Abstract
Probiotic bacteria alleviate many gastrointestinal symptoms, but the current trend of combining bacteria for additional benefit may make their effects more complex. We characterize four probiotics and their combination in terms of pathogen adhesion, barrier function, cell death, and inflammatory response in Helicobacter pylori-infected epithelial cells. H. pylori-infected Caco-2 cells were pretreated with Lactobacillus rhamnosus GG, Lactobacillus rhamnosus Lc705, Propionibacterium freudenreichii subsp. shermanii Js, Bifidobacterium breve Bb99, or all four organisms in combination. We evaluated the adhesion of H. pylori by in situ immunofluorescence; epithelial barrier function by measurement of transepithelial resistance; apoptosis by measurement of caspase 3 activation; cell membrane leakage by measurement of lactate dehydrogenase release; and inflammation by measurement of interleukin-8 (IL-8), IL-10, prostaglandin E2 (PGE2), and leukotriene B4 (LTB4) release. All probiotics inhibited H. pylori adhesion. L. rhamnosus GG, L. rhamnosus Lc705, P. freudenreichii subsp. shermanii Js, and the combination inhibited H. pylori-induced cell membrane leakage. L. rhamnosus GG, L. rhamnosus Lc705, and the combination initially improved epithelial barrier function but increased the H. pylori-induced barrier deterioration after incubation for 24 to 42 h. L. rhamnosus GG, L. rhamnosus Lc705, and P. freudenreichii subsp. shermanii Js inhibited H. pylori-induced IL-8 release, whereas L. rhamnosus GG, L. rhamnosus Lc705, and B. breve Bb99 suppressed PGE2 release. None of these anti-inflammatory effects persisted when the probiotics were used in combination. The combination thus increased the levels of IL-8, PGE2, and LTB4 released from H. pylori-infected epithelial cells. The proinflammatory actions of the individual components dominated the anti-inflammatory effects when the probiotic bacteria were used in combination. Our results stress that the therapeutic response can be optimized if probiotic strains are characterized before they are used in combination.
Helicobacter pylori infection is the single most important risk factor worldwide for the development of gastritis and gastric and duodenal ulcers (12, 28) and of related malignancies (9, 17). The infection, usually acquired in early childhood, is persistent unless it is actively treated with antimicrobials (7). H. pylori adheres to epithelial cells by injecting toxins, such as the cytotoxin-associated antigen (CagA) and vacuolating toxin (VacA), to activate proinflammatory signaling cascades and cause cell death (7). H. pylori-infected epithelial cells secrete cytokines, chemokines (5), and eicosanoids (38) to promote local inflammation and tissue damage. These mediators attract and activate neutrophils and monocytes/macrophages at the site of infection (35). In addition, H. pylori infection disturbs epithelial barrier functions (41, 42, 43, 51). Prolonged perturbation of the barrier function and chronic inflammation not only predispose the individual to functional disorders but also contribute to the development of cancer (38).
Probiotics are nonpathogenic microorganisms that, when administered in adequate amounts, confer a health benefit on the host (20). Probiotic bacteria are noninvasive, yet they need to interact with gastrointestinal (GI) epithelial cells to elicit their immunomodulatory effects (4). Probiotics have been shown to induce various epithelial cell responses by competing with pathogenic bacteria for host adhesion binding sites, improving epithelial cell barrier function, and stimulating the host immune response in general (11).
Probiotic bacteria have successfully been used for the prevention and treatment of GI infections and diseases, such as enteropathogenic Escherichia coli infections and rotavirus infections (18, 46). Probiotics have also been shown to have beneficial effects against H. pylori infections (10, 14). These promising observations have initiated a trend of combining probiotic bacteria for their pronounced or synergistically beneficial effects. The use of combinations of probiotic bacteria has shown some degree of benefit, for example, in alleviating antibiotic-associated symptoms during H. pylori eradication treatment (36), relieving irritable bowel syndrome (21), and preventing relapses of ulcerative colitis and pouchitis (13, 32). It has previously been shown that the individual constituents of the probiotic combination(s) differ in their abilities to induce immunomodulatory effects in intestinal cells and blood cells (15, 25). However, neither of those studies was conducted with a stimulus with live pathogenic bacteria.
In order to find out the synergistic, additive, or even counterbalancing effects of probiotic bacteria used in combination, we compared the effects of four probiotic bacteria, Lactobacillus rhamnosus GG (ATCC 53103), Lactobacillus rhamnosus Lc705 (DSM 7061), Propionibacterium freudenreichii subsp. shermanii Js (DSM 7067), and Bifidobacterium breve Bb99 (DSM 13692), and all four bacteria in combination on the epithelial adhesion of H. pylori, the epithelial barrier function in H. pylori-infected epithelial cells, and H. pylori-induced immunoinflammatory responses and cell death. The selection of this specific probiotic combination was based on its effects in previous clinical studies (21, 36, 45, 55, 56).
MATERIALS AND METHODS
Caco-2 cell culture.
Caco-2 cells (HTB 37) obtained from the American Type Culture Collection (Manassas, VA) were cultured at +37°C in Dulbecco's modified Eagle's medium (DMEM; GIBCO BRL, Grand Island, NY) supplemented with 10% fetal calf serum (Biological Industries, Kibbutz Beit Haemek, Israel) and antibiotics (penicillin G, 100 U/ml; amphotericin B, 250 ng/ml; streptomycin, 100 μg/ml; GIBCO). Cells were seeded on standard 96-well plates (Nalge Nunc International, Naperville, IL), microporous inserts (pore diameter, 0.4 μm; Transwell; Corning Costar, Corning, NY), or 12-well plates (Corning Costar) at an initial density of 1 × 105 cells/cm2. Confluent monolayers differentiated by contact inhibition for 15 to 21 days were cultured in medium without antibiotics for 24 h before each experiment. The pH values of the supernatants from 12-well plates were monitored with a pH meter throughout the experiments (from 1 to 48 h).
Bacterial cultures.
Lyophilized H. pylori NCTC 11637 GagA+ VacA+ (German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany) was suspended in phosphate-buffered saline (PBS) and was grown on brucella agar (BBL, Sparks, MD) enriched with 7% (vol/vol) horse serum (BioTrading Benelux B.V., Mijdrecht, The Netherlands) at +37 ± 1°C for 4 to 6 days under microaerophilic conditions (Anaerocult C; Merck, Darmstadt, Germany). The bacterial suspension was stored in 15% sterile glycerol (J. T. Baker, Deventer, The Netherlands) in 0.5-ml aliquots at −80°C. Before the experiments, H. pylori was subcultured twice on brucella agar as described above and was aseptically harvested and centrifuged (550 × g). The bacterial pellets were suspended in DMEM and kept under microaerophilic conditions until use (a maximum of 20 min). The H. pylori concentrations were determined by plating methods.
Both L. rhamnosus GG and L. rhamnosus Lc705 were grown in MRS broth (Lab M) at +37 ± 1°C under aerobic conditions for 18 to 20 h. P. freudenreichii subsp. shermanii Js was grown in broth for propionibacterial strains (Valio Ltd., Helsinki, Finland) at +30 ± 1°C for 48 h. B. breve Bb99 was grown in MRS broth enriched with 1% l-cysteine hydrochloride monohydrate (Merck) under anaerobic conditions at +37 ± 1°C for 24 h. E. coli strain DH5α was grown in Luria-Bertani broth under aerobic conditions at +37 ± 1°C for 18 h. All probiotic bacteria were subcultured three times, harvested, and centrifuged (4,500 × g). The pellets were washed with PBS and resuspended at 109 CFU/ml, as estimated by plating methods, in DMEM with 5% fetal calf serum. The L. rhamnosus GG and L. rhamnosus Lc705 concentrations were analyzed by aerobic and anaerobic plating methods in MRS broth at +37 ± 1°C for 72 h. The P. freudenreichii subsp. shermanii Js concentration was defined by the anaerobic plating method in buffered propionibacterium agar at +30 ± 1°C for 6 days (54).
H. pylori adhesion.
The adhesion of H. pylori was analyzed by using a modified method of Nozawa et al. (39). Briefly, Caco-2 cells were allowed to differentiate on standard 96-well plates. Cell monolayers were pretreated with the individual probiotics or their combination in fresh culture medium at +37°C under a 5% CO2 atmosphere for 1 h. The culture medium was then replaced with a 100-μl aliquot of H. pylori in fresh culture medium. The plates were incubated at +37°C under a 5% CO2 atmosphere for 90 min and washed twice with PBS to remove the nonadherent H. pylori cells. Cells with adherent H. pylori were fixed with ice-cold 10% formalin (Sigma-Aldrich, St. Louis, MO) at +4°C for 1 h. After three washes with PBS, 70 μl of rabbit anti-H. pylori antibody (Dako A/S, Glostrup, Denmark) in PBS (1:30) was added to each well. After a 1-h incubation at room temperature (+20 ± 2°C) and three washes with 1% bovine serum albumin-PBS, 70 μl of secondary antibody (goat anti-rabbit immunoglobulin G; Alexa Fluor 488; Molecular Probes, Eugene, OR) in PBS (1:500) was added to each well. The plates were incubated 1 h at +37°C under protection from light and then washed four times. The fluorescence was then measured with a Victor2 multilabel counter 1420 (Perkin-Elmer, Boston, MA) by using excitation and emission wavelengths of 485 nm and 535 nm, respectively.
Epithelial cell integrity.
Bacteria were added at the desired concentrations to the apical compartments of cell culture inserts, and the cultures were incubated at +37°C under a 5% CO2 atmosphere for 42 h. Transepithelial electrical resistance (TER), which was used as an index of epithelial integrity, was measured with an EVOM epithelial voltohmmeter with a “chopstick” electrode (World Precision Instruments, Stevenage, United Kingdom). TER across the monolayers was measured at the indicated time points over 42 h of incubation, and the inserts were maintained at a constant temperature (+37°C) under a 5% CO2 atmosphere. Measurements are expressed in Ω/cm2 after subtraction of the mean resistance of the cell-free inserts. Prior to experimentation, the Caco-2 monolayers were allowed to differentiate for 21 days to acquire a mean baseline TER of 950 Ω/cm2.
Measurement of epithelial cell leakage and apoptosis.
The release of lactate dehydrogenase (LDH), which indicates cell membrane damage, and caspase 3, which indicates apoptosis, was measured 8 and 24 h after H. pylori infection. The release of LDH into the culture medium was quantified by using a kit from Roche Molecular Biochemicals (Mannheim, Germany). Cell culture supernatants were collected at 8 or 24 h after H. pylori infection, centrifuged to remove particulate matter, and assayed according to the manufacturer's instructions. Total LDH release was evaluated by lysing the cell monolayer with 10% sodium dodecyl sulfate. The activation of caspase 3 was measured with a kit from Molecular Probes. Cells from the 12-well experiments were lysed and assayed according to the manufacturer's instructions. The fluorescence was measured at excitation and emission wavelengths of 355 nm and 460 nm, respectively, after 1 h of incubation at room temperature.
Cytokine and eicosanoid measurements.
The levels of interleukin-8 (IL-8) and IL-10 and the proinflammatory eicosanoids prostaglandin E2 (PGE2) and leukotriene B4 (LTB4) in the culture supernatants were analyzed with enzyme-linked immunosorbent assay (ELISA) kits, according to the manufacturers' instructions. The assays' detection limits were 1 pg/ml for the IL-8 and IL-10 ELISAs (both from CLB, Sanquin, Amsterdam, Netherlands) and 15 pg/ml and 6 pg/ml for the PGE2 and LTB4 assays (both from Cayman Chemical, Ann Arbor, MI), respectively.
Statistical analysis.
Statistical differences were analyzed by one-way analyses of variance and Bonferroni multiple-comparison testing. P values of <0.05 were considered significant. Calculations were performed with GraphPad Prism software (San Diego, CA).
RESULTS
Probiotic strains inhibit H. pylori adhesion to intestinal epithelial cells.
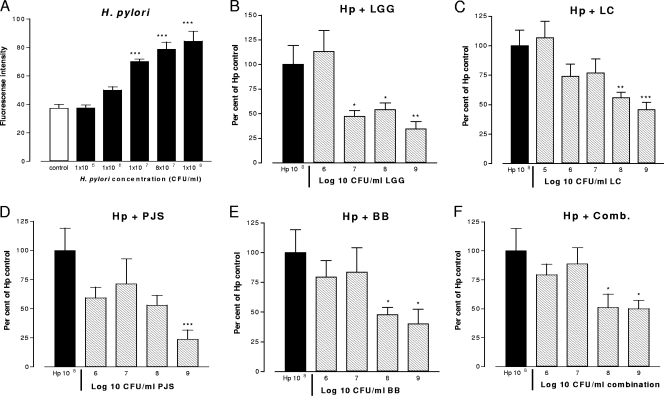
The ability of probiotic strains to interfere with the adhesion of H. pylori to Caco-2 cells was determined by in situ immunofluorescence after preincubation with the probiotic strains. The concentration dependence of H. pylori adherence was evaluated by incubating Caco-2 cells with increasing concentrations of H. pylori (Fig. 1A). The level of adhesion of H. pylori at 108 CFU/ml was assigned a value of 100%, and the effects of preincubation with the probiotic strains were compared to this control level (Fig. 1B to F). L. rhamnosus GG at a concentration of 107 CFU/ml already inhibited the adhesion of H. pylori by 53% (P < 0.05) (Fig. 1B). At this concentration, none of the other probiotics inhibited H. pylori adherence. L. rhamnosus Lc705, B. breve Bb99, and the combination were effective at concentrations 1 order of magnitude greater (Fig. 1C, E, and F), whereas P. freudenreichii subsp. shermanii Js inhibited H. pylori adherence only at the highest concentration, 109 CFU/ml (P < 0.001) (Fig. 1D). E. coli DH5α did not have any effect on H. pylori adhesion when E. coli was used over a concentration range of 105 to 109 CFU/ml (data not shown). Maximal inhibitory effects were seen with L. rhamnosus GG and P. freudenreichii subsp. shermanii Js at concentrations of 109 CFU/ml, which decreased the levels of H. pylori adhesion by 66% and 76%, respectively. For all bacteria, the pH of the coculture medium remained stable (pH 6.9 to 7.3) throughout the incubation.
FIG. 1.
Cells cultured on 96-well plates and allowed to differentiate for 15 days were incubated with H. pylori at the indicated concentrations with and without a 1-h preincubation with the indicated concentrations of the probiotic strains. The level of adhesion of H. pylori at 108 CFU/ml was assigned a value of 100%, and the effects of the probiotics were compared with this control value. All bacteria were suspended in fresh culture medium, and control cultures received fresh culture medium instead of treatment. Data are the means ± standard errors of the means (n = 5 to 7). *, P < 0.05 compared to the results obtained with H. pylori alone; **, P < 0.01 compared to the results obtained with H. pylori alone; ***, P < 0.001 compared to the results obtained with H. pylori alone. Hp, H. pylori; LGG, L. rhamnosus GG; Lc705, L. rhamnosus Lc705; PJS, P. freudenreichii subsp. shermanii Js; BB, B. breve Bb99; Comb., all four probiotic strains in combination.
Probiotics differentially modulate the acute and delayed barrier function of H. pylori-infected epithelial cells.
H. pylori-infected cells were pretreated for 2 h with control medium, the individual probiotics, or the combination of probiotics (Fig. 2). Without probiotic pretreatment, H. pylori dose dependently decreased TER after 18 h. In contrast, L. rhamnosus GG and L. rhamnosus Lc705 increased TER in uninfected cells at 18 h (Fig. 2A and B). When H. pylori-infected epithelial cells were pretreated with L. rhamnosus GG, L. rhamnosus Lc705, or the combination of the four probiotics, an increase in barrier function (P < 0.001) was observed during the first 8 h. After 18 h of incubation, however, this effect was counteracted, with a TER decline and the potentiation of H. pylori-induced barrier deterioration. None of the probiotics or the probiotic combination protected against H. pylori-induced barrier function decline.
FIG. 2.
TER from 0 to 42 h after preincubation with and without probiotic strains. Cells were allowed to differentiate for 21 days on semipermeable inserts in 12-well plates. The indicated concentrations of H. pylori with and without a 1-h probiotic pretreatment were added to the apical compartments of culture inserts, and TER across the epithelial monolayer was measured. All bacteria were suspended in fresh culture medium, and control cultures received fresh culture medium instead of treatment. Data are the means ± standard errors of the means (n = 6). *, P < 0.01 compared to the results for the uninfected control. Hp, H. pylori; LGG, L. rhamnosus GG; Lc705, L. rhamnosus Lc705; PJS, P. freudenreichii subsp. shermanii Js; BB, B. breve Bb99; Comb., all four probiotic strains in combination.
Probiotics alleviate acute H. pylori-induced cell membrane damage but aggravate it at later stages of infection.
The levels of acute and delayed cell membrane damage in the cell monolayers were measured by determination of the release of LDH and by the detection of apoptosis by determination of the level of activation of caspase 3 at 8 and 24 h. H. pylori increased the level of LDH released from the epithelial cells when it was measured at 8 h (P < 0.01) (Fig. 3), suggesting the induction of acute cell membrane leakage. Pretreatment with L. rhamnosus GG, L. rhamnosus Lc705, P. freudenreichii subsp. shermanii Js, or the combination (Fig. 3A to C and E) counteracted this H. pylori-induced effect. The amount of LDH released from probiotic-pretreated uninfected cells was similar to that released from the untreated controls. At 8 h of incubation, no significant effects on caspase 3 activity were observed, suggesting that neither H. pylori nor the probiotic pretreatments promote acute apoptotic effects (Fig. 3A to E).
FIG. 3.
Effects of probiotics and H. pylori on LDH release and caspase 3 activity at 8 h. Caco-2 cells cultured on 12-well plates, allowed to differentiate for 21 days, and pretreated for 1 h with the probiotics at the indicated concentrations were infected with H. pylori at a concentration of 108 CFU/ml. The LDH activity in the conditioned medium was evaluated and compared to that for untreated cells. Caspase 3 activity was evaluated from cell lysates. All bacteria were suspended in fresh culture medium, and control cultures received fresh culture medium instead of treatment. Data are the means ± standard errors of the means (n = 4 to 5). ***, P < 0.001 compared to the results obtained with the uninfected control; ⧫⧫⧫, P < 0.001 for H. pylori not pretreated versus the results for pretreated H. pylori. Hp, H. pylori; LGG, L. rhamnosus GG; Lc705, L. rhamnosus Lc705; PJS, P. freudenreichii subsp. shermanii Js; BB, B. breve Bb99; Comb., all four probiotic strains in combination.
After 24 h of incubation, each of the probiotic bacteria and their combination potentiated the release of LDH from H. pylori-infected Caco-2 cells (Fig. 4A, B, and E). However, no cell detachment or structural protein loss, as measured by the sustained expression of actin (3, 22), was evident at any time point or with any treatment throughout the study (data not shown). In order to exclude potential pH-dependent activity on H. pylori or epithelial cell viability, the experiments were carried out at neutral pH. The pH of the culture supernatants was repeatedly measured throughout the experiments (data not shown). Despite the aggravation of H. pylori-induced membrane damage, P. freudenreichii subsp. shermanii Js alone increased the level of epithelial cell apoptosis (P < 0.01), as measured by determination of the level of caspase 3 activity, after 24 h of incubation (Fig. 4C). Our results suggest that H. pylori induces acute cell membrane damage that is preventable by probiotic pretreatment. Probiotic pretreatment aggravated H. pylori-evoked delayed cell membrane damage.
FIG. 4.
Effects of probiotics and H. pylori on LDH release and caspase 3 activity at 24 h. Caco-2 cells cultured on 12-well plates, allowed to differentiate for 21 days, and pretreated for 1 h with the probiotics at the indicated concentrations were infected with H. pylori at a concentration of 108 CFU/ml. The LDH activity in the conditioned medium was evaluated and compared to that for untreated cells. Caspase 3 activity was evaluated from cell lysates. All bacteria were suspended in fresh culture medium, and control cultures received fresh culture medium instead of treatment. Data are the means ± standard errors of the means (n = 4 to 5). *, P < 0.05 compared to the results for the uninfected control; ***, P < 0.001 compared to the results for the uninfected control; ⧫, P < 0.05 for H. pylori not pretreated versus the results for pretreated H. pylori; ⧫⧫, P < 0.01 for H. pylori not pretreated versus the results for pretreated H. pylori; ⧫⧫⧫, P < 0.001 for H. pylori not pretreated versus the results for pretreated H. pylori. Hp, H. pylori; LGG, L. rhamnosus GG; Lc705, L. rhamnosus Lc705; PJS, P. freudenreichii subsp. shermanii Js; BB, B. breve Bb99; Comb., all four probiotic strains in combination.
Strain-dependent effects on inflammatory responses as a result of differential penetrance of the combination of probiotics in H. pylori-infected epithelial cells.
The effects of the probiotics on H. pylori-induced inflammation were evaluated by measuring the IL-8, IL-10, PGE2, and LTB4 concentrations at the end of the study period. H. pylori dose dependently increased the release of IL-8, PGE2, and LTB4 (Fig. 5 to 7) from epithelial cells. The basal level of IL-10 released from the Caco-2 cells was undetectable by ELISA but was measurable after incubation with H. pylori (1.9 pg/ml at 108 CFU/ml and 2.0 pg/ml at 107 CFU/ml).
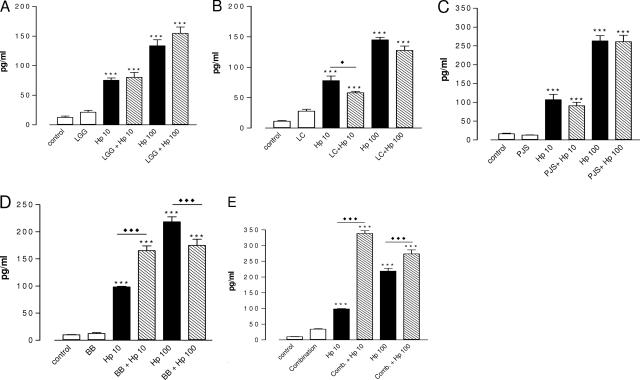
FIG. 5.
Levels of IL-8 in the culture medium after 42 h of H. pylori infection with and without probiotic pretreatment. Cells cultured on semipermeable inserts in 12-well plates were allowed to differentiate for 21 days. After 1 h of pretreatment with and without probiotics at a concentration of 108 CFU/ml, H. pylori was added at 107 CFU/ml (Hp 10) or 108 CFU/ml (Hp 100). All bacteria were suspended in fresh culture medium, and control cultures received fresh culture medium instead of each treatment. Data are the means ± standard errors of the means (n = 6). *, P < 0.05 compared to the results for the uninfected control; ***, P < 0.001 compared to the results for the uninfected control; ⧫, P < 0.05 for H. pylori not pretreated versus the results for pretreated H. pylori; ⧫⧫, P < 0.01 for H. pylori not pretreated versus the results for pretreated H. pylori; open bars, apical compartment; shaded bars, basal compartment. Hp, H. pylori; LGG, L. rhamnosus GG; Lc705, L. rhamnosus Lc705; PJS, P. freudenreichii subsp. shermanii Js; BB, B. breve Bb99; Comb., all four probiotic strains in combination.
FIG. 7.
Levels of LTB4 in the culture medium after 42 h of H. pylori infection with and without probiotic pretreatment. Cells cultured on semipermeable inserts in 12-well plates were allowed to differentiate for 21 days. After 1 h of pretreatment with and without probiotics at a concentration of 108 CFU/ml, H. pylori was added at 107 CFU/ml (Hp 10) or 108 CFU/ml (Hp 100). All bacteria were suspended in fresh culture medium, and control cultures received fresh culture medium instead of treatment. Data are the means ± standard errors of the means (n = 6). ***, P < 0.001 compared to the results for the control; ⧫, P < 0.05 for H. pylori not pretreated versus the results for pretreated H. pylori; ⧫⧫⧫, P < 0.001 for H. pylori not pretreated versus the results for pretreated H. pylori. Hp, H. pylori; LGG, L. rhamnosus GG; Lc705, L. rhamnosus Lc705; PJS, P. freudenreichii subsp. shermanii Js; BB, B. breve Bb99; Comb., all four probiotic strains in combination.
Pretreatment with L. rhamnosus GG, L. rhamnosus Lc705, or P. freudenreichii subsp. shermanii Js inhibited the secretion of IL-8 from infected cells but had no effect on that from uninfected cells. In contrast, B. breve Bb99 and the combination of probiotics induced a massive increase in the level of secretion of IL-8 compared to that for the control cell monolayer or the H. pylori-infected monolayer. The B. breve Bb99 strain also substantially potentiated the release of IL-8 from H. pylori-infected cells. Of the effects of all the probiotic strains, only the effect of B. breve Bb99, although it was diminished, persisted when the probiotics were used in combination. B. breve Bb99 pretreatment also enhanced the release of IL-10 from H. pylori-infected cells (2.6 pg/ml at 108 CFU/ml and 3.5 pg/ml at 107 CFU/ml). This anti-inflammatory effect was lost when the probiotics were used in combination (data not shown).
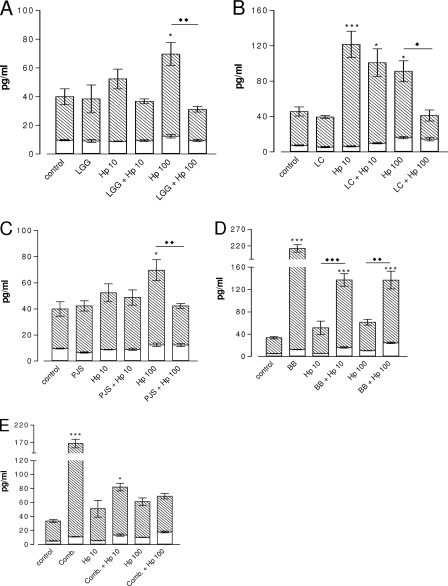
H. pylori dose dependently increased the levels of PGE2 (Fig. 6) and LTB4 (Fig. 7) released from Caco-2 cells. Treatment of H. pylori-uninfected cells with L. rhamnosus GG, L. rhamnosus Lc705, and P. freudenreichii subsp. shermanii Js also induced the production of PGE2 (Fig. 6A to C). This effect was not seen with B. breve Bb99 or the combination (Fig. 6D to E). However, pretreatment of H. pylori-infected (108 CFU/ml) cells with L. rhamnosus Lc705, P. freudenreichii subsp. shermanii Js, or B. breve Bb99 attenuated H. pylori-induced PGE2 production (Fig. 6B to D). An opposite and enhancing effect was observed with L. rhamnosus GG and the combination treatment (Fig. 6A and E). Thus, none of the anti-inflammatory activity of L. rhamnosus Lc705, P. freudenreichii subsp. shermanii Js, or B. breve Bb99 was reflected when they were used in the combination.
FIG. 6.
Levels of PGE2 in the culture medium after 42 h of H. pylori infection with and without probiotic pretreatment. Cells cultured on semipermeable inserts in 12-well plates were allowed to differentiate for 21 days after they reached confluence. After 1 h of pretreatment with and without probiotics at a concentration of 108 CFU/ml, H. pylori was added at 107 CFU/ml (Hp 10) or 108 CFU/ml (Hp 100). All bacteria were suspended in fresh culture medium, and control cultures received fresh culture medium instead of treatment. Data are the means ± standard errors of the means (n = 6). *, P < 0.05 compared to the results for the control; **, P < 0.01 compared to the results for the control; ***, P < 0.001 compared to the results for the control; ⧫⧫, P < 0.01 for H. pylori not pretreated versus the results for pretreated H. pylori; ⧫⧫⧫, P < 0.001 for H. pylori not pretreated versus the results for pretreated H. pylori. Hp, H. pylori; LGG, L. rhamnosus GG; Lc705, L. rhamnosus Lc705; PJS, P. freudenreichii subsp. shermanii Js; BB, B. breve Bb99; Comb., all four probiotic strains in combination.
A decrease in the amount of the H. pylori-induced chemotactic proinflammatory eicosanoid LTB4 released was evident by pretreatment with L. rhamnosus Lc705. In contrast, the amount of LTB4 released from cells infected with the lower concentration of H. pylori was increased by 1.7-fold with B. breve Bb99 (Fig. 7D) and by 3.5-fold with the combination pretreatments (Fig. 7E). The anti-inflammatory effect of L. rhamnosus Lc705 was lost upon treatment with combination of probiotics, which also persistently increased the amount of LTB4 released from cells infected with the higher concentration of H. pylori. The probiotics had no effect on the release of LTB4 from uninfected cells.
DISCUSSION
Our results show that in in vitro Caco-2 cell line experiments, all probiotics decreased the level of H. pylori adhesion to intestinal epithelial cells. Both L. rhamnosus strains, P. freudenreichii subsp. shermanii Js, and the combination inhibited the H. pylori-induced acute membrane leakage. Simultaneously, both L. rhamnosus strains and the combination transiently improved the epithelial barrier function. The inflammatory effects prevailed when the probiotics were used in combination, since the H. pylori-induced IL-8 release was inhibited by L. rhamnosus GG, L. rhamnosus Lc705, and P. freudenreichii subsp. shermanii Js; only the IL-8 secretion-inducing effect of B. breve Bb99 was seen in the combination. Moreover, H. pylori-induced PGE2 release was inhibited by L. rhamnosus Lc705, P. freudenreichii subsp. shermanii Js, and B. breve Bb99 and was enhanced by L. rhamnosus GG. Again, only the effect of L. rhamnosus GG prevailed in the combination. Furthermore, despite the inhibition of H. pylori-induced LTB4 release by L. rhamnosus Lc705 and B. breve Bb99, pretreatment with the combination of probiotics increased only the amount of LTB4 released.
The H. pylori adherence-inhibiting effects of probiotics are considered important. In earlier in vitro studies, several probiotic strains were shown to inhibit the adhesion capacity of H. pylori (6, 26, 31, 34, 37, 48, 51). In our study, all probiotics were able to decrease the level of adhesion of H. pylori significantly. Several previous studies have shown that L. rhamnosus GG, L. rhamnosus Lc705, P. freudenreichii subsp. shermanii Js, and some B. breve strains adhere to epithelial cells with different affinities (2, 8, 33, 52, 53). However, we found that both L. rhamnosus strains, L. rhamnosus GG and L. rhamnosus Lc705, reduced the level of H. pylori adhesion to fairly similar degrees. Thus, our results suggest that the H. pylori adhesion inhibition is possibly unrelated to the adhesion potential of the probiotic strain. Midolo et al. (31) studied the inhibition of H. pylori by several nonpathogenic strains and also by lactic, acetic, and hydrochloric acids. Six of the Lactobacillus strains tested inhibited H. pylori adhesion, and the effects were only partly explained by organic acid production. Furthermore, Coconnier et al. (6) found the anti-Helicobacter substance(s) in L. acidophilus LB to be different from lactic acid. Our experiments, conducted at neutral pH, now provide results showing that the antiadhesive activities of probiotics against H. pylori are also unrelated to the production of organic acids. Thus, the inhibition of H. pylori adhesion observed is likely due to the adhesion of probiotic bacteria by competition for binding sites on epithelial cells. However, it must be stressed that the adhesion of H. pylori has been better studied under more acidic conditions and that Caco-2 cells are not able to produce mucus, which is an important factor when the adhesion of H. pylori is considered. Since the effects of each probiotic strain on barrier function, cell leakage, and the inflammatory response to H. pylori were different, our results suggest that these are independent of pathogen adherence and are in strong disagreement with adherence inhibition being a good marker for anti-H. pylori activity.
The Caco-2 cell line is a well-characterized model of the gut epithelium and is capable of differentiation and polarization (16), and it releases several inflammatory mediators upon treatment with H. pylori (23). It also provides a suitable and frequently cited method for studying the effects of infectious agents as well as probiotic bacteria (47). When Caco-2 cells are allowed to differentiate for 21 days, as in our study, they reflect more enterocytes than colonocytes. We measured the differentiated Caco-2 cell monolayer's resistance, TER, as an indicator of GI-epithelial barrier function. The Lactobacillus rhamnosus strains (L. rhamnosus GG and L. rhamnosus Lc705) acutely tightened the barrier, whereas after longer exposure times, these treatments potentiated the decline in barrier function in H. pylori-infected epithelial cells. A similar acute enhancement has been reported for the probiotic mixture VSL#3 (40). Moreover, B. breve strain C50 and Streptococcus thermophilus strain 065 decreased the integrity of the epithelial cell monolayer under inflammatory conditions (30). Our results show that probiotics have different time-dependent effects on the intestinal barrier function and present a possible rationale that can be used to explain the various outcomes observed in previous studies. In particular, strains able to acutely enhance epithelial permeability increase the level of H. pylori-infected cell layer dysfunction later on. This effect may be due to the greater initial stimulation of the epithelial cell responses by these bacteria, which may cause epithelial injury. A similar effect was evident in the combination group, suggesting the persistence of activity when probiotic bacteria are used in combination.
Apoptosis and cell membrane integrity are crucial components of epithelial barrier function. We therefore investigated the effects of probiotics on apoptosis and cell membrane leakage in cells infected with H. pylori. We observed a biphasic response to probiotics similar to that seen from the TER measurements. Moreover, our data show that although probiotic bacteria are able to prevent acute H. pylori-induced membrane damage, conceivably by inhibiting its adherence to epithelial cells, their copresence increases cell membrane leakage. On the basis of the fact that no cell detachment or any decreased yield of structural proteins that suggested necrotic cell death was observed (3, 22), we propose that the increased cell membrane damage is linked to the inability to promote the barrier function at later stages. With L. rhamnosus GG, P. freudenreichii subsp. shermanii Js, and the combination, the LDH release correlated well with the increased decline in the barrier function of H. pylori-infected cells. In accordance with the findings of a recent study (57), in which L. rhamnosus GG prevented the cytokine-induced apoptosis of human intestinal epithelial cells and mouse colonocytes, we found that both of the Lactobacillus strains studied, L. rhamnosus GG and L. rhamnosus Lc705, prevented caspase 3 activation when the cells were infected with H. pylori. The potent capacity of P. freudenreichii subsp. shermanii Js to induce apoptosis is consistent with the findings of previous work by Jan et al. (19), who showed that related Propionibacterium strains induced apoptosis in Caco-2 cells.
We found that the Lactobacillus strains (L. rhamnosus GG and L. rhamnosus Lc705) and the propionibacterium P. freudenreichii subsp. shermanii Js alleviated H. pylori-induced IL-8 production. L. rhamnosus GG was recently reported to have similar anti-inflammatory action in terms of tumor necrosis factor alpha in H. pylori-treated macrophages (44). Surprisingly, B. breve Bb99 greatly induced IL-8 production from cells with or without H. pylori exposure. In epithelial cells not infected with H. pylori, this enhancing effect persisted when probiotics were used in combination. In infected cells, however, no such superinduction was evident. Our results are consistent with those presented in a report by Morita et al. (33), who showed that specific B. breve strains may have proinflammatory effects. Our results are also in accordance with those presented elsewhere that nonpathogenic bacteria suppressed IL-8 production in colon epithelial cells stimulated with tumor necrosis factor alpha (1, 27, 29). Furthermore, there is clinical evidence that L. rhamnosus GG and the combination of probiotics modulate immune responses differently (45, 55, 56).
Given the role of eicosanoids in the pathogenicity of H. pylori and as positive regulators for the persistence of inflammation (38), we investigated the effects of the probiotics on H. pylori-induced PGE2 and LTB4 release. L. rhamnosus Lc705, P. freudenreichii subsp. shermanii Js, and B. breve Bb99 were able to suppress the release of PGE2 induced by H. pylori, even though L. rhamnosus Lc705 and P. freudenreichii subsp. shermanii Js alone activated PGE2 production. Consistent with a previous report of COX-2 induction by L. rhamnosus GG (24), we found that L. rhamnosus GG enhanced the secretion of PGE2 with or without H. pylori, suggesting that H. pylori infection has an additive effect. Again, none of the anti-inflammatory actions of the components persisted when the probiotics were used in combination.
Taken together, our results show that probiotics show striking differences in their modulation of cellular responses with or without a stimulus with a live pathogen. Our results also suggest that the proinflammatory effects prevailed when the probiotics were used in combination. Our study stresses the importance of the characterization of the individual strain to optimize the therapeutic responses of probiotics used in combination. Moreover, our data provide evidence that these characterizations be conducted in the presence of a relevant pathological stimulus, such as H. pylori in our study, since without the stimulus, the effects may be the opposite and may thus lack any predictive therapeutic value.
Acknowledgments
We are grateful to Lahja Eurajoki for expert technical assistance.
The study was supported by the Foundation for Nutrition Research, the Finnish Cultural Foundation, and the Finnish Cultural Foundation of Satakunta.
Footnotes
Published ahead of print on 25 June 2008.
REFERENCES
- 1.Bai, A. P., Q. Ouyang, W. Zhang, C. H. Wang, and S. F. Li. 2004. Probiotics inhibit TNF-α-induced interleukin-8 secretion of HT29 cells. World J. Gastroenterol. 10:455-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernet, M. F., D. Brassart, J. R. Neeser, and A. L. Servin. 1993. Adhesion of human bifidobacterial strains to cultured human intestinal epithelial cells and inhibition of enteropathogen-cell interactions. Appl. Environ. Microbiol. 59:4121-4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bizik, J., E. Kankuri, A. Ristimäki, A. Taieb, H. Vapaatalo, W. Lubitz, and A. Vaheri. 2004. Cell-cell contacts trigger programmed necrosis and induce cyclooxygenase-2 expression. Cell Death Differ. 11:183-195. [DOI] [PubMed] [Google Scholar]
- 4.Blum, S., Y. Delneste, S. Alvarez, D. Haller, P. F. Perez, C. Bode, W. P. Hammes, A. M. A. Pfeifer, and E. J. Schiffrin. 1999. Interactions between commensal bacteria and mucosal immunocompetent cells. Int. Dairy J. 9:63-68. [Google Scholar]
- 5.Bodger, K., and J. E. Crabtree. 1998. Helicobacter pylori and gastric inflammation. Br. Med. Bull. 54:139-150. [DOI] [PubMed] [Google Scholar]
- 6.Coconnier, M. H., V. Lievin, E. Hemery, and A. L. Servin. 1998. Antagonistic activity against Helicobacter infection in vitro and in vivo by the human Lactobacillus acidophilus strain LB. Appl. Environ. Microbiol. 64:4573-4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunn, B. E., H. Cohen, and M. J. Blaser. 1997. Helicobacter pylori. Clin. Microbiol. Rev. 10:720-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elo, S., M. Saxelin, and S. Salminen. 1991. Attachment of Lactobacillus casei strain GG to human colon carcinoma cell line Caco-2: comparison with other dairy strains. Lett. Appl. Microbiol. 13:154-156. [Google Scholar]
- 9.Eurogast Study Group. 1993. An international association between Helicobacter pylori infection and gastric cancer. Lancet 341:1359-1362. [PubMed] [Google Scholar]
- 10.Felley, C., and P. Michetti. 2003. Probiotics and Helicobacter pylori. Best Pract. Res. Clin. Gastroenterol. 17:785-791. [DOI] [PubMed] [Google Scholar]
- 11.Forestier, C., C. De Champs, C. Vatoux, and B. Joly. 2001. Probiotic activities of Lactobacillus casei rhamnosus: in vitro adherence to intestinal cells and antimicrobial properties. Res. Microbiol. 152:167-173. [DOI] [PubMed] [Google Scholar]
- 12.Fox, J. G., and T. C. Wang. 2001. Helicobacter pylori—not a good bug after all. N. Engl. J. Med. 345:829-832. [DOI] [PubMed] [Google Scholar]
- 13.Gionchetti, P., F. Rizzello, U. Helwig, A. Venturi, K. M. Lammers, P. Brigidi, B. Vitali, G. Poggioli, M. Miglioli, and M. Campieri. 2003. Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology 124:1202-1209. [DOI] [PubMed] [Google Scholar]
- 14.Hamilton-Miller, J. M. 2003. The role of probiotics in the treatment and prevention of Helicobacter pylori infection. Int. J. Antimicrob. Agents 22:360-366. [DOI] [PubMed] [Google Scholar]
- 15.Hart, A. L., K. Lammers, P. Brigidi, P. Vitali, F. Rizzello, P. Gionchetti, M. Campieri, M. A. Kamm, S. C. Knight, and A. J. Stagg. 2004. Modulation of human dendritic cell phenotype and function by probiotic bacteria. Gut 53:1602-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hidalgo, I. J., T. J. Raub, and R. T. Borchardt. 1989. Characterization of the human colon carcinoma cell line (Caco-2) as model system for intestinal epithelial permeability. Gastroenterology 96:736-749. [PubMed] [Google Scholar]
- 17.IARC. 1994. Schistosomes, liver flukes and Helicobacter pylori, p. 1-241. Monographs on the evaluation of carcinogenic risks to humans. IARC Science, Lyon, France. [PMC free article] [PubMed]
- 18.Isolauri, E., P. V. Kirjavainen, and S. Salminen. 2002. Probiotics: a role in the treatment of intestinal infection and inflammation? Gut 50(Suppl. iii):54-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jan, G., A. S. Belzacq, D. Haouzi, A. Rouault, D. Métivier, G. Kroemer, and C. Brenner. 2002. Propionibacteria induce apoptosis of colorectal carcinoma cells via short-chain fatty acids acting on mitochondria. Cell Death Differ. 9:179-188. [DOI] [PubMed] [Google Scholar]
- 20.Joint FAO/WHO Working Group. 2002. Report on drafting guidelines for the evaluation of probiotics in food. FAO and WHO, London, Ontario, Canada.
- 21.Kajander, K., K. Hatakka, T. Poussa, M. Färkkilä, and R. Korpela. 2005. A probiotic mixture alleviates symptoms in irritable bowel syndrome patients: a controlled 6-month intervention. Aliment. Pharmacol. Ther. 22:387-394. [DOI] [PubMed] [Google Scholar]
- 22.Kankuri, E., D. Cholujova, M. Comajova, A. Vaheri, and J. Bizik. 2005. Induction of hepatocyte growth factor/scatter factor by fibroblast clustering directly promotes tumor cell invasiveness. Cancer Res. 65:9914-9922. [DOI] [PubMed] [Google Scholar]
- 23.Kim, J. M., J. S. Kim, H. C. Jung, I. S. Song, and C. Y. Kim. 2002. Up-regulation of inducible nitric oxide synthase and nitric oxide in Helicobacter pylori-infected human gastric epithelial cells: possible role of interferon-gamma in polarized nitric oxide secretion. Helicobacter 7:116-128. [DOI] [PubMed] [Google Scholar]
- 24.Korhonen, R., O. Kosonen, R. Korpela, and E. Moilanen. 2004. The expression of COX-2 protein induced by Lactobacillus rhamnosus GG, endotoxin and lipoteichoic acid in T84 epithelial cells. Lett. Appl. Microbiol. 39:19-24. [DOI] [PubMed] [Google Scholar]
- 25.Lammers, M., U. Helwig, E. Swennen, F. Rizzello, A. Venturi, E. Caramelli, M. A. Kamm, P. Brigidi, P. Gionchetti, and M. Campieri. 2002. Effect of probiotic strains on interleukin 8 production by HT29/19A cells. Am. J. Gastroenterol. 97:1182-1186. [DOI] [PubMed] [Google Scholar]
- 26.Lorca, G. L., T. Wadström, G. F. Valdez, and Å. Ljungh. 2001. Lactobacillus acidophilus autolysins inhibit Helicobacter pylori in vitro. Curr. Microbiol. 42:39-44. [DOI] [PubMed] [Google Scholar]
- 27.Ma, D., P. Forsythe, and J. Bienenstock. 2004. Live Lactobacillus reuteri is essential for the inhibitory effect on tumor necrosis factor alpha-induced interleukin-8 expression. Infect. Immun. 72:5308-5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marshall, B. J., and J. R. Warren. 1984. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet i:1311-1315. [DOI] [PubMed] [Google Scholar]
- 29.McCracken, V. J., T. Chun, M. E. Baldeón, S. Ahrné, G. Molin, R. I. Mackie, and H. R. Gaskins. 2002. TNF-α sensitizes HT-29 colonic epithelial cells to intestinal lactobacilli. Exp. Biol. Med. 227:665-670. [DOI] [PubMed] [Google Scholar]
- 30.Menard, S., C. Candalh, J. C. Bambou, K. Terpend, N. Cerf-Bensussan, and M. Heyman. 2004. Lactic acid bacteria secrete metabolites retaining anti-inflammatory properties after intestinal transport. Gut 53:821-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Midolo, P. D., J. R. Lambert, R. Hull, F. Luo, and M. L. Grayson. 1995. In vitro inhibition of Helicobacter pylori NCTC 11637 by organic acids and lactic acid bacteria. J. Appl. Bacteriol. 79:475-479. [DOI] [PubMed] [Google Scholar]
- 32.Mimura, T., F. Rizzello, U. Helwig, G. Poggioli, S. Schreiber, I. C. Talbot, R. J. Nicholls, P. Gionchetti, M. Campieri, and M. A. Kamm. 2004. Once daily high dose probiotic therapy (VSL#3) for maintaining remission in recurrent or refractory pouchitis. Gut 53:108-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morita, H., F. He, T. Fuse, A. C. Ouwehand, H. Hashimoto, M. Hosoda, K. Mizumachi, and J. I. Kurisaki. 2002. Adhesion of lactic acid bacteria to Caco-2 cells and their effect on cytokine secretion. Microbiol. Immunol. 46:293-297. [DOI] [PubMed] [Google Scholar]
- 34.Mukai, T., T. Asasaka, E. Sato, K. Mori, M. Matsumoto, and H. Ohori. 2002. Inhibition of binding of Helicobacter pylori to the glycolipid receptors by probiotic Lactobacillus reuteri. FEMS Immunol. Med. Microbiol. 32:105-110. [DOI] [PubMed] [Google Scholar]
- 35.Mumy, K. L., and B. A. McCormick. 2004. Events at the host-microbial interface of the gastrointestinal tract. II. Role of the intestinal epithelium in pathogen-induced inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 288:G854-G859. [DOI] [PubMed] [Google Scholar]
- 36.Myllyluoma, E., L. Veijola, T. Ahlroos, S. Tynkkynen, E. Kankuri, H. Vapaatalo, H. Rautelin, and R. Korpela. 2005. Probiotic supplementation improves tolerance to Helicobacter pylori eradication therapy—a placebo-controlled, double-blind randomized pilot study. Aliment. Pharmacol. Ther. 21:1263-1272. [DOI] [PubMed] [Google Scholar]
- 37.Nam, H., M. Ha, O. Bae, and Y. Lee. 2002. Effect of Weissella confusa strain PL9001 on the adherence and growth of Helicobacter pylori. Appl. Environ. Microbiol. 68:4642-4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naumann, M., and J. Crabtree. 2004. Helicobacter pylori-induced epithelial cell signaling in gastric carcinogenesis. Trends Microbiol. 12:29-36. [DOI] [PubMed] [Google Scholar]
- 39.Nozawa, Y., K. Nishihara, R. M. Peek, M. Nakano, T. Uji, H. Ajioka, N. Matsura, and H. Miyake. 2002. Identification of a signalling cascade for interleukin-8 production by Helicobacter pylori in human gastric epithelial cells. Biochem. Pharmacol. 64:21-30. [DOI] [PubMed] [Google Scholar]
- 40.Otte, J. M., and D. K. Podolsky. 2004. Functional modulation of enterocytes by gram-positive and gram-negative microorganisms. Am. J. Physiol. Gastrointest. Liver Physiol. 286:G613-G626. [DOI] [PubMed] [Google Scholar]
- 41.Pai, R., T. L. Cover, and A. S. Tarnawski. 1999. Helicobacter pylori vacuolating cytotoxin (VacA) disorganizes the cytoskeletal architecture of gastric epithelial cells. Biochem. Biophys. Res. Commun. 262:245-250. [DOI] [PubMed] [Google Scholar]
- 42.Papini, E., B. Satin, N. Norais, M. de Bernard, J. L. Telford, and R. Rappuoli. 1998. Selective increase of the permeability of polarized epithelial cell monolayers by Helicobacter pylori vacuolating toxin. J. Clin. Investig. 102:813-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pelicic, V., J. M. Reyrat, L. Sartori, C. Pagliaccia, R. Rappuoli, J. L. Telford, C. Montecucco, and E. Papini. 1999. Helicobacter pylori VacA cytotoxin associated with the bacteria increases epithelial permeability independently of its vacuolating activity. Microbiology 145:2043-2050. [DOI] [PubMed] [Google Scholar]
- 44.Peña, J. A., and J. Versalovic. 2003. Lactobacillus rhamnosus GG decreases TNF-α production in lipopolysaccharide-activated murine macrophages by a contact-independent mechanism. Cell. Microbiol. 5:277-285. [DOI] [PubMed] [Google Scholar]
- 45.Pohjavuori, E., M. Viljanen, R. Korpela, M. Kuitunen, M. Tiittanen, O. Vaarala, and E. Savilahti. 2004. Lactobacillus GG effect in increasing IFN-gamma production in infants with cow's milk allergy. J. Allergy Clin. Immunol. 114:131-136. [DOI] [PubMed] [Google Scholar]
- 46.Servin, A. L. 2004. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol. Rev. 28:405-440. [DOI] [PubMed] [Google Scholar]
- 47.Servin, A. L., and M. H. Coconnier. 2003. Adhesion of probiotic strains to the intestinal mucosa and interaction with pathogens. Best Pract. Res. Clin. Gastroenterol. 17:741-754. [DOI] [PubMed] [Google Scholar]
- 48.Sgouras, D., P. Maragkoudakis, K. Petraki, B. Martinez-Gonzalez, E. Eriotou, S. Michopoulos, G. Kalantzopoulos, E. Tsakalidou, and A. Mentis. 2004. In vitro and in vivo inhibition of Helicobacter pylori by Lactobacillus casei strain Shirota. Appl. Environ. Microbiol. 70:518-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smoot, D. T., Z. Wynn, T. B. Elliott, C. R. Allen, G. Mekasha, T. Naab, and H. Ashktorab. 1999. Effects of Helicobacter pylori on proliferation of gastric epithelial cells in vitro. Am. J. Gastroenterol. 94:1508-1511. [DOI] [PubMed] [Google Scholar]
- 50.Terrés, A. M., J. M. Pajares, A. M. Hopkins, A. Murphy, A. Moran, A. W. Baird, and D. Kelleher. 1998. Helicobacter pylori disrupts epithelial barrier function in a process inhibited by protein kinase C activators. Infect. Immun. 66:2943-2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsai, C. C., L. F. Huang, C. C. Lin, and H. Y. Tsen. 2004. Antagonistic activity against Helicobacter pylori infection in vitro by a strain of Enterococcus faecium TM39. Int. J. Food Microbiol. 96:1-12. [DOI] [PubMed] [Google Scholar]
- 52.Tuomola, E. M., and S. J. Salminen. 1998. Adhesion of some probiotic and dairy Lactobacillus strains to Caco-2 cell cultures. Int. J. Food Microbiol. 41:45-51. [DOI] [PubMed] [Google Scholar]
- 53.Tuomola, E. M., A. C. Ouwehand, and S. J. Salminen. 1999. The effect of probiotic bacteria on the adhesion of pathogens to human intestinal mucus. FEMS Immunol. Med. Microbiol. 26:137-142. [DOI] [PubMed] [Google Scholar]
- 54.Vedamuthu, E. R., M. Raccach, B. A. Glatz, E. W. Seitz, and M. S. Reddy. 1992. Acid producing microorganism, p. 225-238. In C. Vanderzant and D. F. Splittstoesser (ed.), Compendium of methods for the microbiological examination of foods. APHA, Washington, DC.
- 55.Viljanen, M., E. Pohjavuori, T. Haahtela, R. Korpela, M. Kuitunen, A. Sarnesto, O. Vaarala, and E. Savilahti. 2005. Induction of inflammation as a possible mechanism of probiotic effect in atopic eczema-dermatitis syndrome. J. Allergy Clin. Immunol. 115:1254-1259. [DOI] [PubMed] [Google Scholar]
- 56.Viljanen, M., M. Kuitunen, T. Haahtela, K. Juntunen-Backman, R. Korpela, and E. Savilahti. 2005. Probiotic effects on faecal inflammatory markers and on faecal IgA in food allergic atopic eczema/dermatitis syndrome infants. Pediatr. Allergy Immunol. 16:65-71. [DOI] [PubMed] [Google Scholar]
- 57.Yan, F., and B. Polk. 2002. Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. J. Biol. Chem. 277:50959-50965. [DOI] [PMC free article] [PubMed] [Google Scholar]