Abstract
In recent clinical trials, a herpes simplex virus (HSV) recombinant glycoprotein D (gD) vaccine was more efficacious in woman than in men. Here we report six HLA-DR-restricted T-cell gD epitope peptides that bind to multiple HLA-DR (DR1, DR4, DR7, DR13, DR15, and DRB5) molecules that represent a large proportion of the human population. Four of these peptides recalled naturally primed CD4+ T cells in up to 45% of the 46 HSV-seropositive, asymptomatic individuals studied. For the gD49-82, gD77-104, and gD121-152 peptides, the CD4+ T-cell responses detected in HSV-seropositive, asymptomatic women were higher and more frequent than the responses detected in men. Immunization of susceptible DRB1*0101 transgenic mice with a mixture of three newly identified, gender-dependent, immunodominant epitope peptides (gD49-82, gD77-104, and gD121-152) induced a gender- and CD4+ T-cell-dependent immunity against ocular HSV type 1 challenge. These results revealed a gender-dependent T-cell response to a discrete set of gD epitopes and suggest that while a T-cell epitope-based HSV vaccine that targets a large percentage of the human population may be feasible with a limited number of immunodominant promiscuous HLA-DR-restricted epitopes, gender should be taken into account during evaluations of such vaccines.
The vast majority of the world's human population is infected with herpes simplex virus type 1 (HSV-1) and/or HSV-2, which cause diseases in every stage of life, ranging from fatal disseminated disease in newborns to skin lesions (cold sores), genital ulcerations, blinding eye lesions, and fatal encephalitis in adults (21, 43, 47, 85). More than 450,000 people in the United States have a history of recurrent ocular herpes disease that can lead to sight-threatening herpetic stromal keratitis caused by an immunopathological response in the cornea (2, 25, 31, 66). Recurrent genital herpes disease also has an immunopathological course that leads to the development of genital lesions, ulcerations, and scarring (2). The rapid spread of genital herpesvirus infection, which occurs mostly during unrecognized or asymptomatic shedding, is reflected in more than one million new cases per year in the United States (44).
Although costly drug therapy (e.g., acyclovir and derivatives) can limit morbidity and mortality from active disease, it cannot cure infection (38). Thus, developing an effective immunotherapeutic vaccine against herpesvirus infection must be an important global health priority (52, 53, 55, 85, 86). Such a vaccine would be a cost-effective approach and would be beneficial not only in developed nations but also in underdeveloped regions such as some sub-Saharan African regions, where 70% of high-risk, human immunodeficiency virus (HIV)-negative individuals and 85% of HIV-infected individuals are seropositive for herpesvirus (46, 61, 75). However, progress toward a clinical herpes vaccine faces significant challenges including the identification of protective antigens (Ags).
Of the 11 HSV glycoproteins (33), glycoprotein D (gD) is a leading vaccine candidate (20, 53, 72, 73, 85). gD has been successful in eliciting T-cell-mediated protective immunity in mouse, rabbit, and guinea pig herpes models (13, 15, 53). Moreover, in the most recent clinical trial, immunization with a recombinant gD vaccine protected previously uninfected women while failing to protect men or HSV-1-seropositive women (8, 68, 69). In part, the results of this clinical trial stimulated us to look for the gender-dependent immunological response we report here. The immune mechanism and the protective gD epitopes that might be targeted during this apparent gender-dependent immunity remain to be elucidated. In this large trial, the HSV-specific antibody response levels induced by gD were found to be similar to those induced by natural HSV infection (8, 68, 69). However, the full breadth and specificities of the T-cell responses induced by these gD vaccines are unknown, mainly because of a lack of information about human T-cell epitopes on gD (13, 21, 68). Mapping of human T-cell epitopes on gD would (i) contribute to a better understanding of the immune correlates of protection and (ii) help in developing effective immunotherapeutic vaccine strategies.
The two main goals of the present study were (i) to identify HSV-1 gD-derived human CD4+ T-cell epitopes, with a special interest in promiscuous HLA-DR-restricted epitopes, that can be recognized by the majority of humans, irrespective of ethnicity, and (ii) to determine if there are gender-dependent gD T-cell responses to these epitopes in HSV-seropositive individuals.
With the computer program TEPITOPE, we previously predicted 12 potential epitope peptides from HSV-1 gD that are specific to 10 HLA-DR molecules representing the majority of the world population (2). Here we demonstrated that 6 of the 12 potential epitope peptides bind in vitro with high affinity to 6 of the 10 HLA-DR molecules tested. Four gD epitope peptides recalled functional HSV-specific gamma interferon (IFN-γ)-producing CD4+ T cells in healthy, HSV-seropositive, asymptomatic individuals and were recognizable by virus-primed CD4+ T cells in HLA-DR*0101 and HLA-DR*0401 transgenic (Tg) mice following ocular infection with HSV. Interestingly, three gD peptides (gD49-82, gD77-104, and gD121-152) that recalled stronger CD4+ T-cell responses in HSV-seropositive women than in men also recalled stronger CD4+ T-cell responses in HSV-infected age-matched female compared to male HLA-DR*0101 and HLA-DR*0401 Tg mice. Finally, a cocktail of three immunodominant, gender-dependent gD peptide epitopes elicited CD4+ T-cell-dependent immunity against lethal herpesvirus infection in HLA-DR*0101 Tg mice.
MATERIALS AND METHODS
Study population.
From August 2003 to October 2007, we screened 283 HSV-1- and/or HSV-2-seropositive individuals. Among these, a cohort of 87 immunocompetent individuals, with an age range of 18 to 63 (median, 31) years, who were seropositive or seronegative for HSV-1 and/or HSV-2 were enrolled in the present study. For the characteristics of this study population with respect to sex, age, and HSV serology, see Table 3. Fifty-seven individuals were white, 30 were nonwhite (African, Asian, Hispanic, or other), 43 were females, and 44 were males. All patients were negative for HIV and hepatitis B virus and had no history of immunodeficiency. Twenty-eight patients were HSV-1 seropositive and HSV-2 seronegative, 10 patients were HSV-2 seropositive and HSV-1 seronegative, 9 patients were both HSV-1 and HSV-2 seropositive, and 40 were HSV seronegative. All of the patients selected for this study were healthy and asymptomatic (i.e., seropositive with no history of recurrent ocular, orofacial, or genital herpes disease). All subjects were enrolled at the University of California Irvine under an institutional review board-approved protocol. All subjects provided written informed consent.
TABLE 3.
Cohort of HSV-seropositive but asymptomatic and HSV-seronegative individuals enrolled in this study
| Characteristica | Value for all subjects whose sera were studied by:
|
|
|---|---|---|
| T-cell proliferation assay (n = 55) | IFN-γ ELISPOT assay (n = 87) | |
| Gender [no. (%)] | ||
| Females | 35 (63.6) | 43 (49.4) |
| Males | 20 (36.4) | 44 (49.6) |
| Race [no. (%)] | ||
| Whites | 35 (63.6) | 57 (65.5) |
| Nonwhites | 20 (36.4) | 30 (34.5) |
| Age (yr) | ||
| Median | 31 | 31 |
| Range | 18-63 | 18-63 |
| HSV status [no. (%)] | ||
| HSV-1 positive | 19 (34.5) | 28 (32.2) |
| HSV-2 positive | 8 (14.5) | 10 (11.5) |
| HSV-1 and -2 positive | 6 (10.9) | 9 (10.3) |
| HSV negative | 22 (40) | 40 (46) |
The HSV-seropositive, asymptomatic individuals enrolled in this study had one or no episodes of recurrent disease/year and were healthy and hepatitis and HIV negative.
HSV-1 and HSV-2 seropositivity screening.
The sera collected from 61 patients were tested for HSV-1 and HSV-2 status with HerpeSelect immunoglobulin G1 and G2 HSV enzyme-linked immunosorbent assay (ELISA) kits (Focus Diagnostics, Cypress, CA) as previously described (30). The sensitivities and specificities of these ELISAs were 91.2% (HSV-1) to 96.1% (HSV-2) and 92.3% (HSV-1) to 97% (HSV-2). Although this assay generally gives a clear-cut result, in some instances the serotyping was also validated by Western blotting as previously described (63).
Bioinformatic analysis.
The full-length gD sequence (26) was loaded into the new prediction software TEPITOPE to predict promiscuous epitopes (19). The TEPITOPE algorithm is a Windows application that is based on 25 quantitative matrix-based motifs that cover a significant part of the human HLA class II peptide binding specificity (58). The algorithm permits the prediction and parallel display of ligands for each of the 25 HLA-DR alleles. The TEPITOPE prediction threshold was set at 5%, and 12 regions predicted to bind a minimum of 50% of the major histocompatibility complex (MHC) class II molecules were picked (Fig. 1). The HLA-DR-restricted peptide epitopes derived from OVA (OVA323-339) (45) and from gB (gB161-175) (ATMYYKDVTVSQVWF) were used for control of specificity. Peptide gD253-278 (LPPELSETPNATQPELAPEDPEDSAL) from region gD257-287 and peptide gB728-761 (NAAMFAGLGAFFEGMGDLGRAVGKVVMGIVGGVV), which were not picked by the TEPITOPE program as containing potential T-cell epitopes, were synthesized and used as negative controls.
FIG. 1.
Illustration of HSV-1 gD showing the relative positions of T-cell epitope peptides used in this study. HSV-1 (strain 17) gD regions carrying potential human class II-restricted T-cell epitopes predicted by the TEPITOPE computer-assisted algorithm based on known HLA-peptide-TCR interactions are shown. The amino acid sequence, in single-letter code, and the peptide positions based on the full-length sequence of gD are shown. The black box represents the transmembrane domain.
Peptides and immunization.
A total of 12 gD peptides were selected by TEPITOPE as previously described (2). Each peptide, consisting of 27 to 34 amino acids, was synthesized as previously described (1, 3-7, 14, 27, 59). gB (ATMYYKDVTVSQVWF) and OVA T helper peptides were used as control peptides. The purity of peptides was 96%, as determined by reversed-phase high-performance liquid chromatography (Vydac C18) and mass spectroscopy (Voyager matrix-assisted laser desorption ionization-time of flight mass spectrometry system). Stock solutions were made at 1 mg/ml in water, except for peptide gD146-179, which was solubilized in 5% dimethyl sulfoxide. All peptides were aliquoted and stored at −20°C until assayed. All in vivo immunization studies were conducted with the immunogen emulsified in CpG1826 adjuvant, which was immediately injected subcutaneously into mice as recently described (10, 12, 53, 62).
Virus.
The McKrae strain of HSV-1 and strain 333 of HSV-2 were used in this study. The virus was triple plaque purified and was prepared as previously described (54, 56). UV-inactivated HSV-1 and HSV-2 were made by exposing the live virus to a Philips 30-W UV bulb for 10 min at a distance of 5 cm. Heat-inactivated virus was made by heating a virus solution at 100°C for 5 min. HSV inactivation was confirmed by the inability to produce plaques when tested on Vero cells as previously described (11, 52).
MHC-peptide binding assays.
HLA-DR molecules were purified from homologous Epstein-Barr virus-infected cell lines by affinity chromatography with monomorphic monoclonal antibody (MAb) L243 coupled to protein A-Sepharose CL-4B gel (Amersham Pharmacia Biotech, Orsay, France) as described previously (70, 71). Binding to HLA-DR molecules was assessed by competitive ELISA as previously reported (71). HLA-DR molecules were diluted with an appropriate biotinylated peptide and serial dilutions of competitor peptides. Samples (100 μl/well) were incubated in 96-well polypropylene plates (Nunc, Roskilde, Denmark) at 37°C for 24 to 72 h. After pH neutralization, samples were applied to 96-well MaxiSorp ELISA plates (Nunc) previously coated with 10 μg/ml MAb L243 for HLA-DR molecules. Bound biotinylated peptides were detected by streptavidin-alkaline phosphatase (Amersham, Little Chalfont, United Kingdom) with 4-methylumbelliferyl phosphate as the substrate (Sigma Chemical Co., St. Quentin Fallavier, France). Fluorescence (excitation at 365 nm, emission at 450 nm) was measured on a Wallac Victor2 1420 multilabel counter (Perkin-Elmer, Courtaboeuf, France). Maximal binding was determined by incubating the biotinylated peptide with the MHC II molecule in the absence of competitor. Data were expressed as the concentration of peptide that prevented binding of 50% of the labeled peptide (IC50). Binding specificity for each HLA II was ensured by the choice of the biotinylated peptides as described previously (71). Unlabeled forms of the biotinylated peptides were used as reference peptides to assess the validity of each experiment. Their IC50 variation did not exceed a factor of 3. Their sequences and IC50s were the following: HA 306-318 (PKYVKQNTLKLAT) for DRB1*0101 (2 nM), DRB1*0401 (42 nM), DRB1*1101 (52 nM), and DRB5*0101 (16 nM); YKL (AAYAAAKAAALAA) for DRB1*0701 (6 nM); A3 152-166 (EAEQLRAYLDGTGVE) for DRB1*1501 (13 nM); MT 2-16 (AKTIAYDEEARRGLE) for DRB1*0301 (305 nM); B1 21-36 (TERVRLVTRHIYNREE) for DRB1*1301 (276 nM); LOL 191-210 (ESWGAVWRIDTPDKLTGPFT) for DRB3*0101 (12 nM); and E2/E168 (AGDLLAIETDKATI) for DRB4*0101.
Virus challenge.
Ocular challenge was performed by dropping of 2 × 105 PFU of HSV-1 (McKrae) in 4 μl tissue culture medium on each eye, and gently rubbing the lid against the eye for 30 s.
PBMC isolation and HLA-DR stereotyping.
Peripheral blood mononuclear cells (PBMC) were prepared as previously described (5, 6, 27), by density gradient centrifugation of leukapheresis blood products provided by healthy individuals and commercially available from General Clinical Research Center, University of California Irvine Medical Center (Orange, CA). The cells were washed in Hanks balanced salt solution and resuspended in complete medium (CM) consisting of RPMI 1640 medium containing 10% fetal bovine serum (FBS) with 15 mM HEPES, 5 × 10−5 M β-mercaptoethanol, 2 mM glutamine, 50 U of penicillin, and 50 μg of streptomycin (GIBCO-BRL, Grand Island, NY). Aliquots of freshly isolated PBMC were cryopreserved in 90% FBS and 10% dimethyl sulfoxide in liquid nitrogen for future experiments. The HLA-DR status of the blood samples was confirmed by indirect staining with 10 μl of anti-HLA-DR MAbs BB7.2 and MA2.1 (1:10 dilution of culture supernatant) (ATCC) at 4°C for 30 min, followed by 30 min of incubation with fluorescein isothiocyanate-conjugated goat anti-mouse antibodies (BD Bioscience, San Jose, CA) and analyzed on a FACScalibur analyzer (BD Bioscience).
HLA-DR Tg mice and immunization.
Six- to eight-week-old HLA-DR1 and HLA-DR4 Tg mice, established on the susceptible BALB/c genetic background, were used in all experiments. HLA identification of HLA-DR1 and HLA-DR4 mice was confirmed by staining with fluorescein isothiocyanate-conjugated anti-mouse HLA-DR1 and HLA-DR4 antibodies at 4°C for 30 min, followed by analysis on a FACScalibur analyzer (BD Bioscience). Groups of five sex- and age-matched mice per strain were immunized subcutaneously with a mixture of four gD peptides (100 μg each) in 20 μg CpG adjuvant on days 0 and 14.
IFN-γ ELISPOT assays.
Human PBMC were cultured in 24-well plates for 5 days in a humidified 5% CO2 atmosphere with heat-inactivated HSV-1 (multiplicity of infection [MOI] = 5) and subsequently analyzed in an IFN-γ enzyme-linked immunospot (ELISPOT) assay. Functional T-cell recognition IFN-γ ELISPOT assays were performed with the human CD4+ T-cell IFN-γ ELISPOT MAb set (BD PharMingen, San Diego, CA). Briefly, on day 5, 96-well multiscreen immunoprecipitation plates were blocked with RPMI 1640 medium supplemented with 10% FBS for 2 h at room temperature. Cells (2 × 105/well) were added in triplicate to MAb-coated plates and incubated with 12 individual gD peptides for an additional 18 to 24 h at 37°C in 5% CO2. Plates were then washed with phosphate-buffered saline and supplemented with a detection peroxidase-labeled antibody, followed by a substrate according to the manufacturer's instructions. The developed spots were counted under a light microscope. For HLA-DR Tg mice, spleen cells were cultured in 24-well plates for 5 days in a humidified 5% CO2 atmosphere with heat-inactivated HSV-1 (MOI = 5) and HSV-2 (MOI = 5), respectively, and subsequently analyzed in an IFN-γ ELISPOT assay. Functional T-cell recognition IFN-γ ELISPOT assays were performed with the mouse IFN-γ ELISPOT MAb pair (BD PharMingen, San Diego, CA). Briefly, on day 4, 96-well multiscreen immunoprecipitation plates were coated by incubation overnight at 4°C with 100 μl (1:250) of anti-IFN-γ capture MAb. Plates were then blocked with RPMI 1640 medium supplemented with 10% FBS for 2 h at room temperature. Cells (5 × 104/well) were added in triplicate to MAb-coated plates and incubated for an additional 18 to 24 h at 37°C in 5% CO2. Plates were then washed with phosphate-buffered saline and supplemented with a detection peroxidase-labeled antibody, followed by a substrate according to the manufacturer's instructions. The developed spots were counted under a light microscope.
T-cell proliferation assay.
We first performed a dose-response study by incubating each peptide at 0.3, 1, 3, 10, and 30 μg/ml with either human- or mouse-derived T cells. Although all doses of the positive peptides induced significant T-cell responses, 1 μg/ml induced the optimal response of human T cells while 30 μg/ml induced optimal T-cell responses in mice (data not shown). Accordingly, the subsequent experiments were carried out with these optimal doses.
Human PBMC were cultured in 96-well plates at 5 × 105/well in CM with 12 individual gD peptides at a 1 μg/ml concentration, with heat-inactivated HSV-1 and HSV-2 (MOI, 0.3), or with phytohemagglutinin (PHA) as a positive control. The PBMC suspensions were incubated for 72 h at 37°C in 5% CO2, and their proliferation was determined in a [3H]thymidine incorporation assay as described previously (1, 3, 4, 7, 14, 59). For HLA-DR Tg mice, 14 days after the second immunization, spleen cells were removed and placed in ice-cold, serum-free HL-1 medium supplemented with 15 mM HEPES, 5 × 10−5 M β-mercaptoethanol, 2 mM glutamine, 50 U of penicillin, and 50 μg of streptomycin (GIBCO-BRL, Grand Island, NY) (1, 3, 4, 7, 14, 59). As for the human T-cell responses described above, we also performed a dose-response study with mouse spleen-derived T cells together with each peptide at 0.1, 0.3, 1, 3, 10, and 30 μg/ml (data not shown). Although all six doses induced T-cell responses of similar magnitudes, the optimal response was obtained with 30 μg/ml. Accordingly, subsequent murine experiments were carried out with the middle dose of 30 μg/ml. The cells were cultured in 96-well plates at 5 × 105/well in CM with 12 individual gD peptides at a 30 μg/ml concentration as previously described (1, 3, 4, 7, 14, 59). The cell suspensions were incubated for 72 h at 37°C in 5% CO2. One microcurie of [3H]thymidine (DuPont NEN, Boston, MA) was added to each well for the last 18 h of culture. The incorporated radioactivity was determined by harvesting cells onto glass fiber filters and counting the radioactivity on a Matrix 96 direct ionization counter (Packard Instruments, Meriden, CT) (1, 3, 4, 7, 14, 59). Results were expressed as the mean counts of cell-associated [3H]thymidine per minute recovered from wells containing Ag minus the mean counts of cell-associated [3H]thymidine per minute recovered from wells without Ag (Δcpm; average of triplicate measurements). The stimulation index (SI) was calculated as the mean counts of cell-associated [3H]thymidine per minute recovered from wells containing Ag divided by the mean counts of cell-associated [3H]thymidine per minute recovered from wells without Ag (average of triplicate measurements). For all experiments, the irrelevant control peptide gB728-761 and the T-cell mitogen concanavalin A (Sigma, St. Louis, MO) were used as negative and positive controls, respectively. Proliferation results were confirmed by repeating each experiment twice. A T-cell proliferative response was considered positive when the Δcpm value was >1,000 and when the SI was >2, as previously reported (5, 6, 16, 27, 29). Three of the peptides (gD49-82, gD77-104, and gD121-152) recalled significantly higher responses in CD4+ T cells from asymptomatic, HSV-seropositive women than in those from men. A pool of these three epitope peptides induced a gender-dependent protective CD4+ T-cell-dependent immunity against HSV challenge in HLA-DR*0101 and HLA-DR*0401 Tg mice.
Statistical analysis.
The figures represent data from two or three independent experiments. The data were expressed as the mean ± the standard error of the mean and were compared by using Student's t test on a STATVIEW II statistical program (Abacus Concepts, Berkeley, CA) and two-way analysis of variance (ANOVA) on a GraphPad Prism 4. Differences were considered significant when P was <0.05. All P values were two tailed unless stated otherwise.
RESULTS
In vitro binding of potential CD4+ T-cell epitope peptides from selected gD to HLA-DR molecules.
With the computer program TEPITOPE, we previously found 12 predicted DR-restricted epitope peptides from HSV-1 gD (2). The predicted peptides were synthesized and tested in vitro for binding to 10 soluble HLA-DR molecules (Table 1). This panel of available HLA-DR molecules consists of the most common HLA class II haplotypes, regardless of ethnicity (32). The HLA-DRB1 locus encodes seven of the HLA-DR molecules (HLA-DRB1*0101, HLA-DRB1*0301, HLA-DRB1*0401, HLA-DRB1*0701, HLA-DRB1*1101, HLA-DRB1*1301, and HLA-DRB1*1501). The HLA-DRB3, HLA-DRB4, and HLA-DRB5 molecules were encode by a second HLA-DRB locus. The relative binding capacity (nanomolar IC50) for each peptide was calculated as the concentration of competitor peptide required to inhibit 50% of the binding of an allele-specific biotinylated peptide (indicator peptide) as described in Materials and Methods. Based on an upper threshold of 100 nM, which characterizes high-affinity peptide binders (17, 32), six peptides (gD1-29, gD49-82, gD96-123, gD121-152, gD176-206, and gD200-234) bound to five or more different HLA-DR molecules, five peptides (gD22-52, gD77-104, gD146-179, gD228-257, and gD332-358) bound to four different HLA-DR molecules, and one peptide (gD287-317) did not bind to any of the HLA-DR molecules tested. This suggests that 11 of the 12 predicted peptides contain at least one universal T-cell epitope or several overlapping epitopes presented by multiple HLA-DR molecules.
TABLE 1.
HSV-1 gD-derived human epitope binding to soluble HLA-DR molecules
| Epitope | HLA-DR and HLA-DP binding capacitya (IC50 [nM])
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HLA-DR1 B1*0101 (12.9)d | HLA-DR3 B1*0301 (11) | HLA-DR4 B1*0401 (45.2) | HLA-DR7 B1*0701 (29.0) | HLA-DR11 B1*1101 (19.4) | HLA-DR13 B1*1301 (6) | HLA-DR15 B1*1501 (8) | HLA-DRB3 B3*0101 (51.4) | HLA-DRB4 B5*0101 (45.8) | HLA-DRB5 B4*0101 (23.9) | |
| gD1-29c | 58b | 79 | 58 | 374 | 648 | >100,000 | 10,954 | 535 | >100,000 | 7 |
| gD22-52 | 3 | 2,492 | 63 | 224 | 25 | >100,000 | 787 | 5979 | 397 | 58 |
| gD49-82c | 3 | 1,249 | 93 | 173 | 120 | >100,000 | 18 | 5,000 | 170 | 66 |
| gD77-104c | 22 | 2,349 | NDe | 4 | 300 | ND | 25 | >100,000 | ND | 1 |
| gD96-123c | 3 | ND | 61 | 37 | 598 | 4,762 | 167 | >100,000 | 1,672 | 102 |
| gD121-152c | 53 | 66 | 8 | 19 | 289 | 160 | 2 | 226 | 319 | 134 |
| gD146-179 | 40 | 1,0247 | 632 | 316 | 175 | >100,000 | 35 | 2,020 | 743 | 85 |
| gD176-206c | 54 | 1,342 | 955 | 21 | 5 | 200 | 76 | 25,000 | 1,803 | 91 |
| gD200-234c | 4 | 307 | 40 | 200 | 44 | 2,049 | 13 | 41 | 3,742 | 68 |
| gD228-257 | 1,162 | 2,392 | 9,920 | 20 | 39 | 1,587 | 2 | >100,000 | 1,163 | 22 |
| gD287-317 | 3,162 | 19,494 | 600 | 2,449 | 25,000 | >100,000 | 6,788 | 5,000 | 3,256 | 4,500 |
| gD332-358 | 150 | 1,643 | 5,872 | 274 | 5 | 56 | 950 | 2,307 | 703 | 31 |
Competitive binding of synthetic gD peptides bearing potential HLA class II epitopes to solubilized HLA-DR and HLA-DP molecules was tested in vitro. Results are expressed as means of HLA binding capacities determined from three independent experiments.
Shown in bold are IC50s of <100 nM, which indicate gD peptides with high HLA binding capacities.
One of six MHC class II-restricted peptide sequences that bind to at least five HLA-DR and two HLA-DP4 polymorphic molecules.
Values in parentheses show percent population coverage (allelic frequencies in a Caucasian population). HLA-DP4 alleles (DP401 and DP402) are carried by 75% of individuals and are the most frequent HLA II alleles worldwide. Two gD peptides (gD121-152 and gD176-206) bind with high affinity to the HLA-DP401 molecule, whereas three peptides (gD49-82, gD96-123, and gD121-152) bind to HLA-DP402.
ND means binding of the peptide was not determined due to lack of its solubility.
The projected population coverage of each of the 12 gD epitope peptides was calculated on the basis of the phenotypic frequencies of the HLA-DR haplotypes (Table 2), assuming that these molecules are representative of all subtypes of the same haplotype. A combination of the six promiscuous epitope peptides that bound to five or more HLA-DR molecules would be predicted to cover a large proportion of the human population, regardless of ethnicity.
TABLE 2.
Expected and actual population coverages of HLA-DR binding gD epitope peptides
| Peptide | No. of DR molecules bounda | Population coverage (%)
|
|
|---|---|---|---|
| Projectedb | Actualc | ||
| gD1-29 | 4 | 56.0 | 41.8 |
| gD22-52 | 4 | 53.8 | 28.4 |
| gD49-82 | 4 | 40.6 | 44.8 |
| gD77-104 | 4 | 52.8 | 39.9 |
| gD96-123 | 4 | 60.1 | 18.1 |
| gD121-152 | 5 | 72.8 | 34.5 |
| gD146-179 | 3 | 31.6 | 20.1 |
| gD176-206 | 5 | 64.6 | 32.7 |
| gD200-234 | 6 | 65.5 | 24.5 |
| gD228-257 | 4 | 52.5 | 22.2 |
| gD287-317 | 0 | NDd | 17.2 |
| gD332-357 | 3 | 40.9 | 25.4 |
The panel of HLA-DR molecules is represented by seven molecules encoded by the HLA-DRB1 locus and three molecules encoded by the HLA-DRB3, HLA-DRB4, and HLA-DRB5 loci.
Average projected population coverage was calculated considering the phenotype frequencies of the HLA-DR molecules which bind the corresponding peptide epitope.
Actual population coverage was calculated on the basis of both Tp and IFN-γ responses corresponding to the peptide epitope.
ND, not done.
Recall of gD epitope-specific CD4+ T cells in asymptomatic, HSV-seropositive individuals.
The cohorts of 55 (22 seronegative and 33 seropositive) individuals used to study T-cell proliferative responses (Tp-cell responses) to the 12 gD epitopes are shown in Table 3, column 2. All 33 seropositive individuals who had well-defined clinical histories with one or no episodes of recurrent disease/year (i.e., “protected asymptomatic” or “low-recurrent disease”) were healthy and hepatitis B virus and HIV seronegative. At the time of the study, none of them had had any active clinical ocular or genital herpes symptoms for at least 1 year. Based on their ethnic backgrounds, the 55 individuals were divided into 33 Caucasians (i.e., white) and 20 non-Caucasians (i.e., nonwhites or Asians, blacks, Hispanics, and members of other ethnic minorities).
The six highest Tp-cell responses were recalled by gD1-29, gD22-52, gD49-82, gD77-104, gD121-152, and gD176-206 (Fig. 2A). These six peptides also had medium to strong binding to multiple HLA-DR molecules. This suggests that each of these peptides bears a minimum of one promiscuous T-cell determinant or several overlapping determinants recognized by human T cells from multiple ethnicities. In contrast, gD96-123 and gD200-234 peptides induced weaker Tp-cell responses, despite their high-affinity binding to four HLA-DR molecules. Ten of the 12 gD peptides induced similar Tp-cell responses in white and nonwhite populations. Only gD77-104 and gD176-206 appeared to induce higher Tp-cell responses in white than in nonwhite individuals (P < 0.05). No differences in Tp-cell responses were detected in individuals seropositive for HSV-1 compared to HSV-2 or compared to HSV-1/HSV-2 (data not shown). None of the 12 gD peptides induced positive proliferative response of CD4+ T cells from the 22 HSV-seronegative individuals, regardless of gender (Fig. 3D). As a control, CD4+ T cells from all 55 individuals produced positive responses to the mitogens LeuA and PHA (i.e., SIs of 32 to 45 and Δcpm values of 54 × 103 to 62 × 103). However, CD4+ T cells alone, without Ag stimulation, produced low background responses (i.e., SIs of 3.8 to 4.3 and Δcpm values of 3 × 103 to 5 × 103).
FIG. 2.
CD4+ T-cell proliferative response to HSV-1 gD-derived epitopes. (A) Fresh peripheral blood-derived CD4+ T cells were isolated and stimulated in vitro with 12 individual gD peptides in a 6-day proliferation assay as described in Materials and Methods. The Tp-cell responses of Caucasian (white) and non-Caucasian (nonwhite) ethnic groups against each peptide, assessed by a [3H]thymidine incorporation assay, were compared. Proliferative responses were expressed as Δcpm values (counts per minute of cells incubated with peptide − counts per minute of cells without peptide). The mean of each group is shown as a solid horizontal line. An analysis of T-cell responses in 22 seronegative individuals is shown. A positive T-cell proliferative response was defined on the basis of the responses of HSV-seronegative control individuals, with a Δcpm value of ≥1,000 and an SI (counts per minute of cells incubated with peptide/counts per minute of cells without peptide) of ≥2. The values at the bottom of each data set (i.e., 30/35) are the mean number of responders/total number of individuals tested. The value at the top of each data set (i.e., 11.5) is the mean SI of each of the two groups, i.e., white or nonwhite. The P values compare the Tp-cell responses between white and nonwhite groups by one-way ANOVA. An asterisk in parentheses indicates a peptide that induced higher T-cell proliferation compared with HSV-seronegative control individuals (i.e., significantly higher SI and Δcpm value). (B) Analysis of HLA-DR restriction and functional frequencies of HSV-1 gD peptide-specific CD4+ T cells. Blocking of PBMC proliferation in response to the gD1-29 and gD49-82 peptides by specific anti-HLA-DR MAb.
FIG. 3.
Gender differences in Tp-cell responses to HSV-1 gD epitope peptides detected in healthy, asymptomatic, HSV-seropositive individuals. (A and D) Peptide-specific Tp-cell responses in HSV-1- and/or HSV-2-seropositive, asymptomatic, healthy individuals were examined as described in the legend to Fig. 2. The P values in panel A indicate statistically significant differences in Tp-cell responses between men and women (P < 0.05, ANOVA test). Gender dependence of IFN-γ-producing CD4+ T cells specific to 12 gD peptides (B) and to an irrelevant CD4+ T-cell epitope peptide from OVA (OVA323-339) (C) was detected by ELISPOT in HSV-seropositive, asymptomatic individuals. PBMC derived from HSV-1/HSV-2-seropositive, asymptomatic men and women were stimulated with 12 gD peptides for 5 days, and IFN-γ ELISPOT assays were performed as described in Materials and Methods.
The HLA-DR dependence of the peptide-specific Tp-cell responses was confirmed in a representative HLA-DR blocking experiment, as shown in Fig. 2B. Blockage of HLA-DR molecules resulted in a >95% reduction (P < 0.005) in Tp-cell responses to either 10 or 1 μg/ml gD1-29 or gD49-82 peptide. However, blockage of HLA-DQ molecules did not affect Tp-cell responses induced by either gD1-29 or gD49-82 peptide (e.g., at 10 μg/ml, the gD1-29-specific Tp responses were Δcpm values of 32.9 × 103 and 32.1 × 103 in the presence and absence of anti-HLA-DQ MAb, respectively).
Gender-dependent CD4+ T-cell responses to three gD peptide epitopes in asymptomatic, HSV-seropositive individuals.
IFN-γ secretion is a marker of T-cell activation that is frequently dissociated from the Tp-cell response (5, 6, 27). In addition, HSV-1 and -2 are susceptible to IFN-γ interference (2). To determine whether the T-cell response to the 12 gD peptides is gender dependent, gD epitope-specific IFN-γ-producing CD4+ T-cell responses were examined in a larger cohort of age-matched, HSV-seropositive, asymptomatic individuals composed 43 women and 44 men (Table 3, column 3). A preliminary analysis of IFN-γ-producing CD4+ T cells revealed that 95 or more spots/104 CD4+ T cells gave a 97% probability of defining a positive response. Of the 960 separate ELISPOT wells used to screen 40 HSV-seronegative individuals, only 32 wells (3%) exceeded 95 spot-forming cells (SFC)/104 CD4+ T cells. Of the 1,128 separate ELISPOT assays used to screen the 47 seropositive individuals, 192 (17%) had readings above 95 SFC/104 CD4+ T cells. A value of 95 SFC/104 CD4+ T cells was therefore used as the cutoff point for subsequent analyses.
Three epitope peptides (gD49-82, gD77-104, and gD121-152) induced significantly higher CD4+ Tp responses in HSV-seropositive women than in men (P < 0.05) (Fig. 3A). All three of the epitope peptides that induced higher Tp responses in women than in men and two additional epitope peptides (gD200-234 and gD228-257) induced significantly higher levels of IFN-γ-producing CD4+ T-cell responses in women than in men (P < 0.05) (Fig. 3B). The largest gender-dependent difference appeared to occur with gD77-104 (P < 0.0001). The levels of IFN-γ production in response to PHA were similar in men and women (data not shown). To ascertain the epitope specificity of gender-dependent CD4+ T cells detected in seropositive individuals, we used an irrelevant HLA-DR-restricted peptide epitope derived from ovalbumin (OVA), i.e., OVA323-339 (45). The geometric mean of T-cell proliferation in response to the OVA323-339 peptide was low and similarly not significant in both males and females. The IFN-γ response was also HSV epitope specific, since no IFN-γ production was induced when HSV-specific T-cell lines were incubated with the CD4+ Th OVA323-339 peptide (Fig. 3C). Taken together, these results suggest that, compared to those from men, CD4+ T cells from HSV-seropositive, asymptomatic women responded to the gD49-82, gD77-104, and gD121-152 epitope peptides with higher frequency and magnitude.
Gender-dependent CD4+ T-cell responses confirmed in HLA-DRB1*0101 and HLA-DRB1*0401 Tg mice.
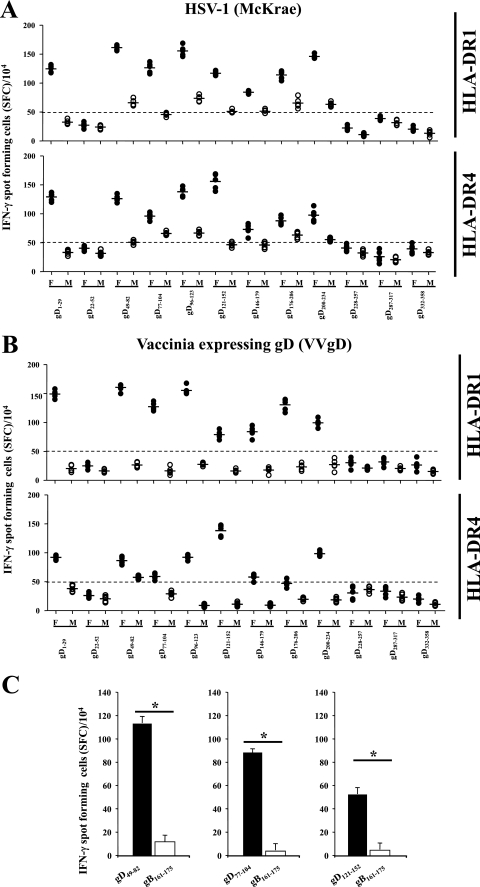
Fourteen days after ocular infection with HSV-1 (2 × 105 PFU/eye, strain McKrae) or intraperitoneal injection of vaccinia virus expressing HSV-1 gD (VVgD), splenocyte CD4+ T cells from HLA-DR1 and HLA-DR4 Tg mice were recalled in vitro with the 12 individual epitope peptides and IFN-γ ELISPOT assays as described above. Studies similar to those described above with humans determined that the cutoff for a positive response in mice was 50 spots/104 cells (data not shown). The results were similar for HLA-DR1 and HLA-DR4 mice, as well as with HSV-1 and VVgD (Fig. 4). In all instances in which a positive IFN-γ response was detected, female mice had a higher response than age-matched male mice. In addition, the same eight peptides produced a positive IFN-γ response regardless of the mouse strain or HSV-1 versus VVgD infection. Similar to the case for humans, gender-dependent IFN-γ-producing CD4+ T-cell responses against the gD1-29, gD49-82, gD77-104, and gD121-152 peptides were detected in HLA-DR Tg mice (Fig. 4A and B). However, the HLA-DR Tg mice revealed a gender-dependent T-cell response against four additional peptides: gD96-123, gD146-179, gD176-206, and gD200-234. Interestingly, although a gender-dependent IFN-γ-producing CD4+ T-cell response to gD228-257 was seen in humans, this epitope peptide did not induce any significant response in mice. The IFN-γ responses against gD peptides detected in HSV-1-infected HLA Tg mice were specific, since no IFN-γ production was observed when gender-dependent gD49-82-, gD77-104-, and gD121-152-specific T-cell lines were incubated in vitro with a heterologous HLA-DR-restricted T-cell epitope from HSV-1 gB (i.e., gB161-175) (Fig. 4C).
FIG. 4.
Gender difference in gD epitope-specific CD4+ T-cell responses detected in HLA-DR1 B1*0101 and HLA-DR4 B1*0401 Tg mice. Age-matched HLA-DR1 B1*0101 (HLA-DR1) and HLA-DR4 B1*0401 (HLA-DR4) Tg mice were infected ocularly with HSV-1 (McKrae) (A) or immunized intraperitoneally with VVgD (B). Spleens were harvest on day 14 postinfection and stimulated with each of the 12 individual peptides for 5 days, and peptide-specific IFN-γ-producing CD4+ T cells were detected as described in Materials and Methods. (C) CD4+ T-cell lines specific to gender-dependent gD49-82, gD77-104, and gD121-152 were established from HSV-1-infected HLA Tg mice and restimulated in vitro with either the corresponding specific gD peptide or a heterologous HLA-DR-restricted T-cell epitope from HSV-1 gB (i.e., gB161-175). The numbers of SFC producing IFN-γ are shown for each peptide as mean values ± standard deviations from two independent experiments. The P values are for comparisons of the IFN-γ-producing CD4+ T-cell responses of age-matched females and males by one-way ANOVA.
The IFN-γ production was abrogated by anti-HLA-DR, but not by anti-HLA-A2.1, MAbs (i.e., in HLA-DR1, IFN-γ ELISPOT response against gD49-82 peptide; 152 spot-forming cells [SFC] without MAb reduced to 33 SFC and 148 SFC when anti-HLA-DR and anti-HLA-A, -B, and -C were added, respectively). The IFN-γ response was gD peptide specific, since no IFN-γ production was observed in response to a heterologous gB CD4+ Th peptide (Fig. 4C). Overall these results confirm that, similar to humans, a gender difference in CD4+ T-cell responses was detected in age-matched HLA-DR1 and HLA-DR4 Tg mice against the gD1-29, gD49-82, gD77-104, and gD121-152 peptides following either HSV infection or VVgD immunization.
Immunization with a pool of immunodominant gD epitope peptides induced CD4+ T-cell-dependent protective immunity against lethal herpesvirus infection in HLA-DR*0101 Tg mice.
Three epitope peptides (gD49-82, gD77-104, and gD121-152) were selected as the “best” overall on the basis of three criteria: (i) high-affinity binding to multiple HLA class II molecules, including HLA-DRB1*0101 (Table 1); (ii) strong recall of CD4+ Tp-cell and IFN-γ responses in HSV-seropositive, asymptomatic individuals (Fig. 2 and 3); and (iii) high recall of IFN-γ-producing CD4+ T cells in HLA-DRB1*0101 Tg mice (Fig. 4). A pool of these three peptides was then used to immunize susceptible HLA-DR1 Tg mice against a lethal HSV-1 challenge. The previously described protective epitope gD1-29 (20, 21, 53, 68, 86), which exhibited similar properties, was excluded from these experiments. A pool of the gD146-179, gD287-317, and gD332-358 subdominant peptides was used as a control.
Three groups of 20 HLA-DR*0101 Tg female mice were immunized with (i) a pool of the gD49-82, gD77-104, and gD121-152 peptides emulsified in CpG1826 adjuvant; (ii) a pool of the gD146-179, gD287-317, and gD332-358 peptides emulsified in CpG1826 adjuvant; or (iii) the CpG1826 adjuvant alone (mock-immunized control). Two weeks after the second immunization, mice in each group were challenged ocularly with a lethal dose of 5 × 105 PFU of HSV-1 (McKrae strain). All of the mice that died following the challenge did so between days 8 and 14 postinfection. The HLA-DR*0101 Tg mice immunized with the immunodominant CD4+ T-cell epitope peptide pool were protected against death, compared to those immunized with the subdominant CD4+ T-cell epitope peptide pool (95% versus 25% survival, P < 0.05) or adjuvant alone (0% survival) (Table 4). When the mice immunized with the immunodominant CD4+ T-cell epitope peptide pool were depleted of CD4+ T cells prior to virus challenge, survival was significantly decreased (Table 4). In contrast, depletion of CD8+ T cells had no significant effect. Depletion of either T-cell type had no significant impact on the mock immunized or subdominant immunized groups. Thus, with this immunization regimen, CD4+ T cells were required and CD8+ T cells were not sufficient for protective immunity against lethal ocular HSV-1 challenge.
TABLE 4.
Immunization with HLA-DR-restricted gD epitope peptides in CpG1826 adjuvant confers protective immunity to a lethal HSV-1 challenge in HLA-DR*0101 Tg mice
| Immunization groupa | No. of survivors/no. challengede (% survivors)
|
P value vs controlf | ||
|---|---|---|---|---|
| Untreatedb | CD4 depletedc | CD8 depletedd | ||
| 1 | 19/20 (95) | 0/20 (0) | 18/20 (90) | <0.005 |
| 2 | 5/20 (25) | 3/20 (15) | 3/20 (15) | >0.005 |
| Controlg | 0/20 (0) | 0/20 (0) | 0/20 (0) | |
HLA-DR*0101 Tg mice, 60 per group, were immunized subcutaneously on day 0 and day 21 with 100 μg each of immunodominant gD49-82, gD121-152, and gD200-234 pooled as a group of three peptides and emulsified in CpG1826 adjuvant (group 1) or with 100 μg each of subdominant gD146-179, gD287-317, and gD332-358 pooled as a group of three peptides (group 2). Control mice received CpG1826 adjuvant alone.
Twenty immunized HLA-DR*0101 Tg mice were left untreated.
Twenty immunized HLA-DR*0101 Tg mice were depleted of CD4+ T cells with a MAb specific to mouse CD4.
Twenty immunized HLA-DR*0101 Tg mice were depleted of CD8+ T cells with a MAb specific to mouse CD8.
Two weeks after the last immunization, all animals received a lethal ocular challenge with 5 × 105 PFU of HSV-1 (strain McKrae).
The P values shown are for comparisons of immunized versus control mice by ANOVA.
Twenty control mice were treated similarly to those in groups 1 and 2, with an irrelevant isotype control MAb.
To assess a gender dependence in protective immunity induced by gD peptides, 10 age-matched HLA-DR*0101 Tg female and male mice were immunized with the individual gD49-82, gD77-104, and gD121-152 peptides emulsified in CpG1826 adjuvant. Two weeks after the second immunization, both genders were similarly challenged ocularly with a lethal dose of HSV-1 as described above. The HLA-DR*0101 Tg female mice immunized with the immunodominant CD4+ T-cell gD49-82, gD77-104, and gD121-152 peptides showed better protection against death compared to male mice immunized with the same epitopes (65% versus 35% survival for gD49-82, 60% versus 25% survival for gD77-104, and 55% versus 20% survival for gD121-152, respectively). This result indicates, similar to their T-cell immunogenicity, that there was also a gender-dependent protective immunity induced by the immunodominant CD4+ T-cell gD49-82, gD77-104, and gD121-152 peptides.
DISCUSSION
We report here the identification of a panel of HLA-DR-restricted epitope peptides derived from HSV-1 gD, a glycoprotein that produces protective immunity against herpesvirus infection and disease in animal models and humans (20, 21, 53, 68, 86). These epitopes previously identified by screening HSV-1 gD for peptides that contain the HLA-DR supertype binding motif (2). We demonstrated that 11 of the 12 predicted peptides bound with high affinity to three or more different types of HLA-DR molecules. Three of them (i.e., gD49-82, gD77-104, and gD121-152) recalled significantly higher responses in CD4+ T cells from asymptomatic, HSV-seropositive women than in those from men. A pool of these three epitope peptides induced a gender-dependent protective CD4+ T-cell-dependent immunity against HSV challenge in HLA-DR*0101 and HLA-DR*0401 Tg mice.
In humans, HSV-specific CD4+ T cells play a crucial role in controlling both primary and recurrent infections (23, 60). In the late 1980s and early 1990s, Zarling and coworkers generated several human CD4+ T-cell clones from HSV-1-seropositive, asymptomatic individuals by stimulating peripheral blood lymphocytes (PBL) with a recombinant vaccinia virus expressing HSV-1 gD (83, 84). Five of these CD4+ T-cell clones lysed autologous HSV-1-infected lymphoblastoid cell lines, and their cytotoxicity was restricted to HLA class II molecules. Later, it was demonstrated that some of these HSV-1-specific T-cell clones were directed against both gD-1 and gD-2 by using target cells infected with wild-type HSV strains, a gC deletion mutant of HSV-1, and an HSV-1 × HSV-2 recombinant virus (81). In another study, purified HSV-1 gD, expressed in mammalian cells, stimulated proliferation of, and interleukin-2 (IL-2) production by, PBL of HSV-seropositive individuals, indicating the presence of memory T cells to gD in HSV-seropositive individuals (82). In addition, T-cell clones generated by stimulation of PBL with HSV-1 were found to proliferate in response to stimulation with gD-1 in the absence of exogenous IL-2 and to lyse HSV-1- or HSV-1/HSV-2-infected autologous target cells (83).
HSV-specific cytotoxic CD4+ clones have been recovered ex vivo from HSV-2 lesions of five patients by Corey, Koelle, and coworkers (40, 41). Cunningham and coworkers have also reported that HSV gD is a major target for CD4+ cytotoxic T lymphocytes with human epidermal keratinocytes as targets (49, 50). Although the above findings demonstrated that gD is a major target for human HSV-specific CD4+ T cells, no specific gD minimal epitopes have ever been defined. Therefore, to our knowledge, the present study reports the first six immunodominant HLA-DR-restricted human CD4+ T-cell epitopes on gD. However, results reported here do not imply that these are the only human CD4+ T-cell epitopes present on gD. Cunningham and collaborators have recently reported finding three epitopes on HSV-2 gD by the complementary overlapping peptide method, with some but not all predicted by the TEPITOPE algorithm (32nd Annual International Herpesvirus Workshop, 2007). Our approach to epitope identification is complementary to Cunningham's work, and because it was tailored specifically for HLA-DR epitopes, it could represent a model system that could be applied to any HLA-DR haplotype and to any other herpesvirus structural or regulatory protein. The minimum requirements needed to utilize this approach are (i) existing sequence data for the proteins in question; (ii) access to the genome (DNA or RNA) of the pathogen for the purpose of generating recombinant vaccinia virus vectors; (iii) HSV-seropositive, asymptomatic individuals; and (iv) HLA Tg mice. Moreover, the present strategy accurately identified regions bearing all of the previously reported gD epitopes: gD1-23 (24, 28, 36), gD241-260 (34, 48, 80), and gD290-314 (24). Therefore, our approach to epitope identification, as well as testing epitope peptides for their T-cell antigenicity, immunogenicity, and protective efficacy, should be nearly universal, regardless of the protein studied.
Recently, we reported four immunodominant H2d-restricted CD4+ T-cell epitope peptides on HSV-1 gD (2). Among these, the peptide gD49-82, a naturally processed epitope, protects H2d mice against lethal ocular HSV-1 challenge (2). We have also recently found that the gD49-82 epitope contains an 8-mer, gD53-61, that represents an immunodominant HLA-A2.1-restricted human CD8+ T-cell epitope (18). Therefore, a minimum of two human epitopes, the gD53-61 CD8+ T-cell epitope and the CD4+ T-cell epitope, are nested within the gD49-82 peptide and this epitope peptide represents an excellent candidate for inclusion in a CD4+ and CD8+ T-cell epitope-based human vaccine.
Humans are not immunologically naive and often develop memory T cells that cross-react with and respond to unrelated pathogens or Ags, a phenomenon termed heterologous immunity (reviewed in references 64 and 76). Interestingly, although an IFN-γ-producing CD4+ T-cell response specific to gD228-257 was detected in HSV-seronegative individuals, no significant T-cell responses specific to this epitope peptide were detected in HSV-infected HLA-DR mice. This result suggests that T cells specific to gD228-257 detected in humans might cross-react with and respond to a yet-to-be-identified, unrelated-pathogen-derived epitope, a recently described phenomenon termed heterologous immunity (reviewed in references 64 and 76). The processing and presentation to T cells of the gD228-257 epitope could also be different between HLA Tg mice and humans. Therefore, some apparently gD-specific CD4+ T cells identified in this study might have arisen due to cross-reaction with an unrelated pathogen. Such scenarios have been reported in murine CD4+ T-cell responses to cytomegalovirus (22, 64, 76) and may be true for HSV (22, 64, 76, 77). To help ascertain whether the detected CD4+ T-cell responses against the gD epitope peptides were primed by HSV, the epitope mapping was extended to pathogen-free HLA-DR1 and HLA-DR4 Tg mice that were infected with either HSV-1 or VVgD. The results suggested that CD4+ T cells from both HLA-DR1- and HLA-DR4-infected Tg mice also recognized five of the six epitopes that were reactive to human CD4+ T cells (gD1-29, gD49-82, gD77-104, gD121-152, and gD200-234). In addition, HLA-DRB1*0101 and DRB1*0401 Tg mice also mounted CD4+ T-cell responses against three epitope peptides not recognized by humans. This may reflect the high level of infection in this mouse system. The ability of CD4+ T cells generated by gD49-82, gD77-104, and gD121-152, epitope peptides to protect against HSV challenge in HLA-DRB1*0101 and DRB1*0401 Tg mice is also a critical parameter. These mouse studies suggest that it is very likely that the gD1-29, gD49-82, gD77-104, gD121-152, and gD200-234 epitope peptides detected in humans were not cross-reactive with nonherpesvirus Ags or pathogens but rather were induced following HSV-1 or HSV-2 infection. This does not exclude the involvement of other infectious agents in shaping herpesvirus CD4+ T-cell responses. It also remains to be determined (i) whether the gender-dependent T-cell responses to gD epitopes will translate in a serotype-specific protective immunity against HSV-1 and/or HSV-2 and (ii) whether a therapeutic immunization with gD epitopes will protect latently HSV-1- and/or HSV-2-infected HLA-DR Tg male and female mice.
gD is a leading herpes vaccine candidate Ag that has been successful in eliciting protective immunity in women but failed to protect men (8, 68, 69). However, the gD T-cell epitopes that might be targeted during this apparent gender-dependent protective immunity have not been reported. In this study, there were gender disparities in the CD4+ T-cell responses of HSV-seropositive individuals to a discrete set of gD epitopes. Indeed, HSV-seropositive women exhibited higher magnitudes of CD4+ T-cell responses to three gD epitopes (gD49-82, gD77-104, and gD121-152) compared to age- and ethnicity-matched, HSV-seropositive men. In addition, a gender- and T-cell-dependent protective immunity was induced in susceptible HLA-DR Tg mice by the same three immunodominant CD4+ T-cell peptide epitopes (gD49-82, gD77-104, and gD121-152). Thus, results reported here are in agreement with the gender-dependent protective immunity reported in the above-cited clinical trial and both suggest that sex factors may affect the host's immune response to herpesvirus epitopes. Although the present study was mainly focused on CD4+ epitopes, the possible involvement of gD CD8+ T-cell epitopes in shaping this gender-dependent protective immunity has not been ruled out. Altogether, these data indicate that gender should be taken into account during the evaluation of T-cell epitope-based herpes vaccines. Boosting the recombinant gD immunization with a discrete set of gD T-cell epitopes may be considered particularly for women, while boosting the immunization of men with a different, nonoverlapping set of gD epitopes may be warranted.
The first indication of a gender difference in herpes immunity was reported by Cantin and coworkers, who showed that female mice have low-grade infection and disease compared with strain- and age-matched male mice (35). Besides a possible association with the IFN-γ molecule (35), the immune mechanisms contributing to gender-dependent susceptibility to herpesvirus infection and disease have not been reported. A recent study by Diamond and coworkers demonstrated a higher frequency of memory T cells specific to human cytomegalovirus—another virus of the herpesvirus family—that produce higher levels of Th1 cytokines in women than in men (74). On the contrary, Klein and coworkers showed that women maintain lower levels of varicella-zoster virus-specific memory T cells compared to men (37). Although the above studies suggest that gender differences in memory T-cell responses exist for herpesviruses other than HSV-1 and HSV-2, no specific gender-associated Ags or epitopes have ever been defined. Identification of the viral Ags or epitopes recognized by T cells from HSV-seropositive men and women—either vaccinated individuals or individuals with naturally acquired herpes immunity (i.e., seropositive, asymptomatic individuals)—would facilitate the process of designing an efficient vaccine. It is possible that gender-dependent CD4+ T-cell responses detected in this study reside at the Ag-presenting cell (APC) level rather than at the T-cell level. Indeed, in other systems, female mice that are more susceptible to experimental allergic encephalomyelitis preferentially mount Th1 immune responses and their APCs produce IL-12 but not IL-10 (78). In contrast, the APCs derived from resistant male mice produce IL-10 but not IL-12. Activation of T cells with APCs derived from the opposite sex demonstrated that these cytokines were derived from the respective APC populations (78). Other studies have demonstrated that gender differences in T-cell responses are mediated, in part, by differences in sex hormone-immune interactions (37, 42, 51, 57, 74, 78). Kovats and coworkers were the first to demonstrate that sex hormones such as estrogen play a crucial role in regulating dendritic cell phenotypic and functional differentiation (42, 51, 57). Whether an increase in dendritic cell phenotypic and functional maturation leads to the strong gD epitope-specific CD4+ T-cell responses detected in seropositive women or to an increase in the function of gD-specific memory T cells remains to be determined. Nevertheless, regardless of the mechanisms, our findings are the first demonstrating that a discrete set of gD CD4+ T-cell epitope peptides are highly targeted in women compared to men and were able to elicit a protective immunity in female HLA-DR Tg mice but not in age-matched male mice. Altogether, these data suggest that gender restrictions to herpes vaccines may be overcome by focusing the immune response toward selected sets of epitopes.
The vast majority of the world's human population is infected with HSV-1 and/or HSV-2 and may benefit from therapeutic vaccination approaches designed to induce vigorous HSV-specific cellular immunity at a higher magnitude and breadth than the suboptimal natural immunity. Although the primary goals of this study were to evaluate the antigenicity of the HSV-1 CD4+ T-cell epitope peptides and the gender-specific responses to these epitopes, a secondary but important goal is to develop the knowledge of protective herpesvirus T-cell epitopes required for the development of a lipopeptide-based vaccine. Focusing on the immune response toward such epitopes could be of value in the case of HSV-1 and HSV-2 infections, where T cells directed against the immunodominant epitopes might have been inactivated and T cells specific for subdominant epitopes might have escaped T-cell tolerance. A question of practical importance is the translation of the current immunological findings for the development of an epitope-based lipopeptide vaccine for a genetically heterogeneous human population. (1, 4, 7, 53, 59, 85, 86). Accordingly, a herpes vaccine will need to induce responses against a number of promiscuous epitopes that are recognized in the context of many different HLA alleles. Several studies have demonstrated that the majority of HLA-DR molecules can be grouped into broad supertypes with overlapping peptide binding specificities (65). In the case of the HLA-DR molecules, a single superfamily encompassing DRB1 alleles that are expressed in the majority of humans has been defined (67). HLA-DR supertypes have been studied in detail, and several CD4+ T-cell epitopes that belong to these supertypes have been identified and validated (9). Broad population coverage can be established, provided that epitopes corresponding to multiple HLA supertype families are incorporated into the vaccine. From the standpoint of vaccine development, the panel of immunodominant gD peptides identified in this study has the potential to induce CD4+ T-cell responses in up to 92.9% of an average human population. The fact that each of the peptides was antigenic in the human cohort studied is most likely a result of focusing the screening exclusively on peptides capable of binding multiple HLA-DR alleles. Similar correlations have been found between promiscuous HLA-DR binding capacity and antigenicity for HIV peptides (79). Because of their apparent promiscuousness, these CD4+ T-cell epitope peptides should prove to be useful in vaccines for the heterogeneous human population. To maximize efficacy, a multiepitope-based herpes vaccine should probably include several T-cell epitopes from several different structural glycoproteins and regulatory proteins, each chosen to represent the HLA supertypes known to provide recognition in a large proportion of the global population, regardless of race and ethnicity.
In the past 2 decades, there has been increasing recognition of a worldwide pandemic of herpesvirus infection despite the widespread use of antiviral drug therapies (reviewed in reference 39). Although drug therapy can limit morbidity and mortality from active herpes disease, it cannot cure infection. In addition, considering cost, toxicity, and viral escape issues, the usefulness of drug treatment might be limited, especially in underdeveloped regions such as parts of sub-Saharan Africa, where 70% of high-risk, HIV-negative individuals and 85% of HIV-infected individuals are seropositive for herpesvirus (46, 61, 75). Developing an effective immunoprophylactic or immunotherapeutic vaccine against herpesvirus infection must be an important global health priority. The use of multivalent epitope vaccines is a promising approach to fighting herpesvirus diseases, since it allows for directing the immune response to protective epitopes that might have a greater impact on disease outcome. The HLA-DR-restricted HSV-1 gD epitopes reported here meet these criteria and hence should be considered in the design of an effective immunotherapeutic and immunoprophylactic herpes vaccine for humans.
Acknowledgments
This work was supported by Public Health Service grants EY14900, EY15225, and EY16663; the Discovery Eye Foundation; the Henry L. Guenther Foundation; and a Research to Prevent Blindness Challenge grant. L. BenMohamed is an RPB Special Award Investigator.
Footnotes
Published ahead of print on 30 July 2008.
REFERENCES
- 1.BenMohamed, L., Y. Belkaid, E. Loing, K. Brahimi, H. Gras-Masse, and P. Druilhe. 2002. Systemic immune responses induced by mucosal administration of lipopeptides without adjuvant. Eur. J. Immunol. 32:2274-2281. [DOI] [PubMed] [Google Scholar]
- 2.BenMohamed, L., G. Bertrand, C. D. McNamara, H. Gras-Masse, J. Hammer, S. L. Wechsler, and A. B. Nesburn. 2003. Identification of novel immunodominant CD4+ Th1-type T-cell peptide epitopes from herpes simplex virus glycoprotein D that confer protective immunity. J. Virol. 77:9463-9473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.BenMohamed, L., H. Gras-Masse, A. Tartar, P. Daubersies, K. Brahimi, M. Bossus, A. Thomas, and P. Druilhe. 1997. Lipopeptide immunization without adjuvant induces potent and long-lasting B, T helper, and cytotoxic T lymphocyte responses against a malaria liver stage antigen in mice and chimpanzees. Eur. J. Immunol. 27:1242-1253. [DOI] [PubMed] [Google Scholar]
- 4.BenMohamed, L., R. Krishnan, C. Auge, J. F. Primus, and D. J. Diamond. 2002. Intranasal administration of a synthetic lipopeptide without adjuvant induces systemic immune responses. Immunology 106:113-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.BenMohamed, L., R. Krishnan, J. Longmate, C. Auge, L. Low, J. Primus, and D. J. Diamond. 2000. Induction of CTL response by a minimal epitope vaccine in HLA A*0201/DR1 transgenic mice: dependence on HLA class II restricted TH response. Hum. Immunol. 61:764-779. [DOI] [PubMed] [Google Scholar]
- 6.BenMohamed, L., A. Thomas, M. Bossus, K. Brahimi, J. Wubben, H. Gras-Masse, and P. Druilhe. 2000. High immunogenicity in chimpanzees of peptides and lipopeptides derived from four new Plasmodium falciparum pre-erythrocytic molecules. Vaccine 18:2843-2855. [DOI] [PubMed] [Google Scholar]
- 7.BenMohamed, L., S. L. Wechsler, and A. B. Nesburn. 2002. Lipopeptide vaccines—yesterday, today, and tomorrow. Lancet Infect. Dis. 2:425-431. [DOI] [PubMed] [Google Scholar]
- 8.Bernstein, D. 2005. Glycoprotein D adjuvant herpes simplex virus vaccine. Expert Rev. Vaccines 4:615-627. [DOI] [PubMed] [Google Scholar]
- 9.Bertoni, R., J. Sidney, P. Fowler, R. W. Chesnut, F. V. Chisari, and A. Sette. 1997. Human histocompatibility leukocyte antigen-binding supermotifs predict broadly cross-reactive cytotoxic T lymphocyte responses in patients with acute hepatitis. J. Clin. Investig. 100:503-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bettahi, I., G. Dasgupta, O. Renaudet, A. A. Chentoufi, X. Zhang, D. Carpenter, S. Yoon, P. Dumy, and L. BenMohamed. 27 June 2008. Antitumor activity of a self-adjuvanting glyco-lipopeptide vaccine bearing B cell, CD4+ and CD8+ T cell epitopes. Cancer Immunol. Immunother. [Epub ahead of print.] doi: 10.1007/s00262-008-0537-y. [DOI] [PMC free article] [PubMed]
- 11.Bettahi, I., A. B. Nesburn, S. Yoon, X. Zhang, A. Mohebbi, V. Sue, A. Vanderberg, S. L. Wechsler, and L. BenMohamed. 2007. Protective immunity against ocular herpes infection and disease induced by highly immunogenic self-adjuvanting glycoprotein D lipopeptide vaccines. Investig. Ophthalmol. Vis. Sci. 48:4643-4653. [DOI] [PubMed] [Google Scholar]
- 12.Bettahi, I., X. Zhang, R. E. Afifi, and L. BenMohamed. 2006. Protective immunity to genital herpes simplex virus type 1 and type 2 provided by self-adjuvanting lipopeptides that drive dendritic cell maturation and elicit a polarized Th1 immune response. Viral Immunol. 19:220-236. [DOI] [PubMed] [Google Scholar]
- 13.Blaney, J. E., Jr., E. Nobusawa, M. A. Brehm, R. H. Bonneau, L. M. Mylin, T. M. Fu, Y. Kawaoka, and S. S. Tevethia. 1998. Immunization with a single major histocompatibility complex class I-restricted cytotoxic T-lymphocyte recognition epitope of herpes simplex virus type 2 confers protective immunity. J. Virol. 72:9567-9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bossus, M., L. Benmohamed, A. Londono, B. Barbier, A. Tartar, P. Druilhe, and H. Gras-Masse. 1997. Improved detection of human antibodies to a Plasmodium antigen using a peptide modified with Aib residues. J. Pept. Sci. 3:47-53. [DOI] [PubMed] [Google Scholar]
- 15.Bourne, N., F. J. Bravo, M. Francotte, D. I. Bernstein, M. G. Myers, M. Slaoui, and L. R. Stanberry. 2003. Herpes simplex virus (HSV) type 2 glycoprotein D subunit vaccines and protection against genital HSV-1 or HSV-2 disease in guinea pigs. J. Infect. Dis. 187:542-549. [DOI] [PubMed] [Google Scholar]
- 16.Brahimi, K., E. Badell, J.-P. Sauzet, L. BenMohamed, P. Daubersies, C. Guérin-Marchand, G. Snounou, and P. Druilhe. 2001. Human antibodies against Plasmodium falciparum liver-stage antigen 3 cross-react with Plasmodium yoelii preerythrocytic-stage epitopes and inhibit sporozoite invasion in vitro and in vivo. Infect. Immun. 69:3845-3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castellino, F., and R. N. Germain. 2007. Chemokine-guided CD4+ T cell help enhances generation of IL-6RαhighIL-7Rαhigh prememory CD8+ T cells. J. Immunol. 178:778-787. [DOI] [PubMed] [Google Scholar]
- 18.Chentoufi, A. A., X. Zhang, K. Lamberth, G. Dasgupta, I. Bettahi, A. Nguyen, M. Wu, X. Zhu, A. Mohebbi, S. Buus, S. L. Wechsler, A. B. Nesburn, and L. BenMohamed. 2008. HLA-A*0201-Restricted CD8+ cytotoxic T lymphocyte epitopes identified from herpes simplex virus glycoprotein D. J. Immunol. 180:426-437. [DOI] [PubMed] [Google Scholar]
- 19.Consogno, G., S. Manici, V. Facchinetti, A. Bachi, J. Hammer, B. M. Conti-Fine, C. Rugarli, C. Traversari, and M. P. Protti. 2003. Identification of immunodominant regions among promiscuous HLA-DR-restricted CD4+ T-cell epitopes on the tumor antigen MAGE-3. Blood 101:1038-1044. [DOI] [PubMed] [Google Scholar]
- 20.Cooper, D., J. C. Mester, M. Guo, F. Nasar, V. Souza, S. Dispoto, M. Sidhu, M. Hagen, J. H. Eldridge, R. J. Natuk, and M. W. Pride. 2006. Epitope mapping of full-length glycoprotein D from HSV-2 reveals a novel CD4+ CTL epitope located at the transmembrane-cytoplasmic junction. Cell. Immunol. 239:113-120. [DOI] [PubMed] [Google Scholar]
- 21.Corey, L., A. G. Langenberg, R. Ashley, R. E. Sekulovich, A. E. Izu, J. M. Douglas, Jr., H. H. Handsfield, T. Warren, L. Marr, S. Tyring, R. DiCarlo, A. A. Adimora, P. Leone, C. L. Dekker, R. L. Burke, W. P. Leong, and S. E. Straus. 1999. Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: two randomized controlled trials. JAMA 282:331-340. [DOI] [PubMed] [Google Scholar]
- 22.Cornberg, M., A. T. Chen, L. A. Wilkinson, M. A. Brehm, S. K. Kim, C. Calcagno, D. Ghersi, R. Puzone, F. Celada, R. M. Welsh, and L. K. Selin. 2006. Narrowed TCR repertoire and viral escape as a consequence of heterologous immunity. J. Clin. Investig. 116:1443-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cunningham, A. L., R. J. Diefenbach, M. Miranda-Saksena, L. Bosnjak, M. Kim, C. Jones, and M. W. Douglas. 2006. The cycle of human herpes simplex virus infection: virus transport and immune control. J. Infect. Dis. 194(Suppl. 1):S11-S18. [DOI] [PubMed] [Google Scholar]
- 24.Damhof, R. A., J. W. Drijfhout, A. J. Scheffer, J. B. Wilterdink, G. W. Welling, and S. Welling-Wester. 1993. T cell responses to synthetic peptides of herpes simplex virus type 1 glycoprotein D in naturally infected individuals. Arch. Virol. 130:187-193. [DOI] [PubMed] [Google Scholar]
- 25.Dana, M. R., Y. Qian, and P. Hamrah. 2000. Twenty-five-year panorama of corneal immunology: emerging concepts in the immunopathogenesis of microbial keratitis, peripheral ulcerative keratitis, and corneal transplant rejection. Cornea 19:625-643. [DOI] [PubMed] [Google Scholar]
- 26.Danve-Szatanek, C., M. Aymard, D. Thouvenot, F. Morfin, G. Agius, I. Bertin, S. Billaudel, B. Chanzy, M. Coste-Burel, L. Finkielsztejn, H. Fleury, T. Hadou, C. Henquell, H. Lafeuille, M. E. Lafon, A. Le Faou, M. C. Legrand, L. Maille, C. Mengelle, P. Morand, F. Morinet, E. Nicand, S. Omar, B. Picard, B. Pozzetto, J. Puel, D. Raoult, C. Scieux, M. Segondy, J. M. Seigneurin, R. Teyssou, and C. Zandotti. 2004. Surveillance network for herpes simplex virus resistance to antiviral drugs: 3-year follow-up. J. Clin. Microbiol. 42:242-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daubersies, P., A. W. Thomas, P. Millet, K. Brahimi, J. A. Langermans, B. Ollomo, L. BenMohamed, B. Slierendregt, W. Eling, A. Van Belkum, G. Dubreuil, J. F. Meis, C. Guerin-Marchand, S. Cayphas, J. Cohen, H. Gras-Masse, and P. Druilhe. 2000. Protection against Plasmodium falciparum malaria in chimpanzees by immunization with the conserved pre-erythrocytic liver-stage antigen 3. Nat. Med. 6:1258-1263. [DOI] [PubMed] [Google Scholar]
- 28.DeFreitas, E. C., B. Dietzschold, and H. Koprowski. 1985. Human T-lymphocyte response in vitro to synthetic peptides of herpes simplex virus glycoprotein D. Proc. Natl. Acad. Sci. USA 82:3425-3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fidock, D. A., H. Gras-Masse, J. P. Lepers, K. Brahimi, L. BenMohamed, S. Mellouk, C. Guerin-Marchand, A. Londono, L. Raharimalala, J. F. Meis, et al. 1994. Plasmodium falciparum liver stage antigen-1 is well conserved and contains potent B and T cell determinants. J. Immunol. 153:190-204. [PubMed] [Google Scholar]
- 30.Field, P. R., D. W. Ho, W. L. Irving, D. Isaacs, and A. L. Cunningham. 1993. The reliability of serological tests for the diagnosis of genital herpes: a critique. Pathology 25:175-179. [DOI] [PubMed] [Google Scholar]
- 31.Gahery, H., N. Daniel, B. Charmeteau, L. Ourth, A. Jackson, M. Andrieu, J. Choppin, D. Salmon, G. Pialoux, and J. G. Guillet. 2006. New CD4+ and CD8+ T cell responses induced in chronically HIV type-1-infected patients after immunizations with an HIV type 1 lipopeptide vaccine. AIDS Res. Hum. Retrovir. 22:684-694. [DOI] [PubMed] [Google Scholar]
- 32.Gahery, H., S. Figueiredo, C. Texier, S. Pouvelle-Moratille, L. Ourth, C. Igea, M. Surenaud, J. G. Guillet, and B. Maillere. 2007. HLA-DR-restricted peptides identified in the Nef protein can induce HIV type 1-specific IL-2/IFN-γ-secreting CD4+ and CD4+/CD8+ T cells in humans after lipopeptide vaccination. AIDS Res. Hum. Retrovir. 23:427-437. [DOI] [PubMed] [Google Scholar]
- 33.Ghiasi, H., R. Kaiwar, A. B. Nesburn, S. Slanina, and S. L. Wechsler. 1994. Expression of seven herpes simplex virus type 1 glycoproteins (gB, gC, gD, gE, gG, gH, and gI): comparative protection against lethal challenge in mice. J. Virol. 68:2118-2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grammer, S. F., A. Sette, S. Colon, L. Walker, and R. Chesnut. 1990. Identification of an HSV-1/HSV-2 cross-reactive T cell determinant. J. Immunol. 145:2249-2253. [PubMed] [Google Scholar]
- 35.Han, X., P. Lundberg, B. Tanamachi, H. Openshaw, J. Longmate, and E. Cantin. 2001. Gender influences herpes simplex virus type 1 infection in normal and gamma interferon-mutant mice. J. Virol. 75:3048-3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heber-Katz, E., S. Valentine, B. Dietzschold, and C. Burns-Purzycki. 1988. Overlapping T cell antigenic sites on a synthetic peptide fragment from herpes simplex virus glycoprotein D, the degenerate MHC restriction elicited, and functional evidence for antigen-Ia interaction. J. Exp. Med. 167:275-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klein, N. P., T. H. Holmes, M. A. Sharp, T. C. Heineman, M. R. Schleiss, D. I. Bernstein, G. Kemble, A. M. Arvin, and C. L. Dekker. 2006. Variability and gender differences in memory T cell immunity to varicella-zoster virus in healthy adults. Vaccine 24:5913-5918. [DOI] [PubMed] [Google Scholar]
- 38.Koelle, D. M. 2006. Vaccines for herpes simplex virus infections. Curr. Opin. Investig. Drugs 7:136-141. [PubMed] [Google Scholar]
- 39.Koelle, D. M., and L. Corey. 2008. Herpes simplex: insights on pathogenesis and possible vaccines. Annu. Rev. Med. 59:381-395. [DOI] [PubMed] [Google Scholar]
- 40.Koelle, D. M., L. Corey, R. L. Burke, R. J. Eisenberg, G. H. Cohen, R. Pichyangkura, and S. J. Triezenberg. 1994. Antigenic specificities of human CD4+ T-cell clones recovered from recurrent genital herpes simplex virus type 2 lesions. J. Virol. 68:2803-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koelle, D. M., J. M. Frank, M. L. Johnson, and W. W. Kwok. 1998. Recognition of herpes simplex virus type 2 tegument proteins by CD4 T cells infiltrating human genital herpes lesions. J. Virol. 72:7476-7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kovats, S., and E. Carreras. 13 February 2008. Regulation of dendritic cell differentiation and function by estrogen receptor ligands. Cell. Immunol. [Epub ahead of print.] doi: 10.1016/j.cellimm.2007.10.008. [DOI] [PMC free article] [PubMed]
- 43.Kruszon-Moran, D., and G. M. McQuillan. 2005. Seroprevalence of six infectious diseases among adults in the United States by race/ethnicity: data from the third national health and nutrition examination survey, 1988-94. Adv. Data 352:1-9. [PubMed] [Google Scholar]
- 44.Lapkus, O., T. M. Elsheikh, B. A. Ujevich, Y. L. Liu, and J. F. Silverman. 2006. Pitfalls in the diagnosis of herpes simplex infection in respiratory cytology. Acta Cytol. 50:617-620. [DOI] [PubMed] [Google Scholar]
- 45.Lee, H. S., Y. H. Chung, T. G. Kim, T. H. Kim, J. B. Jun, S. Jung, S. C. Bae, and D. H. Yoo. 2003. Independent association of HLA-DR and FCγ receptor polymorphisms in Korean patients with systemic lupus erythematosus. Rheumatology (Oxford) 42:1501-1507. [DOI] [PubMed] [Google Scholar]
- 46.Mbopi-Kéou, F. X., G. Gresenguet, P. Mayaud, H. A. Weiss, R. Gopal, M. Matta, J. L. Paul, D. W. Brown, R. J. Hayes, D. C. Mabey, and L. Belec. 2000. Interactions between herpes simplex virus type 2 and human immunodeficiency virus type 1 infection in African women: opportunities for intervention. J. Infect. Dis. 182:1090-1096. [DOI] [PubMed] [Google Scholar]
- 47.McQuillan, G. M., D. Kruszon-Moran, B. J. Kottiri, L. A. Kamimoto, L. Lam, M. F. Cowart, M. Hubbard, and T. J. Spira. 2006. Prevalence of HIV in the US household population: the National Health and Nutrition Examination Surveys, 1988 to 2002. J. Acquir. Immune Defic. Syndr. 41:651-656. [DOI] [PubMed] [Google Scholar]
- 48.MeŸó, G., Z. Majer, E. Vass, M. A. Jimenez, D. Andreu, and F. Hudecz. 2003. Conformational study of linear and cyclic peptides corresponding to the 276-284 epitope region of HSV gD-1. Biophys. Chem. 103:51-65. [DOI] [PubMed] [Google Scholar]
- 49.Mikloska, Z., and A. L. Cunningham. 1998. Herpes simplex virus type 1 glycoproteins gB, gC and gD are major targets for CD4 T-lymphocyte cytotoxicity in HLA-DR expressing human epidermal keratinocytes. J. Gen. Virol. 79(Pt. 2):353-361. [DOI] [PubMed] [Google Scholar]
- 50.Mikloska, Z., A. M. Kesson, M. E. Penfold, and A. L. Cunningham. 1996. Herpes simplex virus protein targets for CD4 and CD8 lymphocyte cytotoxicity in cultured epidermal keratinocytes treated with interferon-gamma. J. Infect. Dis. 173:7-17. [DOI] [PubMed] [Google Scholar]
- 51.Nalbandian, G., and S. Kovats. 2005. Understanding sex biases in immunity: effects of estrogen on the differentiation and function of antigen-presenting cells. Immunol. Res. 31:91-106. [DOI] [PubMed] [Google Scholar]
- 52.Nesburn, A. B., I. Bettahi, G. Dasgupta, A. A. Chentoufi, X. Zhang, S. You, N. Morishige, A. J. Wahlert, D. J. Brown, J. V. Jester, S. L. Wechsler, and L. BenMohamed. 2007. Functional Foxp3+ CD4+ CD25(Bright+) “natural” regulatory T cells are abundant in rabbit conjunctiva and suppress virus-specific CD4+ and CD8+ effector T cells during ocular herpes infection. J. Virol. 81:7647-7661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nesburn, A. B., I. Bettahi, X. Zhang, X. Zhu, W. Chamberlain, R. E. Afifi, S. L. Wechsler, and L. BenMohamed. 2006. Topical/mucosal delivery of sub-unit vaccines that stimulate the ocular mucosal immune system. Ocul. Surf. 4:178-187. [DOI] [PubMed] [Google Scholar]
- 54.Nesburn, A. B., R. L. Burke, H. Ghiasi, S. M. Slanina, and S. L. Wechsler. 1998. Therapeutic periocular vaccination with a subunit vaccine induces higher levels of herpes simplex virus-specific tear secretory immunoglobulin A than systemic vaccination and provides protection against recurrent spontaneous ocular shedding of virus in latently infected rabbits. Virology 252:200-209. [DOI] [PubMed] [Google Scholar]
- 55.Nesburn, A. B., T. V. Ramos, X. Zhu, H. Asgarzadeh, V. Nguyen, and L. BenMohamed. 2005. Local and systemic B cell and Th1 responses induced following ocular mucosal delivery of multiple epitopes of herpes simplex virus type 1 glycoprotein D together with cytosine-phosphate-guanine adjuvant. Vaccine 23:873-883. [DOI] [PubMed] [Google Scholar]
- 56.Nesburn, A. B., S. Slanina, R. L. Burke, H. Ghiasi, S. Bahri, and S. L. Wechsler. 1998. Local periocular vaccination protects against eye disease more effectively than systemic vaccination following primary ocular herpes simplex virus infection in rabbits. J. Virol. 72:7715-7721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paharkova-Vatchkova, V., R. Maldonado, and S. Kovats. 2004. Estrogen preferentially promotes the differentiation of CD11c+ CD11bintermediate dendritic cells from bone marrow precursors. J. Immunol. 172:1426-1436. [DOI] [PubMed] [Google Scholar]
- 58.Panigada, M., T. Sturniolo, G. Besozzi, M. G. Boccieri, F. Sinigaglia, G. G. Grassi, and F. Grassi. 2002. Identification of a promiscuous T-cell epitope in Mycobacterium tuberculosis Mce proteins. Infect. Immun. 70:79-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Perng, G. C., B. Maguen, L. Jin, K. R. Mott, J. Kurylo, L. BenMohamed, A. Yukht, N. Osorio, A. B. Nesburn, G. Henderson, M. Inman, C. Jones, and S. L. Wechsler. 2002. A novel herpes simplex virus type 1 transcript (AL-RNA) antisense to the 5′ end of the latency-associated transcript produces a protein in infected rabbits. J. Virol. 76:8003-8010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Posavad, C. M., D. M. Koelle, and L. Corey. 1998. Tipping the scales of herpes simplex virus reactivation: the important responses are local. Nat. Med. 4:381-382. [DOI] [PubMed] [Google Scholar]
- 61.Rebbapragada, A., C. Wachihi, C. Pettengell, S. Sunderji, S. Huibner, W. Jaoko, B. Ball, K. Fowke, T. Mazzulli, F. A. Plummer, and R. Kaul. 2007. Negative mucosal synergy between herpes simplex type 2 and HIV in the female genital tract. AIDS 21:589-598. [DOI] [PubMed] [Google Scholar]
- 62.Renaudet, O., L. BenMohamed, G. Dasgupta, I. Bettahi, and P. Dumy. 2008. Towards a self-adjuvanting multivalent B and T cell epitope containing synthetic glycolipopeptide cancer vaccine. ChemMedChem 3:737-741. [DOI] [PubMed] [Google Scholar]
- 63.Schmid, D. S., D. R. Brown, R. Nisenbaum, R. L. Burke, D. Alexander, R. Ashley, P. E. Pellett, and W. C. Reeves. 1999. Limits in reliability of glycoprotein G-based type-specific serologic assays for herpes simplex virus types 1 and 2. J. Clin. Microbiol. 37:376-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Selin, L. K., M. A. Brehm, Y. N. Naumov, M. Cornberg, S. K. Kim, S. C. Clute, and R. M. Welsh. 2006. Memory of mice and men: CD8+ T-cell cross-reactivity and heterologous immunity. Immunol. Rev. 211:164-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sette, A., and J. Sidney. 1998. HLA supertypes and supermotifs: a functional perspective on HLA polymorphism. Curr. Opin. Immunol. 10:478-482. [DOI] [PubMed] [Google Scholar]
- 66.Smith, T. J., L. A. Morrison, and D. A. Leib. 2002. Pathogenesis of herpes simplex virus type 2 virion host shutoff (vhs) mutants. J. Virol. 76:2054-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Southwood, S., J. Sidney, A. Kondo, M. F. del Guercio, E. Appella, S. Hoffman, R. T. Kubo, R. W. Chesnut, H. M. Grey, and A. Sette. 1998. Several common HLA-DR types share largely overlapping peptide binding repertoires. J. Immunol. 160:3363-3373. [PubMed] [Google Scholar]
- 68.Stanberry, L. R., S. L. Spruance, A. L. Cunningham, D. I. Bernstein, A. Mindel, S. Sacks, S. Tyring, F. Y. Aoki, M. Slaoui, M. Denis, P. Vandepapeliere, and G. Dubin. 2002. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N. Engl. J. Med. 347:1652-1661. [DOI] [PubMed] [Google Scholar]
- 69.Straus, S. E., L. Corey, R. L. Burke, B. Savarese, G. Barnum, P. R. Krause, R. G. Kost, J. L. Meier, R. Sekulovich, S. F. Adair, et al. 1994. Placebo-controlled trial of vaccination with recombinant glycoprotein D of herpes simplex virus type 2 for immunotherapy of genital herpes. Lancet 343:1460-1463. [DOI] [PubMed] [Google Scholar]
- 70.Texier, C., S. Pouvelle, M. Busson, M. Herve, D. Charron, A. Menez, and B. Maillere. 2000. HLA-DR restricted peptide candidates for bee venom immunotherapy. J. Immunol. 164:3177-3184. [DOI] [PubMed] [Google Scholar]
- 71.Texier, C., S. Pouvelle-Moratille, M. Busson, D. Charron, A. Menez, and B. Maillere. 2001. Complementarity and redundancy of the binding specificity of HLA-DRB1, -DRB3, -DRB4 and -DRB5 molecules. Eur. J. Immunol. 31:1837-1846. [DOI] [PubMed] [Google Scholar]
- 72.van Lint, A., M. Ayers, A. G. Brooks, R. M. Coles, W. R. Heath, and F. R. Carbone. 2004. Herpes simplex virus-specific CD8+ T cells can clear established lytic infections from skin and nerves and can partially limit the early spread of virus after cutaneous inoculation. J. Immunol. 172:392-397. [DOI] [PubMed] [Google Scholar]
- 73.van Lint, A. L., L. Kleinert, S. R. Clarke, A. Stock, W. R. Heath, and F. R. Carbone. 2005. Latent infection with herpes simplex virus is associated with ongoing CD8+ T-cell stimulation by parenchymal cells within sensory ganglia. J. Virol. 79:14843-14851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Villacres, M. C., J. Longmate, C. Auge, and D. J. Diamond. 2004. Predominant type 1 CMV-specific memory T-helper response in humans: evidence for gender differences in cytokine secretion. Hum. Immunol. 65:476-485. [DOI] [PubMed] [Google Scholar]
- 75.Weiss, H. 2004. Epidemiology of herpes simplex virus type 2 infection in the developing world. Herpes 11(Suppl. 1):24A-35A. [PubMed] [Google Scholar]
- 76.Welsh, R. M. 2006. Private specificities of heterologous immunity. Curr. Opin. Immunol. 18:331-337. [DOI] [PubMed] [Google Scholar]
- 77.Welsh, R. M., S. K. Kim, M. Cornberg, S. C. Clute, L. K. Selin, and Y. N. Naumov. 2006. The privacy of T cell memory to viruses. Curr. Top. Microbiol. Immunol. 311:117-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wilcoxen, S. C., E. Kirkman, K. C. Dowdell, and S. A. Stohlman. 2000. Gender-dependent IL-12 secretion by APC is regulated by IL-10. J. Immunol. 164:6237-6243. [DOI] [PubMed] [Google Scholar]
- 79.Wilson, C. C., B. Palmer, S. Southwood, J. Sidney, Y. Higashimoto, E. Appella, R. Chesnut, A. Sette, and B. D. Livingston. 2001. Identification and antigenicity of broadly cross-reactive and conserved human immunodeficiency virus type 1-derived helper T-lymphocyte epitopes. J. Virol. 75:4195-4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yamashita, K., and E. Heber-Katz. 1989. Lack of immunodominance in the T cell response to herpes simplex virus glycoprotein D after administration of infectious virus. J. Exp. Med. 170:997-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yasukawa, M., and Y. Kobayashi. 1985. Inhibition of herpes simplex virus replication in vitro by human cytotoxic T cell clones and natural killer cell clones. J. Gen. Virol. 66(Pt. 10):2225-2229. [DOI] [PubMed] [Google Scholar]
- 82.Zarling, J. M., P. A. Moran, L. Brewer, R. Ashley, and L. Corey. 1988. Herpes simplex virus (HSV)-specific proliferative and cytotoxic T-cell responses in humans immunized with an HSV type 2 glycoprotein subunit vaccine. J. Virol. 62:4481-4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zarling, J. M., P. A. Moran, R. L. Burke, C. Pachl, P. W. Berman, and L. A. Lasky. 1986. Human cytotoxic T cell clones directed against herpes simplex virus-infected cells. IV. Recognition and activation by cloned glycoproteins gB and gD. J. Immunol. 136:4669-4673. [PubMed] [Google Scholar]
- 84.Zarling, J. M., P. A. Moran, L. A. Lasky, and B. Moss. 1986. Herpes simplex virus (HSV)-specific human T-cell clones recognize HSV glycoprotein D expressed by a recombinant vaccinia virus. J. Virol. 59:506-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang, X., A. Issagholian, E. A. Berg, J. B. Fishman, A. B. Nesburn, and L. BenMohamed. 2005. Th-cytotoxic T-lymphocyte chimeric epitopes extended by Nɛ-palmitoyl lysines induce herpes simplex virus type 1-specific effector CD8+ Tc1 responses and protect against ocular infection. J. Virol. 79:15289-15301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhu, X., T. V. Ramos, H. Gras-Masse, B. E. Kaplan, and L. BenMohamed. 2004. Lipopeptide epitopes extended by Nɛ-palmitoyl lysine moiety increases uptake and maturation of dendritic cell through a Toll-like receptor 2 pathway and triggers a Th1-dependent protective immunity. Eur. J. Immunol. 34:3102-3114. [DOI] [PubMed] [Google Scholar]