Abstract
The DNA polymerase delta processivity factor Proliferating Cell Nuclear Antigen (PCNA) promotes the DNA damage-induced degradation of the replication initiation factor Cdt1 via the CRL4Cdt2 E3 ubiquitin ligase complex. Here we demonstrate that PCNA promotes the ubiquitylation and degradation of the CDK inhibitor p21 in cells irradiated with low dose of ultraviolet (UV) by a similar mechanism. Human cells that are depleted of Cul4, DDB1 (damage-specific DNA-binding protein-1), or the DCAF Cdt2, are deficient in the UV-induced ubiquitylation and degradation of p21. Depletion of mammalian cells of PCNA by siRNA, or mutations in p21 that abrogate PCNA binding, prevent UV-induced p21 ubiquitylation and degradation, indicating that physical binding with PCNA is necessary for the efficient ubiquitylation of p21 via the CRL4Cdt2 ubiquitin ligase. Cdt2 functions as the substrate recruiting factor for p21 to the rest of the CRL4 ubiquitin ligase complex. The CRL4Cdt2 E3 ubiquitin ligase ubiquitylates p21 both in vivo and in vitro, and its activity is dependent on the interaction of p21 with PCNA. Finally, we show that the CRL4Cdt2 and the SCFSkp2 ubiquitin ligases are redundant with each other in promoting the degradation of p21 during an unperturbed S phase of the cell cycle.
Keywords: CRL4, DDB1, PCNA, p21 ubiquitylation, Cdt2, DCAFs
The eukaryotic cell cycle is tightly controlled, with DNA being replicated with high fidelity once per cell cycle. Cells evolved multiple mechanisms that recognize DNA damage such as those induced by chemotherapeutics, ultraviolet (UV) or γ irradiation, or other cellular stresses. In response to these cellular stresses, cells halt DNA replication by arresting the cell cycle until the damage is repaired. Levels of the CDK inhibitor p21CIP1/WAF1 increase in response to DNA damage primarily due to its transcriptional up-regulation by the tumor suppressor p53. p21 inhibits the kinase activity of Cdk2, thereby preventing progression into or through the S phase of the cell cycle (Brugarolas et al. 1995). Although p21 accumulates after genotoxic stresses, it is degraded in response to DNA damage induced by low-dose UV irradiation, and the ability to degrade p21 under these conditions is critical for optimal DNA repair (Bendjennat et al. 2003; Chen et al. 2004; Lee et al. 2006). Cells irradiated with low-dose UV arrest, nevertheless, as Cdk2 activity is inhibited due to enhanced inhibitory phosphorylation at Tyr15 (Bendjennat et al. 2003).
Several studies have shown how p21 is degraded during an unperturbed cell cycle: At the G1/S transition and during the S phase of the cell cycle, the SCFSkp2 ubiquitin ligase targets p21 for degradation (Yu et al. 1998; Bornstein et al. 2003; Sarmento et al. 2005; Wang et al. 2005), while the CDK inhibitor is ubiquitylated via the APC/CCdc20 ubiquitin ligase during G2/M (Amador et al. 2007). Our understanding of p21 degradation following UV irradiation, on the other hand, remains rather confusing. While some studies argue that this process is proteasome-dependent but ubiquitin-independent, others have suggested the involvement of both components. For example, using mouse embryonic fibroblasts (MEFs) deficient in Skp2, Bendjennat et al. (2003) demonstrated that p21 fails to degrade after low-dose UV irradiation, suggesting that the SCFSkp2 ubiquitin ligase mediates p21 ubiquitylation and its subsequent degradation in irradiated cells. Lee et al. (2006), on the other hand, showed that Skp2 is not required for p21 degradation after UV irradiation and further argued that the degradation of p21 is ubiquitin-independent. A recent study further demonstrated that activation of ATR by a low dose of UV triggers the phosphorylation of p21 at Ser114 by GSK3β and results in proteasomal-dependent, but ubiquitin-independent degradation of p21 (Lee et al. 2007). These results are consistent with the finding that an ATR-dependent pathway is essential for triggering the down-regulation of p21 after UV irradiation in mammalian cells (Bendjennat et al. 2003). Another study suggested that the down-regulation of p21 in response to UV irradiation is facilitated by the ubiquitin ligase activity of the Cul4-RING ubiquitin ligase and its substrate recognition subunit DDB2 (CRL4Ddb2) on p53 (Stoyanova et al. 2008). Thus, the involvement of the ubiquitin system, in general, and Skp2 or other ubiquitin ligases in particular, in degrading p21 in UV-irradiated cells remains to be clarified.
Following UV irradiation, as well as in S phase of the cell cycle, the CRL4Cdt2 E3 ligase (composed of the Cul4A/B, DDB1 [damage-specific DNA-binding protein-1], and the DCAF subunit Cdt2) promotes the ubiquitylation and degradation of the replication factor Cdt1 in cooperation with Proliferating Cell Nuclear Antigen (PCNA) (Higa et al. 2003, 2006; Hu et al. 2004; Arias and Walter 2006; Hu and Xiong 2006; Jin et al. 2006; Ralph et al. 2006; Sansam et al. 2006; Senga et al. 2006). The ability to degrade Cdt1 under these conditions is critical for preventing rereplication and the induction of irradiation-induced early G2/M checkpoint (Sansam et al. 2006). Cdt1 interacts with PCNA via a conserved PCNA-interacting motif (PIP Box) in the N-terminal end of Cdt1, and this interaction is required for Cdt1 ubiquitylation via the CRL4Cdt2 E3 ligase (Arias and Walter 2006; Higa et al. 2006; Senga et al. 2006). Since p21 is known to associate with PCNA through a PIP box in its C-terminal end and is degraded after UV irradiation similarly to Cdt1, we hypothesized that PCNA might mediate the degradation of p21 via the CRL4Cdt2 ubiquitin ligase complex by a similar mechanism. In this study, we show that p21 ubiquitylation and degradation in human cells irradiated with a low dose of UV are dependent on the CRL4 E3 ubiquitin ligase and the substrate-specificity factor Cdt2. The CRL4Cdt2 E3 ubiquitin ligase also promotes the destruction of p21 during the S phase of the cell cycle of unperturbed cells in apparent redundancy with the SCFSkp2 ubiquitin ligase. We show that immuno-purified CRL4Cdt2 E3 ubiquitin ligase promotes the in vitro polyubiquitylation of p21, which is enhanced by a phosphomimetic substitution on Ser114 of p21. Finally, we show that the CRL4Cdt2-mediated ubiquitylation and degradation of p21 requires p21 to physically bind PCNA.
Results
Low-dose UV irradiation triggers Skp2-independent proteasomal degradation of p21
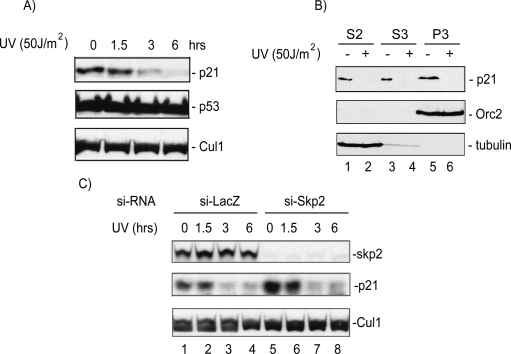
To clarify the role of Skp2 in mediating the UV-induced degradation of p21, we tested whether acute depletion of Skp2 by siRNA would affect UV-induced degradation of p21. In agreement with other reports, irradiation of HeLa cells with low-dose UV caused the degradation of p21, which was maximal 6 h post-irradiation (Fig. 1A). Chromatin-bound p21 was also degraded under these conditions, demonstrating that the degradation of p21 was not limited to soluble pools of the protein (Fig. 1B). The UV-induced degradation of p21 was not limited to HeLa cells, as p21 was efficiently degraded in the osteosarcoma cell line U2OS (Supplemental Fig. S1A,B), and in several other human cell lines as well (data not shown). Although p21 was degraded in response to UV in all the cell lines tested, different cell lines varied with regard to the dose of UV that was necessary to induce maximal p21 degradation (data not shown). The reduction of p21 protein after UV irradiation was not due to transcriptional repression as shown by the lack of a decrease in p21 mRNA levels after UV (Supplemental Fig. S1C) and the fact that exogenous p21 expressed off a heterologous promoter was also degraded (Fig. 4C,D,F, below). The UV-induced proteolysis of p21 was dependent on the proteasome as p21 was not degraded in cells treated with the 26S proteasome inhibitor MG132 (Supplemental Fig. S1A). Consistent with a role of Skp2 in mediating p21 degradation in unstressed cells, acute depletion of Skp2 by siRNA elevated the basal levels of p21 in nonirradiated HeLa cells (Fig. 1C, cf. lanes 1,5). Unlike the results obtained in Skp2-deficient MEFs (Bendjennat et al. 2003), however, acute depletion of Skp2 in HeLa cells had no effect on the UV-induced degradation of endogenous (Fig. 1C) or ectopically expressed p21 (data not shown). Our results are therefore consistent with those reported by Lee et al. (2006) and suggest the existence of yet another proteasome-dependent pathway that is required for the efficient degradation of p21 after low-dose UV irradiation.
Figure 1.
The p21 protein is degraded via a Skp2-independent mechanism in cells irradiated with low-dose UV. (A) Western blot analysis of protein lysate from HeLa cells to test the effect of UV irradiation on p21 degradation, p53 protein level. The CRL1 proteins indicate equal loading. (B) Chromatin-bound p21 is degraded after UV irradiation. Western blot analysis of cytoplasmic (S2), soluble nuclear (S3), and chromatin-bound (P3) lysates of HeLa cells. Anti-Orc2 and anti-tubulin blots demonstrate proper fractionation. (C) A Western blot of HeLa lysates shows that acute silencing of Skp2 by siRNA does not prevent p21 degradation after UV.
Figure 4.
PCNA promotes the polyubiquitylation and degradation of p21 after UV irradiation, and its physical interaction with p21 is necessary for the degradation. (A) si-RNA knockdown of PCNA prevents p21 degradation in UV-irradiated cells. Western blot of lysates of HCT116p53−/− cells transfected with si-GL2 control oligos or siRNA targeting PCNA (si-PCNA) and immunoblotted for the indicated proteins. (B) p21-PCNA does not interact with PCNA. Cell lysates and Flag immunoprecipitates Western blotted for Flag-p21 and PCNA. (C) Western blot of HeLa cells transfected with Flag-p21 or Flag-p21-PCNA with or without exposure to UV. (LC) A cross-reacting band serving as a loading control. (D) Time-course analysis of the degradation of ectopically expressed p21 and p21-PCNA proteins in HeLa cells following UV (20 J/m2). The anti-p21 blot indicates that endogenous wild-type p21 is visibly degraded within 2 h of UV irradiation. Flag-p21-PCNA remains stable for up to 8 h following UV. (E) Quantitation of Flag-p21 proteins displayed in D to determine the rate of degradation of p21 relative to p21-PCNA in response to UV irradiation. (F) Wild-type p21 but not p21-PCNA is efficiently degraded at various low doses of UV irradiation. HCT116p21−/− stably expressing either Flag-p21 or Flag-p21-PCNA harvested 4 h after radiation and immunoblotted for Flag. (*) A cross-reacting band serving as a loading control. (G) Wild-type p21 but not p21-PCNA is efficiently ubiquitylated in vivo and that the ubiquitylation is stimulated upon UV irradiation. (Top) Anti-p21 Western blot analysis of the immunoprecipitated ubiquitylated proteins (with anti-HA antibody) extracted from HCT116p21−/− coexpressing wild-type p21 or p21-PCNA and HA-tagged ubiquitin. (Bottom) Anti-p21 Western blot of the anti-Flag immunoprecipitates demonstrates that p21 and p21-PCNA were expressed to similar levels in these cells. A low exposure of the nonubiquitylated p21 is shown as well. The X-ray films were scanned, and the ratio of the ubiquitylated p21 to that of total p21 (ubiquitylated + nonubiquitylated p21 from the nonsaturating lower-exposure film) is indicated below each lane.
p21 degradation in UV-irradiated cells is inhibited upon depletion of Cul4A/B, DDB1, or Cdt2
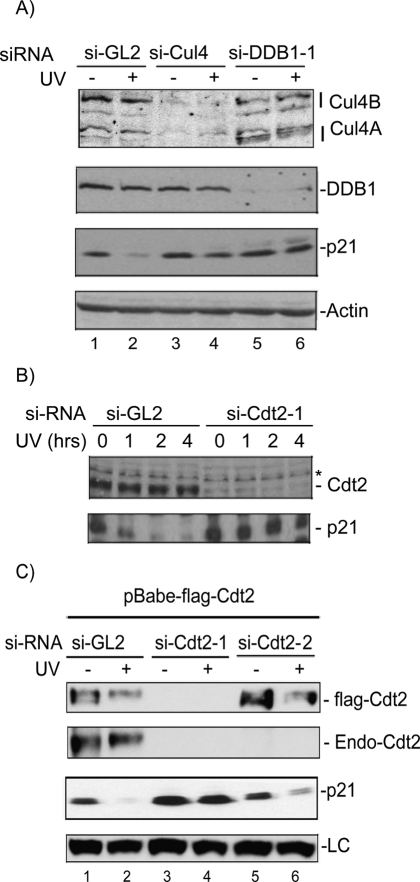
Because p21 associates with PCNA via its PIP motif similarly to Cdt1, we speculated that the ubiquitylation and degradation of p21 after UV irradiation is carried out via the same E3 ubiquitin ligase, which promotes the DNA damage-induced and PCNA-dependent degradation of Cdt1. We first tested whether p21 degradation after UV is dependent on Cul4 proteins. Transient transfection of the human colon cancer cell line HCT116 lacking the p53 gene (HCT116p53−/−) with siRNA oligos targeting both Cul4A and B resulted in a significant reduction in Cul4A and B (Fig. 2A). We used this cell line to avoid indirect effects of the Cul4 on p21 through p53. Silencing Cul4 in this cell line for 48 h produced a small but reproducible increase in the basal levels of p21 (Fig. 2A, lanes 1,3). Significantly, in cells depleted of Cul4A/B, p21 was largely protected from UV-induced degradation (Fig. 2A). The degradation of p21 in response to a low dose of UV was also blocked when we down-regulated DDB1, the Cul4 adaptor protein that bridges the DCAF substrate recognition subunit to the rest of the CRL4 E3 ligase (Hu et al. 2004; Wertz et al. 2004), by two different siRNA oligos (Fig. 2A; Supplemental Fig. S2A). Similar results were observed in several other cell lines (data not shown). We conclude therefore that Cul4 and DDB1 are both required for the efficient degradation of p21 in UV-irradiated cells and that this effect is independent of the tumor suppressor p53.
Figure 2.
The CRL4Cdt2 E3 ubiquitin ligase is required to efficiently degrade p21 in UV-irradiated cells. (A) si-RNA knockdown of Cul4A/B or DDB1 prevents p21 degradation after UV. Western blot from nonirradiated or UV-irradiated (20 J/m2) HCT116p53−/− after knockdown of Cul4A and B (si-Cul4), DDB1 (si-DDB1-1), or control siRNA (si-GL2). Actin is shown as a loading control. (B) siRNA knockdown of the DCAF Cdt2 prevents p21 degradation by UV. Western blot of HCT116p53−/− after knockdown of Cdt2 or control si-GL2, UV-irradiated (20 J/m2) and harvested after the indicated hours post-irradiation. (*) A cross-reacting band in the anti-Cdt2 blot that serves as a loading control. (C) Ectopic expression of si-RNA-resistant Cdt2 restores the UV-induced degradation of p21. U2OS cells stably expressing Flag-epitope-tagged Cdt2 (without the 3′UTR) are transfected with either si-GL2 or siRNA targeting the ORF (si-Cdt2-1), or the 3′UTR of Cdt2 (si-Cdt2-2) and UV-irradiated 6 h prior to harvesting. The anti-Cdt2 blot shows endogenous Cdt2 (Endo-Cdt2). An anti-Flag blot shows the exogenously expressed Cdt2 and its efficient silencing by si-Cdt2-1 but not si-Cdt2-2. Exogenous Cdt2 was not shown in the same blot because it overlaps with a cross-reactive band that runs higher than the endogenous protein. (LC) A cross-reacting band in the anti-p21 blot serving as a loading control.
To address whether the DCAF Cdt2 has a role in the degradation of p21 after low-dose UV irradiation, we measured the steady-state levels of p21 in cells depleted of Cdt2 by siRNA. Down-regulation of Cdt2 with two different siRNA oligos inhibited p21 down-regulation following UV (Fig. 2B; Supplemental Fig. S2B,C). Time-course analysis of p21 levels shows that while p21 is detectably degraded within an hour in control cells, it became resistant to degradation even 4 h post-irradiation in cells depleted of Cdt2 (Fig. 2B). The effect of down-regulation of Cdt2 on p21 degradation in response to UV was specific since depletion of DDB2, an irrelevant DCAF, had no effect on the UV-induced degradation of p21 (Supplemental Fig. S2C). Significantly, in U2OS cells stably expressing Flag-epitope-tagged Cdt2 (without 3′ untranslated region [UTR]), si-Cdt2-1 (targeting the ORF) but not si-Cdt2-2 (targeting the 3′UTR) oligo, inhibits UV-induced degradation of p21 (Fig. 2C). Thus, the inhibition of p21 degradation by siRNA against Cdt2 was a direct consequence of Cdt2 down-regulation and not due to off-target activity of the siRNA duplex. The stabilization of p21 in UV-irradiated cells depleted of Cul4, DDB1, or Cdt2 was not due to secondary effects of the cell cycle since unlike U2OS or HeLa cells, which undergo G2/M-phase accumulation in the absence of Cdt2 (Jin et al. 2006; data not shown), HCT116p53−/− cells depleted of Cdt2, Cul4, or DDB1 did not exhibit significant changes in their cell cycle profiles (Supplemental Fig. S2D). Collectively, these results demonstrate that the CRL4 E3 ubiquitin ligase requires the DCAF, Cdt2 for the efficient degradation of p21 in UV-irradiated cells.
p21 associates with the CRL4Cdt2 ubiquitin ligase complex via the DCAF, Cdt2
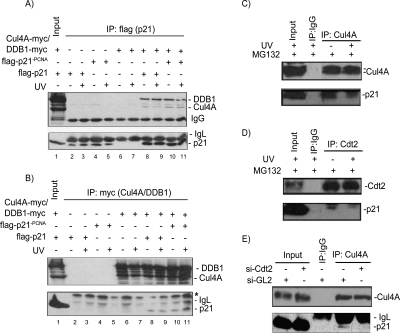
We tested whether p21 associates with components of the CRL4 ubiquitin ligase complex in vivo. When ectopically expressed, both DDB1 and Cul4A readily coimmunoprecipitated with p21 (Fig. 3A, lane 8) and reciprocally, p21 could easily be detected in Cul4A and DDB1 immunoprecipitates (Fig. 3B, lane 8). We observed that DDB1 was enriched in the p21 immunoprecipitates relative to Cul4A (Fig. 3A,B, lane 8), suggesting that this interaction is mediated primarily through DDB1. These results are consistent with the role of DDB1 in binding substrates either directly or through DCAFs and bridging them to the CRL4 E3 ligase (Hu et al. 2004; Wertz et al. 2004). To test the effect of UV irradiation on the association between p21 and Cul4A–DDB1, we treated cells overexpressing these proteins with UV for 6 h in the presence of MG132 to prevent p21 degradation by the 26S proteasome. The association between p21 and Cul4A and DDB1 was not stimulated by UV irradiation under these conditions (Fig. 3A,B, lanes 8,9). The association between p21 and Cul4A was specific since we were only able to detect endogenous p21 in Cul1 and Cul4A, but not in Cul2, Cul3, or Cul5 immunoprecipitates (data not shown). Furthermore, endogenous p21 coimmunoprecipitated with endogenous Cul4A both in nonirradiated and UV-irradiated cells (Fig. 3C), demonstrating that the interactions between p21 and Cul4A and DDB1 were not an artifact of overexpression. Importantly, endogenous p21 coimmunoprecipitated with endogenous Cdt2 (Fig. 3D), suggesting that Cdt2 may promote p21 association with the CRL4 ubiquitin ligase complex. Consistent with this, depletion of HeLa cells of Cdt2 by siRNA significantly diminished Cul4–p21 interaction (Fig. 3E). Therefore, the association of p21 with Cul4 and DDB1 is mediated primarily by its association with Cdt2. This finding is consistent with the proposed role of Cdt2 as the substrate recognition subunit that bridges the substrate to the rest of the CRL4 ubiquitin ligase (Jin et al. 2006).
Figure 3.
p21 interacts with the CRL4Cdt2 ubiquitin ligase in vivo. (A) Ectopically expressed Cul4A-myc and DDB1-myc coimmunoprecipitate with ectopically expressed Flag-p21 or Flag-p21-PCNA. 293T Cells were transiently transfected with plasmids encoding the indicated proteins for 48 h and treated with MG132 for 6 h prior to harvest. Anti-myc Western blot of the anti-Flag immunoprecipitates prepared from 293T cells overexpressing the indicated proteins and shows that Cul4A and DDB1 associate with wild-type p21 and p21-PCNA. An anti-Flag blot demonstrates equal p21 proteins in the anti-Flag immunoprecipitates. (B) The reciprocal of the experiment performed in A, where Flag-p21 (wild type and p21-PCNA) coimmunoprecipitates with CRL4A and DDB1 proteins in the myc IP. (C,D) Endogenous p21 interacts with endogenous Cul4A (C), and Cdt2 (D) in HeLa cells. Lysates of HeLa cells (nonirradiated or UV-irradiated [50 J/m2] and treated with MG132 immediately after irradiation for 6 h prior to harvest) were immunoprecipitated with control anti-IgG (C,D), anti-CRL4A (C), or anti-Cdt2 (D) antibodies. The anti-p21 Western blots show that endogenous p21 is specifically coimmunoprecipitated with Cul4A (C), and Cdt2 (D). (E) Cdt2 mediates the association between p21 and the rest of the CRL4 E3ligase. Proteins extracted from control si-GL2 or si-Cdt2-transfected HeLa cells were immunoprecipitated with the indicated antibodies and immunoblotted with anti-Cul4 or anti-p21. The results show that endogenous p21 protein associates with endogenous Cul4A only in the presence of Cdt2.
The CRL4Cdt2 ubiquitin ligase complex has been shown to target Cdt1 for destruction when it is bound to PCNA on chromatin (Jin et al. 2006). To test whether PCNA plays a role in promoting p21–CRL4Cdt2 association, we created a mutant of p21 with three point substitutions in its PIP box that abrogate its interaction with PCNA (p21-PCNA). A similar PCNA-binding-deficient mutant of p21 has been previously shown to localize correctly to the nucleus and associate with known partners, including CDKs (Cayrol et al. 1998). We confirmed that, in contrast to wild-type p21, p21-PCNA does not associate with endogenous PCNA in HEK293T cells (Fig. 4B). The association of p21 with Cul4A did not depend on the interaction of p21 with PCNA since both DDB1 and Cul4A could still associate with p21-PCNA (Fig. 3A,B, lanes 10,11). Although this outcome can be attributed to the high levels of the proteins in overexpression experiments, they may suggest that residues in p21 independent of the PIP motif contribute to binding to CRL4Cdt2.
PCNA is required for the efficient ubiquitylation and degradation of p21 after UV irradiation
We and others have shown that PCNA is required for the efficient ubiquitylation and degradation of Cdt1 via the CRL4Cdt2 E3 ligase both in human cells and in Xenopus egg extracts (Arias and Walter 2006; Higa et al. 2006; Hu and Xiong 2006; Jin et al. 2006; Senga et al. 2006). We therefore tested whether PCNA plays a role in the CRL4Cdt2-mediated degradation of p21 in response to UV. Down-regulation of PCNA by siRNA significantly increased the basal levels of p21 and inhibited the degradation of p21 after UV irradiation without affecting the steady-state levels of Cul4 (data not shown), DDB1, or Cdt2 (Fig. 4A). We next investigated whether the direct binding of PCNA with p21 is important for the degradation of p21 after UV irradiation. To address this, we transiently overexpressed wild-type p21 or p21-PCNA in mammalian cells and tested the effect of UV on the degradation of p21. Although wild-type p21 was readily degraded in response to low-dose UV irradiation, p21-PCNA failed to degrade in response to UV irradiation (Fig. 4C). Whereas the half-life of exogenously expressed wild-type p21 was <2 h in UV-irradiated cells, p21-PCNA did not show signs of degradation up to 6 h post-irradiation (Fig. 4D,E). HCT116 stably expressing either wild-type p21 or p21-PCNA was also irradiated with various doses of UV. Whereas the wild-type p21 was readily degraded when cells were irradiated with 20 J/m2, p21-PCNA was resistant to degradation even at 100 J/m2 (Fig. 4F). The results demonstrate that the direct interaction of p21 with PCNA is critical for the UV-induced degradation of p21 via the CRL4Cdt2 E3 ubiquitin ligase. To test the role of PCNA on the ability of p21 to be ubiquitylated in vivo, we transiently transfected HCT116 cells lacking the p21 gene (HCT116p21−/−) with either wild-type p21 or p21-PCNA along with Hemagglutinin-tagged (HA) ubiquitin. Forty-eight hours after transfection, cells were directly lysed in boiling lysis buffer to inactivate isopeptidases (Govers et al. 1997). Wild-type p21 was efficiently ubiquitylated even without UV irradiation, while p21-PCNA was not ubiquitylated as well (Fig. 4G). The anti-p21 Western in the lower panel shows that the levels of wild-type p21 or p21-PCNA are roughly comparable. Significantly, while the ubiquitylation of wild-type p21 was stimulated by UV irradiation, we were not able to detect ubiquitylated p21-PCNA even after UV irradiation (Fig. 4G). These results confirm that low-dose UV irradiation stimulates PCNA-dependent p21 ubiquitylation and degradation and suggest that PCNA promotes the ubiquitylation of p21 during the normal cell cycle in the absence of UV irradiation as well (see below and the Discussion).
The CRL4Cdt2 E3 ligase promotes p21 polyubiquitylation in a PCNA-dependent manner in vitro
To test whether the CRL4Cdt2 E3 ubiquitin ligase is capable of ubiquitylating p21 in vitro, we overexpressed Cul4A, DDB1, and Cdt2 along with the Ring finger protein Rbx1/Roc1 in 293T cells. Flag-epitope-tagged Rbx1/Roc1 and Cdt2 associated with overexpressed Cul4A and DDB1 (Fig. 5A), thus confirming the formation of the E3 ubiquitin ligase complex in vivo. We then tested the ability of the CRL4Cdt2 E3 ligase to ubiquitylate in vitro p21 that was separately overexpressed and purified (together with PCNA) from 293T cells. Incubation of the CRL4Cdt2 ubiquitin ligase with E2 and p21 produced high-molecular-weight ubiquitylated p21 species (Fig. 5A). Similar results were obtained when we purified the CRL4 E3 ligase by immuno-precipitating the Cul4A and DDB1 subunits instead (Fig. 5B), thus ruling out the possibility that p21 was ubiquitylated by a contaminating Skp2 in the Rbx1 immunoprecipitates. Dropout experiments confirmed that the in vitro ubiquitylation of p21 by the CRL4Cdt2 ubiquitin ligase requires Cul4A, DDB1, and Rbx1 (data not shown). However, overexpressed Cul4A and DDB1 associated readily with endogenous Cdt2 so that dropping out overexpressed Cdt2 did not affect p21 ubiquitylation in vitro (data not shown). Because the Cul4A associates with many other DCAFs to promote ubiquitin transfer, we tested whether Cdt2 is the relevant DCAF for ubiquitylating p21 in vitro. Initially, we were not able to down-regulate Cdt2 by siRNA and test the ability of overexpressed Cul4A to promote p21 ubiquitylation since overexpression of DDB1 stabilized the Cdt2 protein (data not shown). However, immunoprecipitates of Flag-Cdt2 from 293T cells, without overexpressing any other components of the E3 ligase, purified an E3 that ubiquitylated p21 in vitro (Fig. 5C). This result is consistent with the requirement of Cdt2 in promoting p21 degradation following UV irradiation (Fig. 2B,C).
Figure 5.
CRL4Cdt2 E3 ubiquitin ligase promotes p21 ubiquitylation in vitro. (A,B) The CRL4Cdt2 E3 ubiquitin ligase complex polyubiquitylates p21 in vitro. CRL4Cdt2 enzyme complexes (with the indicated overexpressed proteins on top) were immuno-purified using either (A) anti-Flag or (B) anti-myc antibodies and mixed with separately immuno-purified p21 substrate in an in vitro ubiquitylation reaction. Western blots for DDB1 and CRL4A (anti-myc), Cdt2 (anti-Cdt2) demonstrate the formation of the stable E3 ligase in 293T cells used for the enzyme complex preparation. The anti-PCNA blot shows that PCNA copurified with p21. Anti-p21 immunoblots show the formation of higher p21 molecular weight species corresponding to polyubiquitylated p21. (C) Cdt2 containing E3 ligase is capable of ubiquitylating p21 in vitro. Flag-Cdt2 expressed stably in 293T cells, immunoprecipitated with anti-Flag agarose and incubated with separately purified p21 protein in an in vitro ubiquitylation reaction. Mock IP from control 293T cells were used as a negative control. A high exposure of the anti-p21 blot is shown (right) with ubiquitylated p21 labeled with asterisks (*). The results demonstrate that CRL4Cdt2 is sufficient to polyubiquitylate p21 in vitro.
To explore the role of PCNA in mediating the ubiquitylation of p21 in vitro, we purified the CRL4Cdt2 E3 ligase and p21 (separately) from cells that were depleted of PCNA by siRNA. In the absence of PCNA, the CRL4Cdt2 is largely deficient in promoting p21 polyubiquitylation (Fig. 6A). Furthermore, while the wild-type p21 was ubiquitylated via the CRL4Cdt2 in a time-dependent manner, the p21-PCNA was not (Fig. 6B). Together, these data strongly suggest that PCNA is essential for the in vitro ubiquitylation of p21 via the CRL4Cdt2 ubiquitin ligase. Significantly, whereas the SCFSkp2 efficiently polyubiquitylated wild-type p21 in vitro (Fig. 6C, lane 5), the CRL4Cdt2 did so only when we used a mutant of p21 that carries a phosphomimetic substitution mutation at Ser114 (S114E) as a substrate (Fig. 6C, lane 4), thus explaining the requirement of ATR and the phosphorylation of p21 at Ser114 for the degradation of p21 following UV irradiation (Bendjennat et al. 2003; Lee et al. 2007).
Figure 6.
PCNA is required for the efficient ubiquitylation of p21 in vitro and p21 ubiquitylation is enhanced by phosphomimetic substitution of p21 at Ser114. (A) In vitro ubiquitylation of p21 via the CRL4Cdt2 E3 ligase purified from cells that were depleted of PCNA by siRNA and overexpressing Cul4A, DDB1, Cdt2, and Rbx1 (CRL4Cdt2). The p21 substrate was purified separately from cells also depleted of PCNA. The Western blot demonstrates that p21 is ubiquitylated via the CRL4Cdt2 E3 only when the substrate, p21, copurified with PCNA. The anti-PCNA Western blot shows that PCNA was absent in the substrate preparation from 293T cells that were treated with si-PCNA. (B) Wild-type p21 but not p21-PCNA is ubiquitylated via the CRL4Cdt2 E3 ligase. Time-course analysis of the CRL4Cdt2 E3-mediated ubiquitylation of p21 proteins in vitro. The anti-p21 blot shows that p21 and p21-PCNA are equal in both reaction sets. Time indicates the incubation time in minutes. The anti-PCNA blot shows that PCNA copurifies only with wild type but not the p21-PCNA mutant. (C) In vitro ubiquitylation of wild-type p21 (lanes 1–3) or p21 with a phosphomimetic substitution at Ser114 (p21S114E) (lane 4) via the CRL4Cdt2 ubiquitin ligase. (Lane 5) In vitro ubiquitylation of wild-type p21 via immuno-purified CRL1 E3 (Skp2-dependent ligase) is also shown for comparison. The Western blot was probed for p21 to demonstrate the formation of p21 polyubiquitin chains. The p21S114E runs slightly higher than wild-type protein. A low exposure of the anti-p21 blot demonstrates equal p21 and p21S114E substrates.
The CRL4Cdt2 ligase promotes p21 destruction during the S phase of the cell cycle of unperturbed cells
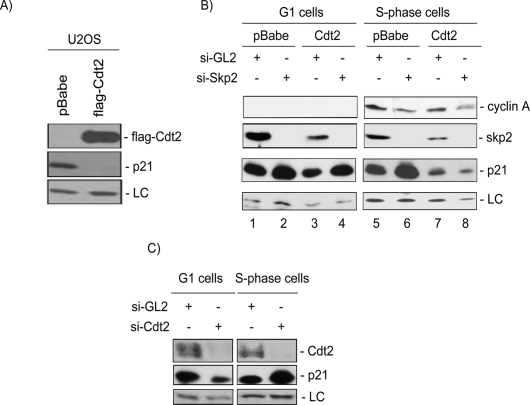
The CRL4Cdt2 ubiquitin ligase complex promotes the degradation of Cdt1 in response to UV and is redundant with SCFSkp2 in mediating Cdt1 degradation during the S phase of the cell cycle (Jin et al. 2006; Nishitani et al. 2006; Senga et al. 2006). The depletion of Cul4, DDB1, Cdt2, or PCNA by siRNA not only prevented the UV-induced degradation of p21 but also stabilized p21 in nonirradiated cells (Figs. 2, 4A; data not shown). Additionally, the in vivo ubiquitylation of p21 demonstrated that wild type but not p21-PCNA was efficiently ubiquitylated in nonirradiated cells (Fig. 4G). These observations raised the possibility that the CRL4Cdt2 ligase might promote the ubiquitylation of p21 in a PCNA-dependent manner in the S phase of nonirradiated cells as well. To test this hypothesis, we examined the steady-state levels of p21 in U2OS cells stably overexpressing a Flag-epitope-tagged version of Cdt2. Consistent with the hypothesis that CRL4Cdt2 may regulate p21 protein levels during the cell cycle, we found that asynchronous U2OS cells overexpressing Cdt2 had significantly lower levels of p21 protein (Fig. 7A) without significant changes in p21 mRNA (data not shown). Similar results were obtained with HCT116p53−/− (data not shown). To test whether Cdt2 controls p21 level specifically in S-phase cells, we synchronized the cells by release from a Nocodazole block. To eliminate the SCFSkp2 ligase that is known to degrade p21 in S phase, the cells were also transfected with siRNA to Skp2 as indicated (Fig. 7B). Consistent with the role of Skp2 in promoting p21 degradation in unperturbed cells (Yu et al. 1998; Bornstein et al. 2003; Sarmento et al. 2005; Wang et al. 2005), si-RNA against Skp2 elevated the basal p21 protein levels during the G1 and S phases of the cell cycle (Fig. 7B, cf. lanes 2,6 and 1,5). Cdt2 overexpression did not significantly decrease p21 in G1 cells or counter p21 stabilization by si-Skp2. However, Cdt2 overexpression significantly reduced p21 protein levels in S-phase cells (Fig. 7B, cf. lanes 5 and 7). Furthermore, the overexpressed Cdt2 in S-phase cells countered the stabilization of p21 by si-Skp2 (Fig. 7B, cf. lanes 6 and 8). Thus, the reduced steady-state levels of p21 protein in Cdt2 overexpressing cells are due to enhanced degradation of p21 protein via the CRL4Cdt2 specifically in S-phase cells. Consistent with this conclusion, siRNA against Cdt2 in HeLa cells specifically stabilized p21 protein in S-phase but not in G1-phase cells (Fig. 7C). Together, these results demonstrate that the CRL4Cdt2 and SCFSkp2 ubiquitin ligases function redundantly to degrade p21 during the S phase of the cell cycle.
Figure 7.
Cdt2 promotes the destruction of p21 in S-phase cells. (A) Stable expression of Cdt2 significantly reduces the steady-state levels of p21 in asynchronous U2OS cells. Soluble protein lysates of Mock or Cdt2-overexpressing U2OS cells were resolved on SDS-PAGE and analyzed for Cdt2 (anti-Flag) and p21 protein levels. (LC) A cross-reacting band in the anti-p21 blot serving as a loading control. (B) Cdt2 specifically targets p21 in S phase of the cell cycle. Control or Cdt2 overexpressing U2OS cells were transfected either with control siRNA (si-GL2) or with siRNA against Skp2. Forty-eight hours after transfections, cells were synchronized with nocodazole, and mitotic cells were collected by shake-off, washed with PBS, and incubated for an additional 8 h (G1 cells) or 16 h (S-phase cells), and the cell lysates were analyzed for the indicated proteins. Cyclin A levels show the synchronization of cells in G1 (left) and S phase (right) of the cell cycle. The results demonstrate that Skp2 down-regulation by siRNA results in the accumulation of p21 in both G1- and S-phase cells. On the other hand, ectopic expression of Cdt2 significantly reduced p21 only in S-phase cells, and that reduction occurred even after down-regulating Skp2 (lanes 7,8). (C) Endogenous Cdt2 promotes the destruction of p21 during S phase of the cell cycle. HeLa cells transfected with either control si-RNA (si-GL2) or siRNA targeting Cdt2 (si-Cdt2) and synchronized in G1 and S phases of the cell cycle as described in B. Protein lysates were subsequently analyzed as in B. The anti-Cdt2 blot demonstrates efficient down-regulation of Cdt2 in cells transfected with si-Cdt2. (LC) A cross-reactive band in the anti-p21 blot used as loading control. The results demonstrate that p21 protein is specifically stabilized in S-phase cells but not in cells in the G1 phase of the cell cycle upon depletion of Cdt2.
Discussion
The CRL4Cdt2 E3 ubiquitin ligase promotes UV-induced ubiquitylation and degradation of p21
In this study, we discovered the E3 ligase that promotes the ubiquitylation and subsequent degradation of the CDK inhibitor p21 in response to low-dose UV irradiation. Although acute depletion of Skp2 in mammalian cells by siRNA elevated the basal levels of p21, it did not interfere with its degradation following irradiation. On the other hand, silencing any of the components of the CRL4Cdt2 E3 ubiquitin ligase by siRNA not only elevated the basal levels of p21, it also blocked p21 degradation following UV (Fig. 2). Although it is known that high levels of p21 inhibit its proteasomal degradation (Cayrol and Ducommun 1998), possibly by inactivating cyclin/CDK complexes, it is unlikely that p21 interferes with its degradation in response to UV irradiation since overexpression of wild-type p21 did not interfere with its degradation following UV radiation (Fig. 4C,D,F). In fact, cells depleted of Skp2 had significantly higher levels of p21, yet p21 was degraded with similar kinetics as in control cells following radiation. Consistent with the requirement for the CRL4Cdt2 in promoting p21 degradation following UV irradiation, immunopurified CRL4Cdt2 E3 ubiquitin ligase was sufficient for ubiquitylating p21 in vitro (Fig. 5). The p21 polyubiquitylation promoted via the CRL4Cdt2 in vitro was most efficient when p21 carried a phosphomimetic substitution at S114 (Fig. 6C). The results are consistent with the findings that p21 degradation following UV irradiation is dependent on ATR and GSK3β-mediated phosphorylation of p21 at S114 (Bendjennat et al. 2003; Lee et al. 2007). Lee et al., however, were not able to demonstrate UV-dependent enhancement of p21 polyubiquitylation in vivo (Lee et al. 2007). One possibility is that prolonged treatment of cells with MG132 prior to and several hours following irradiation may have resulted in the accumulation of ubiquitylated p21 to saturating levels even in nonirradiated cells in their experiments, obscuring the increase in polyubiquitylation following UV. We speculate that p21 phosphorylation at S114 may increase the affinity of the CRL4Cdt2 or PCNA for p21. Alternatively, phosphorylation may induce a conformational change in p21 exposing a lysine residue to serve as a receptor for protein ubiquitylation. Further investigation is required to distinguish between these two possibilities.
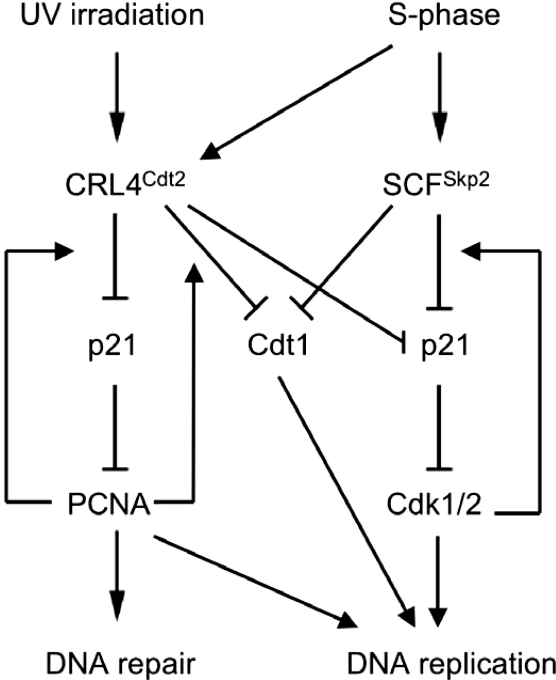
It has been proposed that Cdt2, similar to other DCAFs, may function as a substrate recognition subunit of the CRL4Cdt2 ligase (Jin et al. 2006 and references therein), although there was no formal demonstration that Cdt2 binds the substrate Cdt1. We here show that the association between Cul4 and p21 is lost in cells depleted of Cdt2 by siRNA (Fig. 3E), and thus provide the first example of substrate association with the CRL4 E3 ligase in a Cdt2-dependent manner. The degradation of p21 in response to UV is critical for optimal DNA repair by alleviating the repression of PCNA and promoting translesion DNA synthesis (Bendjennat et al. 2003; Chen et al. 2004; Lee et al. 2006). Thus, our findings suggest that CRL4Cdt2 E3 ubiquitin ligase is critical for the cell’s response to DNA damage, because it not only suppresses replication licensing by targeting Cdt1 for degradation (Arias and Walter 2006; Sansam et al. 2006; Senga et al. 2006), but also promotes DNA repair by promoting the degradation of p21 (Fig. 8).
Figure 8.
Model of the dual role for the CRL4Cdt2 E3 ubiquitin ligase in response to low-dose UV irradiation and during the S phase of the cell cycle. The E3 ubiquitin ligase targets the replication factor Cdt1 for degradation, thereby suppressing replication licensing until the damage is repaired or until the G1 phase of the next cell cycle. Meanwhile, the same E3 ubiquitin ligase targets the Cdk inhibitor p21 for degradation, thereby allowing PCNA to function free of inhibitory p21 in promoting DNA repair or to allow the cells to progress through the S phase in the absence of DNA damage (the latter function being partially redundant with the Skp2-mediated degradation of p21). Both activities are dependent on the physical interaction of both of these substrates to PCNA.
The CRL4Cdt2 ubiquitin ligase functions redundantly with SCFSkp2 in promoting S-phase destruction of p21
In cells depleted of Cul4, DDB1, Cdt2, or PCNA, the UV-induced degradation of p21 was impaired. However, p21 was stabilized in nonirradiated cells as well under these conditions. Moreover, the p21 protein deficient in binding PCNA (p21-PCNA) was ubiquitylated less efficiently than wild-type p21 not only in UV-irradiated cells but also in nonirradiated cells (Fig. 4G), demonstrating that PCNA binding is critical for the in vivo ubiquitylation of p21 in nonirradiated cells. Several other findings supported the conclusion that the CRL4Cdt2 ubiquitin ligase may promote p21 degradation in nonirradiated cells. First, the activity for the CRL4Cdt2 is not strictly dependent on DNA damage elicited by UV irradiation as the enzyme is also active in S phase and promotes the ubiquitylation and degradation of the replication factor Cdt1 (Jin et al. 2006; Senga et al. 2006). The polyubiquitylation of the tumor suppressor protein Merlin by means of CRL4VprBp is another example where the ubiquitin ligase activity is independent of DNA damage and is instead activated by mitogens (Huang and Chen 2008). Second, after incubation of the cells with the proteasome inhibitor MG132 6 h prior to harvesting, the interaction between CRL4Cdt2 and p21 is seen in nonirradiated cells (Fig. 3A–D). Finally, CRL4Cdt2 purified from nonirradiated cells actively ubiquitylated p21 in vitro. Consistent with a role of the CRL4Cdt2 E3 ligase in promoting p21 degradation in unperturbed cells, the steady-state level of p21 was significantly reduced in Cdt2-overexpressing U2OS cells without significant changes in p21 mRNA levels. The decrease in p21 protein in Cdt2-overexpressing cells was most obvious in the S-phase population of cells (Fig. 7B). Significantly, S-phase cells depleted of Cdt2 by siRNA accumulated p21, whereas G1 cells did not (Fig. 7C). Therefore, the CRL4Cdt2 ubiquitin ligase is redundant with SCFSkp2 in promoting the destruction of p21 in S-phase cells in a similar fashion to that observed for the replication factor Cdt1. Interestingly, whereas the CRL4Cdt2-dependent ubiquitylation of p21 depends on p21 interaction with PCNA and is stimulated by GSK3β-dependent phosphorylation on Ser114, SCFSkp2-dependent ubiquitylation of p21 is promoted by p21 binding to and phosphorylation by Cdk1/2 on Ser130 (Bornstein et al. 2003; Wang et al. 2005; Zhu et al. 2005). Thus, it is possible that CRL4Cdt2 preferentially frees PCNA from p21, while SCFSkp2 preferentially targets p21 in complex with Cdk1/2 (see model in Fig. 8). The importance of the CRL4Cdt2-dependent pathway for degrading CDK inhibitors in S phase of the cell cycle is underlined by the independent discovery that this pathway is conserved in Caenorhabditis elegans and of critical importance in cell cycle regulation (E. Kipreos, pers. comm.). Cdt2 could be a major player in shifting the equilibrium toward p21 destruction both during the progression of cells through the S phase of the cell cycle by allowing cyclin–Cdk1/2 to function in the absence of inhibitory p21, and following DNA damage to allow PCNA-dependent DNA repair (Fig. 8).
PCNA: a common cofactor for substrate ubiquitylation via the CRL4Cdt2 ubiquitin ligase?
We demonstrated that the depletion of human cells of PCNA by siRNA stabilized p21 in nonirradiated cells and prevented the down-regulation of p21 following UV. Similarly, a p21 mutant deficient in PCNA binding is resistant to UV-induced degradation and is not well ubiquitylated in nonirradiated cells or post-irradiation. This latter finding clearly demonstrates that the direct binding of p21 to PCNA is critical for its efficient ubiquitylation via the CRL4Cdt2 E3 ligase in vivo. Furthermore, a p21 mutant deficient in PCNA binding was largely resistant to ubiquitylation in vitro (Fig. 6B). The ability of the CRL4Cdt2 ligase to polyubiquitylate p21 in vitro was significantly impaired when both the enzyme and substrate complexes were purified from cells depleted of PCNA by siRNA (Fig. 6A). These results demonstrate that PCNA is an obligatory cofactor for p21 ubiquitylation via the CRL4Cdt2 ubiquitin ligase. The requirement for direct interaction with PCNA as a prerequisite for efficient ubiquitylation is not a unique feature for p21. At least two other substrates, Cdt1 (Arias and Walter 2006; Higa et al. 2006; Hu and Xiong 2006; Jin et al. 2006; Senga et al. 2006) and p53 (Banks et al. 2006), have now been shown to be ubiquitylated via CRL4Cdt2 ubiquitin ligase in a PCNA-dependent manner. In the latter study, however, the p53 ubiquitylation depended on Mdm2 (Banks et al. 2006), and an indirect effect of CRL4Cdt2 E3 ligase on p53 ubiquitylation was not ruled out. Based on in vitro ubiquitylation assays, however, p21 is thus the second PCNA-dependent substrate of the CRL4Cdt2 ubiquitin ligase complex.
The role that PCNA plays in promoting substrate ubiquitylation via the CRL4Cdt2 E3 ubiquitin ligase is not entirely clear. It has been shown the Cdt1–PCNA interaction is necessary to recruit the CRL4Cdt2 ubiquitin ligase to chromatin (Jin et al. 2006). Although it is the docking of Cdt1 onto chromatin-bound PCNA that generates a signal for recruiting the CRL4Cdt2 ligase and ultimately Cdt1 ubiquitylation and destruction (Jin et al. 2006), this may not be the case for all CRL4Cdt2 substrates. Based on overexpression experiments, we show that p21 associates with DDB1 and Cul4A regardless of its ability to bind PCNA (Fig. 3A,B). Nonetheless, because proteins were overexpressed in this experiment, we cannot rule out that for endogenous p21, binding with PCNA may be obligatory before recognition by the CRL4Cdt2 ubiquitin ligase. We are currently investigating the mechanism by which PCNA promotes ubiquitin transfer to various substrates by the CRL4Cdt2 E3 ligase to test whether PCNA is involved in substrate recognition or it stimulates the enzymatic activity of CRL4Cdt2 on certain substrates. It remains to be seen whether PCNA serves as a cofactor only for substrates of CRL4Cdt2 or also for other CRL4-based E3 ubiquitin ligases. The role of PCNA as a cofactor in promoting the activity of CRL4Cdt2 on at least two substrates is intriguing. We speculate that this requirement causes the degradation of Cdt1 or p21 to initiate at sites in the cell where there is a high concentration of PCNA; i.e., chromatin with DNA replication forks or repair complexes. Subsequent recruitment of new substrate to these sites will, of course, eventually decrease the substrate in other parts of the cell.
Materials and methods
Cell lines, antibodies, and reagents
The HCT116p53−/− and HCT116p21−/− cells have been described (Bunz et al. 1998). Other cells were obtained from the American Type Culture Collection (ATCC). The antibodies purchased were p21 (C-19), PCNA (PC10), Cul1 (D-5), Cul4 (C-19), HA (Y-11), and Skp2 (H435) (Santa Cruz Biotechnologies); against Cul4A and DDB2 (Rockland Immunochemicals); and against DDB1 (Invitrogen). The anti-Cdt2 antibody was generated by immunizing rabbits with the C-terminal 50 amino acids that correspond to the human Cdt2 protein fused to GST according to standard procedure. Cells were lysed in a Triton X-100 lysis buffer (50 mM Tris at pH 7.5, 250 mM NaCl, 0.1% Triton X-100, 1 mM EDTA, 50 mM NaF, protease inhibitor cocktail [Sigma]). Chromatin fractionation of HeLa cells was essentially as described (Mendez and Stillman 2000). RNA for Northern blotting was prepared from cells using Trizol reagent (Invitrogen), and Northern Blot analysis was performed according to standard procedures.
Plasmid construction, transient transfections, and stable cell line generation
A mammalian expression plasmid encoding Flag-p21 was prepared by subcloning the full-length human p21 cDNA into pEEFN vector (BamHI, NotI) encoding an N-terminal Flag-epitope upstream of the cloning site. The pEEFN-Flag-p21-PCNA has three point mutations in the PCNA-binding motif (changing Q144, M147, and F150 to A). The plasmid was generated using standard PCR mutagenesis. The Roc1/Rbx1 mammalian expression plasmid was constructed by PCR amplification of the human Roc1 cDNA from PC3 human prostate cancer cells and cloned between the BamHI and NotI sites of the pEEF-N vector to generate a plasmid expressing an N-terminal Flag-tagged Roc-1 protein. The Flag-Cdt2 mammalian expression plasmid was subcloned into the pEEFN vector (BamHI, NotI) and encodes N-terminal Flag-tagged full-length Cdt2. Transient plasmid transfections of HeLa, HCT116, or 293T cells were performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. HCT116p21−/− stably expressing Flag-p21 and Flag-p21-PCNA were established by infecting HCT116p21−/− with retroviruses coding for the indicated proteins. The recombinant virus was prepared by subcloning the p21 and the p21-PCNA from pEEFN vectors into pMSCVpuro vector (Clontech). A similar protocol was used for the generation of stable U2OS, 293T and HCT116p53−/− expressing 3X-Flag-Cdt2. Mock-infected cells with virus containing the empty pBabe vector were used as controls.
Gene silencing by RNAi
siRNA transfection experiments were performed using oligofectamine (Invitrogen) according to the manufacturer’s instructions. siRNA duplexes were purchased from Invitrogen and have the following sequences. The siRNA oligonucleotide sequence used for siRNA interference were Skp2 (si-Skp2), 5′-AAGGGAGUGACAAAGACUUUGdTdT-3′; Cul4A (si-Cul4A), 5′-GACAAUCCGAAUCAGUACCdTdT-3′; Cul4B (si-Cul4B), 5-AGAUAAGGUUGACCAUAUAdTdT-3′; DDB1 (si-DDB1-1), 5′-ACAGAGUGGCGAGAGCAUUdTdT-3′; DDB1 (si-DDB1-2), 5′-CCUGUUGAUUGCCAAAAACdTdT-3′; Cdt2 (si-Cdt2-1), 5′-GAAUUAUACUGCUUAUCGAdTdT-3′; Cdt2 (si-Cdt2-2), 5′-AUACAAGAGUGACUCUAUAdTdT-3′; DDB2 (si-DDB2), 5′-GAGCGAGAUCCGAGUUUACdTdT-3′; PCNA (si-PCNA), 5′-GGAGGAAGCUGUUACCAUAdTdT-3′.
An siRNA oligonucleotide duplex targeting Luciferase (si-GL2) (Saxena et al. 2003), an unrelated gene, was also used as a control. Cells were transfected with the specific siRNA twice and harvested 48 h after the second transfection according to standard procedures.
In vivo and in vitro ubiquitylation assays for p21
Details of the in vivo and in vitro ubiquitylation reactions are in the Supplemental Material. HCT116 and 293T cells were transiently transfected with either Flag-p21 or Flag-p21-PCNA along with HA-ubiquitin. Cells transfected with empty vector and HA-ubiquitin were used as a negative control. Forty-eight hours after transfection, the cells were washed twice with PBS, UV-irradiated with 20 J/m2, and incubated at 37°C in fresh medium. Two hours later, the cells were treated with MG132 (20 μg/mL) for an additional hour. To detect ubiquitylated p21, we used a method that has been developed by Govers et al. (1997) and aimed at immediately inactivating isopeptidases. The procedure for p21 ubiquitin labeling in vitro was essentially as described (Liu et al. 2002; Furukawa et al. 2003).
Acknowledgments
We thank Y. Xiong for reagents. We thank Y. Machida for generating the anti-Cdt2 antibody and the Flag-Cdt2 mammalian expressing plasmid, and members of the Dutta laboratory for helpful suggestions. This work was supported by a fellowship from the Human Frontiers Science Program fellowship to V.A., and grants from the NIH (R37-CA76584, R01-GM57587, and R21-CA125173) to M.P. and (R01-CA89406) to A.D. T.A. was supported by the Cancer Training Grant T32CA009109. K.T. was supported by the Uehara Memorial Foundation Fellowship.
Footnotes
Supplemental material is available at http://www.genesdev.org.
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.1676108.
References
- Amador V., Ge S., Santamaria P.G., Guardavaccaro D., Pagano M. APC/C(Cdc20) controls the ubiquitin-mediated degradation of p21 in prometaphase. Mol. Cell. 2007;27:462–473. doi: 10.1016/j.molcel.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias E.E., Walter J.C. PCNA functions as a molecular platform to trigger Cdt1 destruction and prevent re-replication. Nat. Cell Biol. 2006;8:84–90. doi: 10.1038/ncb1346. [DOI] [PubMed] [Google Scholar]
- Banks D., Wu M., Higa L.A., Gavrilova N., Quan J., Ye T., Kobayashi R., Sun H., Zhang H. L2DTL/CDT2 and PCNA interact with p53 and regulate p53 polyubiquitination and protein stability through MDM2 and CUL4A/DDB1 complexes. Cell Cycle. 2006;5:1719–1729. doi: 10.4161/cc.5.15.3150. [DOI] [PubMed] [Google Scholar]
- Bendjennat M., Boulaire J., Jascur T., Brickner H., Barbier V., Sarasin A., Fotedar A., Fotedar R. UV irradiation triggers ubiquitin-dependent degradation of p21(WAF1) to promote DNA repair. Cell. 2003;114:599–610. doi: 10.1016/j.cell.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Bornstein G., Bloom J., Sitry-Shevah D., Nakayama K., Pagano M., Hershko A. Role of the SCFSkp2 ubiquitin ligase in the degradation of p21Cip1 in S phase. J. Biol. Chem. 2003;278:25752–25757. doi: 10.1074/jbc.M301774200. [DOI] [PubMed] [Google Scholar]
- Brugarolas J., Chandrasekaran C., Gordon J.I., Beach D., Jacks T., Hannon G.J. Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature. 1995;377:552–557. doi: 10.1038/377552a0. [DOI] [PubMed] [Google Scholar]
- Bunz F., Dutriaux A., Lengauer C., Waldman T., Zhou S., Brown J.P., Sedivy J.M., Kinzler K.W., Vogelstein B. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–1501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- Cayrol C., Ducommun B. Interaction with cyclin-dependent kinases and PCNA modulates proteasome-dependent degradation of p21. Oncogene. 1998;17:2437–2444. doi: 10.1038/sj.onc.1202189. [DOI] [PubMed] [Google Scholar]
- Cayrol C., Knibiehler M., Ducommun B. p21 binding to PCNA causes G1 and G2 cell cycle arrest in p53-deficient cells. Oncogene. 1998;16:311–320. doi: 10.1038/sj.onc.1201543. [DOI] [PubMed] [Google Scholar]
- Chen X., Chi Y., Bloecher A., Aebersold R., Clurman B.E., Roberts J.M. N-Acetylation and ubiquitin-independent proteasomal degradation of p21(Cip1) Mol. Cell. 2004;16:839–847. doi: 10.1016/j.molcel.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Furukawa M., He Y.J., Borchers C., Xiong Y. Targeting of protein ubiquitination by BTB-Cullin 3-Roc1 ubiquitin ligases. Nat. Cell Biol. 2003;5:1001–1007. doi: 10.1038/ncb1056. [DOI] [PubMed] [Google Scholar]
- Govers R., van Kerkhof P., Schwartz A.L., Strous G.J. Linkage of the ubiquitin-conjugating system and the endocytic pathway in ligand-induced internalization of the growth hormone receptor. EMBO J. 1997;16:4851–4858. doi: 10.1093/emboj/16.16.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higa L.A., Mihaylov I.S., Banks D.P., Zheng J., Zhang H. Radiation-mediated proteolysis of CDT1 by CUL4–ROC1 and CSN complexes constitutes a new checkpoint. Nat. Cell Biol. 2003;5:1008–1015. doi: 10.1038/ncb1061. [DOI] [PubMed] [Google Scholar]
- Higa L.A., Banks D., Wu M., Kobayashi R., Sun H., Zhang H. L2DTL/CDT2 interacts with the CUL4/DDB1 complex and PCNA and regulates CDT1 proteolysis in response to DNA damage. Cell Cycle. 2006;5:1675–1680. doi: 10.4161/cc.5.15.3149. [DOI] [PubMed] [Google Scholar]
- Hu J., Xiong Y. An evolutionarily conserved function of proliferating cell nuclear antigen for Cdt1 degradation by the Cul4–Ddb1 ubiquitin ligase in response to DNA damage. J. Biol. Chem. 2006;281:3753–3756. doi: 10.1074/jbc.C500464200. [DOI] [PubMed] [Google Scholar]
- Hu J., McCall C.M., Ohta T., Xiong Y. Targeted ubiquitination of CDT1 by the DDB1–CUL4A–ROC1 ligase in response to DNA damage. Nat. Cell Biol. 2004;6:1003–1009. doi: 10.1038/ncb1172. [DOI] [PubMed] [Google Scholar]
- Huang J., Chen J. Oncogene. Vol. 27. 2008. VprBP targets Merlin to the Roc1–Cul4A–DDB1 E3 ligase complex for degradation; pp. 4056–4064. [DOI] [PubMed] [Google Scholar]
- Jin J., Arias E.E., Chen J., Harper J.W., Walter J.C. A family of diverse Cul4–Ddb1-interacting proteins includes Cdt2, which is required for S phase destruction of the replication factor Cdt1. Mol. Cell. 2006;23:709–721. doi: 10.1016/j.molcel.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Lee H., Zeng S.X., Lu H. UV induces p21 rapid turnover independently of ubiquitin and Skp2. J. Biol. Chem. 2006;281:26876–26883. doi: 10.1074/jbc.M605366200. [DOI] [PubMed] [Google Scholar]
- Lee J.Y., Yu S.J., Park Y.G., Kim J., Sohn J. Glycogen synthase kinase 3β phosphorylates p21WAF1/CIP1 for proteasomal degradation after UV irradiation. Mol. Cell. Biol. 2007;27:3187–3198. doi: 10.1128/MCB.01461-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Furukawa M., Matsumoto T., Xiong Y. NEDD8 modification of CUL1 dissociates p120(CAND1), an inhibitor of CUL1–SKP1 binding and SCF ligases. Mol. Cell. 2002;10:1511–1518. doi: 10.1016/s1097-2765(02)00783-9. [DOI] [PubMed] [Google Scholar]
- Mendez J., Stillman B. Chromatin association of human origin recognition complex, cdc6, and minichromosome maintenance proteins during the cell cycle: Assembly of prereplication complexes in late mitosis. Mol. Cell. Biol. 2000;20:8602–8612. doi: 10.1128/mcb.20.22.8602-8612.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitani H., Sugimoto N., Roukos V., Nakanishi Y., Saijo M., Obuse C., Tsurimoto T., Nakayama K.I., Nakayama K., Fujita M., et al. Two E3 ubiquitin ligases, SCF–Skp2 and DDB1–Cul4, target human Cdt1 for proteolysis. EMBO J. 2006;25:1126–1136. doi: 10.1038/sj.emboj.7601002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph E., Boye E., Kearsey S.E. DNA damage induces Cdt1 proteolysis in fission yeast through a pathway dependent on Cdt2 and Ddb1. EMBO Rep. 2006;7:1134–1139. doi: 10.1038/sj.embor.7400827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansam C.L., Shepard J.L., Lai K., Ianari A., Danielian P.S., Amsterdam A., Hopkins N., Lees J.A. DTL/CDT2 is essential for both CDT1 regulation and the early G2/M checkpoint. Genes & Dev. 2006;20:3117–3129. doi: 10.1101/gad.1482106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmento L.M., Huang H., Limon A., Gordon W., Fernandes J., Tavares M.J., Miele L., Cardoso A.A., Classon M., Carlesso N. Notch1 modulates timing of G1–S progression by inducing SKP2 transcription and p27 Kip1 degradation. J. Exp. Med. 2005;202:157–168. doi: 10.1084/jem.20050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena S., Jonsson Z.O., Dutta A. Small RNAs with imperfect match to endogenous mRNA repress translation. Implications for off-target activity of small inhibitory RNA in mammalian cells. J. Biol. Chem. 2003;278:44312–44319. doi: 10.1074/jbc.M307089200. [DOI] [PubMed] [Google Scholar]
- Senga T., Sivaprasad U., Zhu W., Park J.H., Arias E.E., Walter J.C., Dutta A. PCNA is a cofactor for Cdt1 degradation by CUL4/DDB1-mediated N-terminal ubiquitination. J. Biol. Chem. 2006;281:6246–6252. doi: 10.1074/jbc.M512705200. [DOI] [PubMed] [Google Scholar]
- Stoyanova T., Yoon T., Kopanja D., Mokyr M.B., Raychaudhuri P. The xeroderma pigmentosum group E gene product DDB2 activates nucleotide excision repair by regulating the level of p21Waf1/Cip1. Mol. Cell. Biol. 2008;28:177–187. doi: 10.1128/MCB.00880-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Nacusi L., Sheaff R.J., Liu X. Ubiquitination of p21Cip1/WAF1 by SCFSkp2: Substrate requirement and ubiquitination site selection. Biochemistry. 2005;44:14553–14564. doi: 10.1021/bi051071j. [DOI] [PubMed] [Google Scholar]
- Wertz I.E., O’Rourke K.M., Zhang Z., Dornan D., Arnott D., Deshaies R.J., Dixit V.M. Human De-etiolated-1 regulates c-Jun by assembling a CUL4A ubiquitin ligase. Science. 2004;303:1371–1374. doi: 10.1126/science.1093549. [DOI] [PubMed] [Google Scholar]
- Yu Z.K., Gervais J.L., Zhang H. Human CUL-1 associates with the SKP1/SKP2 complex and regulates p21(CIP1/WAF1) and cyclin D proteins. Proc. Natl. Acad. Sci. 1998;95:11324–11329. doi: 10.1073/pnas.95.19.11324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H., Nie L., Maki C.G. Cdk2-dependent inhibition of p21 stability via a C-terminal cyclin-binding motif. J. Biol. Chem. 2005;280:29282–29288. doi: 10.1074/jbc.M407352200. [DOI] [PubMed] [Google Scholar]