Abstract
Elderly individuals are susceptible to pneumococcal infections. Although factors contributing to the increased susceptibility of the elderly to bacterial infections may be several, compromised immune function, a consequence of normal human ageing, is widely accepted to play a role. We evaluated the effect of ageing on the concentrations of naturally acquired antibodies to pneumococcal capsular polysaccharides (PPS) and protein antigens. The concentrations of immunoglobulin G (IgG) and IgM antibodies to the PPS of serotypes 3, 4, 6B, 9V, 14, and 23F and IgG antibodies to the pneumococcal virulence-associated proteins CbpA, LytC, PhtD and its C-terminal fragment (PhtD C), NanA, PspA fam1, and PspA fam2 were measured by enzyme immunoassay in the sera of younger (30 to 64 years of age) and elderly (65 to 97 years of age) adults. The concentrations of anti-PPS IgG against serotypes 3 and 6B, of anti-PPS IgM against serotypes 3, 4, 6B, 9V, and 23F, and of anti-protein IgG against all tested antigens were significantly lower in the elderly than in younger adults. A stronger decline in anti-PPS antibody concentrations was seen with age in women compared to men, while anti-protein antibody concentrations were mainly similar between the genders. Age, gender, and the nature of the antigen have substantial and varying effects on the antibody concentrations in the sera of adults.
Streptococcus pneumoniae causes a wide variety of infections, ranging from common upper respiratory tract infections to rare, severe, and potentially life-threatening conditions, including pneumonia, bacteremia, and meningitis. A major individual risk factor for pneumococcal infections is ageing (40), which can be seen by the increasing incidence of community-acquired pneumonia (CAP) and invasive pneumococcal disease (IPD) in the elderly. S. pneumoniae is an important pathogen in CAP (10), a common disorder among the aged.
Ageing of the immune system contributes to the increased susceptibility to infections in the elderly, although many coexisting chronic illnesses accumulated in elderly people likely act as important underlying cofactors (6). The mechanisms involved in the impaired immune defense are still poorly understood. Ageing is known to have widespread effects on the immune system, including decreases in B- and T-lymphocyte production, as well as perturbations in the function of mature B and T cells (24, 44). These age-associated changes lead up to an impairment of both humoral and cell-mediated immunity, causing a generalized decrease in immune responsiveness. As a consequence, the duration of humoral response is shorter and the quality of produced immunoglobulins is impaired in the aged compared to younger adults (21).
Exposure to S. pneumoniae induces natural antibodies against pneumococcus in the sera of children (29, 42) and adults (11). Existing data on the concentrations of antibodies against pneumococcal antigens acquired during periods of pneumococcal carriage and disease in an unvaccinated elderly population are limited. Concentrations of immunoglobulin G (IgG) antibodies to pneumococcal capsular polysaccharides (PPS) have been found to remain unchanged or decrease by age, depending on the serotype and the study (1, 33, 35). Age-specific development of antibody concentrations to pneumococcal proteins PsaA, PspA, and pneumolysin from young to old has been assessed in a Kenyan study with no decline in ageing adults (20). No previous data are available on the concentrations of IgM antibodies to PPS in the elderly, but a dramatic decline in the numbers of IgM memory B cells has been found with ageing (38).
We determined the concentrations of naturally acquired IgG and IgM antibodies in a large number of sera from younger (30 to 64 years of age) and elderly (≥65 years of age) adults to PPS of six serotypes commonly causing IPD in the elderly. In addition, the concentrations of IgG antibodies to seven essential pneumococcal virulence-associated proteins were analyzed. The antibody results of the elderly were compared to those of the younger adults to evaluate whether any age-associated changes could be demonstrated in the antibody concentrations. We found that age, gender, and the nature of the antigen have substantial and varying effects on the antibody concentrations in the sera of adults.
MATERIALS AND METHODS
Study population and clinical samples.
Serum samples for the purposes of the present study came from the Health 2000 Study, a nationally representative health survey of 9,922 adults aged 18 years or older, carried out in Finland in 2000 to 2001 (http://www.ktl.fi/health2000). A serum sample of each participant aged 30 years or older has been reserved for infectious disease serology. The study protocol was accepted by the project group of the Health 2000 study and evaluated by the ethics committee of the National Public Health Institute. Altogether, 600 randomly picked serum samples were received: 300 samples from younger adults (aged 30 to 64 years; 150 men and 150 women), with a mean age of 48 years, and 300 samples from elderly adults (aged 65 to 97 years; 150 men and 150 women), with a mean age of 77 years. The 300 younger adults were further stratified into three age groups with mean ages of 37 (30 to 44 years; n = 100), 48 (45 to 54 years; n = 100), and 59 (55 to 64 years; n = 100) years. The 300 elderly adults were further stratified into three age groups with mean ages of 69 (65 to 74 years; n = 120), 79 (75 to 84 years; n = 120), and 88 (≥85 years; n = 60) years. None of the participants had received pneumococcal vaccination. Concentrations of IgG antibodies to PPS and protein antigens were measured in all sera available. The concentrations of IgM antibodies to PPS were measured in a subgroup of 240 randomly picked sera from younger (aged 30 to 63 years; 60 men and 60 women) and elderly (aged 65 to 97; 60 men and 60 women) adults.
Measurement of antibodies to PPS and proteins. (i) Antigens for enzyme immunoassay (EIA). (a) Polysaccharide antigens.
The PPS of types 3, 4, 6B, 9V, 14, and 23F were obtained from the American Type Culture Collection (Manassas, VA). These serotypes are important pathogens in the elderly population in Finland (17, 34) and have different immunogenic profiles (39).
(b) Protein antigens.
The recombinant CbpA, LytC, PhtD, and the C-terminal fragment of PhtD (PhtD C) were received from GlaxoSmithKline Biologicals (Rixensart, Belgium). The recombinant PhtD C comprised the C-terminal end of the PhtD protein, the putatively exposed and protection-eliciting part of PhtD. The recombinant PspA family 1 and 2 antigens (PspA1 and PspA2) and NanA were kindly provided by D. Briles and S. Hollingshead from the University of Alabama (Birmingham).
(ii) EIA for serum IgG and IgM anti-PPS antibodies.
A modification of the 22F inhibition EIA method applied by the WHO reference laboratory at the Institute of Child Health (London, United Kingdom) was used to measure the concentrations of anti-PPS IgG and IgM in human sera (http://www.vaccine.uab.edu/). Briefly, purified PPS were mixed with phosphate-buffered saline (PBS), except for capsular PPS of serotype 3 that was mixed with methylated human serum albumin (5 μg/ml), and were adsorbed onto the wells of Maxisorp microtiter plates (Nunc, Roskilde, Denmark) overnight at 22°C or for 5 h at 37°C. The plates were washed between each step four times with PBS containing 0.05% Tween 20 (PBS-T), except before the substrate, when they were washed thrice with PBS-T and twice with purified water. The wells were blocked with 10% fetal bovine serum in PBS (PBS-F) for 1 h at 37°C. Serum samples were diluted in PBS-F containing 10 μg of cell wall polysaccharide (Statens Serum Institut, Copenhagen, Denmark)/ml and 30 μg of PPS of serotype 22F (ATCC)/ml and were incubated overnight at 4°C. 89-SF4 reference serum received from the U.S. Food and Drug Administration was diluted in PBS-F containing 10 μg of cell wall polysaccharide only/ml and incubated overnight at 4°C. The sera were further diluted in the wells of microtiter plates with PBS-F and incubated for 2 h at 37°C. The bound antibodies were detected by using alkaline phosphatase-conjugated anti-human IgG and anti-human IgM (Sigma Immuno Chemicals, St. Louis, MO) diluted in PBS-F and incubated for 2 h at 37°C. The color was developed by using a substrate solution containing 1 mg of p-nitrophenyl phosphate disodium (Sigma Immuno Chemicals, St. Louis, MO) in 1 ml of carbonate buffer (pH 9.8), followed by incubation of the plates for 1 h at 37°C. The optical densities were measured at 405 nm. The results are expressed as micrograms per milliliter calculated on the basis of the assigned IgG and IgM concentrations in reference serum 89-SF4 (28). If the antibody concentration was less than the limit of quantitation, the concentration was assigned a value of half of the quantitation limit. The reproducibility of the EIA was confirmed by using two control sera on each plate. Depending on the PPS antigen used, the interassay coefficient of variation of the control sera for anti-PPS IgG and for anti-PPS IgM ranged from 10 to 20% and from 13 to 22%, respectively.
(iii) EIA for serum IgG anti-protein antibodies.
Briefly, the pneumococcal recombinant protein antigens CbpA, LytC, NanA, PhtD, and PhtD C were mixed with PBS and adsorbed onto the wells of Greiner (Greiner Bio-One, Frickenhausen, Germany) microtiter plates overnight at 4°C. Maxisorp microtiter plates were used for recombinant PspA1 and PspA2 antigens. The plates were washed, and the wells were blocked as described above. Serum samples were diluted in PBS-F and pipetted in duplicates onto the wells of microtiter plates. The plates were incubated, and bound antibodies were detected as described above. The color was developed, and the optical densities were measured as described above. The results for the anti-CbpA, anti-LytC, anti-PhtD, and anti-PhtD C concentrations were calculated in micrograms per milliliter by using the reference serum Lucron H006W11 (Lucron Bioproducts, De Pinte, Belgium) assigned by the method of Zollinger and Boslego (45) to contain 23.49, 3.89, 12.31, and 2.99 μg of anti-CbpA, anti-LytC, anti-PhtD, and anti-PhtD C/ml, respectively. Anti-NanA, anti-PspA1, and anti-PspA2 antibody concentrations were calculated in micrograms per milliliter by using the reference serum Sandoglobulin (Sandoz, Nurnberg, Germany) assigned to contain 14.43, 15.96, and 12.39 μg of anti-NanA, anti-PspA1, and anti-PspA2/ml, respectively. The reference serum was calibrated against a human serum pool provided by David Briles and Susan Hollingshead with assigned antibody concentrations to PspA1 (25), PspA2, and NanA. If the antibody concentration was less than the limit of quantitation, the concentration was assigned a value of half of the quantitation limit. The reproducibility of the EIA was confirmed by using two control sera on each plate. Depending on the protein antigen used, the interassay coefficient of variation of the control sera ranged from 14 to 21%.
Statistical methods.
The results are reported as geometric mean concentrations (GMCs) with 95% confidence intervals (CI). Log-transformed data were used for the statistical comparisons. Antibody concentrations between age and gender were compared by using the Student t test.
RESULTS
Effect of ageing and gender on naturally acquired anti-PPS IgG.
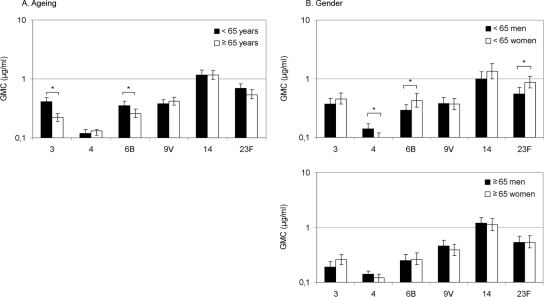
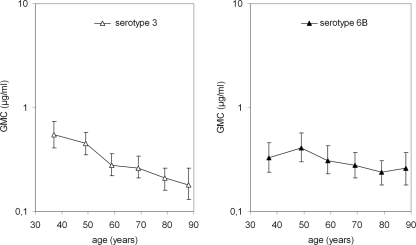
Concentrations of IgG antibodies to PPS, especially to serotype 4, were low in both younger and elderly adults (Fig. 1A). The GMCs of antibodies to serotypes 3 and 6B were significantly lower in elderly than in younger adults (P < 0.001 and P = 0.017, respectively). Stratification of data to six age groups showed a clear decrease in antibody concentration to serotype 3 with ageing and a slight decrease in antibody concentration to serotype 6B with ageing (Fig. 2). For other serotypes tested, the GMCs of antibodies did not differ significantly between younger and elderly individuals. Further stratification of data did not provide additional information; there was variation in antibody concentrations with age (data not shown).
FIG. 1.
Effect of ageing and gender on naturally acquired serum IgG antibodies against PPS in adults. (A) Anti-PPS IgG antibodies in the sera of younger (n = 300) and elderly (n = 300) adults. *, P < 0.05 (Student t test). (B) Anti-PPS IgG antibodies in the sera of younger men (n = 150) and women (n = 150) and in the sera of elderly men (n = 150) and women (n = 150). *, P < 0.05 (Student t test).
FIG. 2.
Effect of ageing on naturally acquired serum IgG antibodies against serotype 3 and 6B PPS in further stratified age groups with mean ages of 37, 48, 59, 69, 79, and 88 years (n = 100, 100, 100, 120, 120, and 60, respectively).
At younger ages, women tended to have higher antibody concentrations than men to all tested serotypes, except serotypes 4 and 9V (Fig. 1B). Younger women had significantly higher concentrations of anti-6B (P = 0.030) and anti-23F (P = 0.007) antibodies than younger men, while men had higher concentrations of anti-4 antibody than women (P = 0.037) (Fig. 1B). In elderly men and women the concentrations of anti-PPS antibodies to all serotypes were similar (Fig. 1B). Elderly women had significantly lower anti-PPS antibody concentrations than younger women to serotypes 3 (P < 0.001), 6B (P = 0.009), and 23F (P = 0.006), while elderly men had lower antibody concentrations than younger men to serotype 3 only (P < 0.001).
Effect of ageing and gender on naturally acquired anti-PPS IgM.
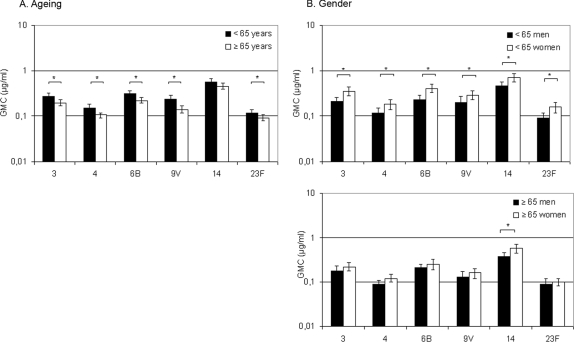
The GMCs of IgM antibodies to PPS were significantly lower in elderly than in younger adults for all tested serotypes, except for type 14 (Fig. 3A). Stratification of data showed an age-dependent decrease in IgM antibody concentrations to all serotypes; types 3, 9V, and 14 represent various effects of ageing on IgM concentration (Fig. 4).
FIG. 3.
Effect of ageing and gender on naturally acquired serum IgM antibodies against PPS in adults. (A) Anti-PPS IgM antibodies in the sera of younger (n = 120) and elderly (n = 120) adults. *, P < 0.05 (Student t test). (B) Anti-PPS IgM antibodies in the sera of younger men (n = 60) and women (n = 60) and in the sera of elderly men (n = 60) and women (n = 60). *, P < 0.05 (Student t test).
FIG. 4.
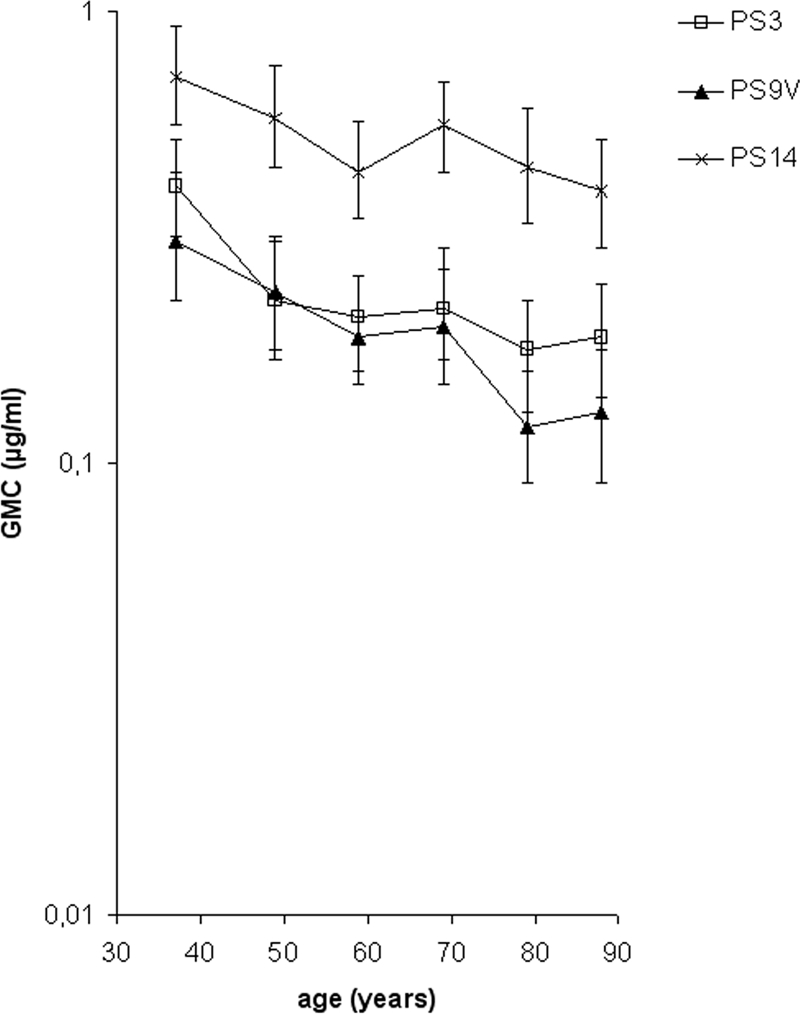
Effect of ageing on naturally acquired serum IgM antibodies against serotype 3, 9V, and 14 PPS in further stratified age groups with mean ages of 37, 48, 59, 69, 79, and 88 years (n = 100, 100, 100, 120, 120, and 60, respectively).
Younger women had significantly higher anti-PPS IgM concentrations than younger men for all serotypes, while in elderly men and women the anti-PPS IgM concentrations were similar, except for serotype 14 (Fig. 3B). In women, the GMCs of anti-PPS IgM antibodies decreased significantly with age for serotypes 3, 4, 6B, 9V, and 23F. In men, the decreases were significant for serotypes 4 and 9V only.
Effect of ageing and gender on naturally acquired anti-protein IgG.
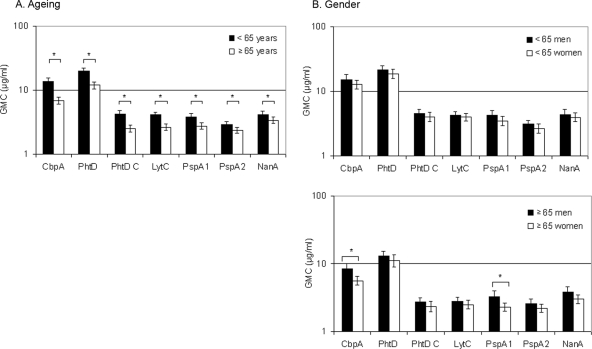
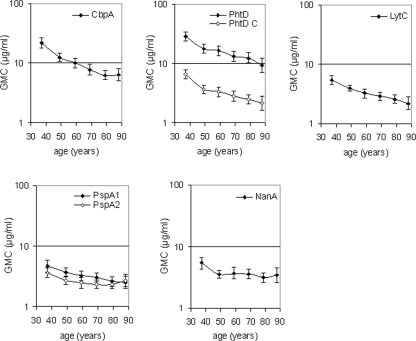
The GMCs of antibodies to pneumococcal virulence-associated proteins were significantly lower in elderly than in younger adults for all antigens tested (Fig. 5A). Stratification of data to six age groups showed a clear decrease in anti-protein antibody concentrations with ageing (Fig. 6).
FIG. 5.
Effect of ageing and gender on naturally acquired serum anti-protein IgG antibodies in adults. (A) Anti-protein IgG antibodies in the sera of younger (n = 300) and elderly (n = 300) adults. *, P < 0.05 (Student t test). (B) Anti-protein IgG antibodies in the sera of younger men (n = 150) and women (n = 150) and in the sera of elderly men (n = 150) and women (n = 150). *, P < 0.05 (Student t test).
FIG. 6.
Effect of ageing on naturally acquired serum antibodies against pneumococcal proteins in further stratified age groups with mean ages of 37, 48, 59, 69, 79, and 88 years (n = 100, 100, 100, 120, 120, and 60, respectively).
In contrast to anti-PPS antibodies, the anti-protein IgG concentrations in younger men and women were similar (Fig. 5B). Elderly women had significantly lower concentrations of antibodies to CbpA (P < 0.001) and PspA1 (P = 0.001) than elderly men (Fig. 5B). Nevertheless, the GMCs in the oldest age group (≥85 years of age) were similar in men and women (data not shown). Elderly women had significantly lower IgG antibody concentrations than younger women to all protein antigens but PspA2. In men, the decreases were significant for CbpA, PhtD, PhtD C, LytC, and PspA1.
DISCUSSION
The objective of the present study was to determine the effect of ageing on serum concentrations of naturally acquired antipneumococcal antibodies. The IgG concentrations especially to pneumococcal virulence-associated proteins, as well as to some PPS, and the IgM concentrations to PPS decrease with increasing age. By subdividing the groups, age-dependent and relatively steady decreases in antibody concentrations were seen. A stronger decline in anti-PPS antibody concentrations was seen with age in women compared to men, while the effects of ageing on anti-protein IgG concentrations were similar between the genders.
Information on the concentration of antibodies to pneumococcus acquired during the periods of pneumococcal carriage and disease in an unvaccinated elderly population is sparse. The concentrations of naturally acquired anti-PPS IgG antibodies have been shown to either decrease or remain unchanged with ageing. In a recent seroepidemiological study (1), the serotype-specific IgG concentrations were shown to remain high in older adults. However, the vaccination status of the subjects was not reported, and the authors suggest that the sustained antibody concentrations in adults aged ≥65 years may partly result from 29% uptake of PPS vaccine in this group. In an analysis of naturally acquired anti-PPS antibodies in Finnish adults reported in 1996 (35), the elderly subjects had significantly lower GMCs of antibodies than the younger persons to five (serotypes 4, 6B, 9V, 19F, and 23F) of six studied serotypes, while in the present study the GMCs of antibodies to two of six serotypes were significantly lower in elderly compared to younger adults. The difference between these two studies may be partly associated with the lower specificity of the EIA method used in 1996 compared to the currently used EIA optimized for PPS specificity by an additional adsorption step with the PPS of serotype 22F (14). In both studies, the lowest GMCs for anti-PPS were detected with serotype 4, which may be explained by poor immunogenicity of this PPS and/or by the low carriage rate of serotype 4 in the adult population (11, 30). The varying effect of ageing on IgG antibody concentrations to different PPS is in agreement with previous studies (33, 35). It may reflect the differences in the antigenic properties of pneumococcal capsular antigens combined with different immune properties of young and elderly adults and/or differences in the prevalence of pneumococcal carriage by various serotypes.
The effect of ageing on serum IgG antibody concentrations was found to be related to the nature of the pneumococcal antigen: anti-protein IgG concentrations against all analyzed pneumococcal proteins decreased significantly with age, while the decrease in anti-PPS IgG was less pronounced and serotype dependent. This finding was interesting, but not surprising. In general, B-cell responses to foreign protein antigens are T cell dependent, performed by B2 cells and modulated by cytokines, while carbohydrate antigens are T cell independent and stimulate B1 cells. Ageing has been suggested to compromise antibody response of B2 but not B1 lymphocytes (15). Accordingly, antibody responses to T-cell-dependent antigens have been shown to be significantly more affected by ageing than those to T-cell-independent antigens (23, 41). Changes in B-cell function during ageing have been largely attributed to underlying deficiencies in T-cell function (24), as well as to changes in the B-cell repertoire (44).
There may be differences between men and women in the immune response to different types of antigens and in the extent that ageing affects these responses. In earlier studies, the IgG antibody concentrations against PPS antigens have been lower in elderly women than in elderly men (32, 35). In the present study, the mean antibody concentrations in elderly women did not differ from those in elderly men, but a more pronounced decrease in the anti-PPS IgG and IgM concentrations was seen in women with ageing. At a younger age, the concentrations of anti-PPS antibodies, especially IgM, were found to be higher in women than in men. This difference may partly reflect the higher exposure by younger women to the pathogen when the household includes young children. However, the difference was not seen in anti-protein antibody concentrations, which tended to be slightly lower in women than in men.
Men generally have a greater risk for pneumococcal diseases at all ages: both IPD (2, 3, 34) and CAP (17) are more common among men than women. The factors contributing to the greater susceptibility of men to IPD or CAP are not fully understood. A recent study in the United States has, however, found a slight but significant increase in the risk of IPD with the pediatric serotypes among elderly women compared to elderly men (9). Whether this reflects differences in exposure to children between elderly men and women remains unclear. Our antibody data are difficult to link with the disease susceptibility: elderly men and women had similar antipneumococcal antibody concentrations, but the decreases in antibody concentrations with ageing were more pronounced in women. An explanation for the differences may partially lie in the functionality of antibodies or in other immunological factors. Also, physiological and lifestyle risk factors for pneumococcal infections, such as coronary artery disease, alcoholism, history of smoking, and smoking-related lung diseases (3, 18, 22, 43), which are more common in men than women, may play an important role. Smoking at all ages is associated with more numerous pneumococcal infections (26) and a higher carrier rate for pneumococci (13), which may in turn result in increased concentrations of antipneumococcal antibodies among smokers, as suggested by our preliminary data (data not shown).
Age-related changes in serum antipneumococcal antibody concentrations were mainly subtle, and their possible effect on the age and/or gender distribution of pneumococcal diseases remains uncertain. Interestingly, while IgG and IgM antibody concentrations specific to pneumococcal antigens tended to decrease with ageing, the serum levels of total IgG and IgM antibodies have been shown to increase and remain unchanged, respectively, with age (12). These data, combined with the previous data showing a more marked reduction with age in functional activity than in concentration of anti-PPS IgG antibodies after immunization with PPS vaccine (PPSV) (8, 33), suggest a significant total effect of ageing on functional antibody response to pneumococcus. Low concentrations of IgM antibodies to bacterial capsular polysaccharides and a low frequency of IgM memory B cells have been shown to correlate with the susceptibility to infections with encapsulated bacteria (4, 19). IgM memory B cells are suggested to have a function similar to that of mouse B1 lymphocytes in producing natural IgM antibodies to offer immediate protection against most microorganisms and principal protection against encapsulated bacteria (5). A dramatic decline in the number of IgM memory B cells in the aged has been suggested to result in poor humoral immunity against pneumococcal infections (38). The significantly lower concentration of anti-PPS IgM antibodies in the elderly compared to younger adults may thus contribute to the increased incidence of pneumococcal diseases in the elderly. Likewise, the significantly lower concentrations of anti-PPS IgM in younger men compared to younger women could partly explain the greater susceptibility of men to pneumococcal diseases.
Studies of natural immunity against S. pneumoniae offer important background information for pneumococcal vaccine development. The vaccine currently recommended for the prevention of pneumococcal infections in adults is PPSV. The efficacy of PPSV against IPD in adults is well documented, but the duration of protective immunity has proven to be shorter in elderly adults than in younger adults (36). In pneumococcal conjugate vaccines (PCV), the PPS antigens have been covalently coupled to a carrier protein to improve the immunogenicity of PPS antigens in children. Early studies using experimental PCV to immunize elderly adults found antibody responses induced by PCV and PPSV comparable (27, 37), but recent studies have demonstrated the superiority of the commercial 7-valent PCV (7, 16). The current infant vaccine covers only 56% of the serotypes causing IPD in the elderly in the United States (31). Pneumococcal proteins are currently being evaluated as a potential alternative approach for antipneumococcal immunization with broader coverage. Several candidate proteins have proved to be strongly protective against pneumococcal carriage and disease in animal models, but the protective value of the antibodies induced by these proteins in humans is unclear. Whether the decline in naturally acquired anti-protein antibody concentrations with ageing found in the present study holds true also for vaccine-induced anti-protein antibodies and whether these antibodies are protective against pneumococcal infections in humans remain to be determined.
In conclusion, we have analyzed the age- and gender-specific concentrations of natural antipneumococcal antibodies with ageing in Finnish adults. We found that particularly the IgG antibody concentrations to pneumococcal protein antigens and the IgM antibody concentrations to PPS antigens decrease significantly with increasing age. A stronger decline in anti-PPS antibody concentrations was seen with age in women than in men. These findings suggest an impairment of the immune defenses consistent with the increasing incidence of pneumococcal infections in the elderly. The antibody concentrations do not, however, tell the whole truth, and it is therefore important to also study the possible age-associated changes in functionality of these antibodies.
Acknowledgments
The present study was supported by the Academy of Finland, the Päivikki and Sakari Sohlberg Foundation, and the Program of Healthy Aging of the National Public Health Institute.
We thank Jan Poolman and coworkers from GlaxoSmithKline Biologicals (Rixensart, Belgium) and D. Briles and S. Hollingshead from the University of Alabama (Birmingham) for providing the pneumococcal protein antigens for EIA. We thank Sinikka Grönholm, Teija Jaakkola, Leena Saarinen, and Pekka Väisänen for technical assistance with the antibody measurements. We thank Esa Ruokokoski and Arja Vuorela for skilled data management and Sirkka Rinne, Pirkko Alha, and Harri Rissanen for assistance with the Health 2000 Study samples.
H.K. has provided consultancies on advisory boards for Sanofi Pasteur and GSK Bio; has had travels paid for by Sanofi Pasteur, GSK Bio, and Wyeth Vaccines as an invited speaker or expert at symposia; and has received honoraria from Sanofi Pasteur, GSK Bio, and Wyeth Vaccines.
Footnotes
Published ahead of print on 2 July 2008.
REFERENCES
- 1.Balmer, P., R. Borrow, J. Findlow, R. Warrington, S. Frankland, P. Waight, R. George, N. Andrews, and E. Miller. 2007. Age-stratified prevalences of pneumococcal-serotype-specific immunoglobulin G in England and their relationship to the serotype-specific incidence of invasive pneumococcal disease prior to the introduction of the pneumococcal 7-valent conjugate vaccine. Clin. Vaccine Immunol. 14:1442-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruce, M. G., S. L. Deeks, T. Zulz, D. Bruden, C. Navarro, M. Lovgren, L. Jette, K. Kristinsson, G. Sigmundsdottir, K. B. Jensen, O. Lovoll, J. P. Nuorti, E. Herva, A. Nystedt, A. Sjostedt, A. Koch, T. W. Hennessy, and A. J. Parkinson. 2008. International circumpolar surveillance system for invasive pneumococcal disease, 1999-2005. Emerg. Infect. Dis. 14:25-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burman, L. A., R. Norrby, and B. Trollfors. 1985. Invasive pneumococcal infections: incidence, predisposing factors, and prognosis. Rev. Infect. Dis. 7:133-142. [DOI] [PubMed] [Google Scholar]
- 4.Carsetti, R., M. M. Rosado, S. Donnanno, V. Guazzi, A. Soresina, A. Meini, A. Plebani, F. Aiuti, and I. Quinti. 2005. The loss of IgM memory B cells correlates with clinical disease in common variable immunodeficiency. J. Allergy Clin. Immunol. 115:412-417. [DOI] [PubMed] [Google Scholar]
- 5.Carsetti, R., M. M. Rosado, and H. Wardmann. 2004. Peripheral development of B cells in mouse and man. Immunol. Rev. 197:179-191. [DOI] [PubMed] [Google Scholar]
- 6.Crossley, K. B., and P. K. Peterson. 1996. Infections in the elderly. Clin. Infect. Dis. 22:209-215. [DOI] [PubMed] [Google Scholar]
- 7.De Roux, A., B. Schmöele-Thoma, G. R. Siber, J. G. Hackell, A. Kuhnke, N. Ahlers, S. A. Baker, A. Razmpour, E. A. Emini, P. D. Fernsten, W. C. Gruber, S. Lockhart, O. Burkhardt, T. Welte, and H. M. Lode. 2008. Comparison of pneumococcal conjugate polysaccharide and free polysaccharide vaccines in elderly adults: conjugate vaccine elicits improved antibacterial immune responses and immunological memory. Clin. Infect. Dis. 46:1015-1023. [DOI] [PubMed] [Google Scholar]
- 8.Devaster, J., I. Leroux-Roels, G. Leroux-Roels, P. Vandepapiliere, Y. Horsmans, I. Henckaerts, and J. Poolman. 2006. Inferior humoral response in elderly versus young adults to the 23-valent polysaccharide vaccine, p. 244. In Program and abstract book of the 5th International Symposium on Pneumococci and Pneumococcal Diseases, Alice Springs, Australia.
- 9.Feikin, D. R., K. P. Klugman, R. R. Facklam, E. R. Zell, A. Schuchat, and C. G. Whitney. 2005. Increased prevalence of pediatric pneumococcal serotypes in elderly adults. Clin. Infect. Dis. 41:481-487. [DOI] [PubMed] [Google Scholar]
- 10.Fine, M. J., M. A. Smith, C. A. Carson, S. S. Mutha, S. S. Sankey, L. A. Weissfeld, and W. N. Kapoor. 1996. Prognosis and outcomes of patients with community-acquired pneumonia: a meta-analysis. JAMA 275:134-141. [PubMed] [Google Scholar]
- 11.Goldblatt, D., M. Hussain, N. Andrews, L. Ashton, C. Virta, A. Melegaro, R. Pebody, R. George, A. Soininen, J. Edmunds, N. Gay, H. Kayhty, and E. Miller. 2005. Antibody responses to nasopharyngeal carriage of Streptococcus pneumoniae in adults: a longitudinal household study. J. Infect. Dis. 192:387-393. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez-Quintela, A., R. Alende, F. Gude, J. Campos, J. Rey, L. M. Meijide, C. Fernandez-Merino, and C. Vidal. 2008. Serum levels of immunoglobulins (IgG, IgA, IgM) in a general adult population and their relationship with alcohol consumption, smoking and common metabolic abnormalities. Clin. Exp. Immunol. 151:42-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenberg, D., N. Givon-Lavi, A. Broides, I. Blancovich, N. Peled, and R. Dagan. 2006. The contribution of smoking and exposure to tobacco smoke to Streptococcus pneumoniae and Haemophilus influenzae carriage in children and their mothers. Clin. Infect. Dis. 42:897-903. [DOI] [PubMed] [Google Scholar]
- 14.Henckaerts, I., D. Goldblatt, L. Ashton, and J. Poolman. 2006. Critical differences between pneumococcal polysaccharide enzyme-linked immunosorbent assays with and without 22F inhibition at low antibody concentrations in pediatric sera. Clin. Vaccine Immunol. 13:356-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu, A., D. Ehleiter, A. Ben-Yehuda, R. Schwab, C. Russo, P. Szabo, and M. E. Weksler. 1993. Effect of age on the expressed B cell repertoire: role of B cell subsets. Int. Immunol. 5:1035-1039. [DOI] [PubMed] [Google Scholar]
- 16.Jackson, L. A., K. M. Neuzil, M. H. Nahm, C. G. Whitney, O. Yu, J. C. Nelson, P. T. Starkovich, M. Dunstan, B. Carste, D. K. Shay, J. Baggs, and G. M. Carlone. 2007. Immunogenicity of varying dosages of 7-valent pneumococcal polysaccharide-protein conjugate vaccine in seniors previously vaccinated with 23-valent pneumococcal polysaccharide vaccine. Vaccine 25:4029-4037. [DOI] [PubMed] [Google Scholar]
- 17.Jokinen, C., L. Heiskanen, H. Juvonen, S. Kallinen, K. Karkola, M. Korppi, S. Kurki, P. R. Ronnberg, A. Seppa, S. Soimakallio, et al. 1993. Incidence of community-acquired pneumonia in the population of four municipalities in eastern Finland. Am. J. Epidemiol. 137:977-988. [DOI] [PubMed] [Google Scholar]
- 18.Koivula, I., M. Sten, and P. H. Makela. 1994. Risk factors for pneumonia in the elderly. Am. J. Med. 96:313-320. [DOI] [PubMed] [Google Scholar]
- 19.Kruetzmann, S., M. M. Rosado, H. Weber, U. Germing, O. Tournilhac, H. H. Peter, R. Berner, A. Peters, T. Boehm, A. Plebani, I. Quinti, and R. Carsetti. 2003. Human immunoglobulin M memory B cells controlling Streptococcus pneumoniae infections are generated in the spleen. J. Exp. Med. 197:939-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laine, C., T. Mwangi, C. M. Thompson, J. Obiero, M. Lipsitch, and J. A. Scott. 2004. Age-specific immunoglobulin G (IgG) and IgA to pneumococcal protein antigens in a population in coastal Kenya. Infect. Immun. 72:3331-3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Linton, P. J., and K. Dorshkind. 2004. Age-related changes in lymphocyte development and function. Nat. Immunol. 5:133-139. [DOI] [PubMed] [Google Scholar]
- 22.Lipsky, B. A., E. J. Boyko, T. S. Inui, and T. D. Koepsell. 1986. Risk factors for acquiring pneumococcal infections. Arch. Intern. Med. 146:2179-2185. [PubMed] [Google Scholar]
- 23.McKearn, J. P., G. W. Miller, and J. Quintans. 1978. The immune response in NZB mice of different ages to thymus-dependent and thymus-independent phosphorylcholine antigens. Immunology 34:1063-1069. [PMC free article] [PubMed] [Google Scholar]
- 24.Miller, R. A. 1996. The aging immune system: primer and prospectus. Science 273:70-74. [DOI] [PubMed] [Google Scholar]
- 25.Nabors, G. S., P. A. Braun, D. J. Herrmann, M. L. Heise, D. J. Pyle, S. Gravenstein, M. Schilling, L. M. Ferguson, S. K. Hollingshead, D. E. Briles, and R. S. Becker. 2000. Immunization of healthy adults with a single recombinant pneumococcal surface protein A (PspA) variant stimulates broadly cross-reactive antibodies to heterologous PspA molecules. Vaccine 18:1743-1754. [DOI] [PubMed] [Google Scholar]
- 26.Nuorti, J. P., J. C. Butler, M. M. Farley, L. H. Harrison, A. McGeer, M. S. Kolczak, R. F. Breiman, et al. 2000. Cigarette smoking and invasive pneumococcal disease. N. Engl. J. Med. 342:681-689. [DOI] [PubMed] [Google Scholar]
- 27.Powers, D. C., E. L. Anderson, K. Lottenbach, and C. M. Mink. 1996. Reactogenicity and immunogenicity of a protein-conjugated pneumococcal oligosaccharide vaccine in older adults. J. Infect. Dis. 173:1014-1018. [DOI] [PubMed] [Google Scholar]
- 28.Quataert, S. A., K. Rittenhouse-Olson, C. S. Kirch, B. Hu, S. Secor, N. Strong, and D. V. Madore. 2004. Assignment of weight-based antibody units for 13 serotypes to a human antipneumococcal standard reference serum, lot 89-S(f). Clin. Diagn. Lab. Immunol. 11:1064-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rapola, S., V. Jantti, R. Haikala, R. Syrjanen, G. M. Carlone, J. S. Sampson, D. E. Briles, J. C. Paton, A. K. Takala, T. M. Kilpi, and H. Kayhty. 2000. Natural development of antibodies to pneumococcal surface protein A, pneumococcal surface adhesin A, and pneumolysin in relation to pneumococcal carriage and acute otitis media. J. Infect. Dis. 182:1146-1152. [DOI] [PubMed] [Google Scholar]
- 30.Regev-Yochay, G., M. Raz, R. Dagan, N. Porat, B. Shainberg, E. Pinco, N. Keller, and E. Rubinstein. 2004. Nasopharyngeal carriage of Streptococcus pneumoniae by adults and children in community and family settings. Clin. Infect. Dis. 38:632-639. [DOI] [PubMed] [Google Scholar]
- 31.Robinson, K. A., W. Baughman, G. Rothrock, N. L. Barrett, M. Pass, C. Lexau, B. Damaske, K. Stefonek, B. Barnes, J. Patterson, E. R. Zell, A. Schuchat, and C. G. Whitney. 2001. Epidemiology of invasive Streptococcus pneumoniae infections in the United States, 1995-1998: opportunities for prevention in the conjugate vaccine era. JAMA 285:1729-1735. [DOI] [PubMed] [Google Scholar]
- 32.Roghmann, K. J., P. A. Tabloski, D. W. Bentley, and G. Schiffman. 1987. Immune response of elderly adults to pneumococcus: variation by age, sex, and functional impairment. J. Gerontol. 42:265-270. [DOI] [PubMed] [Google Scholar]
- 33.Romero-Steiner, S., D. M. Musher, M. S. Cetron, L. B. Pais, J. E. Groover, A. E. Fiore, B. D. Plikaytis, and G. M. Carlone. 1999. Reduction in functional antibody activity against Streptococcus pneumoniae in vaccinated elderly individuals highly correlates with decreased IgG antibody avidity. Clin. Infect. Dis. 29:281-288. [DOI] [PubMed] [Google Scholar]
- 34.Sankilampi, U., E. Herva, R. Haikala, O. Liimatainen, O. V. Renkonen, and M. Leinonen. 1997. Epidemiology of invasive Streptococcus pneumoniae infections in adults in Finland. Epidemiol. Infect. 118:7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sankilampi, U., P. O. Honkanen, A. Bloigu, E. Herva, and M. Leinonen. 1996. Antibody response to pneumococcal capsular polysaccharide vaccine in the elderly. J. Infect. Dis. 173:387-393. [DOI] [PubMed] [Google Scholar]
- 36.Shapiro, E. D., A. T. Berg, R. Austrian, D. Schroeder, V. Parcells, A. Margolis, R. K. Adair, and J. D. Clemens. 1991. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N. Engl. J. Med. 325:1453-1460. [DOI] [PubMed] [Google Scholar]
- 37.Shelly, M. A., H. Jacoby, G. J. Riley, B. T. Graves, M. Pichichero, and J. J. Treanor. 1997. Comparison of pneumococcal polysaccharide and CRM197-conjugated pneumococcal oligosaccharide vaccines in young and elderly adults. Infect. Immun. 65:242-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi, Y., T. Yamazaki, Y. Okubo, Y. Uehara, K. Sugane, and K. Agematsu. 2005. Regulation of aged humoral immune defense against pneumococcal bacteria by IgM memory B cell. J. Immunol. 175:3262-3267. [DOI] [PubMed] [Google Scholar]
- 39.Siber, G. R., C. Priehs, and D. V. Madore. 1989. Standardization of antibody assays for measuring the response to pneumococcal infection and immunization. Pediatr. Infect. Dis. J. 8:S84-S91. [PubMed] [Google Scholar]
- 40.Sims, R. V., E. J. Boyko, G. Maislin, B. A. Lipsky, and J. S. Schwartz. 1992. The role of age in susceptibility to pneumococcal infections. Age Ageing 21:357-361. [DOI] [PubMed] [Google Scholar]
- 41.Smith, A. M. 1976. The effects of age on the immune response to type III pneumococcal polysaccharide (SIII) and bacterial lipopolysaccharide (LPS) in BALB/c, SJL/J., and C3H mice. J. Immunol. 116:469-474. [PubMed] [Google Scholar]
- 42.Soininen, A., H. Pursiainen, T. Kilpi, and H. Kayhty. 2001. Natural development of antibodies to pneumococcal capsular polysaccharides depends on the serotype: association with pneumococcal carriage and acute otitis media in young children. J. Infect. Dis. 184:569-576. [DOI] [PubMed] [Google Scholar]
- 43.Watt, J. P., K. L. O'Brien, A. L. Benin, S. I. McCoy, C. M. Donaldson, R. Reid, A. Schuchat, E. R. Zell, M. Hochman, M. Santosham, and C. G. Whitney. 2007. Risk factors for invasive pneumococcal disease among Navajo adults. Am. J. Epidemiol. 166:1080-1087. [DOI] [PubMed] [Google Scholar]
- 44.Weksler, M. E. 2000. Changes in the B-cell repertoire with age. Vaccine 18:1624-1628. [DOI] [PubMed] [Google Scholar]
- 45.Zollinger, W. D., and J. W. Boslego. 1981. A general approach to standardization of the solid-phase radioimmunoassay for quantitation of class-specific antibodies. J. Immunol. Methods 46:129-140. [DOI] [PubMed] [Google Scholar]