Abstract
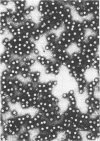
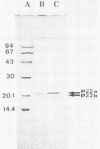
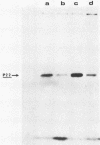
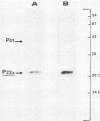
Highly purified hepatitis B virus core particles were obtained in large amounts from the cytoplasm of infected human liver cells. This DNA polymerase-negative core preparation had only hepatitis B core antigen-specific antigenicity and showed a surprising stability. Two forms of a single protein of 22,000 molecular weight, P22, were resolved electrophoretically; the slower moving species, P22a, appeared to be a reduced form of the protein, and the faster moving species, P22b, could have represented a conformational isomer containing an intramolecular disulfide bond(s). The immunological properties and DNA-binding activity of the reduced form, P22a, were examined following separation by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and by transfer onto nitrocellulose membranes (Western blotting). We found that the hepatitis B virus C gene protein shared the antigenic site responsible for both hepatitis B core and e antigen reactivity. We also demonstrated that the core protein(s) bound specifically the genomic hepatitis B virus DNA in comparison with a plasmid DNA (pBR322). This last observation was further substantiated by a radioimmunological method. P22a was also found to be phosphorylated in vitro by the endogenous protein kinase activity, copurified with the hepatitis B core antigen particles. These findings suggest that P22 is a multifunctional protein which is incorporated into core particles within the cytoplasm of the host cell before DNA encapsidation. A critical role of this protein in hepatitis B virus assembly is suggested.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberti A., Pontisso P., Realdi G. Changes in hepatitis B virus DNA-polymerase activity in patients with chronic infection. J Med Virol. 1981;8(4):223–229. doi: 10.1002/jmv.1890080402. [DOI] [PubMed] [Google Scholar]
- Albin C., Robinson W. S. Protein kinase activity in hepatitis B virus. J Virol. 1980 Apr;34(1):297–302. doi: 10.1128/jvi.34.1.297-302.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker L. F., Almeida J. D., Hoofnagle J. H., Gerety R. J., Jackson D. R., McGrath P. P. Hepatitis B core antigen: immunology and electron microscopy. J Virol. 1974 Dec;14(6):1552–1558. doi: 10.1128/jvi.14.6.1552-1558.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Potter V. R. Nuclei from rat liver: isolation method that combines purity with high yield. Science. 1966 Dec 30;154(3757):1662–1665. doi: 10.1126/science.154.3757.1662. [DOI] [PubMed] [Google Scholar]
- Bowen B., Steinberg J., Laemmli U. K., Weintraub H. The detection of DNA-binding proteins by protein blotting. Nucleic Acids Res. 1980 Jan 11;8(1):1–20. doi: 10.1093/nar/8.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budkowska A., Kalinowska B., Nowosławski A. Identification of two HBeAg subspecificities revealed by chemical treatment and enzymatic digestion of liver-derived HBcAg. J Immunol. 1979 Sep;123(3):1415–1416. [PubMed] [Google Scholar]
- Budkowska A., Shih J. W., Gerin J. L. Immunochemistry and polypeptide composition of hepatitis B core antigen (HBc Ag). J Immunol. 1977 Apr;118(4):1300–1305. [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Dane D. S., Cameron C. H., Briggs M. Virus-like particles in serum of patients with Australia-antigen-associated hepatitis. Lancet. 1970 Apr 4;1(7649):695–698. doi: 10.1016/s0140-6736(70)90926-8. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Gerlich W. H., Goldmann U., Müller R., Stibbe W., Wolff W. Specificity and localization of the hepatitis B virus-associated protein kinase. J Virol. 1982 Jun;42(3):761–766. doi: 10.1128/jvi.42.3.761-766.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowans E. J., Burrell C. J., Jilbert A. R., Marmion B. P. Patterns of single- and double-stranded hepatitis B virus DNA and viral antigen accumulation in infected liver cells. J Gen Virol. 1983 Jun;64(Pt 6):1229–1239. doi: 10.1099/0022-1317-64-6-1229. [DOI] [PubMed] [Google Scholar]
- Hoofnagle J. H., Gerety R. J., Barker L. F. Antibody to hepatitis-B-virus core in man. Lancet. 1973 Oct 20;2(7834):869–873. doi: 10.1016/s0140-6736(73)92004-7. [DOI] [PubMed] [Google Scholar]
- Hruska J. F., Robinson W. S. The proteins of hepatitis B Dane particle cores. J Med Virol. 1977;1(2):119–131. doi: 10.1002/jmv.1890010205. [DOI] [PubMed] [Google Scholar]
- Kaplan P. M., Ford E. C., Purcell R. H., Gerin J. L. Demonstration of subpopulations of Dane particles. J Virol. 1976 Mar;17(3):885–893. doi: 10.1128/jvi.17.3.885-893.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan P. M., Greenman R. L., Gerin J. L., Purcell R. H., Robinson W. S. DNA polymerase associated with human hepatitis B antigen. J Virol. 1973 Nov;12(5):995–1005. doi: 10.1128/jvi.12.5.995-1005.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ohori H., Shimizu N., Yamada E., Onodera S., Ishida N. Immunological and morphological properties of HBeAg subtypes (HBeAg/1 and HBeAg/2) in hepatitis B virus core particles. J Gen Virol. 1984 Feb;65(Pt 2):405–414. doi: 10.1099/0022-1317-65-2-405. [DOI] [PubMed] [Google Scholar]
- Ohori H., Yamaki M., Onodera S., Yamada E., Ishida N. Antigenic conversion from HBcAg to HBeAg by degradation of hepatitis B core particles. Intervirology. 1980;13(2):74–82. doi: 10.1159/000149110. [DOI] [PubMed] [Google Scholar]
- Onodera S., Ohori H., Yamaki M., Ishida N. Electron microscopy of human hepatitis B virus cores by negative staining-carbon film technique. J Med Virol. 1982;10(2):147–155. doi: 10.1002/jmv.1890100209. [DOI] [PubMed] [Google Scholar]
- Pasek M., Goto T., Gilbert W., Zink B., Schaller H., MacKay P., Leadbetter G., Murray K. Hepatitis B virus genes and their expression in E. coli. Nature. 1979 Dec 6;282(5739):575–579. doi: 10.1038/282575a0. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Pillot J., Petit M. A. Immunochemical structure of the hepatitis B surface antigen vaccine--I. Treatment of immobilized HBsAg by dissociation agents with or without enzymatic digestion and identification of polypeptides by protein blotting. Mol Immunol. 1984 Jan;21(1):53–60. doi: 10.1016/0161-5890(84)90089-0. [DOI] [PubMed] [Google Scholar]
- Price P., Ostrove S., Flordellis C., Sells M. A., Thung S., Gerber M., Christman J., Acs G. Characterization of RNA transcripts and virally coded proteins synthesized in mouse fibroblasts transfected with hepatitis B DNA: HBeAg synthesis in HBcAg-negative cells with active core-antigen genes. Biosci Rep. 1983 Nov;3(11):1017–1026. doi: 10.1007/BF01121028. [DOI] [PubMed] [Google Scholar]
- Robinson W. S., Clayton D. A., Greenman R. L. DNA of a human hepatitis B virus candidate. J Virol. 1974 Aug;14(2):384–391. doi: 10.1128/jvi.14.2.384-391.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto Y., Yamada G., Mizuno M., Nishihara T., Kinoyama S., Kobayashi T., Takahashi T., Nagashima H. Full and empty particles of hepatitis B virus in hepatocytes from patients with HBsAg-positive chronic active hepatitis. Lab Invest. 1983 Jun;48(6):678–682. [PubMed] [Google Scholar]
- Takahashi K., Akahane Y., Gotanda T., Mishiro T., Imai M., Miyakawa Y., Mayumi M. Demonstration of hepatitis B e antigen in the core of Dane particles. J Immunol. 1979 Jan;122(1):275–279. [PubMed] [Google Scholar]
- Takahashi K., Imai M., Nomura M., Oinuma A., Machida A., Funatsu G., Miyakawa Y., Mayumi M. Demonstration of the immunogenicity of hepatitis B core antigen in a hepatitis B e antigen polypeptide (P19). J Gen Virol. 1981 Dec;57(Pt 2):325–330. doi: 10.1099/0022-1317-57-2-325. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Machida A., Funatsu G., Nomura M., Usuda S., Aoyagi S., Tachibana K., Miyamoto H., Imai M., Nakamura T. Immunochemical structure of hepatitis B e antigen in the serum. J Immunol. 1983 Jun;130(6):2903–2907. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]