Abstract
A competitive enzyme-linked immunosorbent assay (cELISA) based on a broadly conserved, species-specific, B-cell epitope within the C terminus of Babesia bigemina rhoptry-associated protein 1a was validated for international use. Receiver operating characteristic analysis revealed 16% inhibition as the threshold for a negative result, with an associated specificity of 98.3% and sensitivity of 94.7%. Increasing the threshold to 21% increased the specificity to 100% but modestly decreased the sensitivity to 87.2%. By using 21% inhibition, the positive predictive values ranged from 90.7% (10% prevalence) to 100% (95% prevalence) and the negative predictive values ranged from 97.0% (10% prevalence) to 48.2% (95% prevalence). The assay was able to detect serum antibody as early as 7 days after intravenous inoculation. The cELISA was distributed to five different laboratories along with a reference set of 100 defined bovine serum samples, including known positive, known negative, and field samples. The pairwise concordance among the five laboratories ranged from 100% to 97%, and all kappa values were above 0.8, indicating a high degree of reliability. Overall, the cELISA appears to have the attributes necessary for international application.
Babesiosis is a serious tick-transmitted disease of cattle worldwide, with three well-recognized species each capable of producing disease, although the diseases have somewhat different pathogenicities and clinical sequelae (20). Babesia bigemina and B. bovis are often found together in the tropics and subtropics and are transmitted by Boophilus ticks, while B. divergens occurs in Europe and the Mediterranean and is primarily transmitted by Ixodes ricinus ticks. B. bovis is the most virulent species, but in most areas of endemicity, B. bigemina is more prevalent. Both, however, can cause clinical disease and carrier infections. The proper application of control programs, including the use of attenuated vaccines, relies on the ability to differentiate the species responsible for infection. While stained blood films allow the detection of parasites during acute infection for the diagnosis of acute clinical disease, this method does not lend itself as a sensitive or practical means of identifying persistently infected animals with low levels of parasitemia. Serological testing is used as a tool to determine the babesial infection status of individuals and herds in epidemiologic studies and control programs. Although a number of serologic assays have been developed and used for several years, they all suffer to some extent from limitations in stability, sensitivity, specificity, and the objectivity of reading the results or are cumbersome for use for the testing of large numbers of samples. For these reasons, we developed a competitive enzyme-linked immunosorbent assay (cELISA) based on the ability of serum antibody to inhibit a monoclonal antibody (MAb) directed against a B. bigemina-specific epitope within the C terminus of rhoptry-associated protein 1a (RAP-1a). In a previous study, we demonstrated the high specificity afforded by use of a purified recombinant B. bovis RAP-1 C-terminal construct as the antigen and the inhibition of binding of MAb to a RAP-1-specific epitope by serum antibodies. In addition, we established the ability of the assay to detect both relatively early and long-term carrier infections (7). Finally, we validated the use of the assay for international application (8). On the basis of that experience we investigated a similar approach for the detection of B. bigemina-infected cattle.
RAP-1 from B. bigemina, first described as a 58-kDa surface protein (13, 14), is a product of a multigene family (15, 18) with four nonallelic genes containing dimorphic regions that encode both B-cell and T-cell epitopes (10). A conserved B-cell epitope within the C terminus of RAP-1a is recognized by MAb 64/04.10.3 (10, 19) and was the target for the development of the cELISA described here. Recently, we determined that the epitope is useful in avoiding cross-reactions with B. bovis (3), which had been a problem when crude antigens were used (4). In the study reported here, we describe a B. bigemina-specific cELISA formatted for ease of distribution and validate the assay for global use through an international interlaboratory comparison.
MATERIALS AND METHODS
Sera.
For the extended analytical validation, 66 known positive and 474 known negative serum samples were evaluated. The known positive sera were classified on the basis of one or more of the following criteria: the samples were from animals that had experienced an experimental infection (34 samples) or were from regions of endemicity in Mexico or Puerto Rico and were positive by a B. bigemina-specific nested PCR (5), as confirmed by indirect immunofluorescence (6) (32 samples). The 474 known negative samples were from the northwest United States, where neither bovine Babesia nor the tick vector Boophilus microplus exists. Additional sera were processed from whole blood collected in Puerto Rico and Mexico; allowed to clot; and then sent to the Animal Disease Research Unit, Agricultural Research Service, U.S. Department of Agriculture, in Pullman, Washington.
For the interlaboratory comparison, a set of 100 defined serum samples was distributed among five laboratories. The sera represented 79 additional known negative samples from the United States, a single sample from an animal experimentally infected with a Mexican B. bigemina isolate, and 20 samples from cattle from Australia that had been immunized with a live B. bigemina “G” vaccine strain at the Tick Fever Research Center in Australia and that were confirmed to be infected by the microscopic detection of parasites in stained blood films. In addition, each of the 20 samples was tested in Australia by using another ELISA format, as described previously (16). All sera for the interlaboratory validation were preserved with sodium azide prior to shipment.
Two additional cattle were experimentally infected with a Puerto Rican field isolate of B. bigemina. The blood stabilate, which contained 1 × 108 infected erythrocytes, was inoculated intravenously, and serum was collected daily through 35 days postinoculation. The clinical disease was mild, and no treatment was necessary. Sera from these two animals along with sera from the cattle experimentally infected with the Australian “G” vaccine strain mentioned above were used to assess the kinetics of seroconversion.
cELISA.
The format of the cELISA was as described previously for use of the assay with B. bovis (8). The epitope recognized by MAb 64/04.10.3 is contained within amino acids 393 and 443 of the C terminus (amino acids 306 to 480) of B. bigemina RAP-1a (GenBank accession no. M60878 [http://www.ncbi.nlm.nih.gov/GenBank/index.html]). The C terminus was subcloned into a pBAD vector (rCT-rap-1aa1) and expressed as a histidine-tagged thioredoxin fusion protein purified on a ProBond resin column (Invitrogen, Carlsbad, CA). The antigen was coated and then dried onto wells of Immulon II plates. The optimal concentrations of antigen and MAb were determined by block titration. A plate washer was used at each wash step of the protocol. Prior to use, 200 μl of a blocking buffer (phosphate-buffered saline containing 0.2% Tween 20 and 20% nonfat dry milk) was added to each well, and the plates were incubated at room temperature for 1 h on a rotating platform. Each incubation step was accomplished on a rotating platform. After aspiration of the blocking buffer, 100 μl of undiluted serum was added to each well (which contained the principal test sera or control sera) and the plates were incubated at room temperature for 30 min. After aspiration of the serum from each well, 100 μl (5 ng/well) of MAb 64/04.10.3 was added and the plates were incubated at room temperature for 15 min. Each plate was then washed three times with 200 μl of wash buffer (blocking buffer minus the nonfat dry milk), followed by the addition of 100 μl of wash buffer containing a 1/4,000 dilution of conjugate (horseradish peroxidase-labeled goat anti-mouse immunoglobulin G; Kirkegaard & Perry Laboratories, Gaithersburg, MD) (each lot of new conjugate was titrated to obtain the optimal dilution). After incubation at room temperature for 15 min, each plate was washed three times, as described above, and then allowed to set for 30 to 60 s in wash buffer before a final three washes. After equal volumes of 3,3′,5,5′-tetramethylbenzidine and H2O2 were combined, according to the manufacturer's instructions (Kirkegaard & Perry Laboratories), 100 μl of the substrate was added to each well and the plates were incubated at room temperature in the dark for 15 min, followed by the addition of 50 μl of stop buffer (2 N H2SO4). By using a microtiter plate reader, the mean optical density (OD) at 450 nm was determined for all test wells and for duplicate wells of a positive control serum sample and a negative control serum sample pooled from five animals known to be negative. The percent inhibition for each test sample was determined by using the mean of each duplicate well compared to the mean of duplicate control wells and the following formula: 1 − [(OD of sample − OD of buffer)/(OD of negative control − OD of buffer)] × 100.
Interlaboratory comparison.
An aliquot of each of the 100 defined serum samples was provided to each of three laboratories in different countries as well as to another laboratory in the United States and the laboratory of the Animal Disease Research Unit, Agricultural Research Service. In addition to the defined sera, antigen plates from the same lot and conjugates, substrates, and other reagents from the same lot and/or shipment from the same vendors were also provided. All laboratories used the same protocol described above and recorded the ODs at 450 nm with plate readers. All samples were coded so that the assay in each laboratory was run in a blind fashion.
Statistical analysis.
To accurately assess the assay for its diagnostic specificity, sensitivity, and predictive values, the results for the 66 known positive and the 474 known negative samples were subjected to receiver operating characteristic (ROC) analysis, which was performed with MedCalc statistical software (version 9.3.7.0; http://www.medcalc.be); and a frequency distribution graph was generated. The concordance among the laboratories was established by using Cohen's kappa values (1). Hartley's test for homogeneity (9) and a two-way analysis of variance of the ODs among the five laboratories were also included, and statistical significance was established at a P value of <0.05.
RESULTS
Specificity, sensitivity, and predictive values.
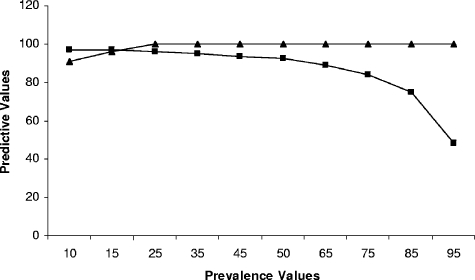
By using a hypothetical prevalence rate of 25%, ROC analysis identified the threshold inhibition value for the definition of a negative result to be 16%. With this value, the assay had a specificity of 98.3% and a sensitivity of 94.7% (Fig. 1A). The area under the ROC curve was 0.992, indicating that the assay had an excellent ability to discriminate between positive and negative samples (Fig. 1B). To increase the specificity to 100% without dramatically decreasing the sensitivity, we raised the threshold inhibition value to 21%. As a result, the sensitivity was reduced from 94.7% to 87.2%. Figure 1C shows the frequency distribution graph for the cELISA obtained with 66 known positive and 474 known negative samples and the associated threshold levels of 16% and 21%. The increase in the inhibition threshold from 16% to 21% resulted in a reduction of the negative predictive value in an area with a prevalence of 25% from 98.2% to 95.9% but increased the positive predictive value from 94.9% to 100%. At 21% inhibition, the negative predictive value varied from 97.0% in an area with a prevalence of 10% to 48.2% in an area with a prevalence of 95%. The positive predictive values were 90.7% and 95.7% in areas with prevalence rates of 10% and 15%, respectively, and 100% under all higher-prevalence conditions (Fig. 2).
FIG. 1.
ROC analysis of the cELISA with 66 known positive and 474 known negative serum samples. (A) Plot-versus-criterion graph identifying 16% inhibition as the suggested threshold, with a specificity (dashed line with 95% confidence intervals) of 98.3% and a sensitivity (solid line with 95% confidence intervals) of 94.7%; (B) ROC plot with an area under the curve of 0.992 (0.00665) and a 95% confidence interval between 0.980 and 0.997; (C) graph of distributions of the known negative (left cluster) and known positive (right cluster) serum samples by using 16% and 21% inhibition as threshold values.
FIG. 2.
Negative (squares) and positive (triangles) predictive values associated with performance of the cELISA with samples from areas with different B. bigemina disease prevalence levels. The values were determined by ROC analysis based on a 21% inhibition threshold.
Seroconversion kinetics.
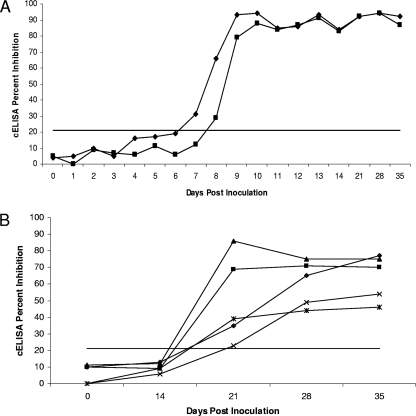
Two cattle were intravenously inoculated with approximately 108 infected erythrocytes of a Puerto Rican field isolate of B. bigemina, and five cattle were subcutaneously inoculated with approximately 106 infected erythrocytes of the Australian “G” vaccine strain. Sera were collected through 35 days postinoculation and were assessed by the cELISA by using 21% inhibition as the threshold value (Fig. 3). Both animals infected with the Puerto Rican isolate seroconverted by 8 days postinoculation (Fig. 3A), while the five cattle infected with the vaccine strain seroconverted at between days 14 and 21 postinoculation (Fig. 3B). In addition, sera from animals infected with the vaccine strain did not inhibit the binding of MAb 64/04.10.3 to the same extent as the sera from animals infected with the Puerto Rican isolate (Fig. 3), consistent with a strain-associated difference in immunogenicity.
FIG. 3.
Kinetics of seroconversion during an initial exposure of cattle to approximately 108 infected erythrocytes of a Puerto Rican field isolate of B. bigemina delivered intravenously (A) or approximately 106 Australian “G” vaccine strain-infected erythrocytes delivered subcutaneously (B). Twenty-one percent inhibition was used as the threshold.
Comparison among laboratories.
A pairwise comparison was done to determine the interlaboratory repeatability of the assay. The concordances and kappa values are shown in Table 1. The rates of agreement ranged from 97% to 100%, and all kappa values were above 0.8, indicating that the assay was reliable.
TABLE 1.
Pairwise concordance of B. bigemina RAP-1a cELISA results among laboratories
| Laboratories whose results were compared | % Concordance | Kappa valuea |
|---|---|---|
| 1 vs 2 | 98 | 0.91 |
| 1 vs 3 | 97 | 0.85 |
| 1 vs 4 | 98 | 0.91 |
| 1 vs 5 | 97 | 0.86 |
| 2 vs 3 | 99 | 0.95 |
| 2 vs 4 | 100 | 1.00 |
| 2 vs 5 | 99 | 0.96 |
| 3 vs 4 | 99 | 0.95 |
| 3 vs 5 | 99 | 0.96 |
| 4 vs 5 | 98 | 0.91 |
Kappa values above 0.70 denote satisfactory reliability.
Although there were significant (P < 0.05) differences between the ODs obtained among the five laboratories and therefore there was a lack of homogeneity, it did not affect the status of a given sample. However, after the analyses were performed in each of the five laboratories by use of the 21% threshold, disparate results were recorded for 5 of the 20 samples (Table 2). All samples with disparate results were from the group of 20 samples from animals immunized with the “G” attenuated live vaccine strain in Australia. The samples were collected at 82 days postinoculation; and disparate results were obtained between three of five laboratories for sample C-8618, two of five laboratories for C-8630 and C-8658, one of five laboratories for C-8672, and four of five laboratories for C-8681. All but 4 of the 12 samples with disparate results had levels of inhibition of between 16% and 21%.
TABLE 2.
Percent inhibition associated with samples giving discordant results between laboratories
| Sample | % Inhibitiona
|
||||
|---|---|---|---|---|---|
| Lab 1 | Lab 2 | Lab 3 | Lab 4 | Lab 5 | |
| C-8618 | 9 | 19 | 20 | 27 | 26 |
| C-8630 | 28 | 17 | 11 | 28 | 23 |
| C-8658 | 14 | 32 | 17 | 23 | 47 |
| C-8672 | 18 | 34 | 21 | 41 | 33 |
| C-8681 | 4 | 11 | 14 | 14 | 33 |
Inhibition of <21% was the threshold used for a negative result.
DISCUSSION
There is a need for a standardized diagnostic assay that can be used globally to detect B. bigemina-infected cattle. Other serological methods that have been developed and utilized in the past include an indirect immunofluorescence assay (6) and a number of ELISA-based formats (4, 17). Although these assays allow the detection of infected cattle, they all have limitations in specificity, sensitivity, and reliability to some degree; and the indirect immunofluorescence assay requires subjective interpretation and low throughput. More recently, an ELISA for the detection of B. bigemina was developed by employing crude B. bigemina antigen and two MAbs, each one of which recognized a different epitope on RAP-1a. One MAb was used as a capture antibody and the other was used for detection in a competitive format with test serum (16). This assay eliminated the problem with B. bovis cross-reactivity and was reported to have a specificity of 97.0% and a sensitivity of 95.7%. Interestingly, the capture MAb used in that assay recognizes the same epitope within the C terminus of RAP-1a recognized by MAb 64/04.10.3 used in this study (data not shown). Specificity has been demonstrated with a recombinant form of this C-terminal antigen in an indirect ELISA (3).
We previously described the development of a competitive ELISA for B. bovis that used a similar species-specific epitope within the C terminus of RAP-1 from B. bovis (7) and included an international validation (8). The usefulness of this detection format is evidenced by the recent recognition by the U.S. Department of Agriculture of similar cELISA formats as the official detection tests for the detection of both B. caballi and B. equi (11, 12) and by the recognition by the Office International des Epizooties (OIE) of the cELISA as the international standard assay for the detection of these babesial species. In order to comply with OIE validation standards, we have now defined the optimized format for both the B. bovis and the B. bigemina cELISAs; determined their specificities, sensitivities, and predictive values; and demonstrated their reliabilities. Both assays include purified recombinant antigen dried onto microtiter wells for ease of handling, distribution, and stability. The B. bigemina antigen is based on a broadly conserved, species-specific epitope within the C terminus of RAP-1a of B. bigemina (10). This antigen is recognized by antibodies from infected cattle in diverse geographic locations, and the response to this epitope persists in experimentally infected cattle for at least several months (data not shown).
There was a difference in the kinetics and the levels of antibodies detected in cattle inoculated with the attenuated Australian “G” vaccine strain and the Puerto Rican field isolate. The vaccine strain represented a lower infectious dose than the nonattenuated Puerto Rican isolate and was delivered subcutaneously instead of intravenously, perhaps contributing to the difference in kinetics. Actual transmission by ticks may also affect the kinetics, delaying seroconversion a bit longer. In addition, there are reports that multiply passaged attenuated parasites are less immunogenic (2). This could also have contributed to a decreased antibody response in this cELISA.
Babesiosis is characterized as a persistent infectious disease, and consequently, the sensitivity of a diagnostic assay or the ability to correctly identify true-negative samples is expected to be a greater issue than specificity. In addition, the specificity of this cELISA is inherently high due to the format and use of species-specific reagents. At a cutoff of 21% inhibition, the cELISA has a specificity of 100% and a sensitivity of 87.2%. This translates into positive and negative predictive values of 100% and 95.9%, respectively, in an area with a 25% prevalence and positive and negative predictive values of 100% and 84.0%, respectively, in an area with a 75% prevalence; these prevalence rates are typical of those found in areas of endemicity.
The B. bigemina cELISA has the attributes necessary for worldwide application for the detection of specific antibody, including the use of a dried antigen plate format. The overall accuracy is good and the reliability is excellent, on the basis of the concordance and the kappa values from the five laboratories that independently performed the assay. The majority of the samples that were scored differently between laboratories had inhibition levels between 16% and 21%. This suggests that the cELISA, as is the case in other assays, should be repeated for samples whose results are near the threshold by using another sample collected several days later. In some cases, supplemental diagnostics such as PCR could be used for samples with inconclusive results. Our experience with the cELISA with a limited number of field samples for which the results obtained by PCR and the cELISA have been compared suggests that resampling when the results are negative but near the threshold is important, since there is a chance that animals with very early infections would be PCR positive prior to the presence of detectable antibody (data not shown). Conversely, if the infection was cleared in an animal, the titer would decline and would progress toward and, finally, below the threshold. This is not uncommon for B. bigemina-infected animals, unlike B. bovis-infected cattle, which for the most part remain carriers for life. In particular, 7 of the 20 samples from cattle inoculated with the “G” vaccine strain used in this study were seronegative at 82 days postinoculation. This was confirmed by use of the ELISA used in Australia as well as the cELISA described here. The five samples with disparate results reported here represent samples that were still seropositive by the Australian ELISA, but they could have been from animals that had recently cleared the infection and whose titers were in decline. The accuracy and reliability of the cELISA need to be further determined by application of the assay to large numbers of serum samples collected from well-defined enzootic regions. However, the assay has the potential for use as an international standard and is formatted for ease of distribution and use under a variety of laboratory conditions.
Acknowledgments
This work was supported by grant USDA-ARS-CWU-5348-32000-010-00D.
We thank Paul Lacy, Carey Wilson, and Carmen Rojas-Martínez for excellent technical support and John Vanderschalie from the Washington Animal Disease Diagnostic Laboratory for the provision of negative serum samples. We also thank Russell Bock and Bert De Vos for constructive review of the manuscript.
D.S.A. is associated with VMRD, Inc., a company with a commercial interest in veterinary diagnostics.
Footnotes
Published ahead of print on 16 July 2008.
REFERENCES
- 1.Bakeman, R., and J. M. Gottman. 1986. Observing interaction: an introduction to sequential analysis. Cambridge University Press, Cambridge, United Kingdom.
- 2.Bock, R., L. Jackson, A. De Vos, and W. Jorgensen. 2004. Babesiosis of cattle. Parasitology 129:S247-S269. [DOI] [PubMed] [Google Scholar]
- 3.Boonchit, S., A. Alhassan, B. Chan, X. Xuan, N. Yokoyama, M. Ooshiro, W. L. Goff, S. D. Waghela, G. G. Wagner, and I. Igarashi. 2006. Expression of C-terminal truncated and full-length Babesia bigemina rhoptry-associated protein 1 and their potential use in enzyme-linked immunosorbent assay. Vet. Parasitol. 137:28-35. [DOI] [PubMed] [Google Scholar]
- 4.El-Ghaysh, A., B. Sundquist, D. A. Christensson, M. Hilali, and A. M. Nassar. 1996. Observations on the use of ELISA for detection of Babesia bigemina-specific antibodies. Vet. Parasitol. 62:51-61. [DOI] [PubMed] [Google Scholar]
- 5.Figueroa, J. V., L. P. Chieves, G. S. Johnson, and G. M. Buening. 1992. Detection of Babesia bigemina-infected carriers by polymerase chain reaction amplification. J. Clin. Microbiol. 30:2576-2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goff, W. L., G. G. Wagner, T. M. Craig, and R. F. Long. 1982. The bovine immune response to tick-derived Babesia bovis infection: serological studies of isolated immunoglobulins. Vet. Parasitol. 11:109-120. [DOI] [PubMed] [Google Scholar]
- 7.Goff, W. L., T. F. McElwain, C. E. Suarez, W. C. Johnson, W. C. Brown, J. Normine, and D. P. Knowles. 2003. Competitive enzyme-linked immunosorbent assay based on a rhoptry-associated protein 1 epitope specifically identifies Babesia bovis-infected cattle. Clin. Diagn. Lab. Immunol. 10:38-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goff, W. L., J. B. Molloy, W. C. Johnson, C. E. Suarez, I. Pino, A. Rhalem, H. Sahibi, L. Ceci, G. Carelli, D. S. Adams, T. C. McGuire, D. P. Knowles, and T. F. McElwain. 2006. Validation of a competitive enzyme-linked immunosorbent assay for detection of antibodies against Babesia bovis. Clin. Vaccine Immunol. 13:1212-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hartley, H. O. 1940. Testing the homogeneity of a set of variances. Biometrika 31:249-255. [Google Scholar]
- 10.Hotzel, I., W. C. Brown, T. F. McElwain, S. D. Rodriguez, and G. H. Palmer. 1996. Dimorphic sequences of rap-1 genes encode B and CD4+ T helper lymphocyte epitopes in the Babesia bigemina rhoptry-associated protein-1. Mol. Biochem. Parasitol. 81:89-99. [DOI] [PubMed] [Google Scholar]
- 11.Kappmeyer, L. S., L. E. Perryman, S. A. Hines, T. V. Baszler, J. B. Katz, S. G. Hennager, and D. P. Knowles. 1999. Detection of equine antibodies to Babesia caballi by recombinant B. caballi rhoptry-associated protein 1 in a competitive-inhibition enzyme-linked immunosorbent assay. J. Clin. Microbiol. 37:2285-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knowles, D. P., L. E. Perryman, L. S. Kappmeyer, and S. G. Hennager. 1991. Detection of equine antibody to Babesia equi merozoite proteins by a monoclonal antibody-based competitive inhibition enzyme-linked immunosorbent assay. J. Clin. Microbiol. 29:2056-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McElwain, T. F., L. E. Perryman, W. C. Davis, and T. C. McGuire. 1987. Antibodies define multiple proteins with epitopes exposed on the surface of live Babesia bigemina merozoites. J. Immunol. 138:2298-2304. [PubMed] [Google Scholar]
- 14.McElwain, T. F., L. E. Perryman, A. J. Musoke, and T. C. McGuire. 1991. Molecular characterization and immunogenicity of neutralization-sensitive Babesia bigemina merozoite surface proteins. Mol. Biochem. Parasitol. 47:213-222. [DOI] [PubMed] [Google Scholar]
- 15.Mishra, V. S., T. F. McElwain, J. B. Dame, and E. B. Stephens. 1992. Isolation, sequence and differential expression of the p58 gene family of Babesia bigemina. Mol. Biochem. Parasitol. 53:149-158. [DOI] [PubMed] [Google Scholar]
- 16.Molloy, J. B., P. M. Bowles, P. J. Jeston, A. G. Bruyeres, J. M. Bowden, R. E. Bock, W. K. Jorgensen, G. W. Blight, and R. J. Dalgliesh. 1998. Development of an enzyme-linked immunosorbent assay for detection of antibodies to Babesia bigemina in cattle. Parasitol. Res. 84:651-656. [DOI] [PubMed] [Google Scholar]
- 17.Morzaria, S., J. Katende, A. Kairo, V. Nene, and A. Musoke. 1992. New methods for the diagnosis of Babesia bigemina infection. Mem. Inst. Oswaldo Cruz 87(Suppl. III):201-205. [DOI] [PubMed] [Google Scholar]
- 18.Suarez, C. E., G. H. Palmer, M. Florin-Christensen, S. A. Hines, I. Hötzel, and T. F. McElwain. 2003. Organization, transcription, and expression of rhoptry associated protein genes in the Babesia bigemina rap-1 locus. Mol. Biochem. Parasitol. 127:101-112. [DOI] [PubMed] [Google Scholar]
- 19.Vidotto, O., T. F. McElwain, R. Z. Machado, L. E. Perryman, C. E. Suarez, and G. H. Palmer. 1995. Babesia bigemina: identification of B-cell epitopes associated with parasitized erythrocytes. Exp. Parasitol. 81:491-500. [DOI] [PubMed] [Google Scholar]
- 20.Wright, I. G., and B. V. Goodger. 1988. Pathogenesis of babesiosis, p. 99-118. In M. Ristic (ed.), Babesiosis of domestic animals and man. CRC Press, Boca Raton, FL.