Abstract
Typhoid caused by Salmonella enterica serovar Typhi remains a major health concern worldwide. The emergence of multidrug-resistant strains of Salmonella with increased virulence, communicability, and survivability leading to increased morbidity and mortality has further complicated its management. Currently available vaccines for typhoid have less-than-desired efficacy and certain unacceptable side effects, making it pertinent to search for new immunogens suitable for vaccine formulation. The outer membrane proteins (OMPs) of Salmonella have been considered possible candidates for conferring protection against typhoid. OMPs interface the cell with the environment, thus representing important virulence factors with a significant role in the pathobiology of gram-negative bacteria and bacterial adaptation. An OMP of Salmonella enterica serovar Typhimurium with an apparent molecular mass of 49 kDa that is highly immunogenic, evokes humoral and cell-mediated immune responses, and confers 100% protection to immunized rats against challenge with very high doses (up to 100 times the 50% lethal dose) of Salmonella enterica serovar Typhimurium has been identified. Further, very efficient clearance of bacteria from the reticuloendothelial systems of immunized animals was seen. This protein is recognized by the antibodies present in serum of typhoid patients. When sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel-eluted protein was further analyzed by high-performance liquid chromatography (HPLC) and two-dimensional electrophoresis, two polypeptides with the same molecular weight were resolved. These have different isoelectric points and gave two peaks with different retention times in reverse-phase HPLC. However, only one of the two bands interacted with patient serum. The immunogenicity studies (enzyme-linked immunosorbent assay and delayed-type hypersensitivity [DTH]) indicated that the immunoreactive protein evoked a strong immune response in rats. The N-terminal sequencing and analysis of the homology of this protein with sequences in the protein database of Salmonella resulted in a match with the N-terminal sequences of a protein in Salmonella enterica serovar Typhi (CT18 and Ty2 strains). The homology search further revealed it to be a hypothetical protein, whose gene had unidentified open reading frames in Salmonella serovar Typhi encoding 447 amino acid residues, corresponding to a molecular mass of 49 kDa. The nucleotide sequence of the encoding gene was deduced, and the gene was amplified by PCR using appropriate primers. An amplified 1.3-kb band was purified and sequenced to confirm its identity. These OMPs provide promising targets for the development of a candidate vaccine against typhoid.
Typhoid fever is an acute systemic illness caused by Salmonella enterica serovar Typhi. The organisms are noncapsulated, nonsporulating, facultative anaerobic bacilli, which have characteristic flagellar, somatic, and outer coat antigens (32). Salmonella enterica serovar Typhimurium is its murine/rodent counterpart, causing salmonellosis in mice and rats. Typhoid fever remains a public health concern, with an estimated 22 million cases and 200,000 related deaths occurring worldwide each year (4). Children in areas of endemicity, travelers, and microbiological laboratory technicians are particularly at risk of contracting the disease. The study of different aspects of typhoid, ranging from molecular biology to epidemiology, offers the opportunity of not only making an impact on health biotechnology through the development of new vaccines and diagnostic methods but also acquiring an insight into the basic biological processes involved in the host-bacterium interaction (2). Salmonella enterica serovar Typhimurium serves as a model for understanding the pathogenesis and epidemiology of Salmonella infection.
The outer membrane proteins (OMPs) of Salmonella have been considered possible candidates for conferring protection against typhoid. Over the past years, several Salmonella OMPs have been investigated as potential vaccine candidates, virulence factors, and diagnostic antigens (11) and the molecular structure and function of OMPs and their respective genes (27, 25, 26) have been studied. However, only a small number of OMPs have so far been characterized (15). Study of other gram-negative bacteria demonstrated that porins represent the most abundant class of OMPs that are protective and show some degree of antigenic heterogeneity among different strains (21). However, these are relatively nonspecific and have interspecies cross-reactivity. In our efforts to identify new candidates with potential for vaccine formulation, we have focused our attention on nonporin OMPs. A major nonporin OMP with a molecular mass of 49 kDa (10) from Salmonella serovar Typhimurium that is antigenically conserved (36) has been purified and characterized. We report its antigenic evaluation. Recent advances in bioinformatic analysis tools make it possible to examine all the proteins and genes from any pathogen. A homology search of its N-terminal sequences with the Salmonella database revealed the presence of a putative unidentified protein in Salmonella serovar Typhi. The gene for the Salmonella serovar Typhi protein has been amplified from Salmonella serovar Typhi genomic DNA and cloned. Future studies aim at expression of the protein in Escherichia coli and analysis of gene expression at the translational level and of the protein's contribution to the identification of rational strategies for the design of a potential vaccine.
MATERIALS AND METHODS
Bacterial culture.
A standard strain of Salmonella enterica serovar Typhimurium (wild), obtained from the National Salmonella Phage Typing Centre, Lady Harding Medical College, New Delhi, India, was used for these studies. The bacteria were further characterized at the Microbiology Division of Majeedia Hospital, and their identity was reconfirmed. Cells were grown overnight at 37°C as stationary cultures on nutrient agar (Hi-Media, India) slants (30).
Preparation of OMP fractions.
Isolation of outer membranes was carried out by the method of Foulaki et al. (7) with certain modifications. The bacteria were harvested and washed three times with 10 mM Tris-HCl (pH 7.5), and 1.0 g (wet weight) of bacteria was extracted with 20 ml extraction buffer (10 mM Tris-HCl, pH 7.5, 10 mM EDTA, and 6 M urea) for 1 hour at 4°C. The extract was dialyzed against distilled water for 3 days with frequent changes. The dialyzed material was centrifuged at 6,000 rpm for 1 h at 4°C, and the supernatant containing surface proteins was collected and lyophilized with a lyophilizer (Heto-Holten, Denmark). The crude OMP preparation was stored at −20°C till further use.
Protein estimation.
The protein content in the samples was estimated by the method of Lowry et al. (16) as modified by Dulley and Grieve (5).
SDS-PAGE of proteins.
Protein samples were solubilized in sample buffer (0.625 M Tris-HCl, pH 6.8, 2% sodium dodecyl sulfate [SDS], 10% glycerol, 5% mercaptoethanol) at 100°C for 5 min. SDS-polyacrylamide gel electrophoresis (PAGE) was carried out on 12% separating gel with the discontinuous buffer system as described by Laemmli (14). The gels were stained with 0.1% (wt/vol) Coomassie brilliant blue R-250 (Merck, India) in 45.4% (vol/vol) methanol and 9.2% (vol/vol) glacial acetic acid and destained with 5% (vol/vol) methanol and 7% (vol/vol) glacial acetic acid.
Purification of proteins.
The major OMPs identified from SDS-PAGE gels on the basis of their molecular masses were used as eliciting antigens in our studies. Four of these OMPs, with molecular masses of 15 kDa, 33 kDa, 37 kDa, and 49 kDa, were selected for the present studies. The protein-acrylamide (PA) complexes were prepared according to the method of Tjian et al. (34). The bands corresponding to these proteins were excised directly from the gels, transferred to a centrifuge tube, and pulverized. The pulverized material was lyophilized to get the PA complex in the form of a fine powder. An aliquot of this powder was suspended in phosphate-buffered saline (PBS), emulsified with an equal volume of complete Freund's adjuvant, and injected subcutaneously into mice (25 μg protein in 0.5 ml emulsion/mouse).
Immunization of animals.
Six- to 8-week-old Swiss albino male mice (25 to 30 g), obtained from the Central Animal House Facility of the university, were housed at 25°C in polypropylene cages and fed ad libitum with a pellet diet (Hindustan Lever Ltd.) and water. The studies were conducted according to ethical guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals and were approved by the Institutional Animal Ethics Committee.
Immunization schedule.
Prior to immunization the animals were bled from the tail vein to obtain preimmune sera. The animals received four subcutaneous injections of the PA complex on days 0, 7, 21, and 28 and a booster on day 40; they were bled on day 50, and the sera were kept at −20°C till further use. The control animals received PBS at the same immunization schedule.
Protection studies.
Ten groups of six animals each were used for these studies. Eight groups were immunized subcutaneously with the four selected proteins (two groups for each of the proteins), and two groups of animals served as the control. After 4 weeks of immunization with the PA complexes, the animals were challenged with two different doses (50 times the 50% lethal dose [50×LD50] and 100×LD50) of Salmonella enterica serovar Typhimurium (wild), injected intraperitoneally. The animals were observed for 14 days after the bacterial challenge, and their condition as well as mortality was recorded. The LD50 of the bacteria was determined by the method of Reed and Muench (29).
Bacterial clearance studies.
To evaluate the efficacy of selected proteins in accelerating the clearance of bacteria from the recticuloendothelial system, the livers of the protected animals were excised under aseptic conditions on day 3 postinfection. These were homogenized in PBS, and an aliquot was cultured on nutrient agar plates. Salmonella enterica serovar Typhimurium colonies obtained after overnight incubation at 37°C (identified by a standard dabbing method on TSI Agar plates, where Salmonella imparts a blackish hue to the otherwise pink plates) were counted. The results were expressed as log10CFU/g tissue.
Immunoblot analysis.
To assess the immunogenic role of the 49-kDa OMP during natural infection, its cross-reactivity with antibodies present in a typhoid patient's serum was analyzed. The electroeluted 49-kDa protein was run on SDS-polyacrylamide gel and transferred to a nitrocellulose membrane (Schleicher and Schuell, Dassel, Germany) by the method of Towbin et al. (35) using transfer buffer (25 mM Tris, 192 mM glycine, 0.02% [wt/vol] SDS, 20% [vol/vol] methanol) and a constant current of 0.65 mA/cm2 in the gel for 3 h. The membranes were stained with 0.5% (wt/vol) Ponceau-S (Hi-Media, India) in 1% acetic acid to check the transfer. The blot was destained, baked at 80°C for 2 h, blocked with PBS (8 g NaCl, 0.2 g KCl, 1.44 g Na2HPO4, and 0.096 g KH2PO4 per liter in distilled water), pH 7.4, containing 2% (wt/vol) bovine serum albumin (Hi-Media, India) at room temperature for 2 h, and incubated with the patient's serum (diluted 1:10 in PBS) for 3 h. After a washing, the color was developed by incubating the blot with peroxidase-conjugated goat anti-human immunoglobulin G (1:10,000 dilution in PBS) for 3 h. Diaminobenzidine tetrahydrochloride (Sigma) was used as the substrate for the peroxidase reaction. Between each step the blots were washed three times for 5 min each in PBS-0.05% Tween 20 (PBST).
Preparative SDS-PAGE for isolation of 49-kDa OMP.
A crude OMP preparation was fractionated on a preparative (3-mm-thick, 12%) SDS-PAGE gel using the method of Jacobs and Clad (12) with certain modifications (13) at 100 V until the dye reached the bottom of the gel. The 49-kDa protein band was located by careful calculation of relative mobility values vis-à-vis the values for protein weight markers (9). The 49-kDa OMP was excised, crushed finely, suspended in 2 volumes of elution buffer (0.025 M Tris-HCl, pH 8.3, 0.192 M glycine, 6 M urea), and incubated overnight at 4°C on a rocking platform. The supernatant was collected, and the gel pellet was reextracted. The two supernatants were pooled, dialyzed at 4°C against distilled water to remove urea, and lyophilized. The concentration of eluted protein was estimated by the method of Lowry et al. (16), and its purity was assessed by SDS-PAGE/silver staining (1).
2D electrophoresis.
The gel-eluted 49-kDa OMP was subjected to two-dimensional (2D) electrophoresis analysis using the method of O'Farrell (22). A 7-cm immobilized pH gradient (pH 3 to 10) strip (IPG; Bio-Rad, Hercules, CA) was rehydrated overnight in 100 μl rehydration buffer {8 M urea, 10 mM dithiothreitol, 2% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate, 0.2% Pharmalyte, 0.001% bromophenol blue} containing 100 μg protein. Isoelectric focusing was performed at 4,000 V for 10,000 V-h using a Protean isoelectric focusing cell (Bio-Rad, Hercules, CA). For the second dimension, the IPG strip was equilibrated for 10 min at room temperature by overlaying it with equilibration buffer I (1.5 M Tris-HCl, pH 8.8, 6 M urea, 2% [wt/vol] SDS, 20% [vol/vol] glycerol, 2% [wt/vol] dithiothreitol). It was then equilibrated with buffer II (1.5 M Tris-HCl, pH 8.8, 6 M urea, 2% [wt/vol] SDS, 20% [vol/vol] glycerol, 2.5% [wt/vol] iodoacetamide) for 10 min, followed by soaking in running buffer, and overlaid on 10% SDS-polyacrylamide gel. Electrophoresis was carried out at room temperature at 80 V for 1 h with a Mini-Protean 3 cell (Bio-Rad, Hercules, CA), and gels were either silver stained to visualize the bands or Western blotted.
HPLC of the 49-kDa OMP.
To further ascertain the purity of the eluted protein, reverse-phase high-performance liquid chromatography (HPLC) was carried out with a liquid chromatography system (Waters) equipped with a photodiode array detector and Waters 600 controller pump (33). The chromatography was performed on a C8 column (Waters; Spherisorb; 4.6 mm by 250 mm; particle size, 5 μm). The column was washed with HPLC grade methanol and prerun with acetonitrile-water (30:70) under 2,000 lb/in2 and a flow rate of 0.8 ml/min. After the baseline was stabilized to zero, 10 μl of gel-eluted OMP (30 μg/μl) was injected into the column and eluted with acetonitrile-water (30:70) containing 0.05% trifluoroacetic acid. The eluent was analyzed by recording its absorbance at 220 nm.
ELISA.
The enzyme-linked immunosorbent assay (ELISA) was carried out according to the protocol of Engvall and Perlman (6). The 96-well plates were coated (in duplicate) with 2 μg antigen at 200 μl/well (prepared in 50 mM sodium carbonate buffer, pH 9.6). The plates were incubated overnight at 4°C, washed with 1× PBST, and blocked with 3% bovine serum albumin for 2 h at 37°C. Aliquots (200 μl) of serially diluted typhoid patient's serum (1:50, 1:100, 1:200, 1:400, 1:800, and so on) in PBS were added to wells and incubated at 37°C for 2 h. The wells were washed thoroughly with 1× PBST, and secondary antibody (diluted in 1× PBS buffer) was added to a final volume of 200 μl per well. The plates were incubated at 37°C for 2 h. After the blots were washed with PBST, color was developed by adding 200 μl of the substrate (o-phenyldiamine) and incubating the plates at 37°C for 30 min. The reaction was stopped by addition of 50 μl of 0.5 N H2SO4, and absorbance at 490 nm was recorded.
DTH studies.
The footpad swelling method described by Collins and Mackaness (3) was used to carry out the delayed-type hypersensitivity (DTH) studies. The animals were divided into three groups of six animals each, with one group serving as the control. The test groups received an injection of 50 μg HPLC-purified 49-kDa protein (dissolved in 100 μl of 1× PBS) in their right hind footpads, while the left footpads received an injection of an equal volume of saline and served as the control. The control group of animals was mock immunized in parallel by injection of an equal volume of 1× PBS (100 μl) instead of protein into their right hind footpads and an injection of saline into their left hind footpads. The footpad swelling was measured at different time intervals (3, 6, 24, 48, and 72 h postinjection) with the help of a digital vernier caliper (Guanglu, China). The values obtained for the swelling induced by saline in the left footpads were subtracted from the values obtained for the swelling induced by the test antigen or PBS alone.
Detection of OMP-specific antibodies in serum of immunized animals.
The double-radial-immunodiffusion method of Ouchterlony and Wilson (23) was followed to detect the humoral immune response elicited by OMPs. One gram of agar was added to 100 ml of PBS, and the solution was boiled till the agar was completely dissolved. Ten milliliters of this solution was poured on two leveled horizontal glass plates each to get gels approximately 1 mm thick. The gel plates were allowed to solidify at room temperature and later were kept at 4°C for 1 h in a moist chamber. The wells were punched in the gels using a punching template. The central wells were filled with Salmonella serovar Typhi (wild) cell lysate (25 μg each), while the surrounding wells were filled with different dilutions of the sera obtained from the animals immunized with the immunoreactive and nonimmunoreactive 49-kDa proteins (P49A and P49B, respectively). The plates were incubated for 72 h at 4°C in a moist chamber. The plates were stained with Coomassie blue for visualization. A precipitation reaction would indicate the immunogenicity of P49 to elicit a humoral response.
To detect the protective efficacy of antibodies, 100 μl S. enterica serovar Typhi culture was added to a 96-well plate. To this, 100 μl of serum from the animals immunized with P49A was added. The center received the serum from unimmunized animals. In a second control, E. coli was used in place of Salmonella serovar Typhi. Agglutination of Salmonella serovar Typhi by serum from immunized animals indicates the protective nature of OMP-specific antibodies.
Amino-terminal sequencing.
The gel-eluted 49-kDa OMP was electrophoresed by 2D-PAGE. The band of P49A was electroblotted to a polyvinylidene difluoride membrane sheet (Bio-Rad, Hercules, CA) using 1× CAPS (3-[cyclohexylamino]-1-propanesulfonic acid) (Sigma) buffer (pH 11) in 10% methanol by the electroblotting method of Matsudaira (18). The section of the membrane containing the protein of interest was excised, and N-terminal sequencing was performed with an Applied Biosystems sequencer (courtesy of D. Salunke, National Institute of Immunology, New Delhi, India).
Homology analysis.
The amino-terminal sequence of P49A of Salmonella serovar Typhimurium was subjected to an NCBI BLAST search to identify the degree of homology with any protein in the Salmonella serovar Typhi database. An unknown protein with apparent molecular mass of 49 kDa having very high amino acid homology was found. The data were used to deduce the sequence of the gene encoding the protein. The gene sequence was matched with that of the Salmonella serovar Typhimurium gene for P49A to deduce the degree of homology.
PCR amplification.
The deduced nucleotide sequence of the gene facilitated the design of oligonucleotide primers for isolation of the gene. Restriction mapping by the Webcutter DNA program showed no sites for NcoI and BamHI within the gene. Therefore, the sites for these enzymes were introduced into the forward and reverse primers, respectively, which helped in directional cloning of the gene after PCR amplification. PCR amplification of the gene was carried out using a deoxynucleotide triphosphate mixture (2.5 mM each), polymerase buffer, forward and reverse primers (10 μM, 1 μl each), a DNA template (100 ng/μl), and Taq and Pfu polymerases (3.0 U/μl each). Pfu polymerase was used for its 5′→3′ exonuclease proofreading activity, which resulted in error-free amplification. The amplification was performed in a Techne Gene thermal cycler at a primer annealing temperature of 55°C. The amplified products were analyzed on agarose gel and stored at −20°C till further use.
The PCR product was fractionated on an 0.8% agarose gel, the band of the desired fragment was excised and minced, and the gel pieces were suspended in 10 mM Tris-HCl (pH 8) and kept on a shaker for 1 to 2 h. To this, equilibrated phenol was added, and the mixture was vortexed and incubated at −70°C for 1 h. The frozen mixture was immediately centrifuged at 12,000 rpm in a microcentrifuge for 10 min at 4°C, and the aqueous phase containing eluted DNA was extracted twice with an equal volume of chloroform-isoamyl alcohol (1:24). The DNA was ethanol precipitated, collected by centrifugation, washed with 70% ethanol, dried, and resuspended in Tris-EDTA buffer. The purified product was checked on an 0.8% gel and quantified.
RESULTS
Proteins of the outer membrane of Salmonella serovar Typhimurium.
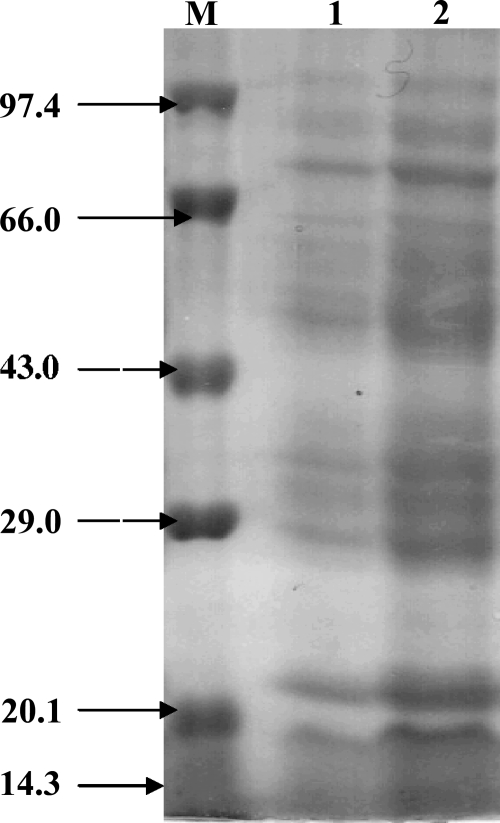
Complex profiles of the proteins present in outer membranes of gram-negative bacteria have been reported. The urea-EDTA extraction method was used for the isolation of OMPs from Salmonella serovar Typhimurium (wild) for its ease, better yields, and reproducibility. The analysis of isolated OMPs by SDS-PAGE showed a complex electrophoretic profile having more than 15 proteins with molecular masses between 15 and 100 kDa (Fig. 1). The molecular masses of 14 main proteins were calculated to be 78, 75, 70, 68, 65, 55, 49, 43, 37, 35, 32, 30, 25, and 15 kDa. The yield of OMPs was approximately 4.5 mg protein/g bacteria (wet weight).
FIG. 1.
SDS-PAGE (12%) analysis of urea-extracted OMPs from Salmonella serovar Typhimurium (wild). Lanes 1 and 2 show 15- and 45-μg OMP fractions, respectively. Lane M, molecular weight markers: phosphorylase b, bovine serum albumin, ovalbumin, carbonic anhydrase, soybean trypsin inhibitor, and lysozyme. The sizes of the marker bands (in thousands) are shown at the left.
Four of the most prominent nonporin OMPs of Salmonella serovar Typhimurium were selected for further studies. The molecular masses of these OMPs were 15 kDa, 33 kDA, 37 kDa, and 49 kDa.
Protection studies.
Studies were carried out to assess the role of the selected proteins in conferring protection against experimentally induced murine salmonellosis. The bands corresponding to these proteins were excised directly from the gel and processed as described in Materials and Methods (“Purification of proteins”), and the PA complexes were used to immunize the animals. The immunized animals were later challenged with Salmonella serovar Typhimurium, and the potential of these proteins for conferring protection against the challenge was assessed. The results of the protection studies are shown in Table 1.
TABLE 1.
Results of the protection studiesa
| Protein | No. (%) of animals that survived at:
|
|
|---|---|---|
| 50×LD50 | 100×LD50 | |
| P15 | 2 (33.3) | 0 (0) |
| P33 | 3 (50) | 2 (33.3) |
| P37 | 4 (66.6) | 3 (50) |
| P49 | 6 (100) | 6 (100) |
| Control | 0 (0) | 0 (0) |
Six mice were immunized with the four selected proteins for 3 weeks, followed by a single booster, before exposing them to two doses (50×LD50 and 100×LD50) of Salmonella serovar Typhimurium (wild). The results are for survival of animals after 14 days of bacterial challenge.
All animals in the control groups started to show the symptoms of salmonellosis, such as ruffled hair, lethargic movements, slow responsiveness to external stimuli, and diminished desire to consume food, at the end of the day 1 postinfection and died after 3 to 4 days. Marginal protection (33.3%) was seen in animals immunized with the 15-kDa protein-polyacrylamide complex (PA15 complex) and challenged with 50×LD50 of bacterium. No protection by this protein was seen when a higher dose (100×LD50) of the bacterium was used. The 33-kDa protein, on the other hand, provided 50% and 33.33% protection under similar conditions. The immunization with the PA37 complex was relatively more effective and conferred a higher degree of protection against the bacterial challenge (66.6% against a bacterial dose of 50×LD50 and 50% protection against 100×LD50). Immunization with the PA49 complex resulted in 100% survival of challenges with Salmonella serovar Typhimurium doses of both 50×LD50 and 100×LD50. As the 49-kDa protein conferred maximum protection, further studies were carried out on this protein.
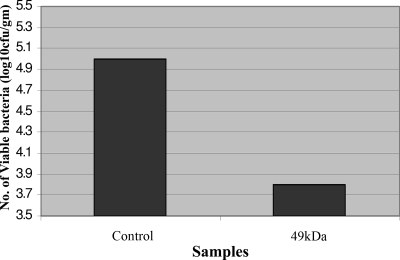
The ability of the PA49 complex to be an effective immunogen was further supported by studying the clearance of bacteria from the reticuloendothelial systems of immunized mice following Salmonella serovar Typhimurium challenge. These mice showed very few viable (approximately 5% compared to the control liver) Salmonella serovar Typhimurium cells in liver when the aliquots of homogenates were cultured overnight on TSI Agar plates. The livers of unimmunized control animals, on the other hand, were highly infected (Fig. 2). Similar results showing accelerated clearance of bacteria were obtained for the spleens of immunized animals also (data not shown).
FIG. 2.
Bacterial clearance from the reticuloendothelial system of mice. Animals were immunized with the 49-kDa protein and exposed to a sublethal dose of Salmonella serovar Typhimurium; livers were removed, homogenized, and cultured on nutrient agar (see Materials and Methods for details); and the colonies obtained were counted.
Purification of 49-kDa OMP.
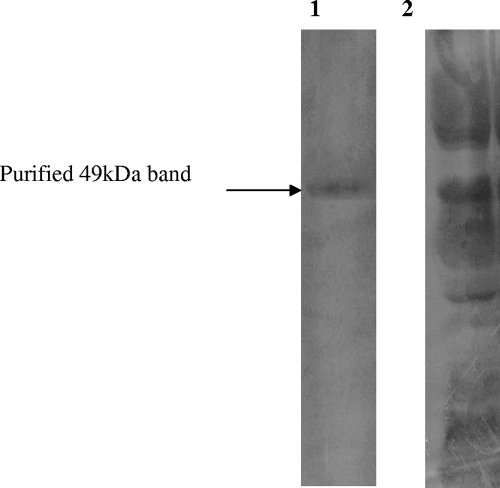
The 49-kDa protein was purified by preparative SDS-PAGE (3-mm-thick, 12% gel). The band of the desired protein was excised and eluted. The identity of the eluted material was confirmed by analytical SDS-PAGE, from which a single band of 49 kDa was obtained (Fig. 3). From 36 mg crude OMP loaded on a preparative PAGE gel, 0.37 mg of the 49-kDa protein was obtained. The 49-kDa protein therefore comprises approximately 0.1% of the total OMP fraction of Salmonella serovar Typhimurium. This fraction was further analyzed and purified by 2D electrophoresis and reverse-phase HPLC.
FIG. 3.
SDS-PAGE showing the purification of the 49-kDa OMP of Salmonella serovar Typhimurium (wild). Lane 1, purified 49-kDa OMP (5 μg); lane 2, total OMP fraction (20 μg) of Salmonella serovar Typhimurium.
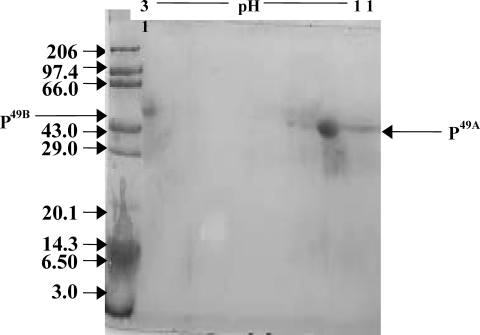
Analysis of the 49-kDa protein by 2D electrophoresis revealed two separate spots having different isoelectric points but the same molecular weight (Fig. 4). Spot 1 (P49A) had a pI in the alkaline range, while spot 2 (P49B) had a PI in the acidic range.
FIG. 4.
2D electrophoretic analysis of the eluted 49-kDa protein of Salmonella serovar Typhimurium. The 1D SDS-PAGE gel-eluted band was further analyzed on 2D gels, from which two bands were resolved at different pIs (P49A has a basic pI, while P49B has an acidic pI). Lane M, molecular weight markers (in thousands).
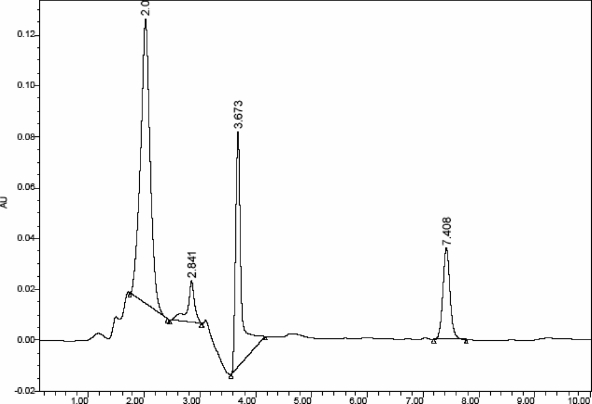
When the SDS-PAGE (one-dimensional [1D]) purified sample was applied to a C8 HPLC column (4.6 mm by 250 mm; particle size, 5 μm) and the eluent was continuously analyzed at 220 nm, two separate peaks were obtained (Fig. 5). The appearance of two peaks (collected at 2.07 and 3.67 min) confirmed the presence of two proteins in the sample. Thus, the HPLC results were in consonance with the 2D gel electrophoresis analysis. Both proteins were collected separately and used for further studies. Taken together, the results of protein analysis also suggest that complete solubilization and almost 100% purity of the proteins have been achieved. The isolated proteins were assessed for their immunogenicity.
FIG. 5.
HPLC analysis of the 49-kDa OMP isolated from Salmonella serovar Typhimurium by preparative SDS-PAGE. The sample (300 μg) was applied to a C8 HPLC column, which was prerun with acetonitrile-water (30:70), and the bound proteins were eluted with the same solvent system. Two major peaks were observed at 2.070 min and 3.673 min. The two minor peaks eluted are insignificant, below the background level.
Immunoreactivity of the 49-kDa OMP of Salmonella serovar Typhimurium.
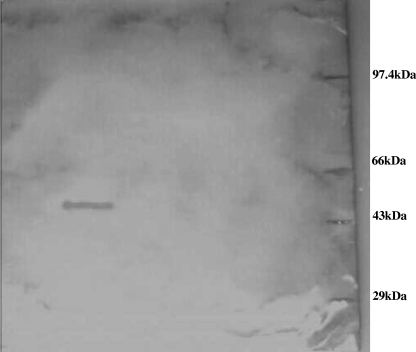
To assess the role of the 49-kDa protein in the pathogenicity of Salmonella during natural infection, the purified 49-kDa protein was run on SDS-PAGE gel, Western blotted, and exposed to the serum of a typhoid patient. As seen in Fig. 6, the protein was recognized by the patient's serum. Further the ELISA data showed that the serum strongly reacted with the purified 49-kDa OMP (Table 2). The results suggest that the 49-kDa protein plays an important role during infection. When the immunoanalysis was performed with 49-kDa proteins resolved by 2D electrophoresis, the serum reacted with only P49A. No cross-reactivity of serum with P49B was seen (Fig. 7A and B). The results of ELISA with the two proteins are given in Table 3.
FIG. 6.
The 49-kDa protein is recognized by the serum of a typhoid patient. P49A was electrophoresed, transferred to nitrocellulose, and exposed to the diluted serum of a typhoid patient (see Materials and Methods for details). The serum recognized the protein, and a distinct band is visible. Positions of molecular mass markers are shown on the right.
TABLE 2.
Immunological reactivity of the 49-kDa protein of Salmonella serovar Typhimurium (wild)
| Well no. | Serum dilution | Precipitin line intensitya |
|---|---|---|
| 1 | None (pure serum) | Intense blue area |
| 2 | 1:10 | ++ |
| 3 | 1:100 | + |
| 4 | 1:1,000 | − |
| 5 | 1:10,000 | − |
| 6 | 1:50,000 | − |
| 7 | NAb (PBS only) | − |
| 8 | NA (PBS at 1:10) | − |
++, significant antigen-antibody reaction; +, visible reaction; −, no visible reaction.
NA, not applicable.
FIG. 7.
The 1D gel-purified 49-kDa protein was subjected to further separation by 2D gel electrophoresis, from which two bands were resolved. These were transferred to nitrocellulose paper and processed. (A) Blot stained with 0.5% Ponceau-S, showing that both bands (corresponding to P49A and P49B) were efficiently transferred. (B) Reaction of the proteins with a typhoid patient's serum (see Materials and Methods for details). As can be seen, only P49A reacts with the serum, and no cross-reactivity with P49B was seen, suggesting that only one of the two proteins (P49A) is immunogenic.
TABLE 3.
ELISA of purified proteins collected showing a strong humoral response in animals immunized with the immunoreactive 49-kDa proteina
| Well contentsa | ELISA result (optical density at 450 nm) for well containing serum at a dilution of:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| None (pure serum) | 1:50 | 1:100 | 1:200 | 1:400 | 1:800 | 1:1,600 | 1:3,200 | 1:6,400 | 1:12,800 | 1:25,600 | |
| A | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| B | 0.883 | 0.877 | 0.864 | 0.855 | 0.852 | 0.844 | 0.840 | 0.824 | 0.807 | 0.801 | 0.745 |
| C | 0.554 | 0.541 | 0.537 | 0.536 | 0.533 | 0.531 | 0.519 | 0.517 | 0.512 | 0.511 | 0.475 |
| D | 0.522 | 0.510 | 0.507 | 0.501 | 0.498 | 0.481 | 0.479 | 0.475 | 0.474 | 0.472 | 0.464 |
A, blank (coated with coating buffer without any peptide); B, coating buffer containing 2 μg of immunoreactive protein P49A; C, 2 μg of the second purified protein (P49B); D, 2 μg of crude OMP (positive control).
DTH response.
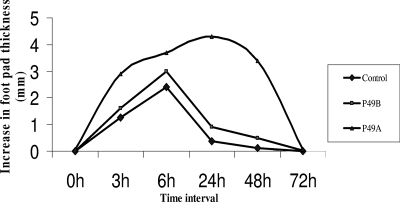
The HPLC-purified proteins were screened for their ability to induce a DTH reaction in mice sensitized earlier with a sublethal dose (0.5×LD50) of Salmonella serovar Typhimurium. The footpad swelling was measured at 3, 6, 24, 48, and 72 h postinjection. Various patterns of swelling were seen in animals injected with different proteins (Fig. 8). The injection of both purified proteins into mice initially induced significant footpad swelling (1.61 mm and 2.89 mm) after 3 h of administration, which further increased at 6 h (2.98 mm and 3.7 mm). However, the response induced by the protein P49B immunization quickly began to decrease, and at 24 h the swelling induced by P49B was only 0.9 mm. On the other hand, the footpad swelling in animals immunized with P49A continued to increase and was 4.3 mm at 24 h and 3.39 mm at 48 h. This swelling subsided to a value of 0.08 mm at 72 h. The results confirmed that only protein P49A was effective in inducing a DTH response in immunized animals.
FIG. 8.
DTH response induced upon injection of the two 49-kDa OMPs purified by HPLC. The values plotted were obtained after subtracting the values of footpad swelling induced in saline-injected feet.
Humoral response.
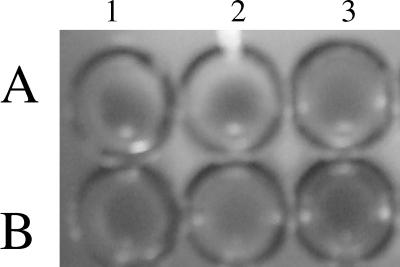
The immunodiffusion experiment showed that P49A evoked a significant humoral response in rats and mice (data not shown), and the serum of immunized animals reacted with the protein. Figure 9 shows the results of the experiment to check the protective nature of anti-P49A antibodies. Wells 1A, 2A, and 3A had the Salmonella serovar Typhi culture, and wells 1B, 2B, and 3B had E. coli cells. To wells 1A, 1B, 2A, and 2B the serum (1:10 dilution) from animals immunized with P49 was added, while wells 3A and 3B received the serum from unimmunized animals. It was found that serum from immunized animals was able to agglutinate Salmonella serovar Typhi (wells 1A and 1B) but not E. coli (wells 1B and 2B). The serum from unimmunized animals did not show any agglutination of either Salmonella serovar Typhi (well 3A) or E. coli (well 3B).
FIG. 9.
Agglutination of Salmonella serovar Typhi cells by anti-P49A antibodies. Wells 1A and 2A have serum from animals immunized with the 49-kDa protein agglutinating from Salmonella serovar Typhi cells. Well 3A contains control serum from an unimmunized animal mixed with Salmonella serovar Typhi cells showing no agglutination. Wells 1B and 2B have serum from immunized animals mixed with E. coli cells, while well 3B has serum from unimmunized animals mixed with E. coli cells. Absence of the agglutination reaction is observed in wells 1B, 2B, and 3B.
Identification of a protein from Salmonella serovar Typhi having homology with P49A.
P49A of Salmonella serovar Typhimurium was electroblotted to a polyvinylidene difluoride membrane, and its N-terminal amino acid sequence was determined. As our aim was to identify a protein that may be used as a potential candidate for the development of a vaccine against typhoid, it was pertinent to look for a protein in Salmonella serovar Typhi that has homology with P49A. A homology search with the N-terminal sequences of P49A was made with the Salmonella serovar Typhi protein database. The sequence showed strong homology with an unidentified protein from Salmonella serovar Typhi (CT18 and Ty2 strains). The NCBI BLAST homology analysis revealed a hypothetical protein reported in the Salmonella serovar Typhi protein database composed of 447 amino acid residues corresponding to a molecular mass of 49 kDa. The sequence of this hypothetical protein is given in Fig. 10. The search for a putative gene coding for this protein in the Salmonella serovar Typhi genome was made.
FIG. 10.
Amino acid sequence of P49A from Salmonella serovar Typhi.
The sequence analysis revealed that the gene is localized at the AL62782 and AE016848 loci of the CT18 and Ty2 strains of Salmonella serovar Typhi, respectively. The gene corresponds to a 1.3-kb fragment with an open reading frame (ORF) of 1,344 bases, encoding a single polypeptide (Fig. 11). In Salmonella serovar Typhimurium the gene is localized at the NC003197 locus of the complete genome of the LT2 strain. When the two retrieved gene sequences were aligned by the Emboss alignment program to find the degree of similarity between the two, 732 nucleotide bases were found to match, revealing 55.32% identity between the two genes (data not shown).
FIG. 11.
Nucleotide sequence of the gene encoding P49A.
Isolation of the gene for the Salmonella serovar Typhi protein.
The PCR amplification of the gene for the Salmonella serovar Typhi protein was carried out using 25-base primers, designed based on the sequence data. Restriction analysis of the 49-kDa gene using the Webcutter DNA program showed no sites for NcoI and BamHI within the gene. These sites were therefore included at the 5′ ends of the forward and reverse primers, respectively, to facilitate the directional cloning of the amplified product. The sequences of the primers used are as follows: forward primer, 5′ CATGCCATGGGAGACGCATCGATAA 3′ (NcoI site underlined); and reverse primer, 5′ CGCGGATCCTTGAGAGGAATTCATT 3′ (BamHI site underlined).
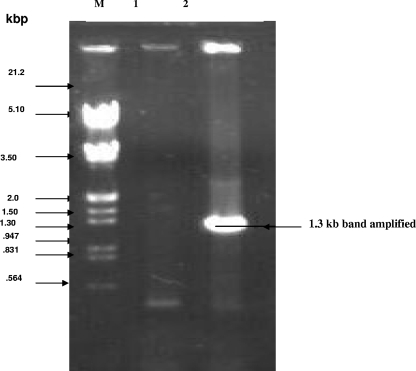
Salmonella serovar Typhi genomic DNA was isolated by standard protocols and used for PCR amplification of the gene. A single band of 1.3 kb was obtained. The amplified product was separated on an 0.8% agarose gel and visualized by staining the gel in ethidium bromide. The fragment was eluted, purified (Fig. 12), and cloned to express the gene product (data not shown).
FIG. 12.
PCR amplification of the 1.3-kb gene for the 49-kDa OMP. Lane M, EcoRI- and HindIII-digested λ DNA; lane 1, PCR of E. coli (control) DNA, showing the absence of the 1.3-kb gene; lane 2, amplification of a specific band in Salmonella serovar Typhi showing the presence of the 1.3-kb gene for the 49-kDa protein.
Sequence analysis of PCR product.
To confirm the identity of the amplified product, it was sequenced from both ends using forward and reverse primers. Sequence analysis of the PCR product revealed a 1,300-bp DNA sequence that matched exactly that of the gene for the hypothetical 49-kDa protein from the Salmonella serovar Typhi database. The sequence analysis thus confirmed the presence of the desired gene in Salmonella serovar Typhi, and the homology analysis revealed 100% homology of the PCR-amplified gene with the reported gene sequence in Salmonella serovar Typhi.
DISCUSSION
We describe the isolation and characterization of an immunogenic protein from the outer membranes of Salmonella serovar Typhimurium (wild). The OMPs of gram-negative bacteria are immunologically important because of their accessibility to the host defense system. However, there has been only relatively limited information about the potential of OMPs to confer protection against salmonellosis. The urea extraction method was used as the method of choice for isolation of OMPs because of the relative ease of working with OMP samples solubilized in urea, which could later be removed easily by dialysis. This method has been used for solubilization of surface proteins of a number of bacterial species (31) and has yielded good results in our laboratory (10).
Salmonella serovar Typhimurium is an intracellular facultative bacterium, and the participation of the cell-mediated immune response of the host is very important for protective immunity. However, recent studies have suggested that the humoral immune responses are equally important (19, 20). Not much is known about the specific immune response in humans, and most of the studies have been carried out using crude antigenic extracts or whole bacteria, as the characterization of OMPs involved in elicitation of the immune response remains undefined (28). Major OMPs identified from SDS-PAGE gels on the basis of their molecular weights were used as eliciting antigens in our studies. The PA complexes were prepared and administered to animals.
The results of protection studies indicated that the selected proteins conferred various degrees of protection (Table 1). The survival of the challenged animals was monitored over a 14-day period postinfection. In the group of animals challenged with 50×LD50 of the bacterium, maximum survival was seen in the animals immunized with P49 (100%), followed by P37 (66.7%), P33 (50%), and P15 (33.3%). The PA49 complex provided 100% protection against an even-higher bacterial challenge, 100×LD50. Protection levels of 50% and 33% in groups of animals immunized with PA37 and PA33 complexes, respectively, were observed. No protection against challenge with 100×LD50 was conferred by PA15. This protection can be attributed to many factors, including elicitation of both specific and nonspecific immune responses. For effectiveness against Salmonella infections, the immunogen has to be able to induce both humoral and cell-mediated immune responses (19). We therefore investigated general and specific immune responses that are important in controlling Salmonella infection.
The immunized animals had significantly lower bacterial translocation to liver as well as to spleen than the control animals. Further, the mean bacterial burdens were considerably lower in the liver homogenates of immunized animals (Fig. 2). The results of such studies indicated that immunization of animals with the 49-kDa protein produced almost 95% inhibition in bacterial translocation to liver compared to that in control unimmunized animals. Earlier studies have also indicated a correlation between protective efficacy and prevention of bacterial translocation (24). The increased bacterial clearance reported in the present study following immunization of animals with selected OMPs can be explained partly by observations that Salmonella serovar Typhimurium infection initially leads to a transient immunosuppression mediated by nitric oxide at least partially. NO is produced in large amounts by macrophages during infection (17). Although T-cell activation occurs during the early stages of infection, it is possible that the lymphocyte response is blocked by NO-induced transient suppression. Eventually the immune system overcomes suppression and achieves bacterial eradication (19).
The preparative electrophoresis technique used by us included separation of target proteins by conventional SDS-PAGE followed by elution of the protein of interest from the gel using a high molar concentration of urea in the elution buffer. This eliminated the need for expensive devices or membranes, and the protein could be purified easily in one step from the crude OMPs. Urea was later removed by dialysis to get the protein sample in a form suitable for structural and in vivo immunological analysis. The proteins seem to retain their correct conformation and could be recognized by the typhoid patient's antibodies. Further, large quantities of OMPs could be obtained by this method in soluble form.
The analysis of the eluted fraction by SDS-PAGE revealed a single band of 49 kDa. However, further analysis by reverse-phase HPLC and 2D electrophoresis revealed the existence of two proteins (P49A and P49B) with the same molecular weight in this preparation. Further, only one of the proteins (P49A) was protective in nature.
Cross-reactivity of the 49-kDa protein with sera of a typhoid patient gave further support to the potential of this protein as a protective antigen. It also showed that this protein (or a similar protein from Salmonella serovar Typhi) plays a role during natural infections leading to typhoid fever. Though an array of different antibodies may be present in a patient's serum, a strong reaction with the 49-kDa protein indicates the immunogenic role of this protein. Further, the analysis also indicated that there might be common epitopes between the 49-kDa proteins of Salmonella serovar Typhi and Salmonella serovar Typhimurium that enable them to evoke an immune response in both humans and mice. The immunoblot analysis with the 2D electrophoresis-separated proteins demonstrated that only P49A was recognized by the typhoid patient's serum. No reactivity of P49B with patient serum was seen. The mapping of epitopes would provide a better understanding of the immunological and molecular basis of Salmonella infection.
The ELISA results indicated that the protein elicits a significant humoral response. The ability of anti-P49A antibodies to agglutinate bacteria confirms its role as a protective antigen and supports its potential for the development of a vaccine against typhoid. The DTH to one or more specific microbial antigens plays an important role in host defense against infectious diseases (8). The results of the studies carried out to follow the induction of the DTH reaction upon immunization of animals with the 49-kDa protein indicated that the protein was highly effective in inducing DTH reactions in immunized animals. The animals exhibited a gradual increase in the swelling, which reached to maximum value at 24 h, registering a decline at 72 h.
The importance of this protein became evident from the protection experiments. When the animals immunized with this protein were challenged with lethal doses of Salmonella serovar Typhimurium, 100% of them were protected against salmonellosis.
The structural and functional roles of individual OMPs (except porins) are largely unknown. The N-terminal sequence analysis of the 49-kDa protein and a homology search of the Salmonella serovar Typhi database identified a hypothetical protein of 447 amino acid residues corresponding to a molecular mass of 49 kDa from Salmonella serovar Typhi. Our sequence analysis demonstrated that the gene encoding the 49-kDa protein is contained within the AL627282 and AE016848 loci of the Salmonella serovar Typhi genome in the CT18 and TY2 strains, respectively. Based on the DNA sequence, the putative ORF of the gene encoding the retrieved protein sequence was identified. The analysis revealed an ORF of 1,344 bases, which resulted in a predicted protein of 447 amino acids. Suitable oligonucleotide primers were designed for amplification of the gene encoding the protein. The NcoI and BamHI restriction sites were engineered into the 5′ ends of forward and reverse primers, respectively. The PCR amplification generated a product of 1.3 kb, which was in agreement with the estimated size of the gene predicted from sequence information. The amplified fragment was purified, and the identity of the PCR product was confirmed by sequence analysis. The gene is being expressed in a heterologous system to get large amounts of recombinant protein for further studies.
To conclude, the present study reports the identification, isolation, and characterization of an immunoreactive protein from Salmonella. The cross-reactivity of the protein with sera from typhoid patients showed the importance of the protein in natural infection and host defense. The protein confers 100% protection against experimental salmonellosis in immunized animals, and the serum of immunized animals can agglutinate bacteria. Thus the protein has strong potential for the development of a subunit vaccine against typhoid.
Acknowledgments
These studies were supported by a research grant from the Department of Science & Technology, Government of India, to S.K.J. N.H. is a UGC-SRF.
We thank T. Hamid and Keyanoosh Zedah for some of the preliminary studies.
Footnotes
Published ahead of print on 23 July 2008.
REFERENCES
- 1.Blum, H., H. Beier, and H. J. Gross. 1987. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 8:93-99. [Google Scholar]
- 2.Calva, E., J. L. Puente, and J. J. Calva. 1988. Research opportunities in typhoid fever: epidemiology and molecular biology. Bioessays 9:173-177. [DOI] [PubMed] [Google Scholar]
- 3.Collins, F. M., and G. B. Mackaness. 1968. Delayed hypersensitivity and Arthus reactivity in relation to host-resistance in Salmonella-infected mice. J. Immunol. 101:830-845. [PubMed] [Google Scholar]
- 4.Crump, J. A., S. P. Luby, and E. D. Mintz. 2004. The global burden of typhoid fever. Bull. W. H. O. 82:346-353. [PMC free article] [PubMed] [Google Scholar]
- 5.Dulley, J. R., and P. A. Grieve. 1975. A simple technique for eliminating interference by detergents in the Lowry method of protein determination. Anal. Biochem. 64:136-141. [DOI] [PubMed] [Google Scholar]
- 6.Engvall, E., and P. Perlman. 1971. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry 8:871-874. [DOI] [PubMed] [Google Scholar]
- 7.Foulaki, K., W. Gruber, and S. Schlecht. 1989. Isolation and immunological characterization of a 55-kilodalton surface protein from Salmonella typhimurium. Infect. Immun. 57:1399-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griffin, F. M., C. Blanco, and S. C. Silverstein. 1975. Characterization of the macrophage receptor for complement and demonstration of its functional independence from the receptor for the Fe portion of immunoglobulin G. J. Exp. Med. 141:1269-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hames, B. D. 1990. An introduction to polyacrylamide gel electrophoresis, p. 1-90. In B. D. Hames and D. Rickwood (ed.), Gel electrophoresis of proteins. A practical approach. IRL Press at Oxford University Press, Oxford, England.
- 10.Hamid, T. 2001. Biological characterization of the outer membrane proteins of S. typhi and S. typhimurium and studies on their role in protection against typhoid. Ph.D. thesis. Jamia Hamdard University, New Delhi, India.
- 11.Isibasi, A., V. Ortiz, M. Vargas, J. Paniagua, C. Gonzalez, J. Moreno, and J. Kumate. 1988. Protection against Salmonella typhi infection in mice after immunization with outer membrane proteins isolated from Salmonella typhi 9,12,d,Vi. Infect. Immun. 56:2953-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobs, E., and A. Clad. 1986. Electroelution of fixed and stained membrane protein from preparative sodium dodecyl sulphate polyacrylamide gels into a membrane trap. Anal. Biochem. 154:583-589. [DOI] [PubMed] [Google Scholar]
- 13.Jeno, P., and M. Horst. 1996. Electroelution of proteins from polyacrylamide gels, p. 207-214. In J. M. Walker (ed.), The protein protocols handbook. Humana Press, Totowa, NJ.
- 14.Laemmli, M. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 227:680-685. [DOI] [PubMed] [Google Scholar]
- 15.Lin, J., S. Huang, and Q. Zhang. 2002. Outer membrane proteins: key players for bacterial adaptation in host niches. Microbes Infect. 4:325-331. [DOI] [PubMed] [Google Scholar]
- 16.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 17.MacFarlane, A. S., M. G. Schwacha, and T. K. Eisenstein. 1999. In vitro blockage of nitric oxide with aminoguanidine inhibits immunosuppression induced by an attenuated strain of Salmonella typhimurium, potentiates Salmonella infection, and inhibits macrophage and polymorphonuclear leukocyte influx into the spleen. Infect. Immun. 67:891-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsudaira, P. 1987. Sequence from picomole quantities of proteins electroblotted onto PVDF membrane. J. Biol. Chem. 262:10035-10038. [PubMed] [Google Scholar]
- 19.Miller, S. I., A. M. Kukral, and J. J. Mekalanos. 1989. A two-component system (phoP-phoQ) controls Salmonella typhimurium virulence. Proc. Natl. Acad. Sci. USA 86:5054-5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mittrucker, H. W., and S. H. F. Kaufmann. 2000. Immune response to infection with Salmonella typhimurium in mice. J. Leukoc. Biol. 67:457-463. [DOI] [PubMed] [Google Scholar]
- 21.Munson, R. S., J. L. Shenep, S. J. Barenkamp, and D. M. Granoff. 1983. Purification and comparison of outer membrane protein P2 from Haemophilus influenzae type b isolates. J. Clin. Investig. 72:677-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Farrell, P. H. 1975. High resolution two-dimensional electrophoresis of proteins. J. Biol. Chem. 250:4007-4021. [PMC free article] [PubMed] [Google Scholar]
- 23.Ouchterlony, O., and L. A. Wilson. 1980. Immunodiffusion and immunoelectrophoresis, p. 32.1-32.5. In D. M. Weir, L. A. Herzerberg, and C. Blackwell (ed.). Handbook of experimental immunology, 4th ed., vol. I. Blackwell, Oxford, United Kingdom. [Google Scholar]
- 24.Perez, C., G. M. Calderon, C. Ximenez, and L. Melendro. 1996. Human cell-mediated immune responses to antigenic fractions of Salmonella typhi. Immunology 89:267-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puente, J. L., V. Alvarez-Scherer, G. Gosset, and E. Calva. 1989. Comparative analysis of the Salmonella typhi and Escherichia coli ompC gene. Gene 83:197-206. [DOI] [PubMed] [Google Scholar]
- 26.Puente, J. L., A. Verdugo-Rodriguez, and E. Calva. 1991. Expression of Salmonella typhi and Escherichia coli OmpC is influenced differently by medium osmolarity; dependence on Escherichia coli OmpR. Mol. Microbiol. 5:1205-1210. [DOI] [PubMed] [Google Scholar]
- 27.Puente, J. L., V. Flores, M. Fernandez, Y. Fuchs, and E. Calva. 1987. Isolation of an ompC-like outer membrane protein gene from Salmonella typhi. Gene 61:75-83. [DOI] [PubMed] [Google Scholar]
- 28.Rajagopalan, P., R. Kumate, and A. N. Maslaviya. 1982. A study of humoral and cell mediated immune response following typhoid vaccination in human volunteers. Clin. Exp. Immunol. 47:275. [PMC free article] [PubMed] [Google Scholar]
- 29.Reed, J., and H. Muench. 1938. A simple method for estimating the fifty percent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 30.Schlecht, S., and O. Westphal. 1966. Wachstum und lipopolysaccaride (O-Antigen)-Gehalt von Salmonella beizuchtung auf Ararnahrboden. Zentrabl. Bakteriol. Parastenkd. Infektionsker. Hyg. Abt. 200:241-259. [PubMed] [Google Scholar]
- 31.Schlecht, S., H. Mossmann, K. H. Wiesmuller, G. Jung, and W. G. Blesser. 1996. Protection against bacterial infection by urea extracts from Salmonella. Zentrabl. Bakteriol. 284:559-564. [DOI] [PubMed] [Google Scholar]
- 32.Shahane, V., V. Muley, A. Kagal, and R. Bharadwaj. 2007. Non-typhoid Salmonellosis: emerging infection in Pune. Indian J. Med. Microbiol. 25:173-174. [DOI] [PubMed] [Google Scholar]
- 33.Stone, K. L., M. B. Crawford, J. M. R. DeAngelis, and K. R. Williams. 1991. Reversed-phase HPLC separation of sub-nanomole amounts of peptides obtained from enzymatic digests, p. 669-677. In C. T. Mant and R. S. Hodges (ed.), High-performance liquid chromatography of peptides and proteins: separation, analysis, and conformation. CRC, Boca Raton, FL.
- 34.Tjian, R., D. Stinchcomb, and R. Losick. 1975. Antibody directed against Bacillus subtilis rho factor purified by sodium dodecyl sulfate slab gel electrophoresis. Effect on transcription by RNA polymerase in crude extracts of vegetative and sporulating cells. J. Biol. Chem. 250:8824-8828. [PubMed] [Google Scholar]
- 35.Towbin, H. T., T. Staehelin, and J. Gordon. 1992. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. 1979. Biotechnology 24:145-149. [PubMed] [Google Scholar]
- 36.Virji, M., K. Zak, and J. E. Heckels. 1986. Monoclonal antibodies to gonococcal outer membrane protein IB: use in investigation of the potential protective effect of antibodies directed against conserved and type-specific epitopes. J. Gen. Microbiol. 132:1621-1629. [DOI] [PubMed] [Google Scholar]