Abstract
Mumps virus infection is a potentially serious viral infection of childhood and early adulthood. In China, live, attenuated S79 mumps virus vaccine has been licensed for pediatric use since 1990. There has been no assessment of its efficacy. Thus, the objective of this study was to determine the effectiveness of live, attenuated S79 mumps virus vaccine against clinical mumps. Cases were selected from the China Information System for Disease Control and Prevention during September 2004 to March 2005. Each case was matched to a control by gender, age, and area of residency. In all, 469 cases and 469 controls were enrolled in the study. Vaccination information was obtained from the Children's EPI Administrative Computerized System. Vaccine effectiveness (VE) was calculated for one or two doses of S79 vaccine, with 95% confidence intervals (CI). VE of mumps virus vaccine for one dose versus none was protection of 86.0% (95% CI, 77.2% to 91.5%) of recipients, and VE was much higher in the first 4 years than in the 5 to 12 years after vaccination. The S79 vaccine can effectively prevent clinical mumps, and a second dose of mumps virus vaccine is necessary for the protection of children in China.
Mumps virus infection, a potentially serious viral infection of childhood and early adulthood, may lead to meningitis (15% of all mumps patients), sensorineural deafness (5 per 100,000), pancreatitis (4%), orchitis (20 to 30% of postpubertal men with mumps), and spontaneous abortion (25% of infected pregnant women, usually in the first trimester of pregnancy) (1, 4, 5, 9, 11, 13, 16, 23, 24). There is still no effective treatment specifically for mumps.
The burden of disease and cost of mumps virus infection led to the development of a specific vaccine in China. This live, attenuated S79 mumps virus vaccine was derived from the Jeryl Lynn strain (isolated in 1979) after three successive passages in primary chicken embryo cell culture. Since 1990, several large domestic manufacturers of biological products (Shanghai, Beijing, and Lanzhou Institute of Biological Products, China) have been licensed to produce S79 strain mumps virus vaccine, and Chinese children have been immunized with more than 1,000,000,000 doses. However, few data are available on the vaccine's safety and efficacy.
In China, 1 dose of S79 live vaccine is recommended in some localities for children more than 8 months old. An assessment of the public health role of the S79 vaccine under the real-world conditions of clinical practice is now needed. Mumps is a great threat to children in Guangzhou, one of the largest and most prosperous cities in China, where 5,171 and 7,934 mumps cases (incidence rate, 70.36/100,00 and 105.53/100,000, respectively) were reported in 2004 and 2005. The S79 vaccine has been used in children since 1995 in Guangzhou, and vaccination has been voluntary. Thus, data were available for us to assess the vaccine effectiveness (VE), and we accordingly carried out a case-control study.
MATERIALS AND METHODS
Study population.
Cases were selected during September 2004 to March 2005 from the China Information System for Disease Control and Prevention, which is a physician-based system for reporting all suspected mumps cases. A case was defined as a person with a clinical diagnosis of mumps. Our case definition included acute onset of unilateral or bilateral tender swelling of the parotid or salivary gland lasting two or more days without any other apparent cause (bacterial infection was excluded by the absence of an increase in the white blood cell count).
Only children (8 months to 12 years old) whose information was found in the Children's Expanded Programmed Immunization (EPI) Administrative Computerized System were enrolled. The Children's EPI Administrative Computerized System, established in Guangzhou in 1997, was designed to manage vaccination information. The system allows health care workers to record, store, retrieve, and analyze all children's vaccination information easily. The demographic and vaccination information of all registered vaccinees could be found in the system. Demographic information included name, parents' names, gender, birth date, place of residence, home telephone number, health condition, etc. Detailed vaccination information included the number and dates of vaccination, vaccine brand name, and batch number.
Controls were confirmed to be children without symptoms of mumps (i.e., no acute onset of unilateral or bilateral tender swelling of the parotid or salivary gland). Confirmation was obtained by telephone or a face-to-face interview with the child's parent or guardian. Controls were matched to cases by gender, age, and community or village of residence.
For each case, three potential controls were randomly selected from the list generated by the Children's EPI Administrative Computerized System. Of the three potential controls, the one with a birth date closest to that of the case was interviewed first. The closeness of the date of birth to that of the case determined the order of the three interviews (for example, the control born on the same day as the case was interviewed first). Finally we selected one control for every case.
Data collection.
The information needed to enroll cases and controls was obtained by trained staff using a questionnaire. The questionnaire was designed by researchers and revised after a pilot investigation in two community hospitals.
Basic information (name, gender, birth date, home telephone number, and address) and S79 mumps virus vaccine vaccination information (total number of doses, dates of vaccination, and vaccine batch numbers) were obtained for both cases and controls.
Statistical analysis.
Data were collected and processed at the Guangzhou Centre for Disease Control and Prevention. Exclusion criteria for cases and controls were an absence of records in the Children's EPI Administrative Computerized System and previous enrollment. For cases, only those vaccinated at least 2 weeks before the onset of disease were considered valid. For controls with valid vaccination, days from birth to vaccination had to be at least 4 weeks earlier than the days from birth to onset for the matched case. Analysis was performed using SPSS statistical software (version 13.0; SPSS, Inc., Chicago, IL). Simple descriptive statistics, such as means, standard deviations, and proportions, were used when appropriate. Student's t test and χ2 tests were used to analyze group differences. VE was analyzed separately for receipt of one dose only and receipt of two doses of the S79 vaccine. VE was analyzed versus time postvaccination. VE was calculated as 1 minus the adjusted matched odds ratio (OR) × 100%, where the OR was the odds of cases developing in the vaccinated group compared with the odds of cases developing in the unvaccinated group.
Cox survival regression was used to calculate the ORs and 95% confidence intervals (CIs) (6, 10). Records (case or control) in the database were stratified according to identification number: 1 was assigned to cases and 0 to controls. Pairs that were discordant for vaccine receipt (e.g., the case received vaccination and the control received no vaccination) were also included. For all analysis, P values that were not more than 0.05 were regarded as significant.
RESULTS
We identified 1,849 children with mumps in Guangzhou between 1 September 2004 and 31 March 2005. Of these, 1,380 (74.6% of the total) were excluded because their records were not found in the Children's EPI Administrative Computerized System. The remaining 469 cases (25.4%) and their 469 controls were included in our analysis.
There was a between-group difference between the gender ratios of cases and all enrollees (male/female ratios, 1.44:1 versus 1.97:1; χ2 = 8.356; P = 0.004), but there was no such difference between ages (8.57 ± 3.10 versus 8.49 ± 3.17 years old; t = 0.497; P > 0.05).
Enrollees had a median age of 8.49 years (range, 10 months to 12 years) and resided in 12 administrative areas in Guangzhou. Cases were 0 to 5 years old (23.9%; n = 112), 6 to 9 years old (42.8%; n = 201), and more than 10 years old (33.3%; n = 156).
Mumps occurred every month, and nearly half of all cases (45.4%) occurred during December (96 cases) and January (117 cases), the months of peak onset. The smallest number occurred in September (33 cases; 7.04%) (Fig. 1).
FIG. 1.
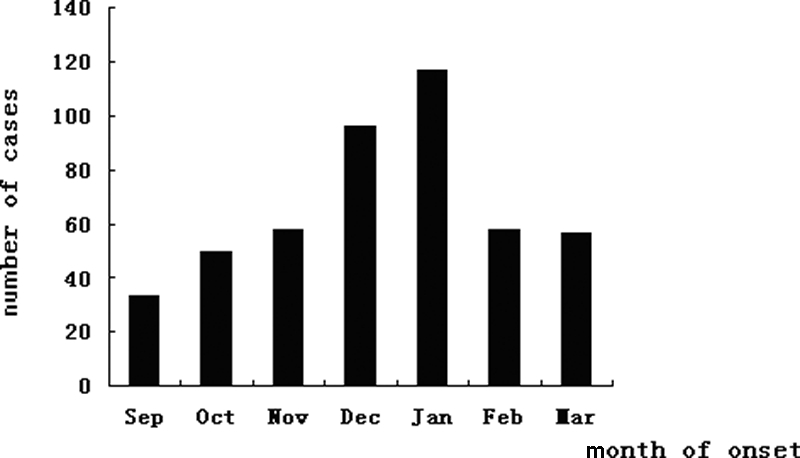
Numbers of mumps cases by month from September 2004 to March 2005.
We identified 1,407 children as potential controls for 469 cases and included 469 controls in the analysis. Age, gender, and place of residence were identical for each pair.
Among 938 study participants, 638 had not been vaccinated, 299 had received 1 valid dose, and 1 child received 2 doses. There were twice as many vaccinated controls as vaccinated cases (43.9% versus 20.0%) (Table 1).
TABLE 1.
Receipt of valid S79 mumps virus vaccine in eligible childrena
| Group | No. of children with indicated vaccination history
|
||
|---|---|---|---|
| 0 dose | 1 dose | 2 doses | |
| Cases | 375 | 94 | 0 |
| Controls | 263 | 205 | 1 |
| Total | 638 | 299 | 1 |
This table includes clinical cases only from September 2004 to March 2005.
In total, 94 of 469 cases received 1 dose of mumps virus vaccine. This group had a median onset age of 7.00 years and an average time from first vaccination to onset of 5.60 years. The median age of controls was 1.50 years (range, 10 months to 15 years) at the time of the first vaccination and 1.54 years at the time of the second vaccination. Vaccination age was statistically different for children of ages 0 to 5 years and those of ages over 6 years (1.06 ± 0.55 versus 1.63 ± 1.28 years; t = 5.533; P = 0.000).
Overall, the VE of the S79 mumps virus vaccine against clinical mumps in children was 86.2%. It was a bit lower for one dose (86.0%) (Table 2) and was not statistically valid for two doses because the sample was too small (98.5%; 95% CI, −1,473,458.0 to 100%).
TABLE 2.
Effectiveness of one dose of S79 mumps virus vaccine in children
| Age (yr) | No. of case (vaccinated)-control (unvaccinated) pairs | No. of case (unvaccinated)-control (vaccinated) pairs | No. of discordant sets | Total no. of cases | VE (%) | 95% CI |
|---|---|---|---|---|---|---|
| 0 | 0 | 1 | 1 | 4 | 98.5 | −1,473,580.0-100 |
| 1 | 0 | 3 | 3 | 6 | 98.5 | −16,377.9-100 |
| 2 | 0 | 5 | 5 | 19 | 98.5 | −1,933.0-100 |
| 3 | 1 | 6 | 7 | 19 | 83.3 | −38.4-98.0 |
| 4 | 3 | 14 | 17 | 39 | 78.6 | 25.4-93.8 |
| 5 | 0 | 17 | 17 | 58 | 98.5 | 24.3-100 |
| 6 | 3 | 15 | 18 | 54 | 80.0 | 30.9-94.2 |
| 7 | 2 | 17 | 19 | 53 | 88.2 | 49.1-97.3 |
| 8 | 4 | 13 | 17 | 38 | 69.2 | 56.0-90.0 |
| 9 | 1 | 17 | 18 | 47 | 94.1 | 55.8-99.2 |
| 10 | 1 | 11 | 12 | 43 | 90.9 | 29.6-98.8 |
| 11 | 2 | 3 | 5 | 26 | 33.3 | −299.0-88.9 |
| 12 | 1 | 3 | 4 | 30 | 66.7 | −220.5-96.5 |
| Overall | 18 | 129 | 147 | 468 | 86.0 | 77.2-91.5 |
No difference in VE was found for those 0 to 5 years old (75.8% to 96.9%) and those more than 6 years old (70.3% to 90.3%). For those between 4 and 10 years old, the seven 95% CIs for the VE of one dose overlapped, and similar VE points could be seen. VE could not be calculated for those of ages 0, 1, 2, 3, 11, and 12 years because of the small sample size.
The number of cases increased as the time after vaccination increased. VE was much higher during the first 4 years than 5 to 12 years after 1 dose of S79 vaccine vaccination (Table 3).
TABLE 3.
Effectiveness of one dose of S79 mumps virus vaccine versus time postvaccination in children
| Time postvaccination (yr) | No. of cases | Accumulative incidence rate (%) | No. of case (vaccinated)-control (unvaccinated) pairs | No. of case (unvaccinated)-control (vaccinated) pairs | No. of discordant sets | VE (%) | 95% CI |
|---|---|---|---|---|---|---|---|
| 1 | 2 | 8.3 | 0 | 203 | 203 | 98.5 | 95.3-99.5 |
| 2 | 5 | 16.7 | 0 | 200 | 200 | 98.5 | 95.2-99.5 |
| 3 | 10 | 25.0 | 1 | 196 | 197 | 99.5 | 96.4-99.9 |
| 4 | 29 | 33.3 | 6 | 182 | 186 | 96.7 | 92.6-98.5 |
| 5 | 45 | 41.7 | 42 | 153 | 195 | 72.5 | 61.4-80.5 |
| 6 | 55 | 50.0 | 43 | 144 | 187 | 70.1 | 58.0-78.8 |
| 7 | 67 | 58.3 | 45 | 134 | 189 | 66.4 | 52.9-76.0 |
| 8 | 76 | 66.7 | 50 | 130 | 180 | 61.5 | 46.7-72.2 |
| 9 | 86 | 75.0 | 50 | 120 | 170 | 58.3 | 42.0-70.0 |
| 10 | 90 | 83.3 | 52 | 117 | 169 | 55.6 | 38.4-67.9 |
| 11 | 92 | 91.7 | 53 | 116 | 169 | 54.3 | 36.8-67.0 |
| 12 | 94 | 100.0 | 54 | 115 | 169 | 53.0 | 35.1-66.0 |
DISCUSSION
An analysis of vaccination information for 469 clinical mumps cases and 469 matched controls (collected from September 2004 to March 2005) revealed that 1 dose of live, attenuated S79 mumps virus vaccine was effective in preventing clinical mumps. The VE of one dose of mumps virus vaccine versus none was protection of 86.0% (95% CI, 77.2% to 91.5%) of recipients and was much higher in the first 4 years than 5 to 12 years after vaccination.
The serological response to the S79 mumps virus vaccine conferred only moderate protection against mumps virus (74.07 to 83.50%), but no further information is available.
The VE was a bit lower in our study (86.0%) than in the previous efficacy trials (89.04%). However, detailed information on those efficacy trials is not available. Those efficacy studies could have overestimated protection because they used poor methodology. The S79 vaccine may also be less effective under field conditions owing to problems with storage (for example, a failure to maintain cold temperatures).
Prelicensing studies normally evaluate protection under the optimal conditions of clinical trials. However, vaccine protection is better estimated under field conditions. Efficacy figures from clinical trials cannot easily be converted to VE because during routine practice not all susceptible children will be immunized before exposure or receive the full immunization series. In addition, the spectrum of vaccine recipients in practice is typically wider than that of the healthy, highly responsive vaccine recipients usually selected for efficacy trials. So, from a public health perspective, the impact of vaccination on outcome in the field should be analyzed (2, 3, 18, 22).
We believe our study, with its large sample size, is the first to accurately determine the VE of the S79 mumps virus vaccine. In the past 10 years, since the S79 vaccine has been in use in China, the VEs of other mumps virus vaccines, but not that of the S79 vaccine, have been evaluated and reported: the Jery1 Lynn and RIT4385 mumps virus vaccines were reported to have VEs of 75 to 91% during mumps outbreaks (7, 12, 17, 19, 21).
The SH (small hydrophobic) protein gene is the most variable part of the mumps virus genome. The distribution of mumps virus genotypes varies extensively both temporally and geographically. Xu and Tang reported in China that the nucleotide sequences of the 1995 wild-type mumps virus were clearly different from those of the vaccine S79 strain (25). Our study found that the S79 strain vaccine is still effective in preventing mumps, partly because the key gene in the wild-type mumps virus has remained almost unchanged.
In our study, the VEs for one dose of mumps vaccine were similar among children of ages 4, 5, 6, 7, 8, 9, and 10 years, which was not noted in previous efficacy trials. However, in our study, younger children received earlier S79 vaccination than the older children.
The waning of immunity with time following vaccination is now a topic of discussion in countries striving for elimination of mumps. In our study, VEs began to decrease from the fifth year after vaccination. Periodic antigenic boosting likely occurs in China owing to circulating wild-type mumps viruses, and this likely complicates the analysis of the waning of vaccine immunity. Cohen et al. found that the VEs of the measles-mumps-rubella vaccine used in England were higher than ours, but the effectiveness of 1 dose also declined from 96% (95% CI, 81% to 99%) in 2-year-olds to 66% (95% CI, 30% to 83%) in 11- to 12-year-olds (7). Nevertheless, there is no statistical difference between their VEs and ours (95% CI for each).
In China, the mumps virus vaccine is not a necessary part of the national immunization schedule. The low coverage of this vaccine (in our study, 43.9%) leaves most infants and children vulnerable to mumps virus infection. Our findings (along with the fact that the vast majority of mumps cases occur in children and one dose is not 100% effective in developing countries) suggest that one dose at a minimum should be administered as early as possible in the first year, and a second dose is recommended in the fifth year after the first dose. Thus, our vaccine can provide as much protection from mumps virus as other mumps virus vaccines (8, 14, 15, 20).
In our study, cases and their matched controls were selected at the same time and from nearby areas to control for seasonal effects and the effects of other risk factors for mumps virus infection.
Our study might have many limitations. Our cases may not be representative of all mumps patients. Nearly one-third of mumps virus infections are subclinical, and the cases in our sample were severe. Some of our cases were not laboratory confirmed, and some of our controls may have been latent or subclinical cases. The VE referred to in our study was against clinical cases or against cases with severe symptoms.
One potential limitation of our study is that existing cases were missing from our computerized system: 74.6% of patients were not found in the computerized system. Excluding these patients might have reduced the generalizability of our findings. There are two main reasons for this absence from the computerized system: address changes and lack of a permanent address. Children with permanent and temporary residences are registered in the Children's EPI Administrative Computerized System. Many children who come from other cities to Guangzhou for treatment give their temporary address at hospital registration, making it impossible to find them later.
The observational nature of case-control studies can result in bias and confounding. We tried to avoid selection bias in controls by using standardized computer methods to locate and enroll them. We also controlled for several possible confounders, such as gender and age. Because of its large size, our study could assess the VE of one dose of S79 mumps virus vaccine. Memory bias was eliminated by using the Children's EPI Administrative Computerized System. Vaccination history was based on computer records rather than parents' or guardians' recall, so the order of onset and vaccination date is explicitly known and valid vaccination is confirmed.
This postlicensing study of the VE of the live, attenuated S79 mumps virus vaccine found that 1 versus 0 doses was effective in preventing mumps and that VE was much higher in the first 4 years than 5 to 12 years after vaccination. Our study indicates that the S79 vaccine can be effective in preventing clinical mumps and a second dose of mumps vaccine is needed to protect children in China.
Acknowledgments
We thank the health care workers and staff who collaborated in this study. We are indebted to all children and their families who participated in the survey.
The work described does not represent the results of a clinical trial.
Footnotes
Published ahead of print on 30 July 2008.
REFERENCES
- 1.Baum, S. G. 2008. Who cares about mumps? You should! Clin. Infect. Dis. 46:1450-1451. [DOI] [PubMed] [Google Scholar]
- 2.Bernaola, E., M. Herranz, N. Clerigue, and G. Gil. 2007. Case-control studies to assess vaccine effectiveness? Yes, but not this way. Clin. Infect. Dis. 45:1240-1241. [DOI] [PubMed] [Google Scholar]
- 3.Brunell, P. 2007. The effectiveness of evaluating mumps vaccine effectiveness. Clin. Infect. Dis. 45:467-469. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2006. Mumps epidemic—Iowa 2006. MMWR Dispatch. 55:1-3. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2006. Mumps epidemic—United kingdom, 2004-2005. MMWR Morb. Mortal. Wkly. Rep. 55:175-177. [PubMed] [Google Scholar]
- 6.Clemens, J., R. Brenner, M. Rao, N. Tafari, and C. Lowe. 1996. Evaluating new vaccines for developing countries. Efficacy or effectiveness? JAMA 275:390-397. [PubMed] [Google Scholar]
- 7.Cohen, C., J. M. White, E. J. Savage, J. R. Glynn, Y. Choi, N. Andrews, D. Brown, and M. E. Ramsay. 2007. Vaccine effectiveness estimates, 2004-2005 mumps outbreak, England. Emerg. Infect. Dis. 13:12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dayan, G. H., M. P. Quinlisk, A. A. Parker, A. E. Barskey, M. L. Harris, J. M. Schwartz, K. Hunt, C. G. Finley, D. P. Leschinsky, A. L. O'Keefe, J. Clayton, L. K. Kightlinger, E. G. Dietle, J. Berg, C. L. Kenyon, S. T. Goldstein, S. K. Stokley, S. B. Redd, P. A. Rota, J. Rota, D. Bi, S. W. Roush, C. B. Bridges, T. A. Santibanez, U. Parashar, W. J. Bellini, and J. F. Seward. 2008. Recent resurgence of mumps in the United States. N. Engl. J. Med. 358:1580-1589. [DOI] [PubMed] [Google Scholar]
- 9.Enders, M., M. Biber, and S. Exler. 2007. Measles, mumps and rubella virus infection in pregnancy. Possible adverse effects on pregnant women, pregnancy outcome and the fetus. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 50:1393-1398. [DOI] [PubMed] [Google Scholar]
- 10.Fu, C., M. Wang, J. Liang, T. He, D. Wang, and J. Xu. 2007. Effectiveness of Lanzhou lamb rotavirus vaccine against rotavirus gastroenteritis requiring hospitalization: a matched case-control study. Vaccine 25:8756-8761. [DOI] [PubMed] [Google Scholar]
- 11.Gershon, A. 2004. Mumps, p. 391-402. In A. Gershon, P. Hotez, and S. Katz (ed.), Krugman's infectious diseases of children, 11th ed. Mosby, Philadelphia, PA.
- 12.Harling, R., J. M. White, M. E. Ramsay, K. F. Macsween, and C. van den Bosch. 2005. The effectiveness of the mumps component of the MMR vaccine: a case control study. Vaccine. 23:4070-4074. [DOI] [PubMed] [Google Scholar]
- 13.Hviid, A., S. Rubin, and K. Mühlemann. 2008. Mumps. Lancet 371:932-944. [DOI] [PubMed] [Google Scholar]
- 14.Katz, S. L. 2006. Has the measles-mumps-rubella vaccine been fully exonerated? Pediatrics 118:1744-1745. [DOI] [PubMed] [Google Scholar]
- 15.López Hernández, B., R. M. Martín Vélez, C. Román García, I. Peñalver Sánchez, and J. A. López Rosique. 2000. An epidemic of mumps: a study of vaccine efficacy. Aten. Primaria 25:148-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacDonald, N., and K. Flegel. 2007. Mumps in young adults: the canary in the coal mine. CMAJ 177:121-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marin, M., P. Quinlisk, T. Shimabukuro, C. Sawhney, C. Brown, and C. W. Lebaron. 2008. Mumps vaccination coverage and vaccine effectiveness in a large outbreak among college students—Iowa, 2006. Vaccine 26:3601-3607. [DOI] [PubMed] [Google Scholar]
- 18.O'Brien, K. L., and O. S. Levine. 2006. Effectiveness of pneumococcal conjugate vaccine. Lancet 368:1469-1470. [DOI] [PubMed] [Google Scholar]
- 19.Peltola, H., P. S. Kulkarni, S. V. Kapre, M. Paunio, S. S. Jadhav, and R. M. Dhere. 2007. Mumps outbreaks in Canada and the United States: time for new thinking on mumps vaccines. Clin. Infect. Dis. 45:459-466. [DOI] [PubMed] [Google Scholar]
- 20.Pugh, R. N., B. Akinosi, S. Pooransingh, J. Kumar, S. Grant, E. Livesley, J. Linnane, and S. Ramaiah. 2002. An outbreak of mumps in the metropolitan area of Walsall, UK. Int. J. Infect. Dis. 6:283-287. [DOI] [PubMed] [Google Scholar]
- 21.Richard, J. L., M. Zwahlen, M. Feuz, H. C. Matter, and Swiss Sentinel Surveillance Network. 2003. Comparison of the effectiveness of two mumps vaccines during an outbreak in Switzerland in 1999 and 2000: a case-cohort study. Eur. J. Epidemiol. 18:569-577. [DOI] [PubMed] [Google Scholar]
- 22.Schaffzin, J. K., L. Pollock, C. Schulte, K. Henry, G. Dayan, D. Blog, and P. Smith. 2007. Effectiveness of previous mumps vaccination during a summer camp outbreak. Pediatrics 120:e862-e868. [DOI] [PubMed] [Google Scholar]
- 23.Stock, I. 2007. Mumps—-infectious disease with various faces. Med. Monatsschr. Pharm. 30:249-256. [PubMed] [Google Scholar]
- 24.WHO. 2005. Global status of mumps immunization and surveillance. Wkly. Epidemiol. Rec. 44:418-424. [PubMed] [Google Scholar]
- 25.Xu, H., and Q. Tang. 1999. Partial nucleotide sequence analysis of vaccine and wild mumps viruses. Chin. J. Microbiol. Immunol. 19:215-218. [Google Scholar]


