Abstract
CD163 is an acute-phase-regulated monocyte/macrophage membrane receptor expressed late in inflammation. It is involved in the haptoglobin-mediated removal of free hemoglobin from plasma, has been identified as a naturally soluble plasma glycoprotein with potential anti-inflammatory properties, and is possibly linked to an individual's haptoglobin phenotype. High levels of soluble CD163 (sCD163) in a malaria episode may therefore downregulate inflammation and curb disease severity. In order to verify this, the relationships between sCD163 levels, malaria severity, and selected inflammatory mediators (tumor necrosis factor alpha [TNF-α], interleukin-6 [IL-6], and IL-10) were assessed by enzyme-linked immunosorbent assay using plasma samples obtained from pediatric malaria patients with uncomplicated malaria (UM [n = 38]), cerebral malaria (CM [n = 52]), and severe malarial anemia (SA [n = 55]) during two consecutive malaria transmission seasons (2002 and 2003). Median sCD163 levels were higher in UM (11.9 μg/ml) patients than in SA (7.7 μg/ml; P = 0.010) and CM (8.0 μg/ml; P = 0.031) patients. Levels of sCD163 were also higher in all patient groups than in a group of 81 age-matched healthy controls. The higher sCD163/TNF-α ratio in UM patients, coupled with the fact that sCD163 levels correlated with TNF-α levels in UM patients but not in CM and SA patients, suggests inflammatory dysregulation in the complicated cases. The study showed that sCD163 levels are elevated during acute malaria. High sCD163 levels in UM patients may be due to the induction of higher-level anti-inflammatory responses, enabling them to avoid disease complications. It is also possible that UM patients simply lost their CD163 receptors from macrophages in inflammatory sites while complicated-malaria patients still had their receptors attached to activated macrophages, reflecting ongoing and higher-level inflammation associated with complicated malaria.
CD163 is a group B cysteine-rich scavenger membrane receptor that is expressed by cells of the monocytic lineage and is involved in the macrophage recognition and uptake of haptoglobin-hemoglobin (Hp-Hb) complexes (11). CD163 is also believed to bind free Hb, though less efficiently than the complex, after total Hp depletion during severe hemolytic episodes (20). Recent studies of rodents have also identified CD163 as an adhesion receptor for erythroblasts, suggesting a possible role for the membrane receptor (5).
CD163 can also be shed as a soluble plasma glycoprotein by mechanisms not yet completely understood, though TIMP3-sensitive metalloproteinases have been implicated in this shedding process (4, 15). Shedding has also recently been linked to oxidative stress that results from inflammation or from induction by 8-iso-prostaglandin F2α (22).
Both membrane-bound CD163 and soluble CD163 (sCD163) have been identified as promoters of anti-inflammation. The membrane-bound receptor indirectly contributes to anti-inflammation by regulating the expression of other inflammatory mediators. CD163 is, for example, known to upregulate the expression of interleukin-10 (IL-10) as well as heme oxygenase-1 (HO-1), both of which exert anti-inflammatory effects during the resolution phase of inflammation (14, 19, 24). Moreover, the expression of membrane-bound CD163 is tightly regulated by inflammatory mediators (9, 25). The soluble receptor is, however, known to have direct anti-inflammatory properties. sCD163 has been identified as an anti-inflammatory mediator that inhibits phorbol ester-induced human T-lymphocyte activation, resulting in the attenuation of the immune response to the phorbol ester (10).
Macrophages that express the CD163 receptor generally appear late in inflammation (7, 25); hence, CD163 shedding is expected to occur during the late phase of the inflammatory cascade. The soluble receptor will then contribute to downregulating proinflammatory cytokine release in order to restore a balance in the levels of pro- and anti-inflammatory mediators. During malaria infections, the balance between pro- and anti-inflammatory cytokines is important in defining the course of the disease, particularly between subjects with complicated malaria and those with uncomplicated malaria (UM) (1, 13, 18). Thus, patients with UM may be expected to have higher levels of sCD163 than patients with complicated malaria. This study examined the relationship between plasma levels of sCD163 and disease severity, as well as the plasma levels of tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), and IL-10 in Ghanaian pediatric malaria patients.
MATERIALS AND METHODS
Patients and sampling.
This study was part of an ongoing study conducted at the Department of Child Health of the Korle-Bu Teaching Hospital, Ghana, and the Noguchi Memorial Institute for Medical Research (NMIMR), Ghana. Plasma samples (frozen at −80°C) obtained from complicated malaria and UM patients between the ages of 0.5 and 12 years who reported for medical care at the hospital between 2002 and 2003 were used in this study. Blood samples were also taken from a group of healthy, age-matched controls recruited from schools in communities in the vicinity of the hospital. The blood samples had earlier been examined for malaria parasites and Hb levels. The inclusion criteria for malaria patients were as previously published (13), with some modifications: fever (>37.5°C), measured within 24 h of admission; asexual parasitemia of >2,500 parasites/μl; and at least one other sign of malaria, such as vomiting, diarrhea, or malaise. Also, patients had to be sickling negative and bacteremia negative and have no other disease. The subjects were divided into groups of patients with cerebral malaria (CM), severe malarial anemia (SA), and UM as follows: CM was defined by an unarousable coma (score of <3 on the Blantyre coma scale) and no other neurological manifestation; SA was defined by a blood hemoglobin concentration of <5 g/dl, full consciousness, and no other cause of anemia; and UM was defined as described for SA, except with the addition of an Hb concentration of >8 g/dl and no other complication of malaria. The study received ethical approval from the Institutional Review Board of the Noguchi Memorial Institute for Medical Research and the Ethical Committee of the University of Ghana Medical School.
Parasitological and hematological measurements.
The hematological parameters, including Hb levels, total white blood cell (WBC) counts, total red blood cell counts, and mean corpuscular volume, were measured with an automated hematological analyzer (model KX-21; Sysmex, Japan). Thick and thin blood films were stained with Giemsa stain and examined for malaria parasites, which were counted against 200 WBCs. Parasite densities were obtained by multiplying the total WBC counts by the number of parasites in 200 WBCs. Sickling tests were done using the sodium metabisulfite test, and sickling-positive patients were excluded from the study.
sCD163 measurement.
Immulon 2 microtiter plates (Thermo Labsystems, Franklin, MA) were coated with polyclonal rabbit anti-CD163 immunoglobulin G (BD Biosciences, San Jose, CA) at 2 μg/ml and incubated overnight at 4°C. The plates were then washed four times with phosphate-buffered saline (PBS)-Tween 20 (0.05%) and blocked with 150 μl/well of 3% bovine serum albumin in PBS, pH 7.2 to 7.4, for an hour. Recombinant human CD163 standards (BD Biosciences, San Jose, CA) diluted serially from 3,000 ng/ml to 1.37 ng/ml and plasma samples diluted 1:100 in 1% bovine serum albumin-PBS were added in duplicate at 100 μl/well and incubated for 90 min. After the wells were washed twice, 100 μl/well of 1 μg/ml mouse monoclonal anti-CD163 antibody (GHI/61) ([r]BD Biosciences, San Jose, CA) was added and incubated for 1 h. The plates were washed four times, and 100 μl/well of peroxidase-labeled goat anti-mouse immunoglobulin (Dako, Carpenteria, CA) was added to each plate and incubated for 1 h. Finally, the plates were washed five times, and 100 μl/well of substrate solution (0.4 mg/ml orthophenyldiamine in 0.025 M citrate, 0.05 M phosphate buffer, pH 5.0, 0.012% H2O2) was added. After 30 min of color development, 50 μl/well of stop solution (1 M H2SO4) was added and the optical density was read at 492 nm with a 620-nm reference wavelength using an enzyme-linked immunosorbent assay (ELISA) plate reader (Thermo Labsystems, Helsinki, Finland).
Cytokine measurements.
IL-6 and IL-10 were detected in plasma by ELISA according to manufacturers' protocols. Briefly, MaxiSorp microtiter plates (Nunc, Roskilde, Denmark) were coated with 4 μg/ml of the respective purified rat anti-human antibodies (BD Biosciences, San Jose, CA) at 50 μl/well and incubated overnight at 4°C. The plates were washed four times with PBS-Tween 20 (0.05%) and blocked with 150 μl/well of 5% heat-inactivated fetal bovine serum (FBS) in PBS for 1 h at room temperature. After the plates were washed twice, recombinant standards (threefold serial dilutions from 4,000 to 1.83 pg/ml; BD Biosciences, San Jose, CA) and undiluted serum samples were added at 50 μl/well and incubated on a shaker for 2 h at room temperature. After the plates were washed four times, 1 μg/ml biotinylated anti-human cytokine antibodies (BD Biosciences, San Jose, CA) in 5% heat-inactivated FBS in PBS were added at 50 μl/well and incubated at room temperature for 45 min. The plates were washed five times, and 0.5 μg/ml streptavidin-peroxidase (1/1,000 dilution of stock in 5% FBS in PBS; KPL, Gaithersburg, MD) was added at 50 μl/well and incubated for 30 min at room temperature. The plates were then washed eight times after the incubation period and developed with orthophenyldiamine substrate for 30 min, and then optical densities were read at 492/620 nm.
For the TNF-α ELISA, Immulon 2 microtiter plates (Thermo Labsystems, Franklin, MA) were used. Purified rat anti-human antibody (2 μg/ml; BD Biosciences, San Jose, CA) was used as the capture antibody, recombinant human TNF-α (twofold serial dilutions from 2,000 to 15 pg/ml; BD Biosciences, San Jose, CA) as the standard, and biotin-conjugated mouse anti-human TNF-α (1 μg/ml; BD Biosciences, San Jose, CA) as the detection antibody. Streptavidin-peroxidase (KPL, Gaithersburg, MD) was added at 0.125 μg/ml (1/4,000 dilution of the stock), and then the plates were washed 10 times before the substrate addition, color development, and optical density reading at 492/620 nm.
Patient management.
The treatment of malaria was carried out in accordance with the existing institutional guidelines at the time. All patients with UM were treated with chloroquine at a total dose of 25 mg/kg body weight, given as single daily doses over 3 days. In the case of treatment failure, the treatment was changed to amodiaquine (10 mg/kg body weight per day as single daily doses for 3 days). Patients with severe malaria were treated with either amodiaquine syrup via a nasogastric tube at the same dosage as stated or intramuscular quinine sulfate (10 mg/kg body weight every 8 hours). The treatment with intramuscular quinine was changed to syrup at the same dosage when patients regained full consciousness or after 72 h (whichever was earlier) to complete a 7-day course. In addition, patients with Hb levels of <5g/dl received blood transfusions.
Statistical analysis.
The Mann-Whitney U test and a one-way analysis of variance (SigmaStat 2.0; Jandel Scientific, San Rafael, CA) were used for all comparisons. Dot plots and line graphs displaying sCD163 levels were created with GraphPad Prism version 4.0 (GraphPad Software, San Diego, CA). Correlation and linear regression analyses were also performed with SPSS version 9.0 (SPSS, San Diego, CA). Differences with a P of <0.05 were considered statistically significant.
RESULTS
Characteristics of patient groups.
The objectives of this study were to compare the plasma levels of sCD163 in patients with UM with the levels in those with complicated pediatric malaria and to correlate sCD163 levels with the levels of the inflammatory mediators TNF-α, IL-6, and IL-10. A total of 52 patients with CM (median age, 4.0 years), 55 with SA (median age, 2.0 years), and 38 with UM (median age, 4.0 years) were included in this study. The median age of the patients in the SA category was significantly lower than those of the other disease categories (P < 0.001). Also, the levels of parasitemia were not statistically different (P = 0.251) among the three study groups (Table 1).
TABLE 1.
Characteristics of patients with UM and complicated malaria
| Disease | No. of patients | Hb concn (g/dl)a | Age (yr)a | Parasite density (103/μl)a |
|---|---|---|---|---|
| UM | 38 | 9.5 (2.3-4.9) | 4.0 (1.0-12.0) | 70.1 (3.74-4,340.2) |
| SA | 55 | 3.9 (8.1-12.2)b | 2.0 (0.5-9.0)b | 52.2 (2.75-567.3)c |
| CM | 52 | 7.6 (5.1-12.7)b | 4.0 (0.5-12.0)b | 98.1 (2.61-1,872.4)c |
Values are reported as medians (minimum to maximum values).
Values are significant at a P of <0.0001 compared to those for UM subjects (one-way analysis of variance).
Values are not significant at a P of 0.251 compared to those for UM subjects (one-way analysis of variance).
sCD163 levels in malaria patients and controls.
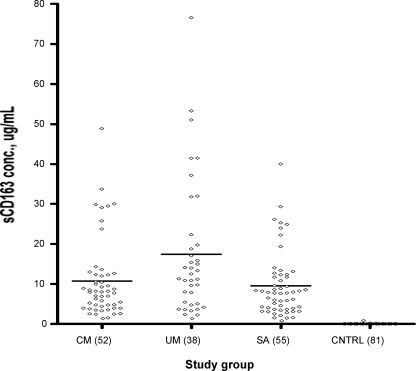
The plasma levels of sCD163 were determined by sandwich ELISA, and Fig. 1 shows the median levels of sCD163 among the three patient categories. The median level of sCD163 was significantly higher in UM patients (11.9 μg/ml; range, 1.5 to 76.6 μg/ml) than they were in CM patients (8.0 μg/ml; range, 1.4 to 48.9 μg/ml; P = 0.031) and SA patients (7.7 μg/ml; range, 0.94 to 40.0 μg/ml; P = 0.01). Compared with patients in the combined SA and CM groups designated the complicated-malaria group, patients with UM still showed significantly higher sCD163 levels (P = 0.007). Levels were, however, not significantly different between patients with SA and CM. When the median sCD163 level of a group of 81 age-matched healthy controls (0.03 μg/ml; range, 0.01 to 0.82 μg/ml) was compared with that of each of the three disease categories, the level was significantly lower (P < 0.0001) in the healthy control group in all cases.
FIG. 1.
Levels of sCD163 in the three patient categories and healthy controls. The levels were determined by sandwich ELISA for 38 patients with UM, 52 with CM, and 55 with SA, alongside 81 healthy controls (CNTRL). The horizontal lines represent the medians of the distributions.
To determine the possible changes in the levels of sCD163 during recovery from malaria, three patients designated A, B, and C whose plasma samples for day 3 and day 14 were available were analyzed for their sCD163 levels. For patient A, the sCD163 level decreased from 7.0 μg/ml on day 0 to 2.5 μg/ml by day 3 and then decreased further to 1.6 μg/ml on day 14. For patient B, the level decreased from 8.6 μg/ml on day 0 to 3.5 μg/ml by day 3 but increased to 4.5 μg/ml on day 14. Similarly, for patient C, the level decreased from 3.8 μg/ml on day 0 to 1.5 μg/ml on day 3 but increased to 3.0 μg/ml on day 14.
sCD163 and disease indicators in patients.
The plasma concentrations of cytokines were compared with sCD163 levels, since CD163 expression is regulated by cytokines. A significant positive correlation was observed between the levels of sCD163 and TNF-α (r = 0.316; P < 0.0001) for all disease categories combined. When correlation analyses were done by disease categories, however, sCD163 levels correlated significantly with TNF-α levels in UM patients (r = 0.426; P < 0.0038) but not those in SA (r = 0.116; P = 0.199) or CM (r = 0.178; P = 0.109) patients (Fig. 2). A linear regression analysis using combined data from all cases showed an association of high levels of sCD163 with serum levels of TNF-α (P < 0.001). Indeed, a unit increase in plasma TNF-α levels corresponded to a 47-fold increase in plasma sCD163 levels. There were no correlations or associations between sCD163 levels and those of IL-6 and IL-10 (data not shown).
FIG. 2.
Correlation between plasma sCD163 levels and TNF levels in CM patients (A), SA patients (B), and UM patients (C). The P values were obtained after a Pearson product correlation test.
Since disease outcome to some extent depends on the balance in the levels of pro- and anti-inflammatory mediators, the ratios of sCD163 to the measured cytokines were compared between disease categories by the Mann-Whitney U test. Median sCD163/TNF-α ratios were significantly higher in UM patients (0.37; range, 0.02 to 3.38) than in SA patients (0.23; range, 0.01 to 2.67; P = 0.014) but not in CM patients (0.32; range, 0.03 to 2.15; P = 0.132). The median ratios between CM and SA patients were not statistically different (P = 0.437). No such associations were found for ratios between sCD163 and IL-6 or IL-10 (data not shown).
Correlation analysis between sCD163 and other clinical parameters showed a positive correlation (r = 0.213; P = 0.005) with Hb levels but not with parasitemia or age.
DISCUSSION
The results of this study reveal an elevation in sCD163 levels in the plasma of both complicated malaria and UM cases compared to the levels in healthy Ghanaian children. The elevation of sCD163 levels in patients with diseases like rheumatoid arthritis and pneumonia has been described previously (15, 16). The significantly higher levels of sCD163 in UM cases compared to complicated-malaria cases may suggest a protective role against malaria for the soluble form of the receptor protein. The differences in sCD163 levels between UM and complicated-malaria cases may be explained by one of two phenomena: either higher anti-inflammatory responses were induced in UM patients, enabling them to avoid disease complications, or UM patients simply lost their CD163 receptors from macrophages in inflammatory sites, while the complicated-malaria patients still had their receptors attached to activated macrophages, reflecting an ongoing and higher level of inflammation associated with complicated malaria.
In our study, sCD163 levels were generally high during acute infection but fell gradually during recovery. This is in agreement with the general observation that sCD163 levels are usually increased under the inflammatory conditions brought about by inflammation but fall gradually during recovery from inflammation and, in this study, after parasite clearance. The levels of sCD163 for patient A decreased further on day 14, but patients B and C recorded slight increases on the same day. These differences in the variation patterns of sCD163 levels 14 days after treatment commenced may reflect a homeostatic adjustment in order to return to normal levels after recovery. These observations are nevertheless inconclusive, and the elucidation of this finding would require a longitudinal study with a larger sample size.
There was a positive significant correlation between plasma levels of sCD163 and those of TNF for UM patients but not for SA and CM patients, as shown in Fig. 2. This may suggest an attempt to counterbalance the effects of the high levels of the proinflammatory TNF by the anti-inflammatory sCD163 in UM patients, a situation which is absent in the severe-malaria (SA and CM) patients. Thus, in the complicated cases (SA and CM), there is a dysregulation of inflammatory mediators.
Dysregulation of inflammatory mediators has been associated with disease severity in malaria (1) and other inflammatory disease states (12). sCD163/TNF-α ratios were also higher in UM patients than in CM and SA patients, suggesting the presence of relatively higher levels of the proinflammatory cytokine TNF-α, especially in SA patients. This also lends support to the reasoning that sCD163 may play an important anti-inflammatory and/or immunoregulatory role in malaria pathology (6, 8). Thus, it may be hypothesized that UM patients are better able to regulate inflammation due to proinflammatory cytokines such as TNF-α by suppressing their production later during infection. That the anti-inflammatory properties of sCD163 may be crucial in protecting against complicated malaria and other inflammation-mediated conditions is also supported by the fact that the soluble receptor occurs in microgram quantities, unlike with many other inflammatory mediators, whose plasma levels are generally much lower.
No associations were observed between sCD163 levels and those of IL-6 or IL-10, though previous studies have reported that CD163 expression on monocytes/macrophages is induced by IL-6 and IL-10 (3, 21). Thus, it may be deduced that IL-6 and IL-10 have a bearing only on the membrane expression and not on the process of sCD163 shedding. The single-point measurements done in this study, however, make such an argument inconclusive. These findings notwithstanding, it may be important to further investigate a possible role for TNF-α in the expression and/or shedding of CD163. Indeed, TNF-α is known to induce the hepatic synthesis of Hp (2), an acute-phase glycoprotein that regulates CD163 expression on monocytes/macrophages. Studies have shown that macrophages are heterogeneous in nature, and while one population is capable of inducing the production of inflammatory mediators such as TNF-α, another population of macrophages induces the production of anti-inflammatory mediators such as sCD163 (23). It is therefore not unusual to have high levels of both mediators in a disease situation, as our data show.
Levels of sCD163 also correlated positively with Hb levels but not with parasitemia or age. Membrane-bound CD163 has been recently identified as an important receptor in erythropoiesis in rodents (5), and since the levels of sCD163 are a reflection of the expression level of the membrane-bound form (17), it may be predictably argued that the severe malaria patients (SA and CM patients) who had relatively low levels of sCD163 would also have low Hb levels due to some impairment or a decreased rate of their erythropoietic process. There is, however, the possibility that this erythropoietic function of CD163 may not be applicable to humans.
In conclusion, sCD163 levels are generally increased during a malaria infection. There are, however, highly elevated levels of sCD163 in the plasma of UM patients relative to levels in complicated-malaria patients, and these high levels may confer relative protection against complicated malaria by regulating inflammation. Additionally, the correlation of sCD163 levels with levels of TNF-α, a key inflammatory mediator in the pathogenesis of malaria, taken together with the timely production of sCD163 late during the inflammatory cascade, suggests a key role for sCD163 in the complex network of inflammatory mediation and/or regulation of the pathogenesis of clinical malaria. sCD163 may therefore be a potential candidate for anti-inflammatory therapy not only for malaria but also for other inflammation-mediated diseases and conditions.
Acknowledgments
This study received financial support from a WHO/TDR/MIM Re-Entry grant (A-10938).
The CD163 standard protein was gracefully provided by Soren K. Moestrup of the Department of Medical Biochemistry, University of Aarhus, Denmark. The members of staff of Immunology Department, NMIMR, are thanked for their excellent technical assistance.
Footnotes
Published ahead of print on 16 July 2008.
REFERENCES
- 1.Akanmori, B. D., J. A. L. Kurtzhals, B. Q. Goka, V. Adabayeri, M. F. Ofori, F. K. Nkrumah, C. Behr, and L. Hviid. 2000. Distinct patterns of cytokine regulation in discrete clinical forms of Plasmodium falciparum malaria. Eur. Cytokine Netw. 11:113-118. [PubMed] [Google Scholar]
- 2.Bowman, B. H. 1993. Haptoglobin, p. 157-167. In B. H. Bowman (ed.), Hepatic plasma proteins. Academic Press, San Diego, CA.
- 3.Buechler, C., M. Ritter, E. Orso, T. Langmann, J. Klucken, and G. Schmitz. 2000. Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and anti-inflammatory stimuli. J. Leukoc. Biol. 67:97-103. [PubMed] [Google Scholar]
- 4.Droste, A., C. Sorg, and P. Hogger. 1999. Shedding of CD163, a novel regulatory mechanism for a member of the scavenger receptor cysteine-rich family. Biochem. Biophys. Res. Commun. 256:110-113. [DOI] [PubMed] [Google Scholar]
- 5.Fabriek, B. O., M. M. Polfliet, R. P. Vloet, R. C. van der Schors, A. J. Ligtenberg, L. K. Weaver, C. Geest, K. Matsuno, S. K. Moestrup, C. D. Dijkstra, and T. K. van den Berg. 2007. The macrophage CD163 surface glycoprotein is an erythroblast adhesion receptor. Blood 109:5223-5229. [DOI] [PubMed] [Google Scholar]
- 6.Frings, W., J. Dreier, and C. Sorg. 2002. Only the soluble form of the scavenger receptor CD163 acts inhibitory on phorbol ester-activated T-lymphocytes, whereas membrane-bound protein has no effect. FEBS Lett. 526:93-96. [DOI] [PubMed] [Google Scholar]
- 7.Goerdt, S., R. Bhardwaj, and C. Sorg. 1993. Inducible expression of MS-1 high-molecular-weight protein by endothelial cells of continuous origin and by dendritic cells/macrophages in vivo and in vitro. Am. J. Pathol. 142:1409-1422. [PMC free article] [PubMed] [Google Scholar]
- 8.Graversen, J. H., M. Madsen, and S. K. Moestrup. 2002. CD163: a signal receptor scavenging haptoglobin-hemoglobin complexes from plasma. Int. J. Biochem. Cell Biol. 34:309-314. [DOI] [PubMed] [Google Scholar]
- 9.Hogger, P., U. Erpenstein, P. Rohdewald, and C. Sorg. 1998. Biochemical characterization of a glucocorticoid-induced membrane protein (RM3/1) in human monocytes and its application as model system for ranking glucocorticoid potency. Pharm. Res. 15:300-306. [DOI] [PubMed] [Google Scholar]
- 10.Hogger, P., and C. Sorg. 2001. Soluble CD163 inhibits phorbol ester-induced lymphocyte proliferation. Biochem. Biophys. Res. Commun. 288:841-843. [DOI] [PubMed] [Google Scholar]
- 11.Kristiansen, M., J. H. Graversen, C. Jacobsen, O. Sonne, H. J. Hoffman, S. K. Law, and S. K. Moestrup. 2001. Identification of the haemoglobin scavenger receptor. Nature 409:198-201. [DOI] [PubMed] [Google Scholar]
- 12.Kulmatycki, K. M., and F. Jamali. 2005. Drug disease interactions: role of inflammatory mediators in disease and variability in drug response. J. Pharm. Pharm. Sci. 8:602-625. [PubMed] [Google Scholar]
- 13.Kurtzhals, J. A. L., V. Adabayeri, B. Q. Goka, B. D. Akanmori, J. O. Oliver-Commey, F. K. Nkrumah, C. Behr, and L. Hviid. 1998. Low plasma concentrations of interleukin 10 in severe malarial anaemia compared with cerebral and uncomplicated malaria. Lancet 351:1768-1772. [DOI] [PubMed] [Google Scholar]
- 14.Lee, T.-S., and L.-Y. Chau. 2002. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat. Med. 8:240-246. [DOI] [PubMed] [Google Scholar]
- 15.Matsushita, N., M. Kashiwagi, R. Wait, R. Nagayoshi, M. Nakamura, T. Matsuda, P. Hogger, P. M. Guyre, H. Nagase, and T. Matsuyama. 2002. Elevated levels of soluble CD163 in sera and body fluids from rheumatoid arthritis patients and inhibition of the shedding of CD163 by TIMP-3. Clin. Exp. Immunol. 130:156-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moller, H. J., H. Aerts, H. Gronbaek, N. A. Peterslund, P. Hyltoft-Petersen, N. Hornung, L. Rejnmark, E. Jabbarpour, and S. K. Moestrup. 2002. Soluble CD163: a marker molecule for monocyte/macrophage activity in disease. Scand. J. Clin. Lab. Investig. 237:29-33. [DOI] [PubMed] [Google Scholar]
- 17.Moller, H. J., N. A. Peterslund, J. H. Graversen, and S. K. Moestrup. 2002. Identification of the haemoglobin scavenger receptor/CD163 as a natural soluble protein in plasma. Blood 99:378-380. [DOI] [PubMed] [Google Scholar]
- 18.Othoro, C., A. A. Lal, B. Nahlen, D. Koech, A. S. Orago, and V. Udhayakumar. 1999. A low interleukin-10/tumor necrosis factor alpha ratio is associated with malarial anaemia in children residing in a holoendemic malaria region in western Kenya. J. Infect. Dis. 179:279-282. [DOI] [PubMed] [Google Scholar]
- 19.Philippidis, P., J. C. Mason, B. J. Evans, I. Nadra, K. M. Taylor, D. O. Haskard, and R. C. Landis. 2004. Hemoglobin scavenger receptor CD163 mediates interleukin-10 release and heme oxygenase-1 synthesis: antiinflammatory monocyte-macrophage responses in vitro, in resolving skin blisters in vivo, and after cardiopulmonary bypass surgery. Circ. Res. 94:119-126. [DOI] [PubMed] [Google Scholar]
- 20.Schaer, D. J., C. A. Schaer, P. W. Buehler, R. A. Boykins, G. Schoedon, A. I. Alayash, and A. Schaffner. 2006. CD163 is the macrophage scavenger receptor for native and chemically modified hemoglobins in the absence of haptoglobin. Blood 107:373-380. [DOI] [PubMed] [Google Scholar]
- 21.Sulahian, T. H., P. Hogger, A. E. Wahner, K. Wardwell, N. J. Goulding, C. Sorg, A. Droste, M. Stehling, P. K. Wallace, P. M. Morganelli, and P. M. Guyre. 2000. Human monocytes express CD163, which is upregulated by IL-10 and identical to p155. Cytokine 12:1312-1321. [DOI] [PubMed] [Google Scholar]
- 22.Timmermann, M., and P. Hogger. 2005. Oxidative stress and 8-iso-prostaglandin F(2alpha) induce ectodomain shedding of CD163 and release of tumor necrosis factor-alpha from human monocytes. Free Radic. Biol. Med. 39:98-107. [DOI] [PubMed] [Google Scholar]
- 23.Verreck, F. A., T. de Boer, D. M. Langenberg, L. van der Zanden, and T. H. Ottenhoff. 2006. Phenotypic and functional profiling of human proinflammatory type-1 and anti-inflammatory type-2 macrophages in response to microbial antigens and IFN-gamma- and CD40L-mediated costimulation. J. Leukoc. Biol. 79:285-293. [DOI] [PubMed] [Google Scholar]
- 24.Wagener, F. A., H. E. Van Beurden, J. W. Von Den Hoff, G. J. Adema, and C. G. Figdor. 2003. The heme-heme oxygenase system: a molecular switch in wound healing. Blood 102:521-528. [DOI] [PubMed] [Google Scholar]
- 25.Zwadlo, G., R. Voegeli, K. S. Osthoff, and C. Sorg. 1987. A monoclonal antibody to a novel differentiation antigen on human macrophages associated with the down-regulatory phase of the inflammatory process. Exp. Cell Biol. 55:295-304. [DOI] [PubMed] [Google Scholar]