphylococcus aureus. J. Bacteriol. 187:5189-5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall, J. A., and A. M. Pajor. 2007. Functional reconstitution of SdcS, a Na+-coupled dicarboxylate carrier protein from Staphylococcus aureus. J. Bacteriol. 189:880-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ikeda, M., and S. Nakagawa. 2003. The Corynebacterium glutamicum genome: features and impacts on biotechnological processes. Appl. Microbiol. Biotechnol. 62:99-109. [DOI] [PubMed] [Google Scholar]
- 16.Inui, M., H. Kawaguchi, S. Murakami, A. A. Vertès, and H. Yukawa. 2004. Metabolic engineering of Corynebacterium glutamicum for fuel ethanol production under oxygen-deprivation conditions. J. Mol. Microbiol. Biotechnol. 8:243-254. [DOI] [PubMed] [Google Scholar]
- 17.Inui, M., S. Murakami, S. Okino, H. Kawaguchi, A. A. Vertès, and H. Yukawa. 2004. Metabolic analysis of Corynebacterium glutamicum during lactate and succinate productions under oxygen deprivation conditions. J. Mol. Microbiol. Biotechnol. 7:182-196. [DOI] [PubMed] [Google Scholar]
- 18.Inui, M., M. Suda, S. Okino, H. Nonaka, L. G. Puskás, A. A. Vertès, and H. Yukawa. 2007. Transcriptional profiling of Corynebacterium glutamicum metabolism during organic acid production under oxygen deprivation conditions. Microbiology 153:2491-2504. [DOI] [PubMed] [Google Scholar]
- 19.Janausch, I. G., and G. Unden. 1999. The dcuD (former yhcL) gene product of Escherichia coli as a member of the DcuC family of C4-dicarboxylate carriers: lack of evident expression. Arch. Microbiol. 172:219-226. [DOI] [PubMed] [Google Scholar]
- 20.Janausch, I. G., E. Zientz, Q. H. Tran, A. Kröger, and G. Unden. 2002. C4-dicarboxylate carriers and sensors in bacteria. Biochim. Biophys. Acta 1553:39-56. [DOI] [PubMed] [Google Scholar]
- 21.Kalinowski, J., B. Bathe, D. Bartels, N. Bischoff, M. Bott, A. Burkovski, N. Dusch, L. Eggeling, B. J. Eikmanns, L. Gaigalat, A. Goesmann, M. Hartmann, K. Huthmacher, R. Krämer, B. Linke, A. C. McHardy, F. Meyer, B. Möckel, W. Pfefferle, A. Pühler, D. A. Rey, C. Rückert, O. Rupp, H. Sahm, V. F. Wendisch, I. Wiegräbe, and A. Tauch. 2003. The complete Corynebacterium glutamicum ATCC 13032 genome sequence and its impact on the production of l-aspartate-derived amino acids and vitamins. J. Biotechnol. 104:5-25. [DOI] [PubMed] [Google Scholar]
- 22.Kim, O. B., and G. Unden. 2007. The l-tartrate/succinate antiporter TtdT (YgjE) of l-tartrate fermentation in Escherichia coli. J. Bacteriol. 189:1597-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kinoshita, S., S. Udaka, and M. Shimonô. 1957. Studies on the amino acid fermentation. Part 1. Production of l-glutamic acid by various microorganisms. J. Gen. Appl. Microbiol. 3:193-205. [PubMed] [Google Scholar]
- 24.Liebl, W. 2005. Corynebacterium taxonomy, p. 9-34. In L. Eggeling and M. Bott (ed.), Handbook of Corynebacterium glutamicum. CRC Press, Boca Raton, FL.
- 25.Nakata, K., M. Inui, P. B. Kos, A. A. Vertès, and H. Yukawa. 2003. Vectors for the genetics engineering of corynebacteria, p. 175-191. In B. C. Saha (ed.), Fermentation biotechnology. American Chemical Society, Washington, DC.
- 26.Okino, S., M. Inui, and H. Yukawa. 2005. Production of organic acids by Corynebacterium glutamicum under oxygen deprivation. Appl. Microbiol. Biotechnol. 68:475-480. [DOI] [PubMed] [Google Scholar]
- 27.Okino, S., M. Suda, K. Fujikura, M. Inui, and H. Yukawa. 2008. Production of d-lactic acid by Corynebacterium glutamicum under oxygen deprivation. Appl. Microbiol. Biotechnol. 78:449-454. [DOI] [PubMed] [Google Scholar]
- 28.Page, R. D. 1996. TreeView: an application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 12:357-358. [DOI] [PubMed] [Google Scholar]
- 29.Pajor, A. M. 1996. Molecular cloning and functional expression of a sodium-dicarboxylate cotransporter from human kidney. Am. J. Physiol. 270:F642-F648. [DOI] [PubMed] [Google Scholar]
- 30.Pajor, A. M. 2000. Molecular properties of sodium/dicarboxylate cotransporters. J. Membr. Biol. 175:1-8. [DOI] [PubMed] [Google Scholar]
- 31.Pajor, A. M. 1999. Sodium-coupled transporters for Krebs cycle intermediates. Annu. Rev. Physiol. 61:663-682. [DOI] [PubMed] [Google Scholar]
- 32.Pajor, A. M., R. Gangula, and X. Yao. 2001. Cloning and functional characterization of a high-affinity Na+/dicarboxylate cotransporter from mouse brain. Am. J. Physiol. Cell Physiol. 280:C1215-C1223. [DOI] [PubMed] [Google Scholar]
- 33.Pajor, A. M., and N. N. Sun. 2000. Molecular cloning, chromosomal organization, and functional characterization of a sodium-dicarboxylate cotransporter from mouse kidney. Am. J. Physiol. Renal Physiol. 279:F482-F490. [DOI] [PubMed] [Google Scholar]
- 34.Pos, K. M., P. Dimroth, and M. Bott. 1998. The Escherichia coli citrate carrier CitT: a member of a novel eubacterial transporter family related to the 2-oxoglutarate/malate translocator from spinach chloroplasts. J. Bacteriol. 180:4160-4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roessner, U., C. Wagner, J. Kopka, R. N. Trethewey, and L. Willmitzer. 2000. Simultaneous analysis of metabolites in potato tuber by gas chromatography-mass spectrometry. Plant J. 23:131-142. [DOI] [PubMed] [Google Scholar]
- 36.Ronson, C. W., P. M. Astwood, and J. A. Downie. 1984. Molecular cloning and genetic organization of C4-dicarboxylate transport genes from Rhizobium leguminosarum. J. Bacteriol. 160:903-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ronson, C. W., P. M. Astwood, B. T. Nixon, and F. M. Ausubel. 1987. Deduced products of C4-dicarboxylate transport regulatory genes of Rhizobium leguminosarum are homologous to nitrogen regulatory gene products. Nucleic Acids Res. 15:7921-7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 39.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 40.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shaw, J. G., M. J. Hamblin, and D. J. Kelly. 1991. Purification, characterization and nucleotide sequence of the periplasmic C4-dicarboxylate-binding protein (DctP) from Rhodobacter capsulatus. Mol. Microbiol. 5:3055-3062. [DOI] [PubMed] [Google Scholar]
- 42.Shirai, T., K. Fujimura, C. Furusawa, K. Nagahisa, S. Shioya, and H. Shimizu. 2007. Study on roles of anaplerotic pathways in glutamate overproduction of Corynebacterium glutamicum by metabolic flux analysis. Microb. Cell Fact. 6:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Six, S., S. C. Andrews, G. Unden, and J. R. Guest. 1994. Escherichia coli possesses two homologous anaerobic C4-dicarboxylate membrane transporters (DcuA and DcuB) distinct from the aerobic dicarboxylate transport system (Dct). J. Bacteriol. 176:6470-6478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soga, T., Y. Ueno, H. Naraoka, Y. Ohashi, M. Tomita, and T. Nishioka. 2002. Simultaneous determination of anionic intermediates for Bacillus subtilis metabolic pathways by capillary electrophoresis electrospray ionization mass spectrometry. Anal. Chem. 74:2233-2239. [DOI] [PubMed] [Google Scholar]
- 45.Strelkov, S., M. von Elstermann, and D. Schomburg. 2004. Comprehensive analysis of metabolites in Corynebacterium glutamicum by gas chromatography/mass spectrometry. Biol. Chem. 385:853-861. [DOI] [PubMed] [Google Scholar]
- 46.Suzuki, N., N. Okai, H. Nonaka, Y. Tsuge, M. Inui, and H. Yukawa. 2006. High-throughput transposon mutagenesis of Corynebacterium glutamicum and construction of a single-gene disruptant mutant library. Appl. Environ. Microbiol. 72:3750-3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ullmann, R., R. Gross, J. Simon, G. Unden, and A. Kröger. 2000. Transport of C4-dicarboxylates in Wolinella succinogenes. J. Bacteriol. 182:5757-5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vertès, A. A., M. Inui, M. Kobayashi, Y. Kurusu, and H. Yukawa. 1993. Presence of mrr- and mcr-like restriction systems in coryneform bacteria. Res. Microbiol. 144:181-185. [DOI] [PubMed] [Google Scholar]
- 50.Wang, H., Y. J. Fei, R. Kekuda, T. L. Yang-Feng, L. D. Devoe, F. H. Leibach, P. D. Prasad, and V. Ganapathy. 2000. Structure, function, and genomic organization of human Na+-dependent high-affinity dicarboxylate transporter. Am. J. Physiol. Cell Physiol. 278:C1019-C1030. [DOI] [PubMed] [Google Scholar]
- 51.Watson, R. J. 1990. Analysis of the C4-dicarboxylate transport genes of Rhizobium meliloti: nucleotide sequence and deduced products of dctA, dctB, and dctD. Mol. Plant-Microbe Interact. 3:174-181. [DOI] [PubMed] [Google Scholar]
- 52.Winnen, B., J. Felce, and M. H. Saier Jr. 2005. Genomic analyses of transporter proteins in Corynebacterium glutamicum and Corynebacterium efficiens, p. 149-186. In L. Eggeling and M. Bott (ed.), Handbook of Corynebacterium glutamicum. CRC Press, Boca Raton, FL.
- 53.Wittmann, C., J. O. Krömer, P. Kiefer, T. Binz, and E. Heinzle. 2004. Impact of the cold shock phenomenon on quantification of intracellular metabolites in bacteria. Anal. Biochem. 327:135-139. [DOI] [PubMed] [Google Scholar]
- 54.Yamada, K., and K. Komagata. 1972. Taxonomic studies on coryneform bacteria. IV. Morphological, cultural, biochemical, and physiological characteristics. J. Gen. Appl. Microbiol. 18:399-416. [Google Scholar]
- 55.Yarosh, O. K., T. C. Charles, and T. M. Finan. 1989. Analysis of C4-dicarboxylate transport genes in Rhizobium meliloti. Mol. Microbiol. 3:813-823. [DOI] [PubMed] [Google Scholar]
- 56.Yukawa, H., C. A. Omumasaba, H. Nonaka, P. Kós, N. Okai, N. Suzuki, M. Suda, Y. Tsuge, J. Watanabe, Y. Ikeda, A. A. Vertès, and M. Inui. 2007. Comparative analysis of the Corynebacterium glutamicum group and complete genome sequence of strain R. Microbiology 153:1042-1058. [DOI] [PubMed] [Google Scholar]
- 57.Yurgel, S. N., and M. L. Kahn. 2004. Dicarboxylate transport by rhizobia. FEMS Microbiol. Rev. 28:489-501. [DOI] [PubMed] [Google Scholar]
- 58.Zientz, E., I. G. Janausch, S. Six, and G. Unden. 1999. Functioning of DcuC as the C4-dicarboxylate carrier during glucose fermentation by Escherichia coli. J. Bacteriol. 181:3716-3720. [DOI] [PMC free article] [PubMed] [Google Scholar]