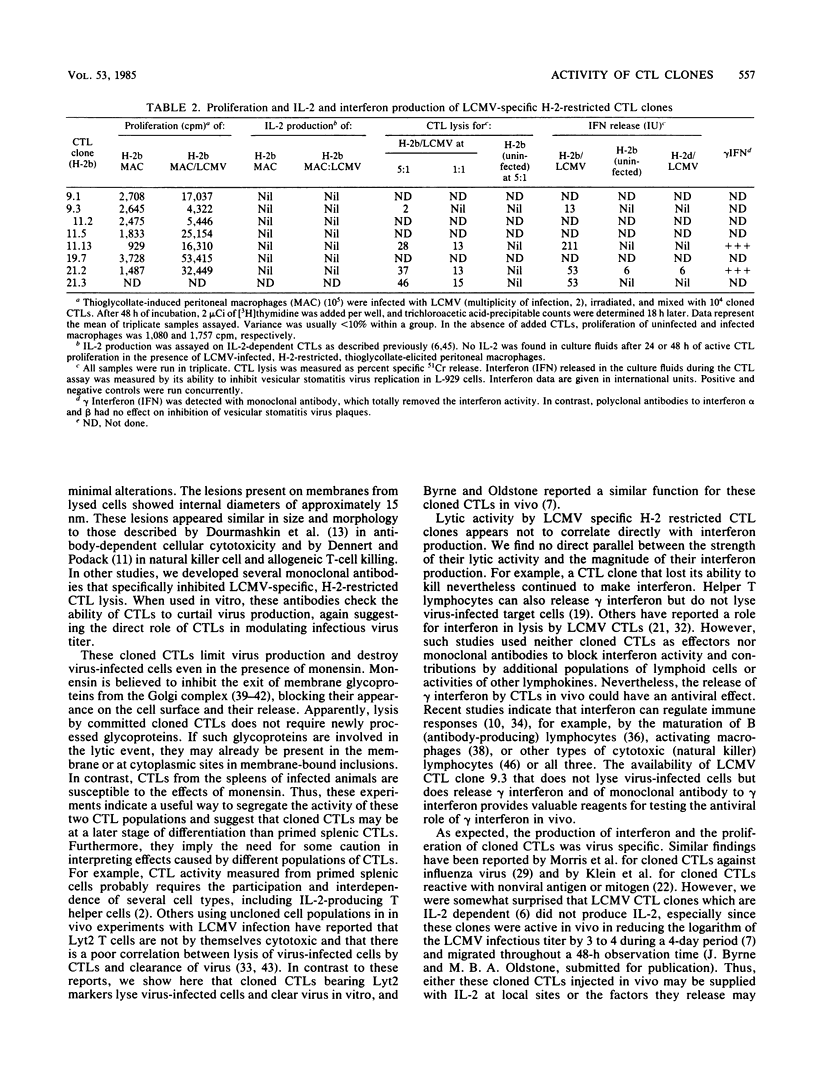
Abstract
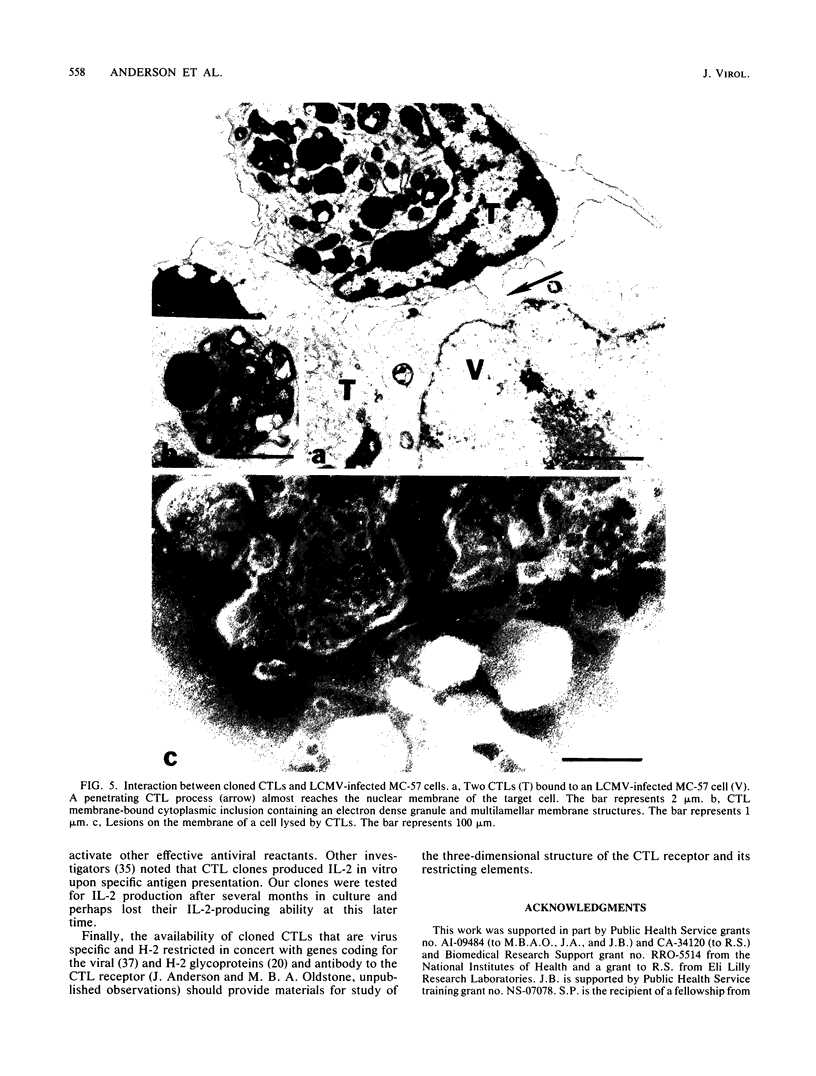
We have generated lymphocytic choriomeningitis virus-specific, H-2-restricted cytotoxic thymus-derived lymphocyte (CTL) clones. By using these reagents in several in vitro assays with infected target cells, we show that CTLs by themselves prevent the release of infectious virus into culture fluids and significantly lower the titers of infectious virus previously produced. This ability of cloned CTLs is not influenced by monensin. However, monensin does abrogate the ability of CTLs from spleens of mice primed 6 to 8 days previously with virus to kill virus-infected syngeneic targets. When tested for the participation of lymphokines in this system, the CTLs proliferate when reacted with syngeneic lymphocytic choriomeningitis virus-infected macrophages but fail to make interleukin-2. These CTLs make gamma interferon when reacted with syngeneic virus-infected targets. However, the production of interferon does not directly correlate with CTL-mediated killing. The number of H-2K and D molecules expressed on the target cell surface is not altered during the course of lymphocytic choriomeningitis virus infection. Electron microscopy shows finger-like projections of the CTL clone thrust into the infected cell and lesions bearing an internal diameter of approximately 15 nm in those membranes, illustrating the lytic process.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed R., Salmi A., Butler L. D., Chiller J. M., Oldstone M. B. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984 Aug 1;160(2):521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrus L., Granelli-Piperno A., Reich E. Cytotoxic T cells both produce and respond to interleukin 2. J Exp Med. 1984 Feb 1;159(2):647–652. doi: 10.1084/jem.159.2.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basham T., Merigan T. C. Immunoregulation by gamma-interferon? Nature. 1982 Oct 28;299(5886):778–778. doi: 10.1038/299778a0. [DOI] [PubMed] [Google Scholar]
- Buchmeier M. J., Lewicki H. A., Tomori O., Oldstone M. B. Monoclonal antibodies to lymphocytic choriomeningitis and pichinde viruses: generation, characterization, and cross-reactivity with other arenaviruses. Virology. 1981 Aug;113(1):73–85. doi: 10.1016/0042-6822(81)90137-9. [DOI] [PubMed] [Google Scholar]
- Buchmeier M. J., Welsh R. M., Dutko F. J., Oldstone M. B. The virology and immunobiology of lymphocytic choriomeningitis virus infection. Adv Immunol. 1980;30:275–331. doi: 10.1016/s0065-2776(08)60197-2. [DOI] [PubMed] [Google Scholar]
- Byrne J. A., Ahmed R., Oldstone M. B. Biology of cloned cytotoxic T lymphocytes specific for lymphocytic choriomeningitis virus. I. Generation and recognition of virus strains and H-2b mutants. J Immunol. 1984 Jul;133(1):433–439. [PubMed] [Google Scholar]
- Byrne J. A., Oldstone M. B. Biology of cloned cytotoxic T lymphocytes specific for lymphocytic choriomeningitis virus: clearance of virus in vivo. J Virol. 1984 Sep;51(3):682–686. doi: 10.1128/jvi.51.3.682-686.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole G. A., Nathanson N., Prendergast R. A. Requirement for theta-bearing cells in lymphocytic choriomeningitis virus-induced central nervous system disease. Nature. 1972 Aug 11;238(5363):335–337. doi: 10.1038/238335a0. [DOI] [PubMed] [Google Scholar]
- Dennert G., Podack E. R. Cytolysis by H-2-specific T killer cells. Assembly of tubular complexes on target membranes. J Exp Med. 1983 May 1;157(5):1483–1495. doi: 10.1084/jem.157.5.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty P. C., Zinkernagel R. M. T-cell-mediated immunopathology in viral infections. Transplant Rev. 1974;19(0):89–120. doi: 10.1111/j.1600-065x.1974.tb00129.x. [DOI] [PubMed] [Google Scholar]
- Dourmashkin R. R., Deteix P., Simone C. B., Henkart P. Electron microscopic demonstration of lesions in target cell membranes associated with antibody-dependent cellular cytotoxicity. Clin Exp Immunol. 1980 Dec;42(3):554–560. [PMC free article] [PubMed] [Google Scholar]
- Durand R. E. Calibration of flow cytometry detector systems. Cytometry. 1981 Nov;2(3):192–193. doi: 10.1002/cyto.990020312. [DOI] [PubMed] [Google Scholar]
- Dutko F. J., Oldstone M. B. Genomic and biological variation among commonly used lymphocytic choriomeningitis virus strains. J Gen Virol. 1983 Aug;64(Pt 8):1689–1698. doi: 10.1099/0022-1317-64-8-1689. [DOI] [PubMed] [Google Scholar]
- Gilden D. H., Cole G. A., Monjan A. A., Nathanson N. Immunopathogenesis of acute central nervous system disease produced by lymphocytic choriomeningitis virus. I. Cyclophosphamide-mediated induction by the virus-carrier state in adult mice. J Exp Med. 1972 Apr 1;135(4):860–873. doi: 10.1084/jem.135.4.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilden D. H., Cole G. A., Nathanson N. Immunopathogenesis of acute central nervous system disease produced by lymphocytic choriomeningitis virus. II. Adoptive immunization of virus carriers. J Exp Med. 1972 Apr 1;135(4):874–889. doi: 10.1084/jem.135.4.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht T. T., Longo D. L., Matis L. A. The relationship between immune interferon production and proliferation in antigen-specific, MHC-restricted T cell lines and clones. J Immunol. 1983 Sep;131(3):1049–1055. [PubMed] [Google Scholar]
- Hood L., Steinmetz M., Goodenow R. Genes of the major histocompatibility complex. Cell. 1982 Apr;28(4):685–687. doi: 10.1016/0092-8674(82)90046-0. [DOI] [PubMed] [Google Scholar]
- Jacobson S., Friedman R. M., Pfau C. J. Interferon induction by lymphocytic choriomeningitis viruses correlates with maximum virulence. J Gen Virol. 1981 Dec;57(Pt 2):275–283. doi: 10.1099/0022-1317-57-2-275. [DOI] [PubMed] [Google Scholar]
- Klein J. R., Raulet D. H., Pasternack M. S., Bevan M. J. Cytotoxic T lymphocytes produce immune interferon in response to antigen or mitogen. J Exp Med. 1982 Apr 1;155(4):1198–1203. doi: 10.1084/jem.155.4.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Lin Y. L., Askonas B. A. Biological properties of an influenza A virus-specific killer T cell clone. Inhibition of virus replication in vivo and induction of delayed-type hypersensitivity reactions. J Exp Med. 1981 Aug 1;154(2):225–234. doi: 10.1084/jem.154.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marker O., Volkert M. Studies on cell-mediated immunity to lymphocytic choriomeningitis virus in mice. J Exp Med. 1973 Jun 1;137(6):1511–1525. doi: 10.1084/jem.137.6.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland H. F. In vitro studies of cell-mediated immunity in an acute viral infection. J Immunol. 1974 Jul;113(1):173–180. [PubMed] [Google Scholar]
- Morris A. G., Lin Y. L., Askonas B. A. Immune interferon release when a cloned cytotoxic T-cell line meets its correct influenza-infected target cell. Nature. 1982 Jan 14;295(5845):150–152. doi: 10.1038/295150a0. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Fujinami R. S., Tishon A., Finney D., Powell H. C., Lampert P. W. Mapping of the major histocompatibility complex and viral antigens on the plasma membrane of a measles virus-infected cell. Virology. 1983 Jun;127(2):426–437. doi: 10.1016/0042-6822(83)90155-1. [DOI] [PubMed] [Google Scholar]
- Palladino M. A., von Wussow P., Pearlstein K. T., Welte K., Scheid M. P. Characterization of interleukin 2-dependent cytotoxic T-cell clones. IV. Production of alpha, beta and gamma interferons and interleukin 2 by Lyt-2+ T cells. Cell Immunol. 1983 Oct 15;81(2):313–322. doi: 10.1016/0008-8749(83)90239-3. [DOI] [PubMed] [Google Scholar]
- Pfau C. J., Gresser I., Hunt K. D. Lethal role of interferon in lymphocytic choriomeningitis virus-induced encephalitis. J Gen Virol. 1983 Aug;64(Pt 8):1827–1830. doi: 10.1099/0022-1317-64-8-1827. [DOI] [PubMed] [Google Scholar]
- Rocklin R. E., Bendtzen K., Greineder D. Mediators of immunity: lymphokines and monokines. Adv Immunol. 1980;29:55–136. doi: 10.1016/s0065-2776(08)60043-7. [DOI] [PubMed] [Google Scholar]
- Roopenian D. C., Widmer M. B., Orosz C. G., Bach F. H. Helper cell-independent cytolytic T lymphocytes specific for a minor histocompatibility antigen. J Immunol. 1983 Feb;130(2):542–545. [PubMed] [Google Scholar]
- Sidman C. L., Marshall J. D., Shultz L. D., Gray P. W., Johnson H. M. Gamma-interferon is one of several direct B cell-maturing lymphokines. 1984 Jun 28-Jul 4Nature. 309(5971):801–804. doi: 10.1038/309801a0. [DOI] [PubMed] [Google Scholar]
- Spitalny G. L., Havell E. A. Monoclonal antibody to murine gamma interferon inhibits lymphokine-induced antiviral and macrophage tumoricidal activities. J Exp Med. 1984 May 1;159(5):1560–1565. doi: 10.1084/jem.159.5.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajiri K., Uchida N., Tanzer M. L. Undersulfated proteoglycans are secreted by cultured chondrocytes in the presence of the ionophore monensin. J Biol Chem. 1980 Jul 10;255(13):6036–6039. [PubMed] [Google Scholar]
- Tartakoff A. M. Perturbation of vesicular traffic with the carboxylic ionophore monensin. Cell. 1983 Apr;32(4):1026–1028. doi: 10.1016/0092-8674(83)90286-6. [DOI] [PubMed] [Google Scholar]
- Tartakoff A., Vassalli P., Détraz M. Comparative studies of intracellular transport of secretory proteins. J Cell Biol. 1978 Dec;79(3):694–707. doi: 10.1083/jcb.79.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen A. R., Volkert M., Bro-Jørgensen K. Virus elimination in acute lymphocytic choriomeningitis virus infection. Correlation with virus-specific delayed-type hypersensitivity rather than cytotoxicity. Scand J Immunol. 1983 Jun;17(6):489–495. doi: 10.1111/j.1365-3083.1983.tb00816.x. [DOI] [PubMed] [Google Scholar]
- Varho M., Lehmann Grube F., Simon M. M. Effector T lymphocytes in lymphocytic choriomeningitis virus-infected mice. Cytolytic activity of Lyt-23 spleen cells in vitro does not correlate with elimination of infectious virus from spleens. J Exp Med. 1981 Apr 1;153(4):992–997. doi: 10.1084/jem.153.4.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignaux F., Gresser I. Differential effects of interferon on the expression of H-2K, H-2D, and Ia antigens on mouse lymphocytes. J Immunol. 1977 Feb;118(2):721–723. [PubMed] [Google Scholar]
- Watson J. Continuous proliferation of murine antigen-specific helper T lymphocytes in culture. J Exp Med. 1979 Dec 1;150(6):1510–1519. doi: 10.1084/jem.150.6.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh R. M., Jr, Buchmeier M. J. Protein analysis of defective interfering lymphocytic choriomeningitis virus and persistently infected cells. Virology. 1979 Jul 30;96(2):503–515. doi: 10.1016/0042-6822(79)90107-7. [DOI] [PubMed] [Google Scholar]
- Welsh R. M., Jr Mouse natural killer cells: induction specificity, and function. J Immunol. 1978 Nov;121(5):1631–1635. [PubMed] [Google Scholar]
- Zinkernagel R. M., Althage A. Antiviral protection by virus-immune cytotoxic T cells: infected target cells are lysed before infectious virus progeny is assembled. J Exp Med. 1977 Mar 1;145(3):644–651. doi: 10.1084/jem.145.3.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. Major transplantation antigens, viruses, and specificity of surveillance T cells. Contemp Top Immunobiol. 1977;7:179–220. doi: 10.1007/978-1-4684-3054-7_5. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. Restriction of in vitro T cell-mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature. 1974 Apr 19;248(5450):701–702. doi: 10.1038/248701a0. [DOI] [PubMed] [Google Scholar]