Abstract
In active tuberculosis (TB), Mycobacterium tuberculosis-specific T cells are compartmentalized more to the site of infection than to the circulating blood. Therefore, an M. tuberculosis-specific enzyme-linked immunospot (ELISPOT) assay with samples from the site of infection may permit a more sensitive or specific diagnosis of active central nervous system (CNS) TB than that achieved by the assay with blood alone. Therefore, we prospectively evaluated the usefulness of circulating and compartmentalized mononuclear cell (MC; i.e., peripheral blood mononuclear cell [PBMC] and cerebrospinal fluid [CSF] MC)-based ELISPOT assays (i.e., the T-SPOT.TB test) for the diagnosis of active TB in patients with suspected CNS TB. The clinical categories of CNS TB were classified as described previously (G. E. Thwaites, T. T. Chau, K. Stepniewska, N. H. Phu, L. V. Chuong, D. X. Sinh, N. J. White, C. M. Parry, and J. J. Farrar, Lancet 360:1287-1292, 2002). Thirty-seven patients with suspected CNS TB were enrolled over a 12-month period. Of these, 31 (84%) showed clinical manifestations of suspected TB meningitis and 6 (16%) gave indications of intracranial tuberculoma with disseminated TB. The final clinical categories of the 37 patients with suspected CNS TB were as follows: 12 (32%) were classified as having CNS TB (7 with confirmed TB, 3 with probable TB, and 2 with possible TB) and 25 (68%) were classified as not having active TB. The sensitivity and specificity of the PBMC ELISPOT assay were 91% (95% confidence interval [CI], 59% to 100%) and 63% (95% CI, 41% to 81%), respectively. By comparison, the sensitivity and specificity of the CSF MC ELISPOT assay were 75% (95% CI, 19% to 99%) and 75% (95% CI, 43% to 95%), respectively. When the ratio of the CSF MC ELISPOT assay results to the PBMC ELISPOT results was 2 or more, the sensitivity and specificity were 50% (95% CI, 7% to 93%) and 100% (95% CI, 74% to 100%), respectively. The ELISPOT assay with PBMCs and CSF MCs is a useful adjunct to the current tests for the diagnosis of CNS TB.
The diagnosis of central nervous system (CNS) tuberculosis (TB) remains a serious clinical problem (1). The signs and symptoms, the results of routine analyses of cerebrospinal fluid (CSF), and the radiologic findings for patients with CNS TB are often inadequate as a guide to the initiation of empirical therapy (1). Therefore, a rapid, sensitive, and specific test for the diagnosis of CNS TB is urgently required. Several newly developed assays for the diagnosis of TB based on Mycobacterium tuberculosis-specific antigens encoded by genes in the RD1 region gave promising results for the detection of latent TB infection and active pulmonary TB (10). However, data on the usefulness of these assays for the diagnosis of CNS TB in actual clinical practice are limited.
The clinical use of the immunodiagnosis of TB is limited in regions where TB has intermediate to high levels of endemicity because it cannot differentiate active TB from latent TB infection (6). Recently, it has been shown that mononuclear cells (MCs) compartmentalized in infected sites such as pleural fluid (8, 14) or bronchoalveolar lavage fluid (3, 5) have higher gamma interferon responses than peripheral blood mononuclear cells (PBMCs). We aimed to demonstrate the proof of concept for the ability of the enzyme-linked immunospot (ELISPOT) assay to differentiate active TB from latent TB using compartmentalized lymphocytes. Therefore, we prospectively evaluated the usefulness of circulating and compartmentalized MC-based ELISPOT assays for the diagnosis of active TB in patients with suspected CNS TB.
MATERIALS AND METHODS
All adult patients with suspected CNS TB were prospectively enrolled at the Seoul National University Hospital, Seoul, Republic of Korea, and the Seoul National University Bundang Hospital in Gyunggi Province, Republic of Korea, between September 2006 and August 2007. The microbiologic and pathological specimens (i.e., sputum, biopsy materials, and CSF samples) used for the diagnosis of CNS TB were processed by standard techniques and procedures, as described previously (6). The tuberculin skin test (TST) was performed by the Mantoux technique, as described previously (6). Decisions regarding antituberculous therapy were the responsibility of the primary care physicians. The results of the ELISPOT assays were not concealed from the attending physicians (Fig. 1). This investigation was approved by the institutional review boards of our hospitals.
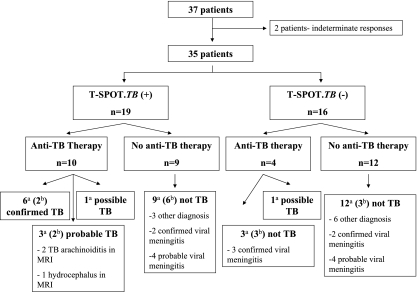
FIG. 1.
Schematic flowchart of the relationship of the ELISPOT assay results to empirical anti-TB therapy and the final classification of CNS TB. Of the total of 37 patients with suspected CNS TB, 2 patients gave indeterminate results by the PBMC ELISPOT assay. Data for the remaining 35 patients (11 with CNS TB plus 24 with not-active TB) were included in the final analysis of the PBMC ELISPOT assay. Of the 21 patients who agreed to additional CSF sampling, the CSF MCs from 5 patients gave indeterminate results by the ELISPOT assay. Thus, the CSF MC/PBMC ratio was assessed for the remaining 16 patients (4 with CNS TB plus 12 with not active TB) with suspected TB meningitis. a, data for 35 patients were included in the final analysis of the PBMC ELISPOT assay; b, data for 16 patients were included in the final analysis of the CSF MC ELISPOT assay. MRI, magnetic resonance imaging.
All cases were independently classified by the study investigators, without knowledge of the results of the ELISPOT assays, on the basis of clinical, histopathological, radiological, and microbiological information collected during at least 3 months of follow-up. The clinical categories of patients with suspected CNS TB were described in previous work (6, 12, 13). Briefly, patients were classified as having confirmed TB if their clinical specimens were positive for M. tuberculosis on culture or when an M. tuberculosis-specific PCR assay was positive. Patients were classified as having probable TB if the clinical picture of meningitis associated with changes in the CSF was consistent with TB meningitis, with or without brain magnetic resonance imaging findings suggesting TB meningitis and a successful response to antituberculous therapy. Patients were classified as having possible TB if they did not fulfill the criteria mentioned above but active TB could not be excluded. Patients were classified as having “not active TB” when some other diagnosis was made or there was clinical improvement without antituberculous therapy 3 months after admission, because untreated TB meningitis would almost always be fatal by that time (12). To classify the diagnostic performances of the ELISPOT assay and TST, confirmed, probable, and possible TB were used as the reference standards for CNS TB and “not-active TB” was used as the reference standard for not-active CNS TB.
ELISPOT assays (T-SPOT.TB; Oxford Immunotec, Abingdon, United Kingdom) with PBMCs from venous blood samples and MCs from CSF samples were performed as described previously (6). Briefly, 250,000 PBMCs or 250,000 CSF MCs were immediately separated from 8-ml samples of peripheral venous blood and 10- to 15-ml samples of CSF, respectively. The cells were plated (2.5 × 105 cells/well) on plates precoated with anti-human gamma interferon antibody. The cells were cultured for 18 h, and the spots were counted with an automated microscope (CRL ImmunoSpot S4 core analyzer; Cellular Technology Ltd., Cleveland, OH). We used the criteria for positive, negative, and indeterminate outcomes recommended by the manufacturer.
Statistical analyses were performed by use of SPSS software for Windows (version 12.0; SPSS Inc., Chicago, IL). The categorical variables were compared by means of the Pearson χ2 test or Fisher's exact test, when appropriate. The Mann-Whitney U test was used to compare the continuous variables. All tests of significance were two-tailed; P values of ≤0.05 were considered significant.
RESULTS
Thirty-seven subjects with suspected CNS TB were prospectively enrolled in the study; 10 patients with suspected CNS TB were also included in the previous study (6). Of these, 31 (84%) showed clinical manifestations of suspected TB meningitis and 6 (16%) gave indications of intracranial tuberculoma with disseminated TB. The final clinical categories for the 37 patients with suspected CNS TB were as follows: 7 (19%) were classified as having confirmed TB, 3 (8%) as having probable TB, 2 (5%) as having possible TB, and 25 (68%) as having not-active TB. The baseline clinical characteristics of the patients with CNS TB and not-active TB are shown in Table 1. The PBMC ELISPOT assay and TST were performed for all subjects. Two of the 37 patients (5%; one with confirmed TB and the other with not-active TB) gave indeterminate ELISPOT assay results: for these 2 subjects there were positive responses in the positive control wells, but there were too many background spots in the negative control wells. The CSF MC ELISPOT assay was performed simultaneously with the PBMC ELISPOT assay for 21 of the 31 patients (68%) with suspected TB meningitis who agreed to additional CSF sampling. Five of the 21 patients (24%; 1 with probable TB, 1 with possible TB, and 3 with not-active TB) gave indeterminate ELISPOT assay results: in 1 subject there was a negative response in the positive control well, and in the other 4 there were too many background spots in the negative control wells.
TABLE 1.
Baseline clinical characteristics of 37 patients with suspected CNS TBa
| Characteristic | Patients with CNS TB (n = 12)b | Patients with not-active TB (n = 25) |
|---|---|---|
| Mean ± SD age (yr) | 45.5 ± 16.5 | 39.3 ± 16.5 |
| Male sex | 5 (42) | 15 (60) |
| Suspected infection and infection site | ||
| Suspected TB meningitisc | 8 (67) | 23 (92) |
| Suspected intracranial tuberculoma with disseminated TB | 4 (33) | 2 (8) |
| Underlying condition or illness | ||
| Human immunodeficiency virus infection | 1 (8) | 1 (4) |
| Transplantation | 0 (0) | 2 (8) |
| Hematologic malignancy | 0 (0) | 2 (8) |
| Solid tumor | 0 (0) | 1 (4) |
| Rheumatologic disease | 1 (8) | 1 (4) |
| Diabetes | 1 (8) | 1 (4) |
| No underlying illness | 8 (67) | 17 (68) |
| Immunosuppressive conditiond | 2 (17) | 7 (28) |
| Prior latent tuberculosis treatment | 0 (0) | 0 (0) |
| Prior active tuberculosis treatment | 2 (17) | 0 (0) |
| Tuberculin skin test induration size of ≥10 mm after 48 h | 6 (50) | 8 (32) |
| Results of diagnostic tests for tuberculosis | ||
| Positive AFB stain of CSF sample | 1 (13)e | 0 (0) |
| Positive M. tuberculosis PCR result for CSF sample | 0 (0)e | 0 (0) |
| Positive M. tuberculosis culture result for CSF sample | 3 (38)e | 0 (0) |
| Positive M. tuberculosis PCR or culture result for some other specimen | 4 (100)f | 0 (0) |
Data are presented as the number (percent) of patients unless indicated otherwise.
CNS TB includes cases of confirmed (n = 7), probable (n = 3), and possible (n = 2) TB.
All 31 patients with suspected TB meningitis showed lymphodominant pleocytosis in the CSF examination. The diagnoses for patients with not-active TB (n = 23) included confirmed viral meningitis (n = 7), probable viral meningitis (n = 9), leptomeningeal seeding of lymphoma (n = 2), and other diagnoses (n = 5).
An immunosuppressive condition was defined as an underlying disease such as human immunodeficiency virus infection, malignancy, liver cirrhosis, and chronic renal failure and/or immunosuppressive treatment.
The denominator is eight patients with TB meningitis.
The denominator is four patients with intracranial tuberculoma and disseminated TB.
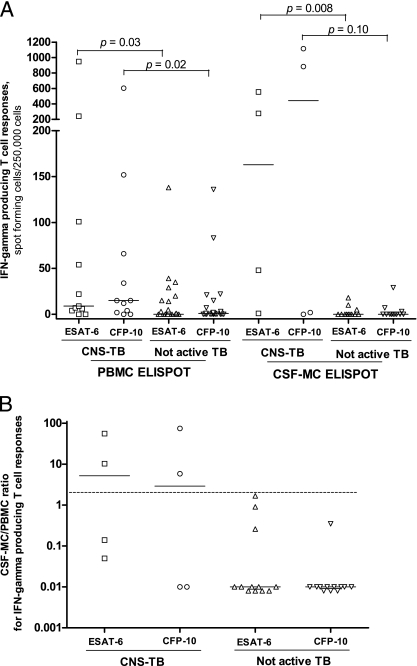
The detailed responses to ESAT-6 and CFP-10 in the PBMC ELISPOT assay and CSF MC ELISPOT assays in patients with suspected CNS TB are shown in Fig. 2 and Fig. 3. The sensitivity and the specificity of the PBMC ELISPOT assay were 91% (10/11 patients; 95% confidence interval [CI], 59% to 100%) and 63% (15/24 patients; 95% CI, 41% to 81%), respectively, while the sensitivity and specificity of the positive TST (induration size, ≥10 mm) were 46% (5/11 patients; 95% CI, 17% to 77%) and 67% (16/24 patients; 95% CI, 45% to 84%), respectively (P = 0.06 for the difference in sensitivity between the TST and the PBMC ELISPOT assay, and P = 0.76 for the difference in specificity between the TST and the PBMC ELISPOT assay). By comparison, the sensitivity and the specificity of the CSF MC ELISPOT assay were 75% (3/4 patients; 95% CI, 19% to 99%) and 75% (9/12 patients; 95% CI, 43% to 95%), respectively (P = 0.57 for the difference in sensitivity between the TST and the CSF MC ELISPOT assay, and P = 0.72 for the difference in specificity between the TST and the CSF MC ELISPOT assay). We considered that the presence of a greater number of ESAT-6- and CFP-10-specific cells in the CSF compared to the number in peripheral blood in patients with CNS TB might help with the differentiation of active TB from not-active TB and confirmed this idea by dividing the CSF MC ELISPOT assay results by the PBMC ELISPOT assay results. The median ratios of the CSF MC ELISPOT assay results to the PBMC ELISPOT assay results in patients with CNS TB were 5.2 (range, 0.05 to 55.6) for ESAT-6 and 2.9 (range, 0.01 to 74.4) for CFP-10, and those in patients with not-active TB were 0.01 (range, 0.01 to 1.67) for ESAT-6 and 0.01 (range, 0.01 to 0.35) for CFP-10. When the cutoff ratio of the CSF MC ELISPOT assay results to the PBMC ELISPOT assay results was set equal to 2 or more, the sensitivity and specificity were 50% (2/4 patients; 95% CI, 7% to 93%) and 100% (12/12 patients; 95% CI, 74% to 100%), respectively (Fig. 2B).
FIG. 2.
Responses to ESAT-6 and CFP-10 according to PBMC ELISPOT assay (A) and CSF MC ELISPOT assay (B) results in patients with suspected CNS TB. Bars, medians; dotted line in panel B, cutoff value; IFN, interferon.
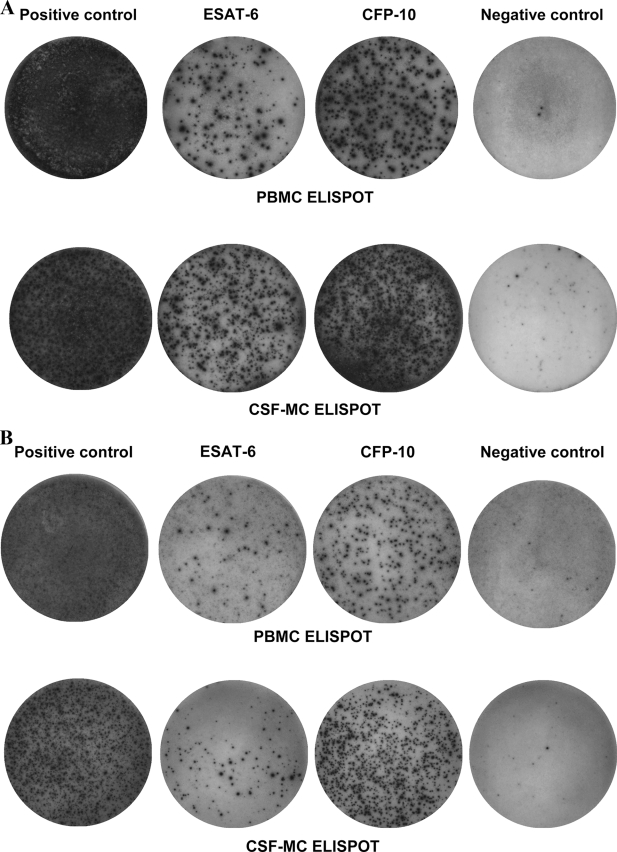
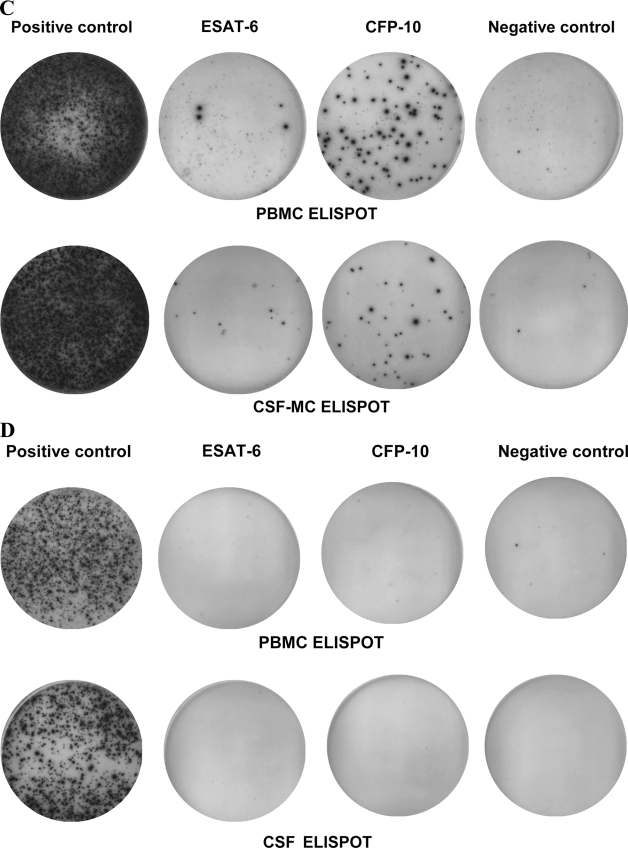
FIG. 3.
Typical results of ELISPOT assay with PBMCs and CSF MCs from patients with tuberculous meningitis (A and B) and aseptic meningitis (C and D). A response was classified as indeterminate if the number of spots for the positive control well was less than 20 or the number of spots for the negative control well was more than 10. A response was classified as positive if the number of spots for ESAT-6 or CFP-10 was six or more after subtraction of the background spots in the negative control well.
DISCUSSION
Conventional tests for the rapid diagnosis of CNS TB are of limited clinical use because the CSF may contain very few bacilli, and invasive procedures are needed to obtain infected tissue from patients with suspected intracranial tuberculomas. In addition, mycobacterial culture can take several weeks, often delaying diagnosis and the initiation of therapy. Nucleic acid amplification tests have been developed with the goal of enabling clinicians to make a rapid diagnosis of TB meningitis (9). However, their overall low sensitivity precludes the use of these tests to rule out TB meningitis (9). Therefore, a rapid, sensitive, and specific test for the diagnosis of CNS TB is urgently required. In the present work, we assessed the clinical usefulness of the newly developed T-cell-based ELISPOT assay with samples from patients with suspected CNS TB. We found that the PBMC ELISPOT assay had a 91% sensitivity for the diagnosis of active TB in patients with suspected CNS TB. This finding is consistent with that from our previous report that showed that the sensitivity of the ELISPOT assay was 94% for the diagnosis of extrapulmonary TB (6). Our findings thus suggest that a negative result by the PBMC ELISPOT assay is a useful adjunct for the exclusion of TB in patients with suspected CNS TB.
In the current study, 9 (38%) of the 24 subjects without active TB who gave valid PBMC ELISPOT assay results were positive by the PBMC ELISPOT assay. Thus, the clinical use of this assay is limited in regions where the endemicity of TB is intermediate to high because it does not differentiate active TB from latent TB infection (6). However, in active TB, M. tuberculosis-specific T cells are recruited to the site of the infection (2-5, 8, 14). Therefore, the enumeration of effector T cells at the site of infection by the ELISPOT assay may permit a more specific diagnosis of active TB than the enumeration of effector T cells in the blood alone (8). Wilkinson et al. (14) and Jafari et al. (5) demonstrated that the ELISPOT assay performed with pleural effusion MCs and bronchoalveolar lavage fluid, respectively, was highly specific for the diagnosis of active TB. However, Losi et al. found that the specificity of the ELISPOT assay performed with pleural effusion MCs for the diagnosis of active TB was 76% (8). Similarly, Breen and colleagues reported that the specificity of the ELISPOT assay performed with bronchoalveolar lavage fluid for the diagnosis of active TB was 76% (3). These findings are consistent with the specificity of 75% (95% CI, 43% to 95%) for the CSF MC ELISPOT assay for the diagnosis of CNS TB in our study. At the time of this writing, Kösters et al. (7) reported on a case which showed high levels of T-cell responses by the CSF MC ELISPOT assay in a patient with TB meningitis, and Thomas et al. (11) described 11 cases (1 confirmed case of TB and 10 probable cases of TB) and showed that 9 cases had positive T-cell responses by the CSF MC ELISPOT assay. They proposed the possibility that this assay may be a useful adjunct to rule in CNS TB. However, we showed that the ratio of the CSF MC ELISPOT assay results to the PBMC ELISPOT assay results can distinguish between active TB and not-active TB with a high degree of specificity. We have thus demonstrated for patients with suspected TB meningitis the proof of concept that the M. tuberculosis-specific ELISPOT assay performed with samples from the site of infection may permit a more specific diagnosis of active TB than the assay performed with blood alone.
Some may be concerned that 42% of the patients were classified as having probable or possible CNS TB mainly on the basis of the findings of assays with CSF and the patients' clinical responses to anti-TB therapy, without microbiologic confirmation. In addition, the fact that the attending physicians were not blinded to the results of the ELISPOT assay could have affected the choice of empirical antituberculous treatment (Fig. 1). We agree that reference standards often involve some degree of error or user dependence. However, real-world considerations compel us to use practical definitions. Therefore, we applied the predefined strict criteria for culture-negative CNS TB or not-active CNS TB cases and classified them without knowledge of the results of the TST and ELISPOT assay to avoid verification bias. Furthermore, most previous studies of CNS TB have used these clinical criteria for the diagnosis of CNS TB because the low bacillary count in the CSF makes bacteriological confirmation difficult (12, 13). Furthermore, our small number of CNS TB patients limits the reliability of the estimates of the sensitivity of the ELISPOT assay. However, the strict case definition used for “not-active TB” definitely excludes active TB. Thus, we conclude that our study reliably estimates the specificity of the ELISPOT assay for patients with suspected CNS TB. Therefore, we suggest that a twofold or greater number of M. tuberculosis-specific T cells in the CSF compared with the number in blood warrants the administration of empirical anti-TB therapy until definitive results are obtained.
In conclusion, our study suggests that the PBMC ELISPOT assay is a useful adjunct test for ruling out CNS TB and that the ratio of the CSF MC ELISPOT assay results to the PBMC ELISPOT assay results is useful for ruling in CNS TB.
Acknowledgments
No author received financial support.
None of the authors has a potential conflict of interest.
Footnotes
Published ahead of print on 16 July 2008.
REFERENCES
- 1.Baker, C. A., C. P. Cartwright, D. N. Williams, S. M. Nelson, and P. K. Peterson. 2002. Early detection of central nervous system tuberculosis with the Gen-Probe nucleic acid amplification assay: utility in an inner city hospital. Clin. Infect. Dis. 35:339-342. [DOI] [PubMed] [Google Scholar]
- 2.Barry, S. M., M. C. Lipman, B. Bannister, M. A. Johnson, and G. Janossy. 2003. Purified protein derivative-activated type 1 cytokine-producing CD4+ T lymphocytes in the lung: a characteristic feature of active pulmonary and nonpulmonary tuberculosis. J. Infect. Dis. 187:243-250. [DOI] [PubMed] [Google Scholar]
- 3.Breen, R. A., S. M. Barry, C. J. Smith, R. J. Shorten, J. P. Dilworth, I. Cropley, T. D. McHugh, S. H. Gillespie, G. Janossy, and M. C. Lipman. 2008. The clinical application of a rapid lung-orientated TB immunoassay in individuals with possible tuberculosis. Thorax 63:67-71. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch, C. S., Z. Toossi, J. L. Johnson, H. Luzze, P. Peters, M. McHugh, A. Okwera, M. Joloba, P. Mugyenyi, R. D. Mugerwa, P. Terebuh, and J. J. Ellner. 2001. Augmentation of apoptosis and interferon-gamma production at sites of active Mycobacterium tuberculosis infection in human tuberculosis. J. Infect. Dis. 183:779-788. [DOI] [PubMed] [Google Scholar]
- 5.Jafari, C., M. Ernst, B. Kalsdorf, U. Greinert, R. Diel, D. Kirsten, K. Marienfeld, A. Lalvani, and C. Lange. 2006. Rapid diagnosis of smear-negative tuberculosis by bronchoalveolar lavage enzyme-linked immunospot. Am. J. Respir. Crit. Care Med. 174:1048-1054. [DOI] [PubMed] [Google Scholar]
- 6.Kim, S. H., S. J. Choi, H. B. Kim, N. J. Kim, M. D. Oh, and K. W. Choe. 2007. Diagnostic usefulness of a T-cell-based assay for extrapulmonary tuberculosis. Arch. Intern. Med. 67:2255-2259. [DOI] [PubMed] [Google Scholar]
- 7.Kösters, K., R. Rau, A. Bossink, I. Greiffendorf, M. Jentsch, M. Ernst, S. Thijsen, T. Hinks, A. Lalvani, and C. Lange. 12 January 2008. Rapid diagnosis of CNS tuberculosis by a T-cell interferon-γ release assay on cerebrospinal fluid mononuclear cells. Infection [Epub ahead of print.] doi: 10.1007/s15010-007-7316-0. [DOI] [PubMed]
- 8.Losi, M., A. Bossink, L. Codecasa, C. Jafari, M. Ernst, S. Thijsen, D. Cirillo, M. Ferrarese, U. Greinert, L. M. Fabbri, L. Richeldi, and C. Lange; European Tuberculosis Network TBNET. 2007. Use of a T-cell interferon-γ release assay for the diagnosis of tuberculous pleurisy. Eur. Respir. J. 30:1173-1179. [DOI] [PubMed] [Google Scholar]
- 9.Pai, M., L. L. Flores, N. Pai, A. Hubbard, L. W. Riley, and J. M. Colford. 2003. Diagnostic accuracy of nucleic acid amplification tests for tuberculosis meningitis: a systematic review and meta-analysis. Lancet Infect. Dis. 3:633-643. [DOI] [PubMed] [Google Scholar]
- 10.Pai, M., L. W. Riley, and J. M. Colford. 2004. Interferon-gamma assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect. Dis. 4:761-766. [DOI] [PubMed] [Google Scholar]
- 11.Thomas, M. M., T. S. C. Hinks, S. Raghuraman, N. Ramalingam, M. Ernst, R. Nau, C. Lange, K. Kösters, C. Gnanamuthu, G. T. John, B. Marshall, and A. Lalvani. 2008. Rapid diagnosis of Mycobacterium tuberculosis meningitis by enumeration of cerebrospinal fluid antigen-specific T-cells. Int. J. Tuberc. Lung Dis. 12:651-657. [PMC free article] [PubMed] [Google Scholar]
- 12.Thwaites, G. E., T. T. Chau, K. Stepniewska, N. H. Phu, L. V. Chuong, D. X. Sinh, N. J. White, C. M. Parry, and J. J. Farrar. 2002. Diagnosis of adult tuberculous meningitis by use of clinical and laboratory features. Lancet 360:1287-1292. [DOI] [PubMed] [Google Scholar]
- 13.Thwaites, G. E., D. B. Nguyen, H. D. Nguyen, T. Q. Hoang, T. T. Do, T. C. Nguyen, Q. H. Nguyen, T. T. Nguyen, N. H. Nguyen, T. N. Nguyen, N. L. Nguyen, H. D. Nguyen, N. T. Vu, H. H. Cao, T. H. Tran, P. M. Pham, T. D. Nguyen, K. Stepniewska, N. J. White, T. H. Tran, and J. J. Farrar. 2004. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N. Engl. J. Med. 351:1741-1751. [DOI] [PubMed] [Google Scholar]
- 14.Wilkinson, K. A., R. J. Wilkinson, A. Pathan, K. Ewer, M. Prakash, P. Klenerman, N. Maskell, R. Davies, G. Pasvol, and A. Lalvani. 2005. Ex vivo characterization of early secretory antigenic target 6-specific T cells at sites of active disease in pleural tuberculosis. Clin. Infect. Dis. 40:184-187. [DOI] [PubMed] [Google Scholar]