Abstract
Francisella tularensis causes severe pneumonia that can be fatal if it is left untreated. Due to its potential use as a biological weapon, research is being conducted to develop an effective vaccine and to select and study adjuvant molecules able to generate a better and long-lasting protective effect. PorB, a porin from Neisseria meningitidis, is a well-established Toll-like receptor 2 ligand and has been shown to be a promising vaccine adjuvant candidate due to its ability to enhance the T-cell costimulatory activity of antigen-presenting cells both in vitro and in vivo. BALB/c mice were immunized with lipopolysaccharide (LPS) isolated from the F. tularensis subsp. holarctica live vaccine strain (LVS), with or without PorB from N. meningitidis, and the antibody levels induced during the vaccination regimen and the level of protection against intranasal challenge with LVS were determined. Antigen administered alone induced a specific F. tularensis LPS immunoglobulin M (IgM) response that was not maintained over the weeks and that conferred protection to only 25% of the mice. In contrast, F. tularensis LPS given in combination with neisserial PorB induced consistent levels of specific IgM throughout the immunization and increased the proportion of surviving mice to 70%. Postchallenge cytokine analysis showed that interleukin-6 (IL-6), monocyte chemoattractant protein 1, and gamma interferon were markers of mortality and that IL-1β was a correlate of survival, independent of the presence of PorB as an adjuvant. These data indicate that neisserial PorB might be an optimal candidate adjuvant for improving the protective effect of F. tularensis LPS and other subunit vaccines against tularemia, but there is still a need to test its efficacy against virulent type A and type B F. tularensis strains.
Francisella tularensis is a gram-negative facultative intracellular bacterium and the cause of tularemia in humans and other animals (41). The two subspecies of this pathogen, F. tularensis subsp. tularensis (type A) and F. tularensis subsp. holarctica (type B), are both very infectious when they are inhaled, although the former causes the most severe form of the disease. A live vaccine strain (LVS) derived from a virulent type B strain is available and has been shown to be relatively effective as a vaccine against severe human tularemia caused by type A strains; it also provides a good experimental model of tularemia due to its high level of virulence in mice (13, 23, 39). Nonetheless, there are concerns with the use of F. tularensis LVS as a human vaccine because the basis for its attenuation remain unknown. Hence, there is the need to develop subunit vaccines to replace it.
The lipopolysaccharide (LPS) of F. tularensis is considered an atypical endotoxin because it does not induce classic cellular and cytokine responses like those induced by LPSs from other gram-negative bacteria (1, 38). Mice immunized with purified F. tularensis LPS by the intradermal (i.d.), intraperitoneal (i.p.), or subcutaneous (s.c.) route were shown to be fully or partially protected against i.p. and i.d. challenge with LVS and other type B strains (9, 11, 17). Following i.d. or s.c. immunization with purified F. tularensis LPS or immunization with the F. tularensis O antigen conjugated to bovine serum albumin by the s.c. route, only partial protection against type B strains was observed, while no protection against type A strains was granted when the immunization was given by the i.d. or aerosol route (8, 9). Efficacious adjuvants are an important requirement in these vaccines, as carbohydrate- or subunit-based vaccines like LPS or its components alone do not always induce adequate protective immunity against tularemia. Previous reports have shown how subcellular F. tularensis antigen preparations elicited only modest protective responses even when they were combined with potent adjuvants such as immunostimulating complexes (ISCOMs) (19, 42). More recently, Eyles et al. (14) have reported how irradiated LVS combined with ISCOMs and CpG oligonucleotides confers full protection against challenge with a type B strain but not type A F. tularensis, while another study has demonstrated how interleukin-12 (IL-12) enhances the protective activity of inactivated LVS against respiratory challenge with LVS (2). Other than those studies, little effort has been made to assess other effective adjuvants to be included in subcellular or inactivated vaccine preparations.
Toll-like receptors (TLRs) are essential components of innate immunity, and TLR2 is required for the inflammatory responses to F. tularensis both in vitro and in vivo (7, 24, 28). TLR ligands are a group of molecules known to be potential vaccine adjuvants (21, 37). Signaling through TLRs induces host cell proliferation and cytokine secretion, as well as the upregulation of T-cell costimulatory molecules on antigen-presenting cells, and their capacity to stimulate both the innate and the adaptive immune systems makes TLR ligands optimal adjuvant candidates (27, 37). Examples of TLR ligands include monophosphoryl lipid A (TLR4) and CpG oligonucleotides (TLR9), and these molecules are currently being tested as adjuvants in human vaccine trials (29, 44). PorB, one of the porins of the bacterium Neisseria meningitidis, is a potent immunostimulatory TLR2/TLR1 ligand (30, 32) and a component of the outer membrane protein (OMP) preparation originally used as a carrier protein for the Haemophilus influenzae type B vaccine. Upon further characterization, the OMP was shown to have adjuvant activity dependent on stimulation through TLR2 in vitro (25). PorB has been shown to induce the T-cell costimulatory ability of murine B cells and dendritic cells, to stimulate B-cell proliferation in vitro, and to enhance the humoral response to the meningococcal capsule in vivo (26, 30, 40). More recently, this protein was shown to produce an antigen-specific eosinophilic recall response similar to that observed in a secondary helminth infection, indicating further potential for its future use as an adjuvant (4). The features of PorB described above indicated that this protein could successfully be used to enhance the protective capacity of F. tularensis LPS against tularemia.
In the present study, we show how the presence of N. meningitidis PorB is necessary to induce a high percentage of survival in mice vaccinated with F. tularensis LPS by the s.c. route and subsequently challenged with F. tularensis LVS by the respiratory route. Our results suggest an important role for PorB as an adjuvant and how it could potentially be used, when it is conjugated to F. tularensis LPS or other safe subunit vaccine candidates, in a vaccine against tularemia caused by both type A and type B strains of Francisella tularensis.
MATERIALS AND METHODS
Bacteria and growth conditions.
F. tularensis subsp. holarctica LVS (ATCC 29684) was obtained from the CDC, Fort Collins, CO. For LPS extraction, broth cultures of LVS were grown for 3 days in T-soy broth supplemented with 0.1% l-cysteine. For the challenge experiments, LVS was grown as described previously (5). Briefly, after culture on chocolate agar, colonies were scraped off and suspended in sterile phosphate-buffered saline (PBS) to an optical density at 600 nm of 0.3. The numbers of CFU were determined by plating out serial dilutions on chocolate agar. Bacterial suspensions were prepared, and the CFU counts were confirmed for each infection experiment.
Isolation and analysis of LPS from F. tularensis LVS.
A method previously described for Bacteroides fragilis was used as a reference for the extraction of LPS (36). F. tularensis LVS cells grown in T-soy broth with 1% l-cysteine were pelleted by centrifugation, washed once with 0.15 M NaCl, and finally, suspended in a small volume of water. This suspension was extracted with an equal volume of hot phenol at 68°C for 30 min, followed by stirring at 4°C overnight. The aqueous layer was collected after centrifugation and was extracted twice with ethyl ether to remove the residual phenol. The sample was dialyzed extensively with water for 4 days in dialysis tubing with a molecular weight cutoff of 3,500. Contaminating macromolecules were removed by digestion with DNase, RNase, and pronase. The samples were concentrated with an Amicon 8400 stirred cell (Millipore, Billerica, MA). The LPS was precipitated from this suspension by adding ethanol to 80% and was held at −20°C overnight. The precipitate was collected by centrifugation and was allowed to air dry. It was then resuspended in a minimal volume of 50 mM EDTA, 95% ethanol was added to 80%, and the mixture was held at −20°C overnight. The material was pelleted by centrifugation, allowed to air dry after the ethanol was poured off, and resuspended in deoxycholate buffer (0.5 sodium deoxycholic acid, 50 mM glycine, 10 mM EDTA, pH 9.8). A Sephacryl S-300 high-resolution column (GE Healthcare Bio-Sciences, Piscataway, NJ) was equilibrated with the deoxycholate buffer, and 5-ml fractions were collected. These were tested for reactivity with an anti-Francisella tularensis LPS monoclonal antibody specific for the O antigen, ab2033 (Abcam, Inc., Cambridge, MA). The antibody-reactive fractions were combined (molecular weight, ∼30,000 to 100,000), precipitated in 95% ethanol, resuspended in water, and lyophilized. The sample was analyzed for its endotoxin level by a chromogenic Limulus amebocyte lysate assay (Cape Cod Associates, East Falmouth, MA), for its nucleic acid level by 260-nm UV spectrophotometric readings, and for its protein level by a colloidal gold assay (Bio-Rad, Inc., Hercules, CA).
SDS-PAGE and immunoblotting.
Ready Gels (4 to 20%) and a Protean minigel apparatus were used for sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) in Tris-glycine buffer (Bio-Rad, Inc.). The LPS sample was electrophoretically transferred from the Ready Gel to a polyvinylidene difluoride membrane. Western blotting was performed with a goat anti-mouse immunoglobulin G (IgG) immunoblot assay kit (Bio-Rad, Inc.). The primary antibody was a mouse monoclonal antibody (monoclonal antibody FB11) specific for Francisella tularensis LPS diluted 1:2,000.
Isolation of N. meningitidis PorB.
Native PorB was isolated from N. meningitidis strain H44/76, which lacks both PorA and RmpM, as described previously (31). The use of PorB from this mutant strain allowed the purification of PorB without contamination from other OMPs. Briefly, the porin was purified by detergent extraction and column chromatography, and negligible contamination by proteins and lipooligosaccharides was shown by PAGE and silver staining (31).
Mice, s.c. immunization, and intranasal challenge.
Seven-week-old female BALB/c mice were obtained from Jackson Laboratories (Bar Harbor, ME) and were given pelleted food and water ad libitum. All experimental procedures were in compliance with the Institutional Animal Care and Use Committee of the Boston University School of Medicine.
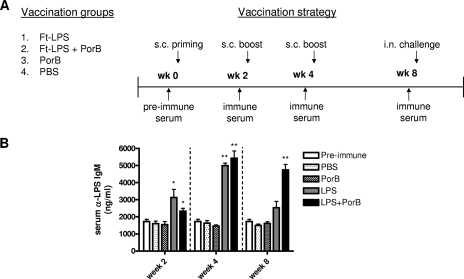
Antigens were administered and serum samples were collected as described in the schematic representation (see Fig. 2). Four groups of 6 to 10 mice each were immunized by the s.c. route with either F. tularensis LPS alone, F. tularensis LPS in combination with neisserial PorB (1:1 ratio), PorB alone, or PBS. The mice were inoculated three times, at 2-week intervals, with 10 μg of each antigen in a total volume of 100 μl/animal. Four weeks after the last immunization, all vaccinated and unvaccinated control mice were anesthetized with ketamine HCl (Fort Dodge Animal Health, Fort Dodge, IA) and xylazine (Lloyd Laboratories, Shenandoah, IA) and infected intranasally with 106 CFU of live LVS in 20 μl of PBS, as described previously (5). All animals were closely observed until they were completely awake from the anesthesia, and survival was recorded for up to 50 days after infection. Changes in body weight were also determined over a time period of 18 days. The data reported in this study are representative of those from two independent experiments.
FIG. 2.
Vaccination and challenge strategy and detection of anti-F. tularensis LPS (Ft-LPS) IgM in mouse serum. (A) Four groups of mice were immunized s.c. three times at 2-week intervals with 10 μg F. tularensis LPS (n = 8), 10 μg F. tularensis LPS plus 10 μg PorB (n = 10), 10 μg PorB (n = 6), or PBS (n = 10). Blood was drawn at 2-week intervals before each inoculation and before challenge, and serum was collected. The mice were challenged 4 weeks after the third vaccination dose with 106 CFU of LVS by the intranasal (i.n.) route. Blood was drawn from the submandibular veins of the mice at different time intervals, and serum was taken and tested for IgM by ELISA. (B) Antibody levels detected at 2-week intervals during the course of vaccination. Data are reported as the amount (ng/ml) of anti-F. tularensis LPS IgM detected in serum. Statistical significance was calculated by the Mann-Whitney test, and the values were found to be either significant (*, P < 0.05) or highly significant (**, P < 0.01).
ELISA and Luminex assays with mouse serum.
Blood was taken from the submandibular vein of mice, stored at room temperature for 10 min, and centrifuged at 1,500 × g for 20 min. The serum phase was collected and stored at −80°C.
To analyze the LPS-specific IgG and IgM antibody levels in mouse sera during the course of vaccination, Immulon IV microtiter plate wells were coated with 100 μl of 0.25 μg/ml F. tularensis LVS LPS in PBS and the plates were incubated at 37°C for 3 h and stored overnight at 4°C. Sera were diluted in 0.05% PBS-Tween 20, added to the previously coated wells, and incubated for 1 h at 37°C. After the plates were washed and dried, alkaline phosphatase-conjugated anti-mouse IgG or IgM (100 μl; Sigma, St. Louis, MO) diluted in PBS-Tween 20 was added, and the plates were incubated for 1 h at 37°C. After the plates were washed and dried once more, the color was developed with 100 μl one-step p-nitrophenyl phosphate (Pierce, Rockford, IL) and the optical density at 405 nm was measured on an ELx800 enzyme-linked immunosorbent assay (ELISA) reader (Bio-Tek Instruments, Inc., Winooski, VT). Colorimetric values were converted to nanograms/milliliter, on the basis of the standard curves for IgG or IgM generated by using known concentrations of IgG and IgM on plates coated with goat anti-mouse IgG and IgM F(ab′)2-specific antibodies (Jackson ImmunoResearch Laboratories Inc., West Grove, PA), as described previously (5, 26). To determine the levels of the cytokines IL-6, IL-1β, gamma interferon (IFN-γ), and monocyte chemoattractant protein 1 (MCP-1) in the sera of vaccinated and challenged mice, a mouse custom multiplex antibody bead kit (BioSource International, Inc., Camarillo, CA) was used and the plates were read by use of a Luminex X-Map 100 platform. The bioassay was performed as indicated by the manufacturer's instructions, and the results were analyzed with SoftMax Pro software (Molecular Devices, Sunnyvale, CA).
Statistical analysis.
Differences between groups were determined by the Mann-Whitney U nonparametric test by using GraphPad Prism (version 4.02) software (San Diego, CA). P values of ≤0.05 were considered significant, and P values of ≤0.01 were considered highly significant.
RESULTS
Characterization of F. tularensis LPS.
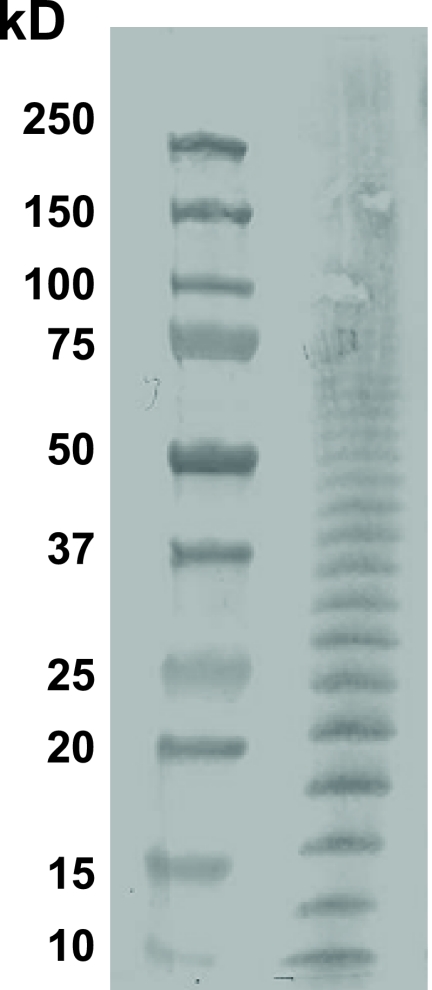
The F. tularensis LPS ladder at 1 mg/ml was fractioned by PAGE with a 4 to 20% gradient (Pierce Scientific, Rockford, IL), and the Western blot was probed with monoclonal antibody ab2033 (Abcam, Inc.) specific for the F. tularensis O antigen (Fig. 1). At 20 mg per ml, the F. tularensis LPS was shown to have minimal endotoxin activity (<0.25 endotoxin units/ml), a protein concentration of less than 4 ng per ml, and less than <1 μg/ml of nucleic acids. The mice tolerated the immunizations with this material well.
FIG. 1.
Ladder of LPS isolated from F. tularensis LVS. F. tularensis LPS is reported as 1 mg/ml fractioned by PAGE with a 4 to 20% gradient. The material is shown as a Western blot probed with a monoclonal antibody specific for the F. tularensis O antigen.
Antibody response after vaccination.
To test if the antibody activity induced by F. tularensis LPS alone or in combination with neisserial PorB would augment protection against intranasal challenge, we designed a mouse vaccination study, as illustrated in Fig. 2A.
Preimmune sera were collected from mice before administration of the first inoculum, and immune sera were taken before administration of the two booster doses at weeks 2 and 4 and before challenge at week 8 (Fig. 2A). ELISAs for F. tularensis LPS-specific IgG and IgM were then performed. Vaccination of the mice did not induce anti-F. tularensis LPS IgG, but the animals were found to produce F. tularensis LPS-specific IgM. After the first vaccination dose at week 2, mice inoculated with F. tularensis LPS and F. tularensis LPS plus PorB developed levels of anti-F. tularensis LPS IgM slightly greater that those at the baseline, with a significant difference for both groups (P < 0.05) compared to the levels in their preimmune sera (Fig. 2B). After the first booster dose at week 4, both groups of mice immunized with either LPS or LPS plus PorB showed increased levels of anti-F. tularensis LPS IgM in their sera compared to the levels in their preimmune sera, and the differences were shown to be highly significant (P < 0.01) (Fig. 2B). Four weeks after administration of the second booster and right before the intranasal challenge, the levels of IgM in the sera of mice vaccinated with LPS returned to levels similar to those at the baseline, while in the sera of animals immunized with LPS plus PorB, the levels remained significantly higher than those detected in preimmune sera (P < 0.01) (Fig. 2B). The serum anti-F. tularensis LPS IgM levels induced by PBS and PorB were comparable to those observed in the preimmune sera (Fig. 2B).
Purified F. tularensis LPS given s.c. in combination with neisserial PorB induces a high level of protection against respiratory tularemia.
Some groups have reported that LPS, its components, or other subunit vaccines are not particularly successful at protecting against respiratory tularemia caused by type A or B strains of F. tularensis (8, 9). Another group used IL-12 in combination with inactivated LVS as a mucosal vaccine in order to achieve successful protection from respiratory challenge with LVS (2). We sought to determine if the addition of neisserial PorB to F. tularensis LPS would improve protection against 106 CFU of LVS given by the intranasal route. All vaccinated animals received the infection dose 4 weeks after the second booster dose, and survival was recorded for 18 days.
All animals injected with PBS succumbed to infection with LVS, and the median time to death was 6.5 days (Fig. 3A). Clinical signs (hunched back, rough fur, decreased response to external stimuli) began as early as day 3 or 4 after infection. To examine if the adjuvant alone would grant a protective effect, mice were also immunized with 10 μg of PorB, and we observed that the disease sign and mortality trends were extremely similar to those seen by the use of PBS as a negative control; the median time to death was 7 days (Fig. 3A). Concomitant with the development of disease signs, mice also showed a progressive loss of body weight of up to 30% or more when they reached the moribund stage (Fig. 3B). Two of eight mice immunized with F. tularensis LPS alone survived intranasal infection with 106 CFU of LVS, and despite the low percentage (25%), the statistical difference in the rate of survival compared to that for the unvaccinated control mice was found to be significant (P < 0.05) (Fig. 3A). Only 3 of 10 mice vaccinated with F. tularensis LPS plus PorB died following challenge, and compared to the rate of survival of the mock-vaccinated mice, the 70% survival rate was found to be highly significant (P < 0.01) (Fig. 3A). All vaccinated mice surviving respiratory challenge developed moderate clinical signs and dramatic body weight loss, which were overcome upon recovery (Fig. 3B). In a preliminary experiment, emulsification with incomplete Freund's adjuvant did not enhance the protective efficacy of F. tularensis LPS, and only 20% of the animals survived the intranasal challenge, similar to the results for mice that received F. tularensis LPS alone (data not shown). No viable organisms were found in the blood of the survivors at 10 and 50 days postinfection, and the histopathology of the lungs of mice killed at day 50 revealed no sign of pneumonic disease, but peribronchial lymphoid areas were detected (data not shown).
FIG. 3.
Survival and body weight loss observed following lethal challenge with LVS. All groups of mice were challenged intranasally with 106 CFU 4 weeks after the last booster. (A) Mock (PBS)-vaccinated mice succumbed to tularemia by 7 days postinfection. Similarly, all animals from the group immunized with PorB died by day 7. Only 25% of the mice from the F. tularensis LPS group were able to recover and survive tularemia, while F. tularensis LPS plus PorB induced protection in 70% of the animals. All mice indiscriminately developed symptoms by day 3 or 4 following intranasal infection. (B) Following intranasal infection, all mice developed visible clinical signs and experienced remarkable weight loss. Animals from groups previously vaccinated with F. tularensis LPS and F. tularensis LPS plus PorB fully regained their body weight by day 18 postchallenge, concomitant with their overcoming of disease signs. The results are expressed as percent survival or grams of body weight at different time intervals. The differences in survival were analyzed by the Mann-Whitney test, and values were found to be either significant (*, P < 0.05) or highly significant (**, P < 0.01).
Levels of proinflammatory cytokines following vaccination and intranasal challenge.
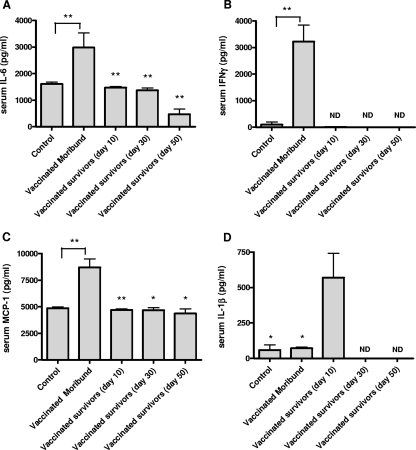
Figure 4 shows the levels of cytokines detected in the sera of mice vaccinated with either LPS or F. tularensis LPS plus PorB and challenged four weeks later with LVS by the intranasal route. We analyzed sera from vaccinated and challenged mice that fully recovered from disease and that were killed at 10, 30, and 50 days postchallenge. We compared their cytokine levels to those of terminally ill (moribund) mice and uninfected controls. Blood and sera were obtained from moribund mice between days 7 and 10 postchallenge. The cytokine levels did not vary between these time periods, and therefore, for simplicity, the values were pooled. The cytokine levels detected in mice immunized with the two vaccine formulations were also grouped together, as no differences were observed. The levels of IL-6 were found to be raised above the baseline levels in vaccinated mice that succumbed to tularemia (moribund), while the survivors had values similar to those observed in the uninfected controls. The differences in IL-6 levels between recovered mice bled at days 10, 30, and 50 days postchallenge and moribund animals bled between days 7 and 10 postchallenge were found to be significant (P < 0.01) (Fig. 4A). A significant difference (P < 0.01) between moribund mice and the uninfected control group was also calculated (Fig. 4A). A similar trend was observed for MCP-1 and IFN-γ levels, which were elevated above the baseline levels in the moribund mice but not in the survivors (Fig. 4B and C). The levels of IFN-γ detected in moribund mice were significantly different from those found in the control group (P < 0.01) (Fig. 4B). The differences in MCP-1 levels between vaccinated mice that became moribund and those that survived challenge were found to be highly significant at day 10 (P < 0.01) and significant at days 30 and 50 (P < 0.05) (Fig. 4C). The levels of MCP-1 were also significantly higher in moribund mice than in the controls (P < 0.01) (Fig. 4C). In contrast, the levels of IL-1β detected in the sera of moribund mice were similar to those found in the sera of the uninfected controls, were increased in the vaccinated survivors at day 10 (P < 0.05), and were undetectable at days 30 and 50 postchallenge (Fig. 4D). A significant difference in the levels of this serum cytokine was also observed between vaccinated survivors at day 10 and the uninfected control group (P < 0.05) (Fig. 4D).
FIG. 4.
Postchallenge cytokine profile detected in the sera of mice at different stages of disease. Following s.c. vaccination and subsequent intranasal infection with LVS, mice (n = 4 to 6) were bled at a terminal disease stage or at different time points after they recovered from symptoms. Mouse preimmune serum was used as a negative control. Data are presented as the quantity (pg) of cytokine per milliliter at different stages of disease. The differences between the moribund mice and the survivors were either significant (*, P < 0.05) or highly significant (**, P < 0.01). ND, not detectable.
DISCUSSION
Tularemia is a severe debilitating disease that occurs naturally in some countries of the Northern Hemisphere. In recent years, there has been an interest in developing vaccines against this bacterial agent due to its potential use as a biological weapon (20). Both live attenuated (33) and killed whole-cell (16) F. tularensis vaccines have been developed and evaluated in studies with human subjects, but there are concerns about their safety and efficacy. The detection of subunit vaccine candidates able to induce an effective and long-lasting protective response has been an important aim in the Francisella research field; however, LPS appears to be the only one able to generate some degree of protection, as reported in studies conducted with mouse models (9, 11, 17). Other proteins, including a 43-kDa OMP and heat shock protein 60, were tested in studies with mice, but the immunity induced by these vaccine candidates did not provide any protection against experimental tularemia (17, 22).
The use of adjuvants to enhance the immunity provided by a subunit vaccine against tularemia, particularly the pneumonic form, is crucial. An adjuvant is a substance that augments the immunogenic activity of an antigen by promoting immunomodulation, stimulating lymphocyte proliferation, generating a depot, and enhancing costimulatory signals (10). Alum is the only FDA-approved adjuvant for human use; thus, there is a need for innovative molecules able to efficaciously stimulate both the innate and the adaptive immune systems (35). TLR ligands have recently become important targets in vaccine adjuvant research due to their ability to successfully combine all the outcomes described above (37). The variety of TLR2 ligands is likely the greatest among TLRs due to the heterodimerization needed for the responses mediated by them (45). The host responses induced by these ligands include IL-10 secretion from dendritic cells (phosphotidylserine from Schistosoma mansoni) (43); dendritic cell maturation; and the production of the proinflammatory cytokines and chemokines tumor necrosis factor alpha, IL-2, IL-6, and macrophage inflammatory protein 2 (peptidoglycan or lipoteichoid acid) (34). More recently, PorB, an OMP of the bacterium N. meningitidis, has been characterized as a potent immunostimulant and a TLR2 ligand. Our laboratory has shown that PorB induces dendritic cell activation and B-cell proliferation, dependent on TLR2 and MyD88 (30, 40), and therefore increases the ability to present antigen and activate specific T cells. It was also reported that PorB augments the humoral response to the meningococcal capsule in the mouse (26). Our previous study, however, did not investigate whether the capsule-PorB conjugate improved survival following in vivo challenge with live meningococci due to the lack of a suitable animal model mimicking N. meningitidis disease. These promising data obtained by the use of neisserial PorB have encouraged us to design experiments to test its adjuvanticity potential in the mouse model and to evaluate if its coadministration with LPS isolated from F. tularensis LVS would enhance the protective ability of this poorly immunogenic antigen.
In this study, groups of mice were vaccinated by the s.c. route with purified F. tularensis LPS alone or combined with neisserial PorB. We also included two control groups, one that received three doses of PBS and one that was given PorB alone, to confirm that this adjuvant would not grant a protective effect on its own. Upon analysis of the anti-LPS antibody response, specific IgM was detected in the sera of mice immunized with PorB as an adjuvant after every immunization dose. The IgM levels detected in this group of animals remained elevated above the baseline levels at week 8 just before intranasal challenge, while mice immunized with F. tularensis LPS alone showed increased levels of this immunoglobulin at week 4, but these levels returned to the baseline levels at week 8. F. tularensis LPS-specific IgG was not detected at any time after vaccination. A similar trend was reported in the work of Dreisbach et al., who described that following the i.p. immunization of mice, LVS LPS primarily induces an IgM response but minimal specific IgG (11). Similarly, Cole et al. showed that only anti-LPS IgM and not IgG was detected in the sera of mice treated with LVS LPS by the i.p. route and how subsequent challenge of the mice with live organisms boosted the anti-LPS IgM response (6).
Despite its low endotoxic activity (1, 38), it has been shown that F. tularensis LPS is able to confer some protection from i.p. challenge with LVS (11, 17). Unfortunately, the i.p. route is an artificial technique for inducing experimental tularemia in animals, so more efforts are needed to develop a vaccine that will be highly efficacious against the more severe pneumonic form of the disease. Mice treated once i.d. with whole LVS LPS did not develop any clinical signs following aerosol challenge with LVS, but they succumbed to infection with virulent type A or B F. tularensis strains (9). It should be noted that the work cited above is based on the administration of a single dose of F. tularensis LPS followed by challenge days later, while our experimental design focused on a more classic immunization regimen, in which three doses of whole purified F. tularensis LPS with or without neisserial PorB were inoculated 2 weeks apart. We observed that s.c. vaccination with F. tularensis LPS alone induced 25% survival from intranasal infection with a high dose of LVS (106 CFU, or 1,000 times the 50% lethal dose). This lack of protection is concomitant with the decreased levels of LPS-specific IgM before challenge (week 8).
Addition of neisserial PorB to the vaccine preparation increased the rate of survival to 70%, potentially correlating with the significantly raised anti-LPS IgM levels compared to those in the preimmune serum controls. Previous studies have shown how B and T lymphocytes are also very important for prolonged survival (11), so both humoral (specific IgM) and cell-mediated immunity might indeed be stimulated by the vaccine preparation comprising F. tularensis LPS plus PorB, and this antigen-adjuvant combination might be crucial for conferring protection. The cellular features of this type of protection are under investigation, and at this stage the key mediators of protection are unknown. Following intranasal challenge, all mice developed moderate disease signs and weight loss by day 4 postchallenge. The animals that eventually succumbed to tularemia all died within 8 days, while survivors overcame the clinical signs and regained body weight gradually and appeared healthy by day 18 postinfection. Ideally, mice immunized with a promising vaccine candidate should not present any indication of illness, but we speculate that future conjugation of F. tularensis LPS to PorB could greatly improve the protective efficacy and, ultimately, raise the rate of survival to 100%. It is known that the covalent conjugation of polysaccharides to proteins induces humoral immune responses with features of T-cell-dependent antigens (15), and this could also be the case for the vaccine candidate that we propose. As mentioned above, PorB has previously been conjugated to the meningococcal capsule and improved the capacity of this antigen to induce a humoral response (26), suggesting that a vaccine prepared by use of this approach might be promising for future testing in mouse models of disease caused by F. tularensis LVS and, most importantly, type A and B strains.
In a recent report, we described how the production of the cytokines IL-6, macrophage inflammatory protein 2, and MCP-1 in lung and spleen tissues was associated with a terminal stage of tularemia, while IL-1β was induced in the tissues of mice that had overcome symptoms and survived the disease when the mouse model of intranasal challenge was used (5). In this study, we analyzed the sera of mice that were vaccinated with either F. tularensis LPS or F. tularensis LPS plus PorB and challenged 4 weeks later with a high dose of F. tularensis LVS for the presence of some of the proinflammatory cytokines mentioned above. Overall, we found trends similar to those observed in the infection model (5), indicating that IL-6, MCP-1, and IFN-γ are markers of mortality and that IL-1β is a correlate of survival. IL-6 is an important marker of sepsis, and our group has previously shown a correlation of IL-6 with mortality following intranasal infection with LVS (5). Similarly, the levels of MCP-1, a chemokine involved in macrophage recruitment, were also increased above those at the baseline. IFN-γ is known to play a pivotal role in protective host responses to F. tularensis in vivo (12, 28), although in the present study, we observed a clear association with mortality. Similar to the findings obtained with our intranasal infection model (5), the IL-1β level was found to be elevated above the baseline level in survivors killed at early but not late time points, suggesting that this cytokine might play a role in clearing infection with or without vaccination. Other groups have reported the production of this cytokine following stimulation of human and murine cells with LVS (3, 18), but our studies reveal the potential involvement of IL-1β in survival. Overall, the serum cytokine pattern observed did not vary between mice vaccinated with F. tularensis LPS plus PorB and those immunized with LPS alone, suggesting that the production of these proinflammatory molecules is independent of the presence of neisserial PorB. More research will be conducted to clarify the mechanisms underlying the production of these cytokines.
In summary, we demonstrate that PorB isolated from Neisseria meningitidis improves the immunogenic and protective capacity of LPS purified from the F. tularensis LVS in a murine model of intranasal challenge and represents a good candidate adjuvant for use in the future development of a subunit vaccine against the pneumonic form of tularemia.
Acknowledgments
We thank Peter Jorth for excellent technical assistance, Matt Rarick for valuable help with the Luminex assays, Frank Gibson III for advice during the LPS isolation process, and Diana Velez for purification of native N. meningitidis PorB.
This work was supported by National Institutes of Health/National Institute of Allergy and Infectious Diseases grant U19 AI056543.
Footnotes
Published ahead of print on 9 July 2008.
REFERENCES
- 1.Ancuta, P., T. Pedron, R. Girard, G. Sandstrom, and R. Chaby. 1996. Inability of the Francisella tularensis lipopolysaccharide to mimic or to antagonize the induction of cell activation by endotoxins. Infect. Immun. 64:2041-2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baron, S. D., R. Singh, and D. W. Metzger. 2007. Inactivated Francisella tularensis LVS protects against respiratory tularemia by intranasal vaccination in an immunoglobulin A-dependent fashion. Infect. Immun. 75:2152-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bolger, C. E., C. A. Forestal, J. K. Italo, J. L. Benach, and M. B. Furie. 2005. The live vaccine strain of Francisella tularensis replicates in human and murine macrophages but induces only the human cells to secrete proinflammatory cytokines. J. Leukoc. Biol. 77:893-897. [DOI] [PubMed] [Google Scholar]
- 4.Burke, J. M., L. M. Ganley-Leal, K. Khatri, and L. M. Wetzler. 2007. Neisseria meningitidis PorB, a TLR2 ligand, induces an antigen-specific eosinophil recall response: potential adjuvant for helminth vaccines? J. Immunol. 179:3222-3230. [DOI] [PubMed] [Google Scholar]
- 5.Chiavolini, D., J. Alroy, C. A. King, P. Jorth, S. Weir, G. Madico, J. R. Murphy, and L. M. Wetzler. 2008. Identification of immunologic and pathologic parameters of death versus survival in respiratory tularemia. Infect. Immun. 76:486-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cole, L. E., K. L. Elkins, S. M. Michalek, N. Qureshi, L. J. Eaton, P. Rallabhandi, N. Cuesta, and S. N. Vogel. 2006. Immunologic consequences of Francisella tularensis live vaccine strain infection: role of the innate immune response in infection and immunity. J. Immunol. 176:6888-6899. [DOI] [PubMed] [Google Scholar]
- 7.Cole, L. E., K. A. Shirey, E. Barry, A. Santiago, P. Rallabhandi, K. L. Elkins, A. C. Puche, S. M. Michalek, and S. N. Vogel. 2007. Toll-like receptor 2-mediated signaling requirements for Francisella tularensis live vaccine strain infection of murine macrophages. Infect. Immun. 75:4127-4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conlan, J. W., H. Shen, A. Webb, and M. B. Perry. 2002. Mice vaccinated with the O-antigen of Francisella tularensis LVS lipopolysaccharide conjugated to bovine serum albumin develop varying degrees of protective immunity against systemic or aerosol challenge with virulent type A and type B strains of the pathogen. Vaccine 20:3465-3470. [DOI] [PubMed] [Google Scholar]
- 9.Conlan, J. W., E. Vinogradov, M. A. Monteiro, and M. B. Perry. 2003. Mice intradermally-inoculated with the intact lipopolysaccharide, but not the lipid A or O-chain, from Francisella tularensis LVS rapidly acquire varying degrees of enhanced resistance against systemic or aerogenic challenge with virulent strains of the pathogen. Microb. Pathog. 34:39-45. [DOI] [PubMed] [Google Scholar]
- 10.Cox, J. C., and A. R. Coulter. 1997. Adjuvants—a classification and review of their modes of action. Vaccine 15:248-256. [DOI] [PubMed] [Google Scholar]
- 11.Dreisbach, V. C., S. C. Cowley, and K. L. Elkins. 2000. Purified lipopolysaccharide from Francisella tularensis live vaccine strain (LVS) induces protective immunity against LVS infection that requires B cells and gamma interferon. Infect. Immun. 68:1988-1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duckett, N. S., S. Olmos, D. M. Durrent, and D. W. Metzger. 2005. Intranasal interleukin-12 treatment for protection against respiratory infection with the Francisella tularensis live vaccine strain. Infect. Immun. 73:2306-2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eigelsbach, H. T., and C. M. Downs. 1961. Prophylactic effectiveness of live and killed tularemia vaccines. Part I. Production of vaccine and evaluation of in the white mouse and guinea pig. J. Immunol. 87:415-425. [PubMed] [Google Scholar]
- 14.Eyles, J. E., M. G. Hartley, T. R. Laws, P. C. F. Oyston, K. F. Griffin, and R. W. Titball. 2008. Protection afforded against aerosol challenge by systemic immunisation with inactivated Francisella tularensis live vaccine strain (LVS). Microb. Pathog. 44:164-168. [DOI] [PubMed] [Google Scholar]
- 15.Finn, A. 2004. Bacterial polysaccharide-protein conjugate vaccines. Br. Med. Bull. 70:1-14. [DOI] [PubMed] [Google Scholar]
- 16.Foshay, L., W. H. Hesselbrock, H. J. Wittenberg, and A. H. Rodenberg. 1942. Vaccine prophylaxis against tularemia in man. Am. J. Public Health 32:1131-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fulop, M., R. Manchee, and R. W. Titball. 1995. Role of lipopolysaccharide and a major outer membrane protein from Francisella tularensis in the induction of immunity against tularemia. Vaccine 13:1220-1225. [DOI] [PubMed] [Google Scholar]
- 18.Gavrilin, M. A., I. J. Bouskl, N. L. Knatz, M. D. Duncan, M. W. Hall, J. S. Gunn, and M. D. Wewers. 2006. Internalization and phagosome escape required for Francisella to induce human monocyte IL-1beta processing and release. Proc. Natl. Acad. Sci. USA 103:141-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Golovliov, I., M. Ericsson, L. Akerblom, G. Sandstrom, A. Tarnvik, and A. Sjostedt. 1995. Adjuvanticity of ISCOMs incorporating a T cell-reactive lipoprotein of the facultative intracellular pathogen Francisella tularensis. Vaccine 13:261-267. [DOI] [PubMed] [Google Scholar]
- 20.Griffin, K. F., P. C. F. Oyston, and R. W. Titball. 2007. Francisella tularensis vaccines. FEMS Immunol. Med. Microbiol. 49:315-323. [DOI] [PubMed] [Google Scholar]
- 21.Guy, B. 2007. The perfect mix: recent progress in adjuvant research. Nat. Rev. Microbiol. 5:505-517. [DOI] [PubMed] [Google Scholar]
- 22.Hartley, M. G., M. Green, G. Choules, D. Rogers, D. G. Rees, S. Newsted, A. Sjostedt, and R. W. Titball. 2004. Protection afforded by heat shock protein 60 from Francisella tularensis is due to copurified lipopolysaccharide. Infect. Immun. 72:4109-4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hornick, R. B., and H. T. Eigelsbach. 1966. Aerogenic immunization of man with live tularemia vaccine. Bacteriol. Rev. 30:532-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katz, J., P. Zhang, M. Martin, S. N. Vogel, and S. M. Michalek. 2006. Toll-like receptor 2 is required for inflammatory responses to Francisella tularensis LVS. Infect. Immun. 74:2809-2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Latz, E., J. Franko, D. T. Golenbock, and J. R. Schreiber. 2004. Haemophilus influenzae type b-outer membrane protein complex glycoconjugate vaccine induces cytokine production by engaging human Toll-like receptor 2 (TLR2) and requires the presence of TLR2 for optimal immunogenicity. J. Immunol. 172:2431-2438. [DOI] [PubMed] [Google Scholar]
- 26.Mackinnon, F. G., Y. Ho, M. S. Blake, F. Michon, A. Chandraker, M. H. Sayegh, and L. M. Wetzler. 1999. The role of B/T costimulatory signals in the immunopotentiating activity of neisserial porin. J. Infect. Dis. 180:755-761. [DOI] [PubMed] [Google Scholar]
- 27.MacLeod, H., and L. M. Wetzler. 2007. T cell activation by TLRs: a role for TLRs in the adaptive immune response. Sci. STKE 402:pe48. [DOI] [PubMed] [Google Scholar]
- 28.Malik, M., C. S. Bakshi, B. Sahay, A. Shah, S. A. Lotz, and T. J. Sellati. 2006. Toll-like receptor 2 is required for control of pulmonary infection with Francisella tularensis. Infect. Immun. 74:3657-3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin, M., S. M. Michalek, and J. Katz. 2003. Role of innate immune factors in the adjuvant activity of monophosphoryl lipid A. Infect. Immun. 71:2498-2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Massari, P., P. Henneke, Y. Ho, E. Latz, D. T. Golenbock, and L. M. Wetzler. 2002. Cutting edge: immune stimulation by neisserial porins is Toll-like receptor 2 and MyD88 dependent. J. Immunol. 168:1533-1537. [DOI] [PubMed] [Google Scholar]
- 31.Massari, P., C. A. King, H. MacLeod, and L. M. Wetzler. 2005. Improved purification of native meningococcal porin PorB and studies on its structure/function. Protein Expr. Purif. 44:136-146. [DOI] [PubMed] [Google Scholar]
- 32.Massari, P., A. Visintin, J. Gunawardana, K. A. Halmen, C. A. King, D. T. Golenbock, and L. M. Wetzler. 2006. Meningococcal porin PorB binds to TLR2 and requires TLR1 for signaling. J. Immunol. 176:2373-2380. [DOI] [PubMed] [Google Scholar]
- 33.McCrumb, F. R. 1961. Aerosol infection of man with Francisella tularensis. Bacteriol. Rev. 25:262-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Michelsen, K. S., A. Aicher, and M. Mohaupt. 2001. The role of Toll-like receptors (TLRs) in bacteria-induced maturation of murine dendritic cells (DCs). Peptidoglycan and lipoteichoic acid are inducers of DC maturation and require TLR2. J. Immunol. 276:25680-25686. [DOI] [PubMed] [Google Scholar]
- 35.O'Hagan, D. T., M. L. MacKichan, and M. Singh. 2001. Recent developments in adjuvants for vaccines against infectious diseases. Biomol. Eng. 18:69-85. [DOI] [PubMed] [Google Scholar]
- 36.Pantosti, A., A. O. Tzianabos, A. B. Onderdonk, and D. L. Kasper. 1991. Immunochemical characterization of two surface polysaccharides of Bacteroides fragilis. Infect. Immun. 59:2075-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pulendran, B. 2004. Modulating vaccine responses with dendritic cells and Toll-like receptors. Immunol. Rev. 199:227-250. [DOI] [PubMed] [Google Scholar]
- 38.Sandstrom, G., A. Sjostedt, T. Johansson, K. Kuoppa, and J. C. Williams. 1992. Immunogenicity and toxicity of lipopolysaccharide from Francisella tularensis LVS. FEMS Microbiol. Immunol. 5:201-210. [DOI] [PubMed] [Google Scholar]
- 39.Saslaw, S., H. T. Eigelsbach, J. A. Prior, H. E. Wilson, and S. Carhart. 1961. Tularemia vaccine study. Part II. Respiratory challenge. Arch. Intern. Med. 107:689-701. [DOI] [PubMed] [Google Scholar]
- 40.Singleton, T. E., P. Massari, and L. M. Wetzler. 2005. Neisserial porin-induced dendritic cell activation is MyD88 and TLR2 dependent. J. Immunol. 174:3545-3550. [DOI] [PubMed] [Google Scholar]
- 41.Tarnvik, A. 1989. Nature of protective immunity to Francisella tularensis. Rev. Infect. Dis. 11:440-451. [PubMed] [Google Scholar]
- 42.Tarnvik, A., M. Ericsson, I. Golovliov, G. Sandstrom, and A. Sjostedt. 1996. Orchestration of the protective immune response to intracellular bacteria: Francisella tularensis as a model organism. FEMS Immunol. Med. Microbiol. 13:221-225. [DOI] [PubMed] [Google Scholar]
- 43.Van der Kleij, D., E. Latz, J. F. Brouwers, Y. C. Kruize, M. Schmitz, E. A. Kurt-Jones, T. Espevik, E. C. De Jong, M. L. Kapsenberg, D. T. Golenbock, A. G. Tielens, and M. Yazdanbakhsh. 2002. A novel host-parasite lipid cross-talk. Schistosomal lyso-phosphatidylserine activates Toll-like receptor 2 and affects immune polarization. J. Biol. Chem. 277:48122-48129. [DOI] [PubMed] [Google Scholar]
- 44.Weeratna, R. D., S. R. Makinen, M. J. McCluskie, and H. L. Davis. 2005. TLR agonists as vaccine adjuvants: comparison of CpG ODN and Resiquimod (R-848). Vaccine 23:5263-5270. [DOI] [PubMed] [Google Scholar]
- 45.Wetzler, L. M. 2003. The role of Toll-like receptors 2 in microbial disease and immunity. Vaccine 21(Suppl. 2):S55-S60. [DOI] [PubMed] [Google Scholar]