Abstract
The aim of this study was to optimize the ability to detect cytomegalovirus (CMV)-specfic cell-mediated immunity (CMI) in human immunodeficiency virus (HIV)-infected individuals by comparing different assays (the lymphocyte proliferation assay [LPA] and assays for gamma interferon [IFN-γ] and interleukin-2 [IL-2] production) and CMV antigenic preparations. Thresholds discriminating positive from negative CMI results were developed with specimens from 36 CMV-seropositive and 21 CMV-seronegative healthy individuals. The analysis showed that the CMI elicited by any of the four CMV whole lysates tested in this study tended to be more robust and sensitive than the responses to the subunit antigens gB and pp65. LPA and inducible IFN-γ but not IL-2 were highly sensitive measures of CMV-specific CMI in HIV-infected and -uninfected individuals. The ability to detect CMV-specific LPA or IFN-γ responses in HIV-infected individuals significantly increased with higher CD4 cell numbers. Nevertheless, the proportion of HIV-infected subjects with CD4 counts of ≥500 cells/μl who had a detectable CMV-specific CMI remained significantly lower than that of healthy adults. The ability to detect CMV-specific CMI in HIV-infected individuals decreased with higher levels of HIV replication, with discriminative thresholds of 103 to 104 HIV RNA copies/ml of plasma, for LPA or inducible IFN-γ production elicited by different antigens. The LPA responses obtained with CMV whole lysate and phytohemagglutinin were significantly correlated in HIV-infected subjects but not uninfected controls, indicating a novel characteristic of the CMI defect caused by HIV. The intrasubject variabilities of the CMV-specific CMI were similar in HIV-infected and -uninfected individuals. These data show that LPA and the inducible IFN-γ production elicited by CMV whole lysates may be used to assess modifications of the immune competency of HIV-infected individuals.
Cytomegalovirus (CMV) is a ubiquitous herpesvirus that generally causes asymptomatic or mildly symptomatic infections in immunocompetent hosts. In contrast, CMV infection in immunocompromised patients carries a high rate of morbidity and mortality. Before the introduction of highly active antiretroviral therapy (HAART), AIDS patients represented the largest group severely affected by CMV, with a 10% annual incidence of sight- or life-threatening disease in this group of patients (7, 14). Although HAART dramatically reduced the incidence of CMV disease in human immunodeficiency virus (HIV)-infected patients (8, 12, 32, 37, 39), CMV invasion of the bloodstream, albeit asymptomatic, retains a significant association with progression to AIDS and death in the era of HAART (5, 8, 37). Currently, transplant recipients comprise the group most likely to have CMV symptomatic disease, with an incidence of 10 to 50%, depending on the type of transplantation, the immunosuppressive regimen used, and the recipient and donor CMV serological status (20, 25, 31, 38). In addition, CMV infection in these patients has severe indirect consequences, including bacterial and fungal superinfection and graft loss (10, 24, 26, 30). Patients deemed at risk of CMV end-organ disease frequently receive prophylactic antiviral therapy, which is both toxic and expensive (11).
There is great interest in identifying immunologic markers of protection against CMV disease, because this could simplify the management and improve the outcome of CMV infection in immunocompromised hosts. However, studies of immune correlates with protection against CMV have yielded conflicting results (3, 4, 6, 9, 13, 17-19, 21, 23, 27, 28, 34, 36). The reasons for the discrepancy in the findings of these studies include the use of different subject populations, different definitions of end points and immunologic markers of CMV-specific memory, and different effector T-cell responses. In addition, the heterogeneity of reagents and techniques may also have contributed to the difference in the results.
The goal of this study was to evaluate the sensitivities and specificities of different measures of CMV-specific cell-mediated immunity (CMI) with different CMV antigenic preparations. We also sought to establish the characteristics of assays used to measure CMV-specific memory, such as the lymphocyte proliferation assay (LPA) and interleukin-2 (IL-2) production or effector production, such as inducible gamma interferon (IFN-γ) production, using peripheral blood mononuclear cells (PBMCs) from HIV-infected and -uninfected subjects.
MATERIALS AND METHODS
Subjects.
A total of 58 HIV-seronegative subjects, including 21 CMV-seronegative subjects, 36 CMV-seropositive subjects, and 1 CMV-indeterminate subject, and 107 HIV-seropositive CMV-seropositive subjects were enrolled at four sites: the University of Colorado Health Sciences Center (Colorado), the University of Texas Medical Branch (UTMB), the University of California at San Diego (San Diego), and Rush Medical School. The HIV-seropositive cohort included 26 subjects with CD4 counts of <100 cells/μl (group A), 28 with CD4 counts of ≥100 cells/μl and <300 cells/μl (group B), 28 with CD4 counts of ≥300 cells/μl and <500 cells/μl (group C), and 25 with CD4 counts of ≥500 cells/μl (group D). The medians of the log10 number of plasma HIV RNA copies/ml for the four groups were 5.1, 3.7, 3.3, and 2.9, respectively. All subjects were >18 years of age, but other demographic or treatment information was not available. Samples from each subject were tested only at the site of enrollment.
Antigens.
CMV lysates and mock-infected controls were manufactured at the Colorado, UTMB, and San Diego sites as described previously. Briefly, the Colorado antigen was prepared with primary human lung fibroblasts infected with CMV AD169. Cell cultures that were 100% infected and uninfected controls were harvested in glycine buffer and inactivated by UV irradiation (35). This antigen was previously shown to equally elicit CD4 and CD8 T-cell proliferation and activation (15). The CMV lysate prepared at UTMB also used CMV AD169 propagated in human fibroblasts, followed by UV inactivation (22). The laboratory in San Diego prepared CMV and control antigens by heat inactivation of CMV AD169-infected and -uninfected human foreskin fibroblasts, respectively. All antigens were manufactured by mycoplasma-free tissue culture. Additional CMV lysate and control antigens were obtained from BioWhittaker Inc. Purified CMV gB and pp65 (Austral Biologicals) and phytohemagglutinin (PHA; Sigma) were also tested.
LPA.
LPA was performed according to the AIDS Clinical Trials Group consensus procedure (http://actg.s-3.com/immlab.htm) with fresh PBMCs from heparinized blood (2). The results are reported as stimulation indices (SIs), which were determined by dividing the median counts per minute obtained in CMV antigen- or mitogen-stimulated wells by the median counts per minute of the appropriate controls. This method was preferred to the analysis of the raw count-per-minute data because we have previously shown that count-per-minute values are instrument dependent and that when data from multiple laboratories are analyzed, SIs are more comparable than counts per minute (2). A value of 1 was imputed when the quotient was less than 1. For gB, pp65, and PHA, the control wells used culture medium alone, whereas for the CMV lysates, the controls consisted of mock-infected cell lysates processed identically to the lysates containing the matched CMV antigen, with the exception that there was no viral infection. A count-per-minute censoring threshold was not used because the PBMCs of HIV-infected subjects are notorious for their poor proliferation.
Inducible cytokines.
The IFN-γ and IL-2 concentrations were measured in PBMC culture supernatants collected 72 and 144 h after stimulation with CMV, PHA, or control antigen. Supernatants from duplicate wells were pooled before they were tested with commercially available kits (Biosource), according to the manufacturer's procedure. CMV-specific cytokine production was calculated by subtracting the cytokine concentration in control antigen-stimulated wells from the concentration measured in CMV- or PHA-stimulated wells and imputing a value of 0 when the difference was negative.
Statistical analysis.
The best cutoff value of an assay for discrimination between CMV-seropositive and -seronegative status was defined as the value that maximized the mean of the estimated sensitivity and specificity (equivalent to the value whose corresponding point on the receiver operating characteristic [ROC] curve is closest to the upper left-hand corner of the plot [1]).
Assay variability was expressed as (i) the median and interquartile ratio (IQR) (across subjects) of the within-subject (across repeated measures over time) estimated standard deviations and (ii) the median and IQR (across subjects) of the proportion of repeated measures for a given subject that were on the same side of a given cutoff value as the majority of repeated measures for that subject.
Correlations were based on the Spearman rank correlation estimator and test.
A two-sample test of the equal mean (across four CMV whole-lysate antigens) correlation between LPA with CMV antigen and LPA with PHA in the HIV-infected versus HIV-uninfected groups was performed by a repeated-measures U-statistic test with a Spearman correlation-type kernel function and a chi-square contrast test (details are available on request.)
Two-sample comparisons of the assay results were assessed by using a Wilcoxon rank sum test of the stochastic equality of the distributions of the assay results for the two groups with different serological status.
The pairwise equality of independent proportions of subjects among the four groups with different CD4 counts and the healthy donors with a given assay result (with blood from their first blood draw only) equal to or greater than a given cutoff was tested by first testing the null hypothesis that all five proportions are equal by use of a 0.05-level exact test, and if and only if this test rejected the null hypothesis, then the method was used at the 0.05 level for pairwise comparisons on the basis of an angular transformation of the proportions and its q distribution (40).
The plasma HIV RNA levels between two groups were compared by use of the Wilcoxon rank sum test, after values below the lower limit of quantification (400 copies/ml) were imputed to 400 and values above the upper limit of quantification (750,000 copies/ml) were imputed to 750,000.
All statistical tests were exploratory at the nominal 5% level of significance.
RESULTS
Performance characteristics of six CMV antigenic preparations for measurement of CMI.
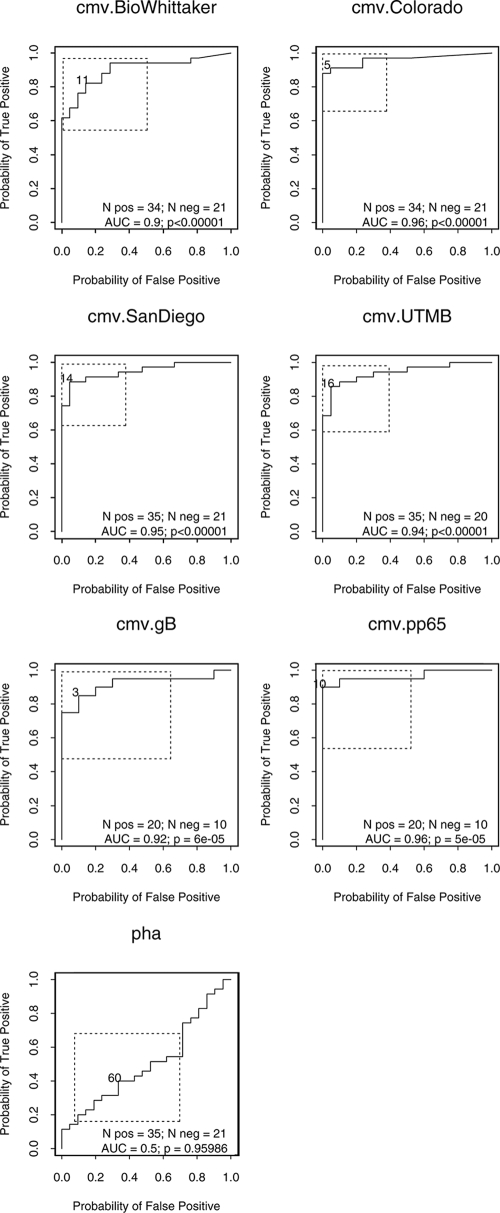
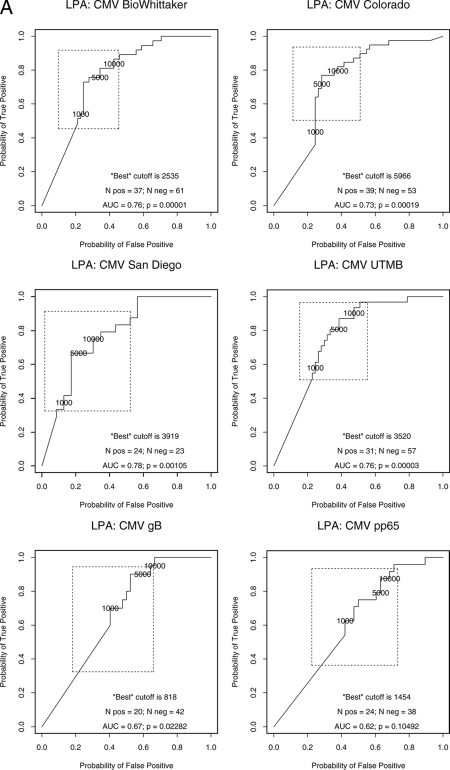
The sensitivities and the specificities of the six CMV antigenic preparations were assessed with PBMCs from 57 healthy donors, including 21 CMV-seronegative and 36 CMV-seropositive adults, after the exclusion of 1 subject with an indeterminate CMV serological status. ROC analysis defined the best SI discriminative thresholds between CMV-seropositive and -seronegative individuals (Fig. 1). The sensitivities and specificities differed somewhat, albeit not significantly, across the antigens at the optimal discriminative thresholds (Table 1). For LPA, the Colorado CMV lysate had the best performance characteristics, on the basis of the mean of the lower bounds of the 95% confidence intervals on sensitivity and specificity, with a sensitivity and a specificity of 91% (95% confidence interval, 76%, 98%) and 95% (95% confidence interval, 76%, 100%), respectively, at an SI of 5.
FIG. 1.
ROC analysis of discriminative LPA thresholds for the detection of CMV immunity with six CMV antigenic preparations and PHA. The data were derived from 36 CMV-seropositive and 27 CMV-seronegative healthy adults. The point closest to the upper left corner indicates the SI value associated with the highest paired sensitivity and specificity for the discrimination between CMV-seropositive and -seronegative individuals. The box around the threshold delineates the 95% joint confidence interval. The PHA ROC curve is shown as a typical example of a measurement lacking discriminative value. pos, positive; neg, negative; AUC, area under the curve.
TABLE 1.
Performance characteristics of six CMV antigens in three CMI assaysa
| Antigen | LPA
|
IFN-γ
|
IL-2
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of samples | SI | Sensib | Specib | No. of samples | Concn (pg/ml) | Sensi | Speci | No. of samples | Concn (pg/ml) | Sensi | Speci | |
| Colorado | 55 | 5 | 91 (76, 98) | 95 (76, 100) | 16 | 3 | 100 (66, 100) | 100 (59, 100) | 16 | 3 | 78 (40, 97) | 71 (29, 96) |
| San Diego | 56 | 12 | 89 (73, 97) | 95 (76, 100) | 16 | 265 | 100 (66, 100) | 100 (59, 100) | 16 | 4 | 100 (66, 100) | 100 (59, 100) |
| UTMB | 55 | 15 | 86 (70, 95) | 95 (75,100) | 16 | 3 | 100 (66, 100) | 100 (59, 100) | 16 | 7 | 67 (30, 93) | 100 (59, 100) |
| BioWhittaker | 55 | 10 | 82 (65, 93) | 86 (64, 97) | 16 | 63 | 100 (66, 100) | 100 (59, 100) | 16 | 1 | 89 (52, 100) | 100 (59, 100) |
| gB | 30 | 3 | 85 (62, 97) | 90 (55, 100) | 13 | 3 | 88 (47, 100) | 100 (48, 100) | 13 | 20 | 38 (9, 76) | 100 (48, 100) |
| pp65 | 30 | 10 | 90 (63, 99) | 100 (69, 100) | 13 | 2 | 100 (63, 100) | 100 (48, 100) | 13 | 6 | 38 (9, 76) | 100 (48, 100) |
Abbreviations: Sensi, sensitivity; Speci, specificity.
The data represent percent sample estimates (95% confidence intervals).
The IFN-γ measurements, performed at 72 and 144 h poststimulation, showed a wide range of responses to the different CMV antigens, varying from undetectable to 12,111 pg/ml. The IFN-γ concentrations in control antigen-stimulated PBMC cultures varied from undetectable to 6.3 pg/ml. After subtraction of the IFN-γ concentrations for the appropriate controls, the CMV-specific IFN-γ levels were not significantly different between the two time points at which the culture supernatants were harvested, and only data for the results obtained at 72 h are shown (Table 1). At the best ROC curve-determined IFN-γ discriminative threshold, all CMV preparations except gB had 100% sensitivity for the identification of CMV-seropositive healthy individuals; the sensitivity of gB was 88%. All CMV preparations had 100% specificity for discriminating CMV-seropositive from CMV-seronegative healthy subjects, although the lower bounds of the 95% confidence intervals were as low as 48% for gB and pp65 (Table 1).
The levels of IL-2 production, measured at 72 and 144 h poststimulation, varied from undetectable to 191 pg/ml. The level of nonspecific IL-2 production measured in medium-stimulated wells was less than 21 pg/ml. CMV-specific IL-2 levels did not appreciably differ between the harvesting times of 72 and 144 h. Overall, the CMV antigen lysates were better stimulators of IL-2 production than purified CMV pp65 or gB, which generated IL-2 levels <50 pg/ml. At the optimal CMV-specific IL-2 discriminative threshold, the CMV San Diego lysate had the best sensitivity and specificity (100% each). The sensitivities of both gB and pp65 tended to be lower than those of the Colorado, San Diego, and BioWhittaker lysates, although the differences did not reach statistical significance. Across all antigens, the sensitivity was as low as 38% (gB and pp65). The specificity was 100% for all antigens except the Colorado antigen (specificity, 71%).
The variability in the SIs determined by LPA was assessed with PBMCs from 30 to 57 CMV-seropositive subjects by the use of two or more observations separated by 1 to 347 days. A qualitative analysis with the median and the IQR (across subjects) of the proportion of repeated measures for a given subject that were on the same side of the ROC curve-derived cutoff value as the majority of repeated measures for that subject showed a high degree of consistency in the results of LPA over time, with at least 75% of the subjects consistently testing above or below the ROC curve-derived optimal SI threshold for all antigens except the San Diego antigen, for which at least 75% of subjects had at least 83% of the test results on the same side of the threshold. The median intrasubject log10 SI standard deviations varied from 0.19 to 0.31 across all antigens.
The variabilities of the CMV-specific IFN-γ and IL-2 measurements were assessed with PBMCs from 9 to 12 CMV-seropositive subjects stimulated with the BioWhittaker, UTMB, or San Diego antigen. The data showed that >75% of the subjects had CMV-specific IFN-γ results reproducibly above or below the ROC curve-derived optimal threshold for all antigenic preparations. The median standard deviations of the CMV-specific IFN-γ concentrations per subject varied from 6 to 70 pg/ml. CMV-specific IL-2 levels were reproducible in >75% of subjects when the UTMB antigen was used. For the BioWhittaker and San Diego antigens, the reproducibility of the results for IL-2 was >50% but <75%. The median standard deviations of the IL-2 concentrations per subject varied from 3 to 5 pg/ml.
CMI associations in healthy individuals.
In vivo CMV infection generates Th1 responses, which protect the host against the viral infection by clearing infected cells. We hypothesized that in a well-functioning immune system, CMV stimulation preferentially expands Th1 effectors, and therefore, immune memory, as determined by LPA and measurement of IL-2 levels, would correlate with the level of IFN-γ production. Indeed, correlation analyses with PBMCs from CMV-seropositive donors showed that the CMV SI significantly increased with higher levels of CMV-specific IFN-γ production at 72 h for all antigens, with correlation coefficients of 0.58 to 0.95 (P < 0.05). High correlation coefficients were also found between the CMV SI and the levels of cytokine production at 144 h, but the number of data points was too small to reach statistical significance. Analyses of the correlation of the CMV SI with the level of IL-2 production showed similar trends, with rho values of 0.52 to 0.63 and P values of 0.02 to 0.09 for all antigens except pp65, which failed to generate detectable levels of CMV-specific IL-2, precluding the ability to detect any associations.
There was also no appreciable association between the SIs obtained with PHA stimulation and those obtained with CMV lysate stimulation, underscoring the specificity of the results of LPA generated with CMV lysates. A weak association was found between the gB and the PHA SIs (rho = 0.42, P = 0.07), and a stronger association was found between the pp65 and the PHA SIs (rho = 0.52, P = 0.02).
CMV-specific CMI in HIV-infected subjects.
All HIV-infected volunteers enrolled in this study were CMV seropositive. The subjects were stratified by CD4 cell numbers into the four groups (groups A to D) described in Materials and Methods. The proportions of responders were calculated by the CMV-specific LPA, the IFN-γ production assay, and the IL-2 production assay by using the best discriminative ROC analysis values for all antigens. However, data are shown only for the antigens with the best ability to discriminate between CMV-seropositive and -seronegative healthy volunteers, i.e., the Colorado antigen for LPA, the San Diego antigen for IL-2 production, and the BioWhittaker antigen for IFN-γ production. This last antigen was chosen from among the multiple CMV antigens that showed 100% discriminative sensitivity and specificity by inducible IFN-γ production, because the results for these antigens were available from a higher number of HIV-infected subjects compared with the number of subjects from whom the results for the other antigens were available.
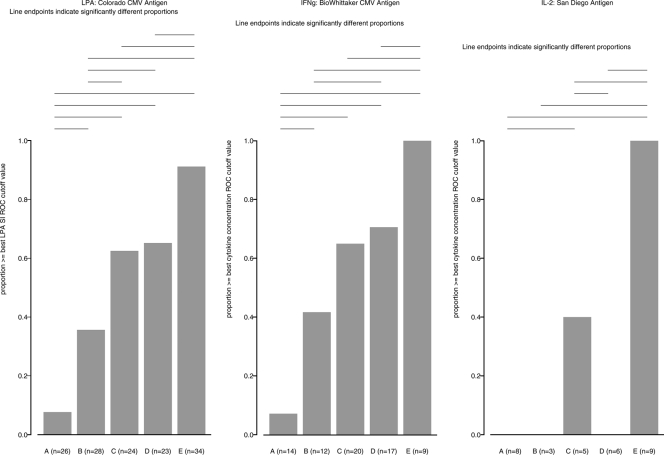
The proportions of subjects with responses higher than the best ROC curve cutoff for a CMV-specific SI increased with the CD4+ cell numbers and were 8%, 36%, 63%, and 65% of responders in groups A, B, C, and D, respectively (Fig. 2A). All HIV-positive groups had significantly lower proportions of CMV LPA-positive subjects than the HIV-negative and CMV-positive controls, of whom 91% were LPA positive. Across the HIV-positive groups, group A, which included the most severely immunocompromised individuals, had a significantly lower proportion of LPA-positive subjects than group B, C, or D. Group B had a significantly lower proportion of responders than groups C and D. The proportions of LPA-positive subjects in groups C and D did not appreciably differ. The median log SIs of the CMV-specific LPA responders in groups A, B, C, and D and the HIV-uninfected group were 0.8, 1.4, 1.6, 1.6, and 1.5, respectively, indicating that PBMCs from HIV-infected subjects with CD4+ cell counts of <100/μl had both qualitative and quantitative proliferative defects. The distribution of the responses across the groups by LPA with the other CMV antigens was not appreciably different from the results obtained with the Colorado lysate, although the magnitudes of the responses varied (data not shown).
FIG. 2.
CMV-specific CMI in HIV-infected subjects with different degrees of immunosuppression compared with that in healthy controls. The data were derived from CMV-seropositive subjects grouped as defined in the text according to their degree of immunosuppression (groups A to D). Group E comprises HIV-uninfected individuals. Bars indicate the proportion of subjects in each group with responses to CMV antigenic stimulation measured by LPA (left graph), inducible IFN-γ production (middle graph), and inducible IL-2 production (right graph). Horizontal lines identify significant differences between the proportion of responders in each group.
The proportions of subjects with responses higher than the best ROC curve cutoff for CMV-specific IFN-γ (BioWhittaker lysate, 72 h) also showed a gradual increase with higher CD4+ cell numbers and were 7%, 42%, 65%, and 71% in groups A, B, C, and D, respectively (Fig. 2B). Similar to the LPA responses, there were significant differences in the proportions of IFN-γ responders in group A compared with those in any of the other groups, in group B compared with those in group D and compared with those in the healthy controls, in group C compared with those in the healthy controls, and in group D compared with those in the healthy controls. The median CMV-specific IFN-γ concentrations among the responders in groups A, B, C, and D and in the healthy controls were 229, 559, 796, 532, and 1,120 pg/ml, respectively. The maximum level of production of CMV-specific IFN-γ among the HIV-positive subjects was 1,312 pg/ml, which was 1 order of magnitude lower than the 12,111-pg/ml maximum concentration observed in HIV-uninfected subjects under the same assay conditions. The results for IFN-γ obtained with the other antigens were not appreciably different from those observed with the BioWhittaker lysate (data not shown).
Very few HIV-positive subjects produced IL-2 in response to stimulation with the CMV San Diego lysate. All the responders belonged to group C, which included 40% CMV-specific IL-2-responders. The maximum level of CMV-specific IL-2 production among the HIV-positive subjects was 13 pg/ml, which was 1 order of magnitude lower than the corresponding value of 191 pg/ml recorded for HIV-negative and CMV-positive subjects. The IL-2 response in HIV-positive individuals in response to the other CMV antigens was also mostly absent and did not have an appreciable relationship with the CD4+ cell count (data not shown).
The variability of the LPA results over time in the HIV-infected subjects was analyzed by comparing the SIs obtained with Colorado CMV lysate-stimulated cells in 21 subjects in group A, 25 in group B, 21 in group C, and 20 in group D, who were repeatedly tested over intervals of 1 to 347 days. The variability was low, with 67% or more of the subjects in all groups having SIs consistently above or consistently below 5, the ROC curve-defined optimal threshold for the Colorado antigen. The median intrasubject log10 SI standard deviations varied from 0.07 to 0.32 across all groups, which was not appreciably different from the values for the healthy controls. Likewise, the variability of the CMV-specific IFN-γ and IL-2 results measured with the BioWhittaker and San Diego CMV lysate-stimulated PBMC cultures, respectively, was low across all groups of HIV-infected individuals, with at least 50% of the subjects testing consistently above or consistently below the ROC curve-defined optimal threshold for each antigen. These data were derived from 11 to 12 subjects for each cytokine (from 4 subjects in group A and 1 subject in group B for each cytokine and from 2 and 5 subjects in group C and 2 and 4 subjects in group D for IL-2 and IFN-γ, respectively). The median standard deviations of the IFN-γ and IL-2 concentrations per HIV-infected subject from all groups varied from 0 to 46 and 0 to 3 pg/ml, respectively. These results were not appreciably different from those for the healthy controls, but due to the limited number of subjects, they must be interpreted with caution.
CMI associations in HIV-infected subjects.
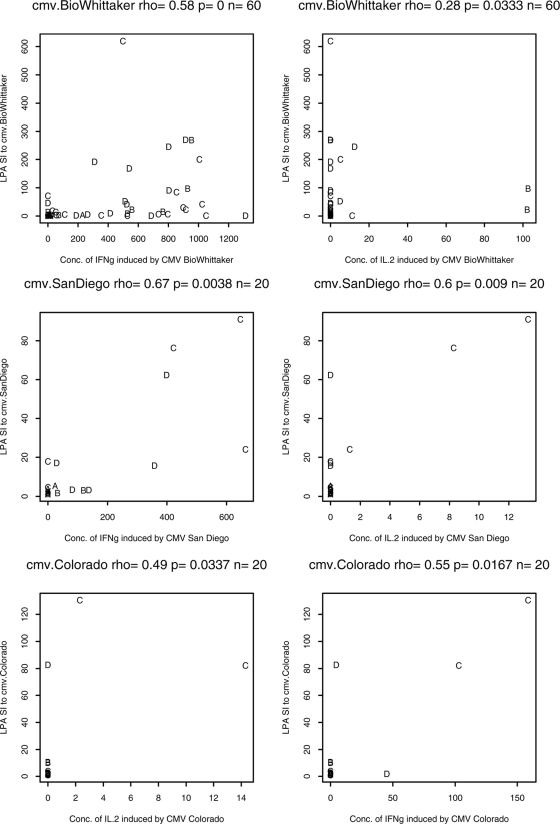
Correlations between the CMV-specific LPA results and the inducible cytokine levels were investigated for the Colorado, BioWhittaker, and San Diego antigens by using pooled data from 20 to 60 HIV-infected subjects (Fig. 3). The LPA responses measured by determination of the SIs were significantly associated with the levels of IFN-γ production (rho = 0.55 to 0.67, P ≤ 0.02) and IL-2 production (rho = 0.28 to 0.6, P ≤ 0.03) for all three antigens. There were no appreciable differences in the correlations of the LPA results and the levels of cytokine production across the four groups of HIV-infected subjects stratified according to their CD4 cell numbers. However, some analyses had data available from only three subjects and the results should be interpreted with caution.
FIG. 3.
Correlation of CMV-specific LPA results with cytokine production in HIV-infected subjects. The graphs show typical representations of correlations between the LPA response and inducible IFN-γ production (upper row) or the LPA response and inducible IL-2 production (lower row) in CMV lysate-stimulated PBMCs from CMV-seropositive HIV-infected subjects. The letters A to D identify the subject groups, as defined in the text.
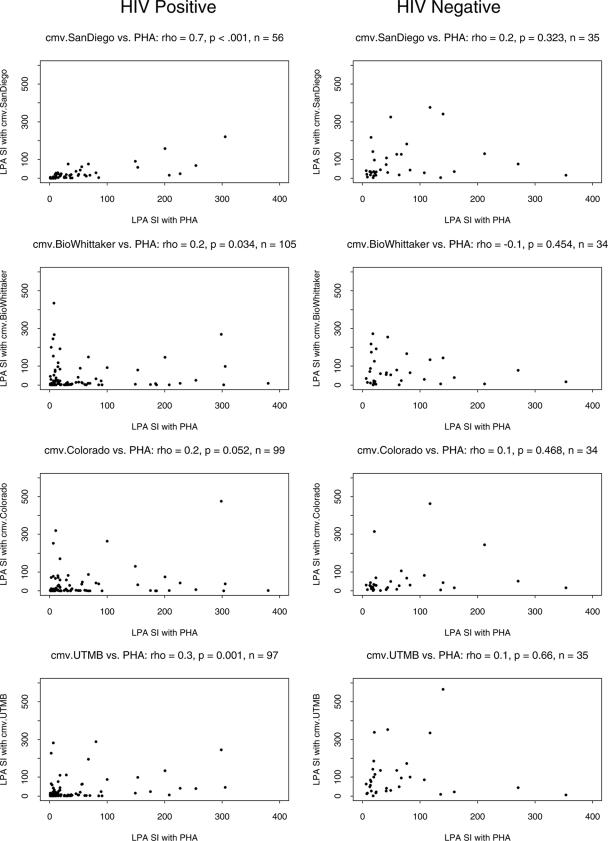
Correlations between CMV- and PHA-driven CMI were investigated for each antigen (Fig. 4). The CMV SI significantly increased with the PHA SI for all antigens (correlation coefficients, 0.20 to 0.69, P ≤ 0.05) except for pp65 (rho = 0.20, P = 0.11). There were no appreciable differences across the groups stratified by CD4 counts, but the numbers of data points per analysis per group were limited, rendering the results less stable. The association between the CMV and PHA SIs for HIV-infected subjects was further supported by an additional analysis that included results for all CMV lysates, which tested the null hypothesis that the correlation between the CMV and PHA SIs was 0. The null hypothesis was rejected on the basis of a P value of 0.05. In contrast, a similar analysis for the data obtained with healthy controls was unable to reject the null hypothesis and had a P value of 0.42. Furthermore, the P value from testing of the null hypothesis that the associations of the CMV SI with the PHA SI were not different for HIV-infected and -uninfected subjects was 0.09. Taken together, these data indicate that the correlation between the SIs obtained by LPA with CMV whole-lysate antigens and PHA is greater for the HIV-positive population than the HIV-negative population.
FIG. 4.
Correlation between CMV and PHA LPA results for HIV-infected subjects (left) and uninfected controls (right). Data obtained with CMV lysates are depicted.
To determine the relationship between CMV-specific CMI and HIV replication, the numbers of plasma HIV RNA copies/ml of the LPA, IFN-γ, or IL-2 responders were compared with those of nonresponders, as defined by the best ROC curve-derived cutoff (Table 2). The data showed that the LPA and IFN-γ responders had significantly fewer HIV plasma RNA copies/ml than the nonresponders. A similar trend was observed when the IL-2 responders were compared with the nonresponders. The differences in HIV plasma RNA levels between the IFN-γ responders and the nonresponders remained significant after adjustment for CD4 cell numbers. The results of similar analyses with data obtained after the stimulation of PBMCs with other CMV antigens (data not depicted) were similar to those displayed in Table 2 for all antigens except pp65, stimulation with which resulted in plasma HIV RNA titers that were not significantly different between the LPA or IFN-γ responders and the nonresponders.
TABLE 2.
Comparison of HIV plasma burden in subjects with and without CMV-specific CMI
| Assay for CMI/antigen | Responders' RNA copies/ml (no. of samples) | Nonresponders' RNA copies/ml (no. of samples) | P valuea | Adjusted P valueb |
|---|---|---|---|---|
| LPA/Colorado | 1,454c (39) | 36,382 (53) | <0.01 | 0.09 |
| IFN-γ production/BioWhittaker | 400 (29) | 36,176 (28) | <0.01 | 0.03 |
P values for the comparison of the HIV plasma titers of the CMI responders versus those of the nonresponders. The data show significantly lower HIV RNA titers in subjects with CMV LPA and inducible IFN-γ responses compared with those of subjects incapable of mounting responses.
P values for the comparison of the HIV plasma titers of the CMI responders versus those of the nonresponders with adjustment for CD4 cell numbers. The data indicate that the association between the HIV RNA copies/ml and CMV CMI response measured as IFN-γ production was maintained after adjustment for CD4 cell numbers
The data represent the median number of HIV RNA copies/ml of plasma in each group.
An ROC analysis was performed to estimate how well plasma HIV RNA discriminates between LPA or IFN-γ responders (Fig. 5). For the CMV-specific LPA, the plasma HIV RNA discriminative threshold varied from 818 copies/ml for CMV gB to 5,966 copies/ml for the Colorado CMV lysate. For CMV-specific IFN-γ, the discriminative threshold varied from 1,079 HIV copies/ml of plasma for CMV gB to 9,132 copies/ml for the Colorado and San Diego CMV lysates. These data show a progressive loss of CMV-specific CMI with higher levels of HIV replication but also indicate that IFN-γ production in response to CMV lysate stimulation may be the most resilient CMV-specific marker of CMI.
FIG. 5.
ROC analysis of plasma HIV RNA thresholds associated with detectable CMV LPA (A) and IFN-γ (IFNg) (B) in HIV-infected subjects. The data were derived from CMV-seropositive HIV-infected subjects. “True positive” means that the number of RNA copies was less than the given RNA copy number cutoff when the CMV-specific LPA (or IFN-γ) was at least at the threshold value determined by the ROC analysis of the HIV-uninfected group. “False positive” means that the number of RNA copies was less than the given RNA copy number cutoff when the CMV-specific LPA (or IFN-γ) was less than the threshold value. The “best” cutoff corresponds to the point closest to the upper left corner and indicates the plasma HIV RNA copies/ml associated with the highest mean sensitivity and specificity for discrimination between CMV-specific CMI responders and nonresponders. The sensitivities and specificities corresponding to benchmark cutoffs of 1,000, 5,000, and 10,000 HIV RNA copies/ml of plasma are also indicated. pos, positive; neg, negative; AUC, area under the curve.
DISCUSSION
This study evaluated several factors critical to the ability of measuring the CMV-specific CMI responses of HIV-infected subjects in vitro. To assess the role of the antigenic composition of the stimulant, PBMCs from HIV-infected and -uninfected subjects were stimulated with different inactivated CMV-infected cell lysates and subunit antigens, including gB, the most abundant CMV surface glycoprotein, and pp65, a major target of cytotoxic T-cell responses (16). There were no appreciable differences across CMV lysates with respect to their ability to elicit LPA and IFN-γ responses for CMV-seropositive HIV-infected or -uninfected subjects. The association of the SI with CMV gB and pp65 stimulation and the SI with PHA stimulation in healthy individuals and the trend toward a lower level of production of IL-2 in response to CMV subunit antigens compared with that in response to lysates, taken together, indicate that CMV lysates offer several advantages over CMV subunit antigens for the detection of CMV-specific CMI in vitro and are the stimulants of choice. CMV whole lysates contain a larger diversity of T-cell epitopes that may contribute to the robustness of CMI. This conclusion is supported by the work of Sylwester et al., who showed that multiple CMV peptides contain T-cell epitopes (33). A limitation of this study was that only one of the CMV lysates, that manufactured by BioWhittaker, is commercially available.
There were differences across CMV antigens with respect to the optimal discriminatory threshold between CMV-seropositive and -seronegative individuals for the LPA response and IFN-γ or IL-2 secretion. For LPA, previous studies typically used an SI of 3 to differentiate the presence and the absence of CMI. All the antigens evaluated in this study except CMV gB had ROC analysis-determined best discriminatory thresholds other than 3, underscoring the importance of establishing antigen-specific criteria during the process of validation of each assay. The results provided by the ROC analysis can be used to emphasize the sensitivity or the specificity of the discriminative threshold if the clinical application may benefit from a higher negative or positive predictive value, respectively.
PBMCs from healthy donors were used to define the assay characteristics of the CMV-specific LPA response and IL-2 production, which predominantly measure CD4+ T-cell memory, and IFN-γ production, which measures effector responses. The level of cytokine production in control antigen-stimulated wells was low, but not negligible, warranting adjustment of the final results by subtracting the values for the wells containing control antigen from those for the wells containing CMV antigens. There were no appreciable differences in the levels of cytokine production between the 72- and 144-h cultures. The CMV-specific LPA response and levels of IFN-γ and IL-2 production were significantly correlated with each other, indicating that they measured the same broad immunologic phenomenon. It is possible that the small subset of subjects in our HIV-uninfected sample for whom we had IL-2 data are not representative of the HIV-uninfected population. With that caveat in mind, however, the results obtained from the IL-2 measurements were less robust than those obtained from either the LPA response or IFN-γ production. Hence, inducible IL-2 measurements may best be used to answer immunologic questions that specifically address CMV-stimulated IL-2 production but not as a broad measure of CMV-specific CMI. In contrast, the results obtained from both the LPA response and IFN-γ production were robust and allowed the highly sensitive and specific discrimination between CMV-seropositive and -seronegative healthy adults, with slightly higher values obtained for IFN-γ production.
The data showed that many HIV-infected individuals were able to mount CMV-specific responses. The LPA response and the level of IFN-γ production of CMV-stimulated PBMCs from HIV-infected subjects clearly increased with their CD4 cell numbers and could be used as a measure of the integrity of the immune system in studies of immune preservation or immune reconstitution. The level of CMV-specific IL-2 production was low and too erratic to be useful as an overall measure of the immune status of these subjects. It is important to underscore that the CMV-specific CMI responses of HIV-infected subjects with all CD4 cell levels, including ≥500 cells/μl, were significantly less frequent than those of healthy individuals. Furthermore, the magnitude of IFN-γ production was also lower in HIV-infected subjects than in healthy individuals. The group of subjects with CD4 cell counts of <100 cells/μl showed the most intense signs of CMV-specific immunodeficiency, as evidenced by <10% responders, according to the LPA response or IFN-γ production. This observation is consistent with the findings of epidemiologic studies showing that CMV end-organ disease predominantly affects HIV-infected subjects with CD4 cell counts of <100 cells/μl (14). There were no appreciable differences in CMV-specific CMI between the groups of HIV-infected subjects in group C (CD4 counts, between 300 and 500 cells/μl) and group D (CD4 counts, ≥500 cells/μl), suggesting that these groups may be consolidated in future analyses.
The ability to detect CMV-specific LPA and inducible IFN-γ responses decreased with higher levels of HIV replication, as determined by measurement of the plasma HIV RNA load, indicating the importance of controlling for HIV replication in analyses of CMI. The association between HIV RNA replication and CMV-specific CMI was maintained after adjustment for CD4 cell numbers, indicating that it was not confounded by CD4 cell numbers. The effect of antiretroviral treatment was not analyzed due to a lack of data. The ROC analysis showed optimal discriminative thresholds of between 818 and 9,132 copies/ml. The thresholds for CMV lysates and IFN-γ production tended to be higher than those for subunit antigens and the LPA response. CMV lysate-stimulated IFN-γ production had the highest HIV RNA discriminative threshold of approximately 5,000 to 10,000 copies/ml plasma. This threshold is similar to the benchmark of 10,000 HIV RNA copies/ml associated with the increased incidence of CMV end-organ disease (8) and is also in agreement with a previously reported association between the CMV-specific IFN-γ responses and protection against CMV end-organ disease (27, 34, 36).
The biologic variability of the CMV LPA response and the level of IFN-γ production in HIV-infected subjects was not appreciably different from that in healthy individuals, suggesting that CMV-specific CMI may be used to monitor changes in the functional immunity of HIV-infected individuals. Data for assessment of the variabilities in the LPA response and the level of IFN-γ production were available for 87 and 12 HIV-infected subjects, respectively. Although the conclusions for the variability in the level of IFN-γ production were derived from data for a limited number of subjects, they are supported by previous findings (34) and by the LPA response data, lending credibility to the results. The low biologic variability of the CMV-specific LPA response or level of IFN-γ production, taken together with the significant association of these CMV-specific CMI markers with the degree of immunosuppression of HIV-infected subjects, indicates that a qualitative improvement of either of these measures could be interpreted with confidence as a sign of immune reconstitution. Conversely, qualitative decreases in the CMV-specific LPA response or level of IFN-γ production indicate progression of the immunodeficiency.
Another observation with potential clinical utility was that the CMV lysate- and PHA-stimulated LPA responses were positively associated in HIV-infected subjects, whereas a similar correlation for healthy individuals was not supported by the data analysis; also, there was some evidence (P = 0.09) that the correlation is greater in the former group. The correlation between the CMV lysate- and PHA-stimulated LPA responses in HIV-infected subjects suggests that their immunologic defects limit both CMV- and PHA-stimulated proliferation, leading to a significant association. The corollary of this observation is that the lifting of the immunologic defects may abrogate the association between the SIs for CMV lysate and PHA stimulation, and this might be used as a measure of immune reconstitution. Further studies are needed to test this hypothesis.
In conclusion, CMV lysates stimulate robust LPA and IFN-γ responses with low intrasubject variability both in healthy and in HIV-infected individuals. IFN-γ assays specific for other microbial agents, such as Mycobacterium tuberculosis, are commercially available for investigation of pathogen-specific CMI. A standardized CMV-specific IFN-γ assay may also find clinical utility, considering that several studies have shown an association between the CMV-specific IFN-γ production and protection against CMV end-organ disease not only in HIV-infected individuals but also in transplant recipients and other immunocompromised hosts (27, 29, 34, 36).
Acknowledgments
We thank the study participants, Graham Ray for assisting with recruitment, and Annie Vazquez for assistance with manuscript preparation.
This study was funded by NIH contract 2U01A38858 27CMV03 and NICHD contract N01-HD-33162.
We do not have any conflicts of interest.
Footnotes
Published ahead of print on 25 June 2008.
REFERENCES
- 1.Agresti, A., and B. A. Coull. 1998. Approximate is better than exact for estimation of binomial porportions. Am. Stat. 52:119. [Google Scholar]
- 2.Betensky, R. A., E. Connick, J. Devers, A. L. Landay, M. Nokta, S. Plaeger, H. Rosenblatt, J. L. Schmitz, F. Valentine, D. Wara, A. Weinberg, and H. M. Lederman. 2000. Shipment impairs lymphocyte proliferative responses to microbial antigens. Clin. Diagn. Lab. Immunol. 7:759-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bunde, T., A. Kirchner, B. Hoffmeister, D. Habedank, R. Hetzer, G. Cherepnev, S. Proesch, P. Reinke, H. D. Volk, H. Lehmkuhl, and F. Kern. 2005. Protection from cytomegalovirus after transplantation is correlated with immediate early 1-specific CD8 T cells. J. Exp. Med. 201:1031-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chalandon, Y., S. Degermann, J. Villard, L. Arlettaz, L. Kaiser, S. Vischer, S. Walter, M. H. Heemskerk, R. A. van Lier, C. Helg, B. Chapuis, and E. Roosnek. 2006. Pretransplantation CMV-specific T cells protect recipients of T-cell-depleted grafts against CMV-related complications. Blood 107:389-396. [DOI] [PubMed] [Google Scholar]
- 5.Deayton, J. R., P. Wilson, C. A. Sabin, C. C. Davey, M. A. Johnson, V. C. Emery, and P. D. Griffiths. 2000. Changes in the natural history of cytomegalovirus retinitis following the introduction of highly active antiretroviral therapy. AIDS 14:1163-1170. [DOI] [PubMed] [Google Scholar]
- 6.Dechanet, J., P. Merville, F. Berge, G. Bone-Mane, J. L. Taupin, P. Michel, P. Joly, M. Bonneville, L. Potaux, and J. F. Moreau. 1999. Major expansion of gammadelta T lymphocytes following cytomegalovirus infection in kidney allograft recipients. J. Infect. Dis. 179:1-8. [DOI] [PubMed] [Google Scholar]
- 7.Drew, W. L. 1988. Cytomegalovirus infection in patients with AIDS. J. Infect. Dis. 158:449-456. [DOI] [PubMed] [Google Scholar]
- 8.Erice, A., C. Tierney, M. Hirsch, A. M. Caliendo, A. Weinberg, M. A. Kendall, and B. Polsky. 2003. Cytomegalovirus (CMV) and human immunodeficiency virus (HIV) burden, CMV end-organ disease, and survival in subjects with advanced HIV infection (AIDS Clinical Trials Group Protocol 360). Clin. Infect. Dis. 37:567-578. [DOI] [PubMed] [Google Scholar]
- 9.Ganepola, S., C. Gentilini, U. Hilbers, T. Lange, K. Rieger, J. Hofmann, M. Maier, U. G. Liebert, D. Niederwieser, E. Engelmann, R. Heilbronn, E. Thiel, and L. Uharek. 2007. Patients at high risk for CMV infection and disease show delayed CD8+ T-cell immune recovery after allogeneic stem cell transplantation. Bone Marrow Transplant. 39:293-299. [DOI] [PubMed] [Google Scholar]
- 10.Hartmann, A., S. Sagedal, and J. Hjelmesaeth. 2006. The natural course of cytomegalovirus infection and disease in renal transplant recipients. Transplantation 82:S15-S17. [DOI] [PubMed] [Google Scholar]
- 11.Hodson, E. M., C. A. Jones, A. C. Webster, G. F. Strippoli, P. G. Barclay, K. Kable, D. Vimalachandra, and J. C. Craig. 2005. Antiviral medications to prevent cytomegalovirus disease and early death in recipients of solid-organ transplants: a systematic review of randomised controlled trials. Lancet 365:2105-2115. [DOI] [PubMed] [Google Scholar]
- 12.Jabs, D. A., J. T. Holbrook, M. L. Van Natta, R. Clark, M. A. Jacobson, J. H. Kempen, and R. L. Murphy. 2005. Risk factors for mortality in patients with AIDS in the era of highly active antiretroviral therapy. Ophthalmology 112:771-779. [DOI] [PubMed] [Google Scholar]
- 13.Jacobson, M. A., H. T. Maecker, P. L. Orr, R. D'Amico, M. Van Natta, X. D. Li, R. B. Pollard, and B. M. Bredt. 2004. Results of a cytomegalovirus (CMV)-specific CD8+/interferon-gamma+ cytokine flow cytometry assay correlate with clinical evidence of protective immunity in patients with AIDS with CMV retinitis. J. Infect. Dis. 189:1362-1373. [DOI] [PubMed] [Google Scholar]
- 14.Jacobson, M. A., and J. Mills. 1988. Serious cytomegalovirus disease in the acquired immunodeficiency syndrome (AIDS). Clinical findings, diagnosis, and treatment. Ann. Intern. Med. 108:585-594. [DOI] [PubMed] [Google Scholar]
- 15.Jesser, R. D., S. Li, and A. Weinberg. 2006. Regulatory T cells generated during cytomegalovirus in vitro stimulation of mononuclear cells from HIV-infected individuals on HAART correlate with decreased lymphocyte proliferation. Virology 352:408-417. [DOI] [PubMed] [Google Scholar]
- 16.Komanduri, K. V., S. M. Donahoe, W. J. Moretto, D. K. Schmidt, G. Gillespie, G. S. Ogg, M. Roederer, D. F. Nixon, and J. M. McCune. 2001. Direct measurement of CD4+ and CD8+ T-cell responses to CMV in HIV-1-infected subjects. Virology 279:459-470. [DOI] [PubMed] [Google Scholar]
- 17.Komanduri, K. V., J. Feinberg, R. K. Hutchins, R. D. Frame, D. K. Schmidt, M. N. Viswanathan, J. P. Lalezari, and J. M. McCune. 2001. Loss of cytomegalovirus-specific CD4+ T cell responses in human immunodeficiency virus type 1-infected patients with high CD4+ T cell counts and recurrent retinitis. J. Infect. Dis. 183:1285-1289. [DOI] [PubMed] [Google Scholar]
- 18.La Rosa, C., A. P. Limaye, A. Krishnan, J. Longmate, and D. J. Diamond. 2007. Longitudinal assessment of cytomegalovirus (CMV)-specific immune responses in liver transplant recipients at high risk for late CMV disease. J. Infect. Dis. 195:633-644. [DOI] [PubMed] [Google Scholar]
- 19.Lilleri, D., G. Gerna, C. Fornara, L. Lozza, R. Maccario, and F. Locatelli. 2006. Prospective simultaneous quantification of human cytomegalovirus-specific CD4+ and CD8+ T-cell reconstitution in young recipients of allogeneic hematopoietic stem cell transplants. Blood 108:1406-1412. [DOI] [PubMed] [Google Scholar]
- 20.Limaye, A. P., R. Bakthavatsalam, H. W. Kim, S. E. Randolph, J. B. Halldorson, P. J. Healey, C. S. Kuhr, A. E. Levy, J. D. Perkins, J. D. Reyes, and M. Boeckh. 2006. Impact of cytomegalovirus in organ transplant recipients in the era of antiviral prophylaxis. Transplantation 81:1645-1652. [DOI] [PubMed] [Google Scholar]
- 21.Manuel, O., M. Pascual, M. Trendelenburg, and P. R. Meylan. 2007. Association between mannose-binding lectin deficiency and cytomegalovirus infection after kidney transplantation. Transplantation 83:359-362. [DOI] [PubMed] [Google Scholar]
- 22.Nokta, M., D. Eaton, O. S. Steinsland, and T. Albrecht. 1987. Ca2+ responses in cytomegalovirus-infected fibroblasts of human origin. Virology 157:259-267. [DOI] [PubMed] [Google Scholar]
- 23.Ozdemir, E., L. S. St. John, G. Gillespie, S. Rowland-Jones, R. E. Champlin, J. J. Molldrem, and K. V. Komanduri. 2002. Cytomegalovirus reactivation following allogeneic stem cell transplantation is associated with the presence of dysfunctional antigen-specific CD8+ T cells. Blood 100:3690-3697. [DOI] [PubMed] [Google Scholar]
- 24.Potena, L., C. T. Holweg, C. Chin, H. Luikart, D. Weisshaar, B. Narasimhan, W. F. Fearon, D. B. Lewis, J. P. Cooke, E. S. Mocarski, and H. A. Valantine. 2006. Acute rejection and cardiac allograft vascular disease is reduced by suppression of subclinical cytomegalovirus infection. Transplantation 82:398-405. [DOI] [PubMed] [Google Scholar]
- 25.Rowshani, A. T., F. J. Bemelman, E. M. van Leeuwen, R. A. van Lier, and I. J. ten Berge. 2005. Clinical and immunologic aspects of cytomegalovirus infection in solid organ transplant recipients. Transplantation 79:381-386. [DOI] [PubMed] [Google Scholar]
- 26.Ruttmann, E., C. Geltner, B. Bucher, H. Ulmer, D. Hofer, H. B. Hangler, S. Semsroth, R. Margreiter, G. Laufer, and L. C. Muller. 2006. Combined CMV prophylaxis improves outcome and reduces the risk for bronchiolitis obliterans syndrome (BOS) after lung transplantation. Transplantation 81:1415-1420. [DOI] [PubMed] [Google Scholar]
- 27.Sacre, K., G. Carcelain, N. Cassoux, A. M. Fillet, D. Costagliola, D. Vittecoq, D. Salmon, Z. Amoura, C. Katlama, and B. Autran. 2005. Repertoire, diversity, and differentiation of specific CD8 T cells are associated with immune protection against human cytomegalovirus disease. J. Exp. Med. 201:1999-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schrier, R. D., W. R. Freeman, C. A. Wiley, J. A. McCutchan, et al. 1995. Immune predispositions for cytomegalovirus retinitis in AIDS. J. Clin. Investig. 95:1741-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sinclair, E., Q. X. Tan, M. Sharp, V. Girling, C. Poon, M. V. Natta, D. A. Jabs, M. Inokuma, H. T. Maecker, B. Bredt, and M. A. Jacobson. 2006. Protective immunity to cytomegalovirus (CMV) retinitis in AIDS is associated with CMV-specific T cells that express interferon-gamma and interleukin-2 and have a CD8+ cell early maturational phenotype. J. Infect. Dis. 194:1537-1546. [DOI] [PubMed] [Google Scholar]
- 30.Singh, N., C. Wannstedt, L. Keyes, M. M. Wagener, T. Gayowski, and T. V. Cacciarelli. 2005. Indirect outcomes associated with cytomegalovirus (opportunistic infections, hepatitis C virus sequelae, and mortality) in liver-transplant recipients with the use of preemptive therapy for 13 years. Transplantation 79:1428-1434. [DOI] [PubMed] [Google Scholar]
- 31.Small, L. N., J. Lau, and D. R. Snydman. 2006. Preventing post-organ transplantation cytomegalovirus disease with ganciclovir: a meta-analysis comparing prophylactic and preemptive therapies. Clin. Infect. Dis. 43:869-880. [DOI] [PubMed] [Google Scholar]
- 32.Smurzynski, M., C. A. Koletar, S. Wu, K. Wu, R. Bosch, and C. Benson. 2006. Opportunistic infections occurring after initiation of randomized HAART regimens in treatment-naive HIV-1-infected patients followed in the ACTG Longitudinal Linked Randomized Trials Study, abstr. 782, p. 336. 13th Conf. Retrovir. Opportunistic Infect.
- 33.Sylwester, A. W., B. L. Mitchell, J. B. Edgar, C. Taormina, C. Pelte, F. Ruchti, P. R. Sleath, K. H. Grabstein, N. A. Hosken, F. Kern, J. A. Nelson, and L. J. Picker. 2005. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J. Exp. Med. 202:673-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weinberg, A., C. Tierney, M. A. Kendall, R. J. Bosch, J. Patterson-Bartlett, A. Erice, M. S. Hirsch, and B. Polsky. 2006. Cytomegalovirus-specific immunity and protection against viremia and disease in HIV-infected patients in the era of highly active antiretroviral therapy. J. Infect. Dis. 193:488-493. [DOI] [PubMed] [Google Scholar]
- 35.Weinberg, A., D. A. Wohl, R. J. Barrett, and C. van der Horst. 2001. Inconsistent reconstitution of cytomegalovirus-specific cell-mediated immunity in human immunodeficiency virus-infected patients receiving highly active antiretroviral therapy. J. Infect. Dis. 184:707-712. [DOI] [PubMed] [Google Scholar]
- 36.Weinberg, A., D. A. Wohl, S. MaWhinney, R. J. Barrett, D. G. Brown, N. Glomb, and C. van der Horst. 2003. Cytomegalovirus-specific IFN-gamma production is associated with protection against cytomegalovirus reactivation in HIV-infected patients on highly active antiretroviral therapy. AIDS 17:2445-2450. [DOI] [PubMed] [Google Scholar]
- 37.Wohl, D. A., D. Zeng, P. Stewart, N. Glomb, T. Alcorn, S. Jones, J. Handy, S. Fiscus, A. Weinberg, D. Gowda, and C. van der Horst. 2005. Cytomegalovirus viremia, mortality, and end-organ disease among patients with AIDS receiving potent antiretroviral therapies. J. Acquir. Immune Defic. Syndr. 38:538-544. [DOI] [PubMed] [Google Scholar]
- 38.Xhaard, A., M. Robin, C. Scieux, R. P. de Latour, S. Deplus, M. C. Mazeron, A. Devergie, H. Esperou, V. Rocha, E. Gluckman, P. Ribaud, and G. Socie. 2007. Increased incidence of cytomegalovirus retinitis after allogeneic hematopoietic stem cell transplantation. Transplantation 83:80-83. [DOI] [PubMed] [Google Scholar]
- 39.Yust, I., Z. Fox, M. Burke, A. Johnson, D. Turner, A. Mocroft, C. Katlama, B. Ledergerber, P. Reiss, and O. Kirk. 2004. Retinal and extraocular cytomegalovirus end-organ disease in HIV-infected patients in Europe: a EuroSIDA study, 1994-2001. Eur. J. Clin. Microbiol. Infect. Dis. 23:550-559. [DOI] [PubMed] [Google Scholar]
- 40.Zar, J. H. 1984. Biostatistical analysis, 2nd ed. Prentice-Hall, Inc., Englewood Cliffs, NJ.