Abstract
The spliceosome is a dynamic, macromolecular complex, which removes non-protein-coding introns from pre-mRNA to form mature mRNA in a process known as splicing. This ribonucleoprotein assembly is comprised of five uridine-rich small nuclear RNAs (snRNAs) as well as over 300 proteins. In humans, several of the known splicing factors are members of the immunophilin superfamily. Immunophilins are peptidyl-prolyl cis-trans isomerases that catalyze the conversion of proteins from cis to trans at Xaa-Pro bonds. Our review of the data portrays a picture of this protein family as activators of spliceosomal proteins by way of folding and transport.
Keywords: cyclophilin, FKBP, spliceosome, cyclosporin, FK506, small nuclear RNAs
INTRODUCTION
Most of the transcripts produced in the eukaryotic cell are comprised of both protein-coding exons and non-protein-coding introns. Following transcription, the introns are removed and the exons ligated together by a dynamic, high molecular weight RNA-protein complex known as the spliceosome [1]. This macromolecular machine is composed of five uridine-rich small nuclear RNAs (snRNAs) each bound to a core set of snRNA-specific proteins as well as numerous splicing factors [2; 3]. Some of these proteins are an essential part of the spliceosome while others perform auxiliary roles in the processing of tissue- and/or developmental stage-specific transcripts.
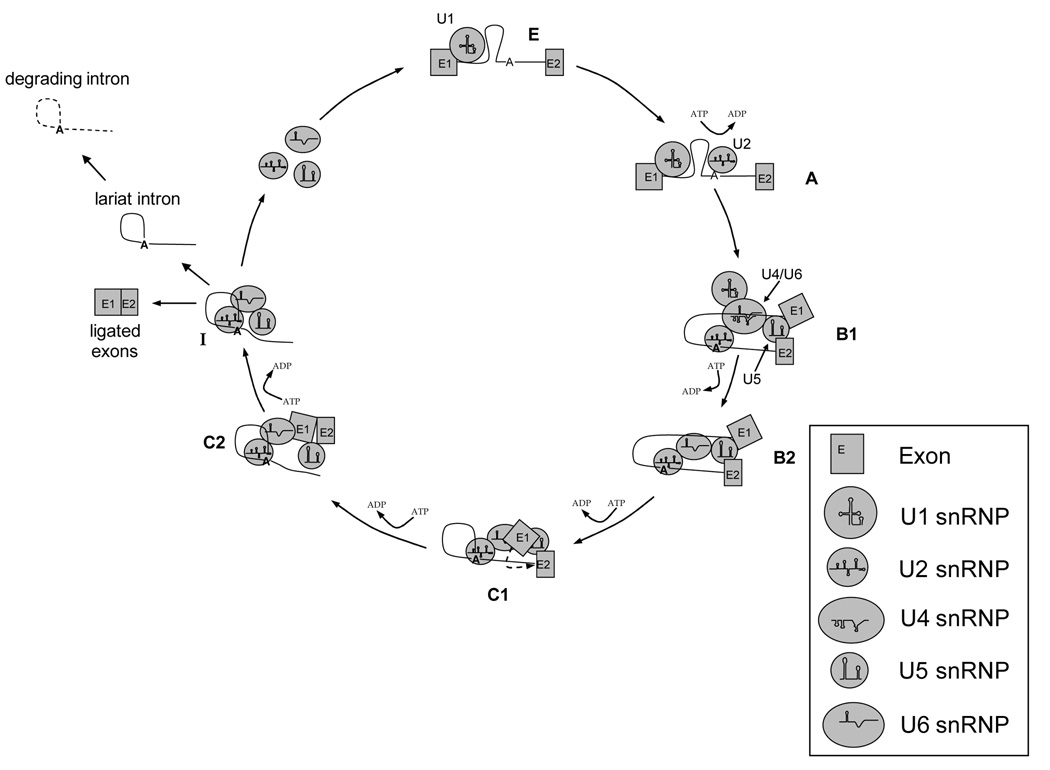
The snRNAs form distinct stem-loop structures that facilitate snRNA-protein and snRNA pre-mRNA interactions [4]. The mechanism of splicing involves a cascade of assembly, trans-esterification and disassembly reactions to produce two ligated exons and an excised intron, which is subsequently degraded [5] (See Figure 1 for a diagrammatic representation of the pre-mRNA splicing process). Following transcription of the pre-mRNA, the U1 small nuclear ribonucleoprotein particle (snRNP) binds to the 5’ exon-intron junction in an ATP independent reaction, creating the E complex [6] (Figure 1). The U1 snRNP promotes the association of U2 with the branch point intronic sequence in an ATP-dependent manner (the A complex) [7], establishing a favorable conformation for the first trans-esterification reaction [8; 9] (Figure 1). Next, a tri-snRNP composed of the U4/U6 di-snRNP and the U5 snRNP interact with the U2 snRNP to produce the B1 complex [10] (Figure 1). The first trans-esterification reaction takes place in the B2 complex, creating a free 5’ exon and a lariat intron/3’ exon [11] (Figure 1). The C1 spliceosome is responsible for the second trans-esterification, where the 3’ end of the intron is truncated, separating the intron from the 3’ exon. At this time, the U5 snRNP holds the two exons in close proximity [9]. Finally, C2 is formed by two ligated exons, an independent lariat intron as well as the U2, U5 and U6 snRNPs [12] (Figure 1). Subsequent to production of the ligated exons, the U2/U6·U5 tri-snRNP complex disassembles into individual snRNPs and are recycled in preparation for the next round of splicing [13].
Figure 1. An overview of pre-mRNA splicing.
Removal of non-protein coding introns from pre-mRNA involves a dynamic, high molecular weight complex known as the spliceosome. This nucleo-protein assemblage is comprised of five uridine rich small nuclear RNAs (snRNAs) as well as over 200 splicing proteins. All small nuclear ribonucleoprotein particles (snRNPs) (U1, U2, U4/U6 di-snRNP and U5) are labeled as they appear. E1 and E2 represent exons, separated by an intron (line) containing the branch point sequence (symbolized by an A). The names of each complex (E, A, B1, B2, C1, C2 and I) are designated in bold. ATP dependent reactions are indicated along the cycle. The degrading intron is symbolized by a dashed line.
In mammalian systems, over 300 proteins participate in splicing, making the spliceosome one of the largest cellular complexes discovered thus far [14; 15]. Many of these proteins have been previously identified and participate in a variety of additional cellular processes including transcription regulation, intra-and inter-cellular transport, apoptosis, kinase activity and protein folding (Figure 2). Proteomics analyses of the human spliceosome identified several splicing factors that are members of the immunophilin family [14; 16]. The inmunophilins represent a diverse group of proteins that are defined by their peptidyl-prolyl cis-trans isomerase (PPIase) activity and are known to bind immunosuppressive drugs [17]. They catalyze the conversion of proteins from cis to trans at Xaa-Pro bonds, which is often a rate-limiting step in protein folding and activity [18]. The first of the immunophilins was isolated in 1984 from bovine thymocytes as a molecule with high affinity for the immunosuppressive drug cyclosporin A (CsA) [19]. It was subsequently discovered that this protein also exhibits PPIase activity [20]. The immunophilin superfamily is classified into three major subfamilies, including the cyclosporin A (CsA) binding proteins (cyclophilins or Cyps) [21], FK506 binding proteins (FKBPs) [22] and parvulins [23]. In addition, other proteins bridge the two subfamilies by possession of both cyclophilin and FKBP domains, like the one identified in the protozoan, Toxoplasma gondii [24]. Immunophilins have been documented in every Domain of life including Archaea, Bacteria and Eukaryota and they are found in a number of cellular compartments including the mitochondria, endoplasmic reticulum, Golgi, nucleus and cytoplasm [17; 25].
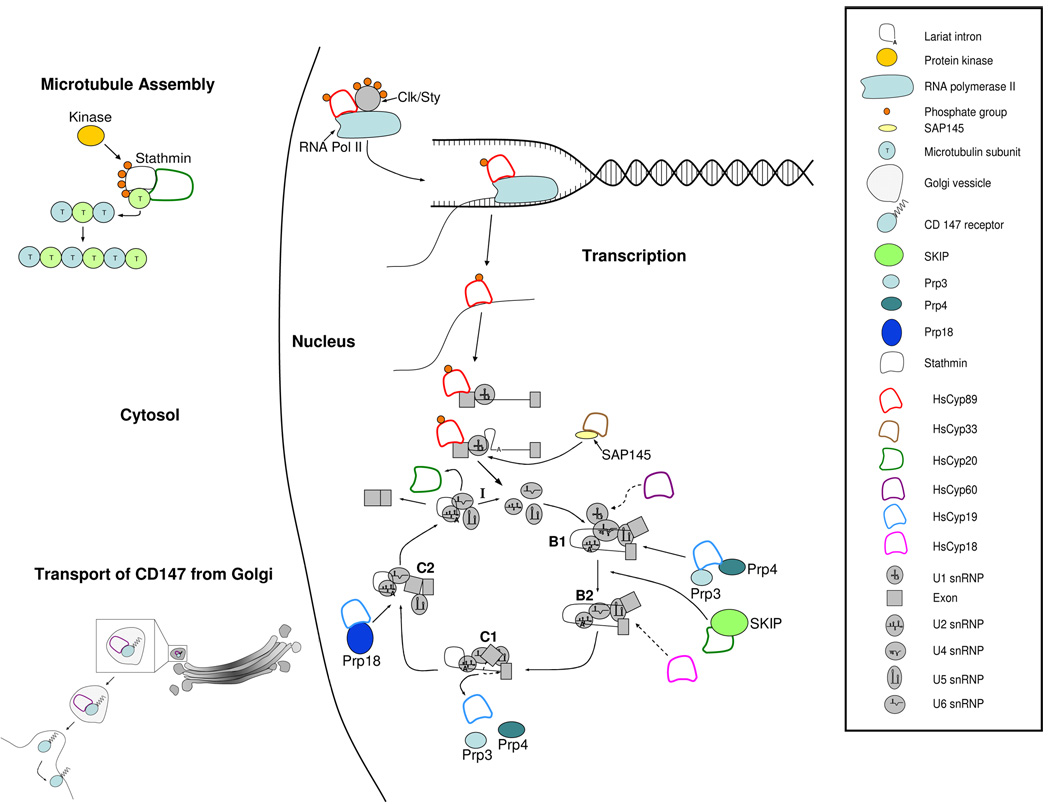
Figure 2. Potential functions of spliceosomal immunophilins in the mammalian cell.
Based on the available data, the hypothesized entry points for each immunophilin into the spliceosome are delineated by arrows. In the cases of PPIL3b and PPIL2, dashed arrows are employed to indicate unknown stages at which these proteins are incorporated into the spliceosome. In addition to their putative roles in splicing, these immunophilins may modulate transcription regulation, microtubule assembly, folding and intra- and inter-cellular transport of proteins from the endoplasmic reticulum, Golgi to locations like the cell membrane.
All immunophilins possess at least one PPIase domain, which modulates most of their cellular functions including folding and chaperone activity [17; 24]. A number of studies have detected the presence of immunophilins in the nucleus. These include PPIG [26], PPIE [27], PPIL1 [28], PPIH [29], PPIL2 [16] and PPIL3a/PPIL3b [16]. These nuclear immunophilins interact directly with splicing factors [29] and make up part of the spliceosome. Some also bind to the carboxyl terminal domain (CTD) of RNA polymerase II (RNA pol II) [26]. The involvement of immunophilins in these processes seems to be an ancient and universal trait due to their presence in the splicing and transcriptional machinery in a multitude of taxa. Furthermore, several studies based on proteomics have identified cyclophilins in spliceosomal complexes (reviewed in [30]; [31]). Interestingly, each of the analyses differs in the number and types of cyclophilins that are found in the spliceosome [31]. For example, Chen and coworkers [31] found six cyclophilins that co-purify with chicken spliceosomes (PPIE, PPIH, serologically defined colon cancer antigen 10 (SDCCAG10), PPIL1, PPIL2 and PPWD1). Rappsilber et al. [14] also identified PPIL3b as part of the human spliceosome, as well as all of the cyclophilins detected by Chen et al. [31] with the exception of PPIH. Another analysis detected PPIE, CYP16, PPIL1, PPIL2, PPIL3b and PPWD1 in human spliceosomes, but not PPIH and SDCCAG10 [16]. The discrepancies among the three studies may be related to species and/or tissue-specific diversity in spliceosomal components. There is precedence for tissue-specific variation in cyclophilin expression [32]. The differences observed may also allude to the dynamic nature in which these proteins associate with the spliceosome.
Given the constitutive and ubiquitous nature of RNA processing, the presence of immunophilins in the splicing machinery may be significant in modulating spliceosomal activity. A number of spliceosomal immunophilins have been identified in several taxa, including matrin-Cyp in Rattus norvegicus [33] and AtCyp59 in Arabidopsis thaliana [34]. The overall theme that emerges from our exploration of the literature is that immunophilins are capable of catalyzing a number of transport and foldase reactions that possibly assist in the assembly/disassembly dynamics of the spliceosome as well as in its coupling to the transcription machinery. Furthermore, it is feasible that the capacity of immunophilins to alter folding may provide accessibility and a mechanism for activating/inactivating spliceosomal proteins. In this review, we focus on the immunophilins of the human and yeast spliceosomes, as prototypic examples of the potential roles that immunophilins play in splicing as well as additional functions that they may possess.
SPLICEOSOMAL IMMUNOPHILINS
PPIH associates with the hPrp4 and hPrp18 splicing factors
PPIH, also known as Snu-Cyp20, USA-Cyp or CypH in humans, is a 19 kDa nuclear protein that contains a single PPIase domain and interacts specifically with the splicing factors hPrp3, hPrp4 and hPrp18 [35; 36; 37; 38] (Figure 2). Both hPrp3 and hPrp4 are human splicing factors that integrate into the U4/U6 di-snRNP and are essential for the initial steps of the splicing reaction [39]. The hPrp18 splicing factor is involved in the assembly of the tri-snRNP and functions in the second trans-esterification reaction [40]. Horowitz and colleagues [29] were able to elute complexes of [3H] CsA, His-tagged PPIH and hPrp4 as well as [3H] CsA, His-tagged PPIH and hPrp18 from affinity columns. This finding suggests that interactions between PPIH and either hPrp4 or hPrp18 do not disrupt the active site for CsA binding [29]. In addition, the crystal structure of PPIH [41] and PPIH in complex with a peptide of hPrp4 [42] reveals that the active site of PPIH remains free and maintains PPIase activity when bound to Prp4 in vitro [42]. Taken together, these data indicate that the PPIase domain of PPIH is available to aid in folding and/or transport of other spliceosomal proteins. Horowitz and coauthors [29] also found that PPIH is part of independent complexes with hPrp3/hPrp4 and hPrp18, respectively and is involved in both trans-esterification steps of splicing as part of each unique assembly. In addition, CsA inhibits both trans-esterification steps of pre-mRNA splicing in vitro and in vivo, an effect that Horowitz and colleagues attribute to the association of PPIH with hPrp4 and hPrp18, respectively [29]. It is possible that inhibition of splicing by CsA is via disruption of the PPIH PPIase active site. Both hPrp4 and hPrp18 contain a highly conserved region of 31 amino acids, which is the common binding site for PPIH [42]. Horowitz et al. [29] propose that PPIH mediates the proper folding and rearrangement of spliceosomal components in the early steps of splicing through an association with hPrp4 and indirectly, hPrp3 and may be involved in chaperone activity or disassembly of the spliceosome as a member of the hPrp18 complex. The fact that PPIH forms independent complexes with two splicing factors may imply that multiple PPIase substrates are present in the human splicing machinery at different times. It is well established that peptidyl-prolyl cis-trans isomerization is a rate-limiting step in protein folding, and it feasible that the introduction of a cyclophilin into the splicing machinery may contribute to the efficiency of the splicing reaction. It is noteworthy that the protein with the highest amino acid identity to PPIH in Saccharomyces cerevisiae lacks the two regions diagnostic for the protein at the N-terminus of the protein and the C-terminus of helix 1 [42]. Interestingly, however, the presence of these regions in the corresponding Cyps from a number of other species including Homo sapiens, Mus musculus, Drosophila melanogaster, Caenorhabditis elegans and Arabidopsis thaliana as well as Schizosaccharomyces pombe and other fungi suggests that these areas may have been deleted from the S. cerevisiae orthologue. If these interactions are missing in Saccharomyces cerevisiae, the question remains whether PPIH is auxiliary or essential to the splicing reaction, or whether one or more PPIases substitutes for PPIH. The presence of multiple spliceosomal immunophilins argues for a possible functional redundancy in the splicing system.
PPIG is a member of the transcription and splicing machinery
Nestel and coworkers [32] demonstrated that PPIG (also known as SR-Cyp, CARS-Cyp, CYPG in humans) interacts with Clk/Sty in yeast two-hybrid screens. Clk/Sty is a member of the SR protein kinase family involved in the regulation of RNA splicing through phosphorylation of splicing factors [43] (Figure 2). PPIG was also identified as a nuclear matrix protein that assembles with splicing factors in nuclear speckles [26], which are known to contain high concentrations of RNA processing components [44]. Nestel and colleagues [32] identified PPIG mRNAs in 13 human tissues and cell types, but could not detect any expressed sequences corresponding to PPIG in natural killer (NK) cells, where the expression of the NK-TR1 cyclophilin is elevated [32]. Nestel et al. [32] postulated that NK-TR1 performs the role(s) of PPIG in NK cells due to the high sequence similarity among both proteins and the absence of PPIG in NK cells.
Bourquin and colleagues [26] showed that the SR domain of PPIG is required for its interaction with the carboxyl terminal domain (CTD) of RNA polymerase II. As a PPIase, PPIG may contribute to the creation of a catalytically active form of RNA polymerase II by folding the proline rich CTD [26] (Figure 2). Although the details of the transition between transcription and splicing remain unknown, the participation of PPIG in both pathways represents an example of the intimate connection between these two cellular functions. The coupling of transcription and pre-mRNA splicing has been well documented [45]. A growing body of evidence indicates that transcription and pre-mRNA splicing at the speckles on the inner side of the nuclear membrane are structurally and functionally linked, possibly for efficiency in facilitating transportation to the cytoplasm. Proteins like PPIG may represent a mediator of these processes.
PPIE contains an RNA binding domain and is a part of the human spliceosome
Mi and colleagues [27] initially detected PPIE (also known as Cyp33 and CypE in humans) in Jurkat human T-cell extracts utilizing CsA affinity columns as a cyclophilin with PPIase activity and an RNA binding domain. Subsequent sequencing of cDNAs with consensus cyclophilin domains led to the identification of the full-length PPIE cDNA [27]. This cyclophilin contains an N-terminal RNA binding domain that is able to bind poly(A) and poly(U) tracts of nucleic acid [27]. Western blots also revealed that PPIE is exclusively present in nuclear fractions [27]. Mi et al. [27] suggest that this protein may bind specific RNAs and aid in folding of additional RNA binding proteins. More recently, two independent analyses of the human spliceosome using different mass spectrometry techniques identified PPIE as a member of the splicing machinery [16]. Consistent with the functions of a number of other immunophilins [32; 29], PPIE may interact with the spliceosome to assist in the proper folding and/or transport of splicing factors; however, further work is needed to delineate the exact role(s) that this protein plays in pre-mRNA splicing.
PPIL1 may catalyze folding of the 35S snRNP complex
PPIL1 is a 20 kDa cyclophilin with ubiquitous expression in adult human tissues [46; 47]. Recent studies using mass spectrometry have shown that PPIL1 is a component of the 45S snRNP (B1) assemblage in active splicesosomes as well as an element of the 35S U5 snRNP [48]. The 45S aggregate contains all five UsnRNAs (U1, U2, U4, U5 and U6) and more than 60 pre-mRNA splicing factors [49]. The 35S U5 snRNP contains a large number of proteins whose association facilitates the formation of the B2 spliceosomal complex (Figure 1) [50]. PPIL1 also binds the highly conserved transcription cofactor, Ski interacting protein (SKIP) (Figure 2) [47; 51]. SKIP associates with the Sloan-Kettering virus oncoprotein (SKI), an essential member of histone deacetylase complexes (HDAC) and repressor of transforming growth factor β (TGFβ) signaling [52].
During splicing, SKIP is recruited before the first catalytic stage of splicing and remains bound through both transesterification reactions [47]. PPIL1 is absent during B1 assembly, where the U4/U6.U5 tri-snRNP first come together [50], but it is incorporated in the formation of B2 (Figure 2). Makarov and colleagues [48] demonstrated that PPIL1 and SKIP also associate with the spliced intron as part of the 35S U5 snRNP. SKIP, an indispensable spliceosomal component and transcriptional coregulator [52], may regulate the efficiency of splicing by serving as a modulator of PPIL1 [3; 51].
These data suggest that PPIL1 plays a role during the activation of the B2 spliceosomal complex as well as the removal of the spliced intron (Figure 2). During the transition from the inactive B1 to an active B2 spliceosome that is poised to undertake the first transesterification reaction, PPIL1 may be recruited by SKIP in an interaction mediated by a region other than the PPIase domain of PPIL1. This would enable the active site of PPIL1 to remain open and facilitate folding of proline rich members of the 45S snRNP [54]. PPIL1 may contribute to the assembly and/or disassembly of the 45S snRNPs complex in a manner similar to the other spliceosomal immunophilins as a chaperone and/or folding enzyme. The presence of PPIL1 as part of the 35S complex also suggests that it may associate with U5 snRNA and assist the release and subsequent dismantling of the 35S snRNP from the post-spliceosomal intron (Figure 2). The discovery that two cyclophilins (PPIG and PPIL1) interact with both the splicing and transcription machinery alludes to a potentially important role for these proteins in coupling the processes of transcription and splicing. These PPIases may bridge the two cellular functions by facilitating conformational changes and/or transporting proteins from the machinery of transcription to that of the spliceosome.
PPIL2 and PPIL3b are components of the human spliceosome
PPIL2 (also known as Cyp60 in humans and CYP60 in yeast) is a 60 kDa human cyclophilin that is localized in both the nucleus and cytoplasm [55]. Although its specific role has yet to be elucidated, PPIL2 was identified as part of the human spliceosome using liquid chromatography tandem mass spectrometry [16] and may serve as a chaperone or protein folding enzyme much the same way as PPIH, PPIG, PPIE and PPIL1 (Figure 2). Zhou et al. [56] identified cDNAs corresponding to PPIL3b (also known as CypJ in humans) from a human fetal brain cDNA library. The PPIL3b gene encodes an alternatively spliced pre-mRNA that produces two isoforms, PPIL3a and PPIL3b [56]. Thus far, only PPIL3b has been detected in human spliceosomes [52]. Although its exact function is unknown, PPIL3b is present in the B2 complex and absent in B1, the U5 snRNP and the U4/U6.U5 tri-snRNP [48; 50; 31] (Figure 2). The lack of PPIL3b in the tri-snRNP and presence in B2 suggests that it is part of the U2 snRNP complex.
CONCLUSION
The current literature reveals that the spliceosomal immunophilins appear to mediate several functions in the removal of introns from pre-mRNA. First, as prolyl isomerases, they seem to aid in folding and activation of proline rich splicing factors, and may be essential in assembly/disassembly of spliceosomal complexes, as well as in coupling transcription and splicing. It remains to be determined whether the immunophilins are essential for splicing reactions to take place, if they possess a more auxiliary role in the splicing of particular transcripts in different tissues and/or developmental stages or both.
The identification of immunophilins in such a ubiquitous and universal assembly as the spliceosome may prompt us to re-evaluate the mechanisms of action of CsA, FK506 and other immunosuppressive drugs that interact with immunophilins. For example, it is possible that some of the side effects resulting from treatments with CsA, FK506 or rapamycin are brought about by sequestration and removal of immunophilins from the spliceosome. In fact, Horowitz and colleagues [29] found that pre-mRNA splicing is inhibited by CsA in vitro and in vivo. The association of drugs to immunophilins may displace these proteins from the splicing complex and prevent the creation of properly folded and active spliceosomes. Displacement of immunophilins from the spliceosome may result in fewer functional splicing complexes and a subsequent reduction in mature mRNAs necessary, for example, in cell cycle control or immune system activation.
Understanding the exact function of the immunophilins in splicing may lead to the creation of novel drugs that act by sequestering an individual immunophilin from the spliceosome at a particular time and/or tissue. It is clear that the highly conserved nature of the PPIase domain hinders the development of ligands that will bind a single immunophilin. Therefore, it is important to target other domains of the immunophilins, as well as unique sequences between protein motifs. In this way, therapeutic agents may be directed against single proteins, rather than all members of a protein family. In addition, it is possible that some of the antigenicity against spliceosomal components in patients with autoimmune diseases resides in the cyclophilins and further investigation of the epitopes responsible for these diseases is warranted. Unfortunately, work describing spliceosomal immunophilins spans a period of time between the mid-to late-1980’s and continues only until about 1998, after which the focus seems to have shifted to other areas. Given the potential of these specific immunophilins as possible drug receptors, further investigation is warranted, specifically in relation to targetting immunophilins in therapeutic strategies. Constant advances in molecular modeling, proteomics and synthetic chemistry are sure to help pave the way for the development of unique and beneficial ligands.
Table 1.
| Human Immunophilin | Putative yeast homologue | Predicted size (kDa) (Human/Yeast) | Known functional domains | Interacting Proteins |
|---|---|---|---|---|
| PPIH, Snu-Cyp20, USA-Cyp, CypH | CYP3 (S. pombe)* | 19/19 | Cyclophilin domain | hPrp4, hPrp18 |
| PPIG, SR-Cyp, CARS-Cyp, CypG | - | 89/- | SR domain Cyclophilin domain Nopp140 related domain |
Clk/Sty, RNA polymerase II |
| PPIE, Cyp33, CypE | - | 33/- | RRM Cyclophilin domain |
hSAP49 |
| PPIL1 | CYP1 (S. pombe) | 20/18 | Cyclophilin domain | 45S snRNP, 35S U5 snRNP, SKIP, stathmin |
| PPIL2, Cyp60 | Cyp60 (R. oryzae) | 60/61 | Ubox domain Cyclophilin domain Uncharacterized conserved region |
CD147, eglin C |
| PPIL3b, CypJ | - | 18/- | Cyclophilin domain | - |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE
- 1.Moore MJ, Sharp PA. Evidence for two active sites in the splicesome provided by stereochemistry of pre-mRNA. Nature. 1993;23:364–368. doi: 10.1038/365364a0. [DOI] [PubMed] [Google Scholar]
- 2.Raker VA, Hartmuth K, Kastner B, Lührmann R. Spliceosomal U snRNP core assembly: Sm proteins assemble onto an Sm site RNA nonanucleotide in a specific and thermodynamically stable manner. Molecular and Cell Biology. 1999;19:6554–6565. doi: 10.1128/mcb.19.10.6554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jurica MS, Licklider LJ, Gygi SR, Grigorieff N, Moore MJ. Purification and characterization of native spliceosomes suitable for three-dimensional structural analysis. RNA. 2002;8:426–439. doi: 10.1017/s1355838202021088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Will CL, Lührmann R. Protein function in pre-mRNA splicing. Current Opinions in Cell Biology. 1997;9:320–328. doi: 10.1016/s0955-0674(97)80003-8. [DOI] [PubMed] [Google Scholar]
- 5.Staley JP, Guthrie C. Mechanical devices of the spliceosome: Motors, clocks, springs and things. Cell. 1998;92:315–326. doi: 10.1016/s0092-8674(00)80925-3. [DOI] [PubMed] [Google Scholar]
- 6.Jamison SF, Crow A, Garcia-Blanco MA. The spliceosome assembly pathway in mammalian extracts. Molecular and Cell Biology. 1992;12:4279–4287. doi: 10.1128/mcb.12.10.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barabino SM, Blencowe BJ, Ryder U, Sproat BS, Lamond AI. Targeted snRNP depletion reveals an additional role of mammalian U1 snRNP in spliceosome assembly. Cell. 1990;63:293–302. doi: 10.1016/0092-8674(90)90162-8. [DOI] [PubMed] [Google Scholar]
- 8.Query CC, Moore MJ, Sharp PA. Branch nucleophile selection in pre-mRNA splicing: evidence for the bulged duplex model. Genes and Development. 1994;8:587–597. doi: 10.1101/gad.8.5.587. [DOI] [PubMed] [Google Scholar]
- 9.Newby MI, Greenbaum NL. Sculpting of the spliceosomal branch site recognition motif by a conserved pseudouridine. Nature Structural and Molecular Biology. 2002;9:958–965. doi: 10.1038/nsb873. [DOI] [PubMed] [Google Scholar]
- 10.Konarska MM, Sharp PA. Interaction between small nuclear ribonucleoprotein particles in formation of the spliceosomes. Cell. 1987;49:763–774. doi: 10.1016/0092-8674(87)90614-3. [DOI] [PubMed] [Google Scholar]
- 11.Sashital DG, Cornilescu G, McManus CJ, Brow DA, Butcher SE. U2-U6 RNA folding reveals a group II intron-like domain and a four-helix junction. Nature Structural and Molecular Biology. 2004;11:1237–1242. doi: 10.1038/nsmb863. [DOI] [PubMed] [Google Scholar]
- 12.Chiara MD, Gozani O, Bennett M, Champion-Arnaud P, Palandjian L, Reed R. Identification of proteins that interact with the exon sequences, splice sites, and the branchpoint sequence during each stage of spliceosomal assembly. Molecular and Cell Biology. 1996;16:3317–3326. doi: 10.1128/mcb.16.7.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohi MD, Ren L, Wall JS, Gould KL, Walz T. Structural characterization of the fission yeast U5.U2/U6 spliceosome complex. Proceedings of the National Academy of Sciences U S A. 2007;104:3195–2000. doi: 10.1073/pnas.0611591104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rappsilber J, Ryder U, Lamond AI, Mann M. Large-scale proteomic analysis of the human spliceosome. Genome Research. 2002;12:1231–1245. doi: 10.1101/gr.473902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valadkhan S. The spliceosome: a ribozyme at heart? Biological Chemistry. 2007;388:693–697. doi: 10.1515/BC.2007.080. [DOI] [PubMed] [Google Scholar]
- 16.Zhou Z, Licklider LJ, Gygi SP, Reed R. Comprehensive proteomic analysis of the human spliceosome. Nature. 2002;419:182–185. doi: 10.1038/nature01031. [DOI] [PubMed] [Google Scholar]
- 17.Galat A. Peptidylprolyl cis/trans isomerases (immunophilins): biological diversity--targets--functions. Current Topics in Medicinal Chemistry. 2003;3:1315–1347. doi: 10.2174/1568026033451862. [DOI] [PubMed] [Google Scholar]
- 18.Baneyx F. Keeping up with protein folding. Microbial Cell Factories. 2004;3:6. doi: 10.1186/1475-2859-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Handschumacher RE, Harding MW, Rice J, Drugge RJ, Speicher DW. Cyclophilin: a specific cytosolic binding protein for cyclosporin A. Science. 1984;226:544–547. doi: 10.1126/science.6238408. [DOI] [PubMed] [Google Scholar]
- 20.Fischer G, Liebold BW, Lang K, Kiefhaber T, Schmid FX. Cyclophilin and peptidyl-prolyl cis-trans isomerase are probably identical proteins. Nature. 1989;337:476–478. doi: 10.1038/337476a0. [DOI] [PubMed] [Google Scholar]
- 21.Pemberton TJ. Identification and comparative analysis of sixteen fungal peptidyl-prolyl cis/trans isomerase repertoires. BMC Genomics. 2006;7:244. doi: 10.1186/1471-2164-7-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ho S, Clipstone N, Timmermann L, Northrop J, Graef I, Fiorentino D, Nourse J, Crabtree GR. The mechanism of action of cyclosporine A and FK506. Clinical Immunology and Immunopathology. 1996;80:S40–S45. doi: 10.1006/clin.1996.0140. [DOI] [PubMed] [Google Scholar]
- 23.Fujiyama S, Yanagida M, Hayano T, Miura Y, Isobe T, Fujimori F, Uchida T, Takahashi N. Isolation and proteomic characterization of human Parvulin-associating preribosomal ribonucleoprotein complexes. Journal of Biological Chemistry. 2002;277:23773–23780. doi: 10.1074/jbc.M201181200. [DOI] [PubMed] [Google Scholar]
- 24.Adams B, Musiyenko A, Kumar R, Barik S. A novel class of dual-family immunophilins. Journal of Biological Chemistry. 2005;280:24308–24314. doi: 10.1074/jbc.M500990200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang P, Heitman J. The cyclophilin. Genome Biology. 2005;6:226. doi: 10.1186/gb-2005-6-7-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bourquin JP, Stagljar I, Meier P, Moosmann P, Silke J, Baechi T, Georgiev O, Schaffner W. A serine/arginine-rich nuclear matrix cyclophilin interacts with the C-terminal domain of RNA polymerase II. Nucleic Acids Research. 1997;25:2055–2061. doi: 10.1093/nar/25.11.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mi H, Kops O, Zimmermann E, Jäschke A, Tropschug M. A nuclear RNA-binding cyclophilin in human T cells. FEBS Letters. 1996;398:201–205. doi: 10.1016/s0014-5793(96)01248-3. [DOI] [PubMed] [Google Scholar]
- 28.Pushkarsky T, Zybarth G, Dubrovsky Ll, Yurchenko V, Tang H, Guo H, Toole B, Sherry B, Bukrinsky M. CD147 facilitates HIV-1 infection by interacting with virus-associated cyclophilin A. Proceedings of the National Academy of Sciences U S A. 2001;98:6360–6365. doi: 10.1073/pnas.111583198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horowitz DS, Lee EJ, Mabon SA, Misteli T. A cyclophilin functions in pre-mRNA splicing. EMBO Journal. 2002;21:470–480. doi: 10.1093/emboj/21.3.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jurica MS, Moore MJ. Pre-mRNA splicing: awash in a sea of proteins. Molecular Cell. 2003;12:5–14. doi: 10.1016/s1097-2765(03)00270-3. [DOI] [PubMed] [Google Scholar]
- 31.Chen YI, Moore RE, Ge HY, Young MK, Lee TD, Stevens SW. Proteomic analysis of in vivo-assembled pre-mRNA splicing complexes expands the catalog of participating factors. Nucleic Acids Research. 2007;35:3928–3944. doi: 10.1093/nar/gkm347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nestel FP, Colwill K, Harper S, Pawson T, Anderson SK. RS cyclophilins: identification of an NK-TR1-related cyclophilin. Gene. 1996;180:151–155. doi: 10.1016/s0378-1119(96)00436-2. [DOI] [PubMed] [Google Scholar]
- 33.Mortillaro MJ, Berezney R. Matrin CYP, an SR-rich cyclophilin that associates with the nuclear matrix and splicing factors. Journal of Biological Chemistry. 1998;273:8183–8192. doi: 10.1074/jbc.273.14.8183. [DOI] [PubMed] [Google Scholar]
- 34.Gullerova M, Barta A, Lorkovic ZJ. AtCyp59 is a multidomain cyclophilin from Arabidopsis thaliana that interacts with SR proteins and the C-terminal domain of the RNA polymerase II. RNA. 2006;12:631–643. doi: 10.1261/rna.2226106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horowitz DS, Kobayashi R, Krainer AR. A new cyclophilin and the human homologues of yeast Prp3 and Prp4 form a complex associated with U4/U6 snRNPs. RNA. 1997;3:1374–1387. [PMC free article] [PubMed] [Google Scholar]
- 36.Teigelkamp S, Achsel T, Mundt C, Göthel SF, Cronshagen U, Lane WS, Marahiel M, Lührmann R. The 20kD protein of human [U4/U6•U5] tri-snRNPs is a novel cyclophilin that forms a complex with the U4/U6-specific 60kD and 90kD proteins. RNA. 1998;4:127–141. [PMC free article] [PubMed] [Google Scholar]
- 37.Pemberton TJ, Rulten SL, Kay JE. Identification and characterisation of Schizosaccharomyces pombe cyclophilin 3, a cyclosporin A insensitive orthologue of human USA-CyP. Journal of Chromatography B, Analytical Technologies in the Biomedical Life Sciences. 2003;786:81–91. doi: 10.1016/s1570-0232(02)00738-9. [DOI] [PubMed] [Google Scholar]
- 38.Kim IS, Yun HS, Kwak SH, Jin IN. The physiological role of CPR1 in Saccharomyces cerevisiae KNU5377 against menadione stress by proteomics. Journal of Microbiology. 2007;45:326–332. [PubMed] [Google Scholar]
- 39.Anthony JG, Weidenhammer EM, Woolford JL., Jr The yeast Prp3 protein is a U4/U6 snRNP protein necessary for integrity of the U4/U6 snRNP and the U4/U6.U5 tri-snRNP. RNA. 1997;3:1143–1152. [PMC free article] [PubMed] [Google Scholar]
- 40.Horowitz DS, Krainer AR. A human protein required for the second step of pre-mRNA splicing is functionally related to a yeast splicing factor. Genes and Development. 1997;11:139–151. doi: 10.1101/gad.11.1.139. [DOI] [PubMed] [Google Scholar]
- 41.Reidt U, Reuter K, Achsel T, Ingelfinger D, Lührmann R, Ficner R. Crystal Structure of the Human U4/U6 Small Nuclear Ribonucleoprotein Particle-specific SnuCyp-20, a Nuclear Cyclophilin. Journal of Biological Chemistry. 2000;275:7439–7442. doi: 10.1074/jbc.275.11.7439. [DOI] [PubMed] [Google Scholar]
- 42.Reidt U, Wahl MC, Fasshauer D, Horowitz DS, Lurhmann R, Ficner R. Crystal structure of a complex between human spliceosomal cyclophilin H and a U4/U6 snRNP-60K peptide. Journal of Molecular Biology. 2003;331:45–56. doi: 10.1016/s0022-2836(03)00684-3. [DOI] [PubMed] [Google Scholar]
- 43.Ngo JC, Chakrabarti S, Ding JH, Velazquez-Dones A, Nolen B, Aubol BE, Adams JA, Fu XD, Ghosh G. Interplay between SRPK and Clk/Sty kinases in phosphorylation of the splicing factor ASF/SF2 is regulated by a docking motif in ASF/SF2. Molecular Cell. 2005;20:77–89. doi: 10.1016/j.molcel.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 44.Spector DL. Nuclear organization of pre-mRNA processing. Current Opinions in Cell Biology. 1993;5:442–447. doi: 10.1016/0955-0674(93)90009-f. [DOI] [PubMed] [Google Scholar]
- 45.Lamond A, Spector D. Nuclear speckles: a model for nuclear organelles. Nature Reviews Molecular Cell Biology. 2003;4:605–612. doi: 10.1038/nrm1172. [DOI] [PubMed] [Google Scholar]
- 46.Ozaki K, Fujiwara T, Kawai A, Shimizu F, Takami S, Okuno S, Takeda S, Shimada Y, Nagata M, Watanabe T, Takaichi A, Takahashi E, Nakamura Y, Shin S. Cloning, expression and chromosomal mapping of a novel cyclophilin-related gene (PPIL1) from human fetal brain. Cytogenetics and Cell Genetics. 1996;72:242–245. doi: 10.1159/000134199. [DOI] [PubMed] [Google Scholar]
- 47.Xu C, Zhang J, Huang X, Sun J, Xu Y, Tang Y, Wu J, Shi Y, Huang Q, Zhang QJ. Solution structure of human peptidyl prolyl isomerase-like protein 1 and insights into its interaction with SKIP. Biological Chemistry. 2006;281:159000–15908. doi: 10.1074/jbc.M511155200. [DOI] [PubMed] [Google Scholar]
- 48.Makarov EM, Makarova OV, Urlaub H, Gentzel M, Will CL, Wilm M, Luhrmann R. Small nuclear ribonucleoproteins remodeling during catalytic activation of the spliceosome. Science. 2002;298:2205–2208. doi: 10.1126/science.1077783. [DOI] [PubMed] [Google Scholar]
- 49.Stevens SW, Ryan DE, Ge HY, Moore RE, Young MK, Lee TD, Abelson J. Composition and functional characterization of the yeast spliceosomal penta-snRNP. Molecular Cell. 2002;9:31–44. doi: 10.1016/s1097-2765(02)00436-7. [DOI] [PubMed] [Google Scholar]
- 50.Makarova OV, Makarov EM, Urlaub H, Will CL, Gentzel M, Wilm M, Lührmann R. A subset of human 35S U5 proteins, including Prp19, function prior to catalytic step 1 of splicing. EMBO Journal. 2004;23:2381–2391. doi: 10.1038/sj.emboj.7600241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Skruzný M, Ambrozková M, Fuková I, Martínková K, Blahůsková A, Hamplová L, Půta F, Folk P. Cyclophilins of a novel subfamily interact with SNW/SKIP coregulator in Dictyostelium discoideum and Schizosaccharomyces pombe. Biochimica et Biophysica Acta. 2001;1521:146–151. doi: 10.1016/s0167-4781(01)00301-3. [DOI] [PubMed] [Google Scholar]
- 52.Folk P, Půta F, Skružný M. Transcriptional coregulator SNW/SKIP: the concealed tie of dissimilar pathways. Cellular and Molecular Life Sciences. 2004;61:629–640. doi: 10.1007/s00018-003-3215-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leong GM, Subramaniam N, Figueroa J, Flanagan JL, Hayman MJ, Eisman JA, Kouzmenko AP. Ski-interacting protein interacts with Smad proteins to augment transforming growth factor-beta-dependent transcription. Journal of Biological Chemistry. 2001;276:18243–18248. doi: 10.1074/jbc.M010815200. [DOI] [PubMed] [Google Scholar]
- 54.Piotukh K, Gu W, Kofler M, Labudde D, Helms V, Freund C. Cyclophilin A binds to linear peptide motifs containing a consensus that is present in many human proteins. Journal of Biological Chemistry. 2005;280:23668–23674. doi: 10.1074/jbc.M503405200. [DOI] [PubMed] [Google Scholar]
- 55.Pushkarsky T, Yurchenko V, Vanpouille C, Brichacek B, Vaisman I, Hatakeyama S, Nakayama KI, Sherry B, Bukrinsky MI. Cell surface expression of CD147/EMMPRIN is regulated by cyclophilin 60. Journal of Biological Chemistry. 2005;280:27866–27871. doi: 10.1074/jbc.M503770200. [DOI] [PubMed] [Google Scholar]
- 56.Zhou Z, Ying K, Dai J, Tang R, Wang W, Huang Y, Zhao W, Xie Y, Mao Y. Molecular cloning and characterization of a novel peptidylprolyl isomerase (cyclophilin)-like gene (PPIL3) from human fetal brain. Cytogenetics and Cell Genetics. 2001;92:231–236. doi: 10.1159/000056909. [DOI] [PubMed] [Google Scholar]