Abstract
The antimicrobial cathelicidin LL-37 is considered to play an important role in the innate immune response to tuberculosis infection. However, little is known about the induction and secretion of this antimicrobial peptide in A549 epithelial cells after infection with Mycobacterium bovis bacillus Calmette-Guérin (BCG), the world's most widely used tuberculosis vaccine. In this study, we investigated the effect of M. bovis BCG on LL-37 mRNA levels in A549 cells by real-time PCR and on protein levels by Western blotting. Treatment of cells with M. bovis BCG upregulates LL-37 mRNA expression in a dose- and time-dependent manner. The quantitative analysis of LL-37 gene expression correlated with our Western blotting results. Moreover, our results demonstrated that treatment of cells with the transcriptional inhibitor actinomycin D effectively inhibited in a concentration-dependent manner the ability of M. bovis BCG to induce LL-37 mRNA expression. Finally, inhibition of the MEK1/2 and p38 mitogen-activated protein kinase (MAPK) signaling pathways reduced M. bovis BCG-mediated LL-37 mRNA expression, a reduction that correlated with the observed high level of downregulation of LL-37 protein induction. Thus, these results indicate that the MEK1/2 and p38 MAPK signaling pathways play a critical role in the regulation of inducible LL-37 gene expression in A549 cells infected with M. bovis BCG.
Tuberculosis is a leading global cause of morbidity and an important cause of death (8). The emergence of multidrug-resistant Mycobacterium tuberculosis strains is alarming and represents a worldwide health care problem (33). This situation underscores the need for more-efficient therapies against human tuberculosis. Recently, it has been demonstrated that the infection of epithelial cells with mycobacteria triggered the induction of antimicrobial peptides (17, 38). Mammalian cells produce different kinds of antimicrobial peptides, such as α-defensin in neutrophils, β-defensins in epithelia, histatins in saliva, and cathelicidin (CAP18 or LL-37) in neutrophils and epithelia (13, 26, 35). The antimicrobial peptides have the property of folding into amphipathic structures that have a positively charged hydrophilic face and a hydrophobic face. The main role of antimicrobial peptides is the direct lysis of mycobacteria through the permeabilization of cellular membranes (14). In addition, these peptides exhibit broad-spectrum antimicrobial activity against microbes, including chemotactic activity for neutrophils, monocytes, and some T cells (6, 7) and induction of interleukin-8 secretion from epithelial cell lines (28). The cationic antimicrobial peptide demonstrating the most significant immunoregulatory potential to date is LL-37 (22, 36). LL-37 is the sole human cathelicidin characterized to date (37). LL-37 is an 18-kDa protein which is the proteolytically processed extracellular form of CAP18, and the last 37 amino acid residues at the C terminus are active against bacteria (3, 12). LL-37 was initially identified in the specific granules of neutrophils, and expression was subsequently identified in various epithelia (1). Its expression is induced during the course of bacterial infection or inflammation in a variety of tissues (5, 10, 32). LL-37 is produced at mucosal surfaces by epithelial cells and is upregulated in response to mycobacterial antigens (16). Although the synthesis and secretion of cationic peptides by epithelia have become recognized as an important mechanism for host defense, whether cathelicidin LL-37 is induced by Mycobacterium bovis BCG in human epithelial cells has not been determined. To our knowledge, this is the first report showing gene induction and secretion of LL-37 in epithelial cells after infection with M. bovis BCG. Elucidation of the effect of M. bovis BCG on LL-37 expression and induction in epithelial cells will aid in the development of novel therapeutic agents for treatment of mycobacterial infections.
MATERIALS AND METHODS
Specific reagents.
U0126, PD98059, and SB203580 were purchased from Calbiochem (La Jolla, CA) and resuspended in sterile dimethyl sulfoxide (DMSO; Sigma). The inhibitors were added 30 min before the infection. Actinomycin D was purchased from Sigma-Aldrich (St. Louis, MO). Anti-human LL-37 antibody was purchased from Santa Cruz Biotechnology, Santa Cruz, CA.
Bacteria.
M. bovis BCG (ATCC 35733) was obtained from the American Type Culture Collection (Manassas, VA). BCG was grown for 15 days in Sauton medium at 37°C. Cultures were harvested by centrifugation and then washed three times in medium. Aliquots of the stock were stored at −80°C until use.
Cell culture.
The human lung epithelial cell line A549 (ATCC CCL 185) was cultured in 75-cm2 culture flasks (Costar) to semiconfluence and maintained at 37°C in a humidified atmosphere of 95% air and 5% CO2 in a Dulbecco's modified Eagle medium (Gibco-BRL, Grand Island, NY) nutrient mixture containing 10% fetal calf serum (HyClone Laboratories, Logan, UT), 100 U/ml penicillin G, and 100 μg/ml streptomycin. Fetal calf serum contained <5 pg lipopolysaccharide/100 ml, as certified by the manufacturer. After reaching confluence, cells were plated in 24-well dishes at a concentration of 106 cells per well. The cells were infected with viable M. bovis BCG at multiplicities of infection (MOIs) of 1:1, 5:1, and 10:1. For all experiments, mycobacteria were added to cells on ice and incubated for 10 min, allowing mycobacteria to settle onto the cells, and then incubated for 18 h at 37°C in 5% CO2. Control cultures with no mycobacteria were always included. Cell viability was assessed by replacing medium and adding 500 μg/ml of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) (Sigma). After incubation for 3 h at 37°C, water-insoluble dark blue formazan crystals that formed from MTT cleaved in actively metabolizing cells were dissolved in lysis buffer containing 20% sodium dodecyl sulfate and 50% dimethylformamide, and the absorbance at 570 nm was determined. To examine the signaling pathway for LL-37 induction, cells were treated with extracellular signal-regulated kinase (ERK)/MEK inhibitors (U0196, and PD98059) and a p38 mitogen-activated protein kinase (MAPK) inhibitor (SB203580). An MTT assay was used to test the viability of cultured cells treated with each inhibitor. No obvious cytotoxicity (<0.5%) was found in cultures treated with these inhibitors at the doses used.
RT-PCR and real-time PCR.
LL-37 gene expression was determined by semiquantitative reverse transcriptase PCR (RT-PCR) analysis or quantitative (real-time) PCR using the β-actin gene as a housekeeping gene. Total RNA was isolated from cells, and samples of total RNA were quantified by measuring the optical density at 260 nm. Reverse transcription of LL-37 mRNA was performed using Superscript III reverse transcriptase with oligo(dT) primers in 20 μl as described by the manufacturer (Invitrogen, Carlsbad, CA). Controls without reverse transcriptase were included in each experiment. First-strand cDNA was amplified by PCR. The following LL-37-specific primers were used: sense, 5′-AGGATTGTGACTTCAAGAAGGACG-3′; antisense, 5′-GTTTATTTCTCAGAGCCCAGAAGC-3′. After an initial denaturing step (90°C for 1 min), the amplification profile was 35 cycles of 1 min of denaturation at 94°C and 2.5 min of annealing and extension at 66°C. The PCR products were separated by electrophoresis on a 2% agarose gel and visualized by ethidium bromide staining. Quantitative PCR was performed using the 5700 sequence detection system (Applied Biosystems) according to the manufacturer's instructions. Briefly, a total of 1 μl of cDNA (described above) was analyzed using the final concentration of 100 nM of primers and 2× Sybr green PCR master mix (Applied Biosystems, Foster City, CA) in a volume of 20 μl. The sequences of forward and reverse primers, as designed by Primer Express (Applied Biosystems) for quantification of LL-37 mRNA were 5′-GAAGACCCAAAGGAATGGCC-3′ and 5′-CAGAGCCCAGAAGCCTGAGC-3′. A standard curve was constructed from serial dilutions of cDNA synthesized from a known quantity of total RNA. Negative controls were included in each real-time PCR run. The results are expressed as means ± standard deviations (SD).
Western blot analysis.
For LL-37 expression analysis, cells were washed with ice-cold phosphate-buffered saline containing 1 mM pervanadate and lysed (in phosphate-buffered saline with 1% Triton X-100, 1 mM EDTA, 1 mM NaVO4, 1 mM NaF) and the cell lysates were clarified by centrifugation. The protein concentration was measured using the Bio-Rad assay kit. Equal amounts of protein from each sample were separated on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels. The material in gels was blotted onto a polyvinylidene difluoride membrane (Bio-Rad Laboratories, FL). The membranes were blocked with 3% bovine serum albumin in Tris-buffered saline buffer, pH 7.5. After washes (0.02% Tween 20, pH 7.5, Tris-buffered saline), immunolabeling was performed using polyclonal rabbit antibody against LL-37 or β-actin overnight. Alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin G (LL-37) or goat anti-mouse immunoglobulin G (β-actin) was applied to the membrane. The peroxidase-positive bands were detected by immersing the blots in a developing solution (73 mM sodium acetate, pH 6.2) containing 0.3% diaminobenzidine tetrahydrochloride and 0.04% H2O2 at room temperature for 5 min. The enzyme reaction was terminated by washing the blots in 0.1 M H2SO4.
Statistical evaluation.
Statistical analysis was performed using Student's t test with Microsoft Excel software. Significance was accepted at P values of <0.05.
RESULTS
Induction of both LL-37 mRNA and protein expression by M. bovis BCG in A549 cells.
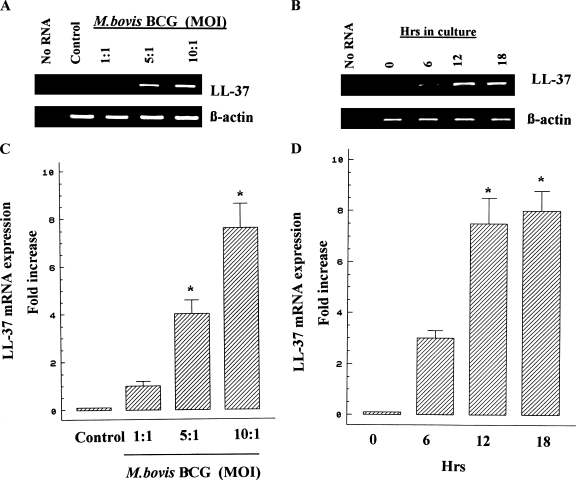
Initially, we examined whether M. bovis BCG infection induces the augmentation of LL-37 mRNA expression in A549 cells. Cells were infected at MOIs of 1:1, 5:1, and 10:1. After 18 h of incubation, RNA was extracted for RT-PCR as described above. The β-actin gene was used as a housekeeping gene control. Figure 1A shows that unstimulated cells hardly showed LL-37 mRNA expression and that LL-37 mRNA levels were upregulated in a dose-dependent manner, indicating that LL-37 mRNA expression is augmented in A549 cells in response to stimulation with M. bovis BCG. To define the kinetics of LL-37 mRNA induction in response to M. bovis BCG, cells were infected with M. bovis BCG for 0, 6, 12, and 18 h. Total RNA was collected and used separately for cDNA synthesis. The cDNA was utilized as templates in the RT-PCRs for LL-37. RT-PCR results showed that the levels of LL-37 mRNA were upregulated in a time-dependent manner (Fig. 1B). To confirm the RT-PCR results, real-time PCR was conducted for LL-37. As indicated in Fig. 1C, the levels of LL-37 mRNA expression were enhanced in response to M. bovis BCG in a dose-dependent manner and LL-37 gene expression in cells stimulated with M. bovis BCG at an MOI of 10 was eightfold higher than that in unstimulated cells (P < 0.05). Figure 1D shows that the levels of LL-37 mRNA were upregulated within 6 h of exposure, with a maximum induction at 12 to 18 h of incubation. In order to determine whether or not transcriptional upregulation of the LL-37 gene is the predominant mechanism through which M. bovis BCG induces LL-37 expression, A549 cells were treated with or without various concentrations of the transcriptional inhibitor actinomycin D and infected with M. bovis BCG at an MOI of 10 for 18 h. Results from all five experiments showed that addition of actinomycin D significantly (P < 0.05) reduced in a concentration-dependent manner the ability of M. bovis BCG to induce LL-37 mRNA expression, indicating that M. bovis BCG upregulates LL-37 expression primarily through transcriptional upregulation of the LL-37 gene in A549 cells (Fig. 2).
FIG. 1.
Induction of LL-37 gene expression by M. bovis BCG in A549 cells. A549 cells were left unstimulated (control) or were stimulated with M. bovis BCG at MOIs of 1:1, 5:1, and 10:1 for 18 h. The kinetics of LL-37 mRNA induction was determined as described above. Total RNA was extracted, and RT-PCR (A and B) or real-time PCR (C and D) was conducted to quantify LL-37 mRNA levels. As a negative control, RNA was omitted from reverse transcription and PCR amplification (no RNA). The β-actin gene was used as a housekeeping gene. In RT-PCR analysis, data shown are representative of four separate experiments. In real-time PCR analysis, results are represented as the means ± SD from at least three independent experiments. *, P < 0.05.
FIG. 2.
Effect of actinomycin D on M. bovis BCG-induced LL-37 mRNA expression. A549 cells were infected with M. bovis BCG at an MOI of 10:1 for 18 h after treatment with or without various concentrations of actinomycin D. Total RNA was extracted, and real-time PCR was conducted to quantify LL-37 mRNA levels, normalized to β-actin. Graphs show means ± SD from at least five independent experiments. *, P < 0.05, for comparison with M. bovis BCG cultures that did not receive actinomycin D.
To determine whether the induction of LL-37 mRNA levels correlated with an increase in protein expression, Western blot analysis was performed. A549 cells were infected with M. bovis BCG at MOIs of 1:1, 5:1, and 10:1. After 18 h of incubation, protein expression was analyzed by Western blotting. As shown in Fig. 3, LL-37 was detected in whole-cell extracts of A549 cells stimulated with M. bovis BCG and was enhanced in response to M. bovis BCG in a dose-dependent manner. In addition, the protein was not observed in unstimulated cells. Taken together, these results suggest that M. bovis BCG induces both LL-37 mRNA and protein expression in A549 cells.
FIG. 3.
Detection of LL-37 in cell extracts of A549 cells infected with M. bovis BCG by Western blot analysis. A549 cells were left unstimulated (control) or were stimulated with M. bovis BCG at MOIs of 1:1, 5:1, and 10:1 for 18 h. Total cell lysates were analyzed by Western blotting to observe LL-37 expression. The amount of protein from the lysates was determined by using the Bio-Rad assay kit, and equal amounts of protein were loaded in all wells. Parallel blotting was performed with an antibody to β-actin. Data from a representative experiment are presented, and similar results were obtained in four independent experiments.
Mechanism of LL-37 mRNA induction by M. bovis BCG in A549 cells.
Since it has been demonstrated that the MEK-ERK signaling pathway is involved in butyrate-mediated cathelicidin induction in colon epithelial cells (25), we examined whether this pathway is also required for M. bovis BCG activity. To do this, cells were treated with U0126, a specific inhibitor of ERK1/2 kinase (MEK1/2). The results demonstrated that M. bovis BCG-induced LL-37 mRNA expression was significantly reduced by the MEK-ERK inhibitor (U0126) in a dose-dependent manner (Fig. 4A). It is important to note that the vehicle DMSO, in an amount equivalent to that contained in 20 μM U0126, had no effect on LL-37 induction (Fig. 4A). To confirm the regulatory role of the MEK-ERK signaling pathway, we also evaluated the effect of a second MEK inhibitor (PD98059, an inhibitor of MEK via upstream-activator-dependent phosphorylation) before M. bovis BCG infection. Results were that M. bovis BCG-mediated LL-37 induction was also significantly reduced by PD98059 in a dose-dependent manner, beginning at 5 μM PD98059 (P < 0.05; Fig. 4B).
FIG. 4.
Effect of specific MAPK inhibitors on M. bovis BCG-induced LL-37 mRNA expression. A549 cells were left unstimulated (control) or were stimulated with M. bovis BCG at an MOI of 10:1 for 18 h after preincubation with or without different concentrations of the MEK inhibitor U0126 (A) or PD98059 (B) or the p38 MAPK inhibitor SB203580 (C), and real-time PCR was conducted to quantify LL-37 mRNA levels, normalized to β-actin. Graphs show means ± SD from at least three independent experiments. *, P < 0.05, for comparison with M. bovis BCG cultures that did not receive an inhibitor.
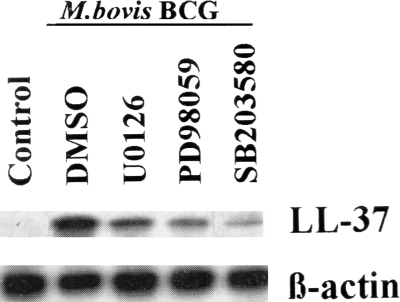
To further investigate the mechanism underlying LL-37 gene activation in response to M. bovis BCG, we tested a specific p38 kinase inhibitor (SB203580). As shown in Fig. 4C, pretreatment with SB203580 inhibited ongoing LL-37 mRNA induction by M. bovis BCG. Taken together, these results indicate that these two MAPK pathways could be involved in M. bovis BCG-mediated LL-37 induction in A549 cells. The regulation of the LL-37 mRNA by the MEK-ERK and the p38 MAPK pathways correlated with reduced expression of the LL-37 protein, as determined by Western blotting (Fig. 5). Our previous results demonstrated that M. bovis BCG activates both ERK1/2 and p38 MAPK signaling pathways in A549 cells (17). In this study, phosphorylation of ERK1/2 by M. bovis BCG in A549 cells was inhibited by the MEK-ERK inhibitors U0126 and PD98059, whereas phosphorylation of p38 MAPK was not affected, showing the specificity and efficacy of U0126 and PD98059 (data not shown).
FIG. 5.
Inhibitors of MAPK block M. bovis BCG-induced LL-37 protein expression. Cells were unstimulated (control) or stimulated with M. bovis BCG at an MOI of 10:1 after preincubation with or without the MEK inhibitor U0126 (20 μM) or PD98059 (10 μM) or the p38 MAPK inhibitor SB203580 (10 μM) or with vehicle (DMSO), and total cell lysates were analyzed by Western blotting to observe LL-37 protein expression. Parallel blotting was performed with an antibody to β-actin. Data from a representative experiment are presented, and similar results were obtained in three independent experiments.
DISCUSSION
The prevalence of tuberculosis is increasing in the world. Consequently, there is an increased need for the development of new antituberculosis treatments, especially for patients who respond poorly to conventional therapy or present resistance to antimycobacterial drugs (9, 34). Since their antimycobacterial activities have demonstrated some therapeutic benefit during tuberculosis infection, antimicrobial peptides have gained the attention of researchers as novel therapeutic agents against human tuberculosis (2, 27). In particular, LL-37 displays broad-spectrum microbicidal activities (3). LL-37 has been recognized as an antimicrobial peptide which protects the skin from bacterial infection (20). Although LL-37 has been identified as an important peptide with a multifunctional role in host defense (4), its particular role in the control of the innate epithelial immune response prompted us to examine the expression of LL-37 by M. bovis BCG. Our results demonstrate both gene expression and protein secretion of LL-37 in epithelial cells after stimulation with M. bovis BCG. We chose to work with epithelial cells, because mycobacteria are transmitted primarily by the respiratory route and alveolar epithelial cells are among the first host cells to encounter mycobacteria (30, 31). We demonstrated that M. bovis BCG induced high levels of LL-37 mRNA in a dose-dependent manner. This observation was extended by using actinomycin D, which showed significantly suppressed M. bovis BCG-induced LL-37 mRNA expression in a dose-dependent manner, suggesting that this expression is at the level of transcription. Moreover, our data were corroborated by Western blotting, which showed that M. bovis BCG induced significant levels of LL-37 protein secretion in a concentration-dependent manner. The augmented expression levels of LL-37 mRNA were almost constant for 12 to 18 h after stimulation with M. bovis BCG. This expression is coincident with the presence of HBD-2, a previously described distinct mammalian antimicrobial peptide produced by the same epithelial cell line after stimulation with M. bovis BCG (18). Since cathelicidins and defensins have synergistic action as antimicrobials (19), it is possible that LL-37 and HBD-2 may act synergistically against mycobacteria.
The underlying mechanisms of cathelicidin expression are only beginning to be understood (24), but recent studies have revealed that cathelicidin expression is regulated via the MAPK signaling pathway. Interestingly, it has been shown that the inhibition of p38 kinase pathway significantly decreased cathelicidin gene expression in human keratinocytes (23). However, Schauber et al. demonstrated that inhibition of the ERK1/2 pathway but not the p38 MAPK pathway blocked sodium butyrate-induced cathelicidin gene expression in colonic, gastric, and hepatic cells (24). In contrast, our present data show that M. bovis BCG-stimulated LL-37 production can be regulated by both the ERK1/2 and p38 MAPK signaling pathways. This apparent conflict may arise because LL-37 expression appears to be differentially regulated among different cell types and dependent on stimuli.
There is now clear evidence that the LL-37 gene has potential binding sites for several transcription factors, including NF-κB, NF-interleukin-6, and AP-1 (29). In addition, it has been reported that activation of AP-1 is regulated via MAPK signaling pathways (11). Thus, we could not exclude the participation of AP-1 in M. bovis BCG-induced LL-37 expression in A549 cells. This interesting point will be explored in future experiments.
It has been demonstrated that Toll-like receptor (TLR) pathways are important for optimal innate immune response against M. tuberculosis (21). Moreover, Liu et al. showed that TLR activation of cells induced killing of M. tuberculosis through LL-37 participation (15). Therefore, it should be possible in future studies to address whether such TLR pathways regulate M. bovis BCG-induced LL-37 expression in epithelial cells.
In summary, we investigated the mechanisms involved in M. bovis BCG-induced LL-37 gene expression in human lung epithelial cell line A549. Our data indicate that M. bovis BCG can induce both LL-37 mRNA and protein expression. Furthermore, we demonstrated that the ERK1/2 and p38 MAPK signaling pathways participate in the regulation of inducible LL-37 gene expression. It remains to be elucidated if M. bovis BCG can strengthen the epithelial defense barrier by upregulating LL-37 mRNA expression in vivo. However, studies on the induction of LL-37 by M. bovis BCG may result in therapeutic approaches that enhance the host immune defense against tuberculosis.
Acknowledgments
This work was supported by the Coordinación General de Posgrado e Investigación de el IPN (grant 20080419). P.M.-S. is a COFAA, EDI, and SNI fellow.
Footnotes
Published ahead of print on 25 June 2008.
REFERENCES
- 1.Agerberth, B., J. Charo, J. Werr, B. Olsson, F. Idali, L. Lindbom, R. Kiessling, H. Jörnvall, H. Wigzell, and G. H. Gudmundsson. 2000. The human antimicrobial and chemotactic peptides LL-37 and alphadefensins are expressed by specific lymphocyte and monocyte populations. Blood 96:3086-3093. [PubMed] [Google Scholar]
- 2.Ashitani, J., I. Mukae, T. Hiratsuka, M. Nakazato, K. Kumamoto, and S. Matsakura. 2002. Elevated levels of α-defensins in plasma and BAL fluid of patients with active pulmonary tuberculosis. Chest 121:519-526. [DOI] [PubMed] [Google Scholar]
- 3.Bals, R., X. Wang, M. Zasloff, and J. M. Wilson. 1998. The peptide antibiotic LL37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc. Natl. Acad. Sci. USA 95:9541-9546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowdish, D. M., D. J. Davidson, Y. E. Lau, K. Lee, M. G. Scott, and R. E. Hancock. 2005. Impact of LL-37 of anti-infective immunity. J. Leukoc. Biol. 77:451-459. [DOI] [PubMed] [Google Scholar]
- 5.Bowdish, D. M. E., D. J. Davidson, and R. E. W. Hancock. 2005. A re-evaluation of the role of host defence peptides in mammalian immunity. Curr. Protein Pept. Sci. 6:35-51. [DOI] [PubMed] [Google Scholar]
- 6.Braff, M. H., M. Zaiou, J. Fierer, V. Nizet, and R. L. Gallo. 2005. Keratinocyte production of cathelicidin provides direct activity against bacterial skin pathogens. Infect. Immun. 73:6771-6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Yang, Q. Chen, A. P. Schmidt, G. M. Anderson, J. M. Wang, J. Wooters, J. Oppenheim, and O. Chertov. 2000. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J. Exp. Med. 192:1069-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dye, C. 2006. Global epidemiology of tuberculosis. Lancet 367:938-940. [DOI] [PubMed] [Google Scholar]
- 9.Dye, C., S. Scheele, P. Dolin, V. Pathania, and M. C. Raviglione. 1999. Consensus statement, global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Survillance and Monitoring Project. JAMA 282:677-686. [DOI] [PubMed] [Google Scholar]
- 10.Hase, K., M. Murakami, M. Iimura, S. P. Cole, Y. Horibe, T. Ohtake, M. Obonyo, R. L. Gallo, L. Eckmann, and M. F. Kagnoff. 2003. Expression of LL-37 by human gastric epithelial cells as a potential host defense mechanism against Helicobacter pylori. Gastroenterology 125:1613-1625. [DOI] [PubMed] [Google Scholar]
- 11.Kida, Y., T. Shimizu, and K. Kuwano. 2002. Opposing roles of activator protein-1 and CCAAT/enhancer binding protein beta in the regulation of inducible granulysin gene expression in a human monocytic cell line, THP-1. Immunology 107:507-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larrick, J. W., M. Hirata, R. F. Balint, J. Lee, J. Zhong, and S. C. Wright. 1995. Human CAP18: a novel antimicrobial lipopolysaccharide-binding protein. Infect. Immun. 63:1291-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laube, D. M., S. Yim, L. K. Ryan, K. O. Kisich, and G. Diamond. 2006. Antimicrobial peptides in the airway. Curr. Top. Microbiol. Immunol. 306:153-182. [DOI] [PubMed] [Google Scholar]
- 14.Lehrer, R., A. Lichtenstein, and T. Ganz. 1993. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu. Rev. Immunol. 11:105-128. [DOI] [PubMed] [Google Scholar]
- 15.Liu, P. T., S. Stenger, H. Li, L. Wenzel, B. H. Tan, S. R. Krutzik, M. T. Ochoa, J. Schauber, K. Wu, C. Meinken, D. L. Kamen, M. Wagner, R. Bals, A. Steinmeyer, U. Zügel, R. L. Gallo, D. Eisenberg, M. Hewison, B. W. Hollis, J. S. Adams, B. R. Bloom, and R. L. Modlin. 2006. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 311:1770-1773. [DOI] [PubMed] [Google Scholar]
- 16.Martineau, A. R., S. M. Newton, K. A. Wilkinson, B. Kampmann, B. M. Hall, N. Nawroly, G. E. Packe, R. N. Davidson, C. J. Griffiths, and R. J. Wilkinson. 2007. Neutrophil-mediated innate immune resistance to mycobacteria. J. Clin. Investig. 117:1988-1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Méndez-Samperio, P., L. Alba, and A. Trejo. 2007. Mycobacterium bovis-mediated induction of human beta-defensin-2 in epithelial cells is controlled by intracellular calcium and p38MAPK. J. Infect. 54:469-474. [DOI] [PubMed] [Google Scholar]
- 18.Méndez-Samperio, P., E. Miranda, and A. Trejo. 2006. Mycobacterium bovis bacillus Calmette-Guérin (BCG) stimulates human β-defensin-2 gene transcription in human epithelial cells. Cell. Immunol. 239:61-66. [DOI] [PubMed] [Google Scholar]
- 19.Nagaoka, I., S. Hirota, S. Yomogida, A. Ohwada, and M. Hirata. 2000. Synergistic actions of antibacterial neutrophil defensins and cathelicidins. Inflamm. Res. 49:73-79. [DOI] [PubMed] [Google Scholar]
- 20.Nizet, V., and R. L. Gallo. 2003. Cathelicidins and innate defense against invasive bacterial infection. Scand. J. Infect. Dis. 35:670-676. [DOI] [PubMed] [Google Scholar]
- 21.Quesniaux, V. J., D. M. Nicolle, D. Torres, L. Kremmer, Y. Guerardel, J. Nigou, G. Puzo, F. Erard, and B. Ryffel. 2004. Toll-like receptor 2 (TLR2)-dependent-positive and TLR2-independent-negative regulation of proinflammatory cytokines by mycobacterial lipomannans. J. Immunol. 172:4425-4434. [DOI] [PubMed] [Google Scholar]
- 22.Ramanathan, B., E. G. Davis, C. R. Ross, and F. Blecha. 2002. Cathelicidins: microbicidal activity, mechanisms of action, and roles in innate immunity. Microbes Infect. 4:361-372. [DOI] [PubMed] [Google Scholar]
- 23.Sayama, K., H. Komatsuzawa, K. Yamasaki, Y. Shirakata, Y. Hanakawa, K. Ouhara, S. Tokumaru, X. Dai, M. Tohyama, P. Ten Dijke, M. Sugai, H. Ichijo, and K. Hashimoto. 2005. New mechanisms of skin innate immunity: ASK1-mediated keratinocyte differentiation regulates the expression of beta-defensins, LL37, and TLR2. Eur. J. Immunol. 35:1886-1895. [DOI] [PubMed] [Google Scholar]
- 24.Schauber, J., K. Iffland, S. Frisch, T. Kudlich, B. Schmausser, M. Eck, T. Menzel, A. Gostner, H. Lührs, and W. Scheppach. 2004. Histone-deacetylase inhibitors induce the cathelicidin LL-37 in gastrointestinal cells. Mol. Immunol. 41:847-854. [DOI] [PubMed] [Google Scholar]
- 25.Schauber, J., R. A. Dorschner, K. Yamasaki, B. Brouha, and R. L. Gallo. 2006. Control of the innate epithelial antimicrobial response is cell-type specific and dependent on relevant microenvironmental stimuli. Immunology 118:509-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Selsted, M. E., and A. J. Ouellette. 2005. Mammalian defensins in the antimicrobial immune response. Nat. Immunol. 6:551-557. [DOI] [PubMed] [Google Scholar]
- 27.Sharma, S., I. Verma, and G. K. Khuller. 2001. Therapeutic potential of human neutrophil peptide 1 against experimental tuberculosis. Antimicrob. Agents Chemother. 45:639-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tjabringa, G. S., J. Aarbiou, D. K. Ninaber, J. W. Drijfhout, O. E. Sorensen, N. Borregaard, K. F. Rabe, and P. S. Hiemstra. 2003. The antimicrobial peptide LL-37 activates innate immunity at the airway epithelial surface by transactivation of the epidermal growth factor receptor. J. Immunol. 171:6690-6696. [DOI] [PubMed] [Google Scholar]
- 29.Whitmarsh, A. J., and R. J. Davis. 1996. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J. Mol. Med. 74:589-607. [DOI] [PubMed] [Google Scholar]
- 30.Wickremasinghe, M. I., L. H. Thomas, C. M. O'Kane, J. Uddin, and J. S. Friedland. 2004. Transcriptional mechanisms regulating alveolar epithelial cell-specific CCL5. secretion in pulmonary tuberculosis. J. Biol. Chem. 279:27199-27210. [DOI] [PubMed] [Google Scholar]
- 31.Wickremasinghe, M. I., L. H. Thomas, and J. S. Friedland. 1999. Pulmonary epithelial cells are a source of IL-8 in the response to Mycobacterium tuberculosis: essential role of IL-1 from infected monocytes in a NF-kappa B-dependent network. J. Immunol. 163:3936-3947. [PubMed] [Google Scholar]
- 32.Woo, J. S., J. Y. Jeong, Y. J. Hwang, S. W. Chae, S. J. Hwang, and H. M. Lee. 2003. Expression of cathelicidin in human salivary glands. Arch. Otolaryngol. Head Neck Surg. 129:211-214. [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization. 2007. Global tuberculosis control, surveillance, planning, and financing. World Health Organization, Geneva, Switzerland.
- 34.World Health Organization. 2000. Anti-tuberculosis drug resistance in the world. Prevalence and trends. WHO/CDS/TB/2000/278. The WHO/IUATLD Global Project on Anti-Tuberculosis Drug Resistance Surveillance, report 2. World Health Organization, Geneva, Switzerland.
- 35.Zaiou, M., and R. L. Gallo. 2002. Cathelicidins, essential gene-encoded mammalian antibiotics. J. Mol. Med. 80:549-561. [DOI] [PubMed] [Google Scholar]
- 36.Zanetti, M. 2004. Cathelicidins, multifunctional peptides of the innate immunity. J. Leukoc. Biol. 75:39-48. [DOI] [PubMed] [Google Scholar]
- 37.Zanetti, M., R. Gennaro, M. Scocchi, and B. Skerlavaj. 2000. Structure and biology of cathelicidins. Adv. Exp. Med. Biol. 479:203-218. [DOI] [PubMed] [Google Scholar]
- 38.Zhu, B. D., Y. Feng, N. Huang, Q. Wu, and B. Y. Wang. 2003. Mycobacterium bovis bacille Calmette-Guérin (BCG) enhances human beta-defensin-1 gene transcription in human pulmonary gland epithelial cells. Acta Pharmacol. Sin. 24:907-912. [PubMed] [Google Scholar]