Abstract
The goal of this study was to determine the magnitude by which acute consumption of fructose in a morning bolus would stimulate lipogenesis (measured by infusion of 13C1-acetate and analysis by GC-MS) immediately and after a subsequent meal. Six healthy subjects [4 men and 2 women; aged (mean ± SD) 28 ± 8 y; BMI, 24.3 ± 2.8 kg/m2; and serum triacylglycerols (TG), 1.03 ± 0.32 mmol/L] consumed carbohydrate boluses of sugars (85 g each) in a random and blinded order, followed by a standardized lunch 4 h later. Subjects completed a control test of glucose (100:0) and a mixture of 50:50 glucose:fructose and one of 25:75 (wt:wt). Following the morning boluses, serum glucose and insulin after 100:0 were greater than both other treatments (P < 0.05) and this pattern occurred again after lunch. In the morning, fractional lipogenesis was stimulated when subjects ingested fructose and peaked at 15.9 ± 5.4% after the 50:50 treatment and at 16.9 ± 5.2% after the 25:75 treatment, values that were greater than after the 100:0 treatment (7.8 ± 5.7%; P < 0.02). When fructose was consumed, absolute lipogenesis was 2-fold greater than when it was absent (100:0). Postlunch, serum TG were 11–29% greater than 100:0 and TG-rich lipoprotein-TG concentrations were 76–200% greater after 50:50 and 25:75 were consumed (P < 0.05). The data demonstrate that an early stimulation of lipogenesis after fructose, consumed in a mixture of sugars, augments subsequent postprandial lipemia. The postlunch blood TG elevation was only partially due to carry-over from the morning. Acute intake of fructose stimulates lipogenesis and may create a metabolic milieu that enhances subsequent esterification of fatty acids flowing to the liver to elevate TG synthesis postprandially.
Introduction
The effects of dietary fructose on lipid and glucose metabolism have been active areas of research for over 40 y (1–11). In controlled feeding studies, fructose has been used to elevate daylong serum triacylglycerol (TG)7 concentrations in healthy (9,11) and diabetic subjects (12), an event that could lead to an accumulation of lipoprotein remnants, which could be atherogenic. The potential for the chronic consumption of sugars to increase lipogenesis has also been the focus of over-feeding studies documenting significant increases in de novo lipogenesis, representing the source of 20–40% of lipoprotein TG fatty acids, concurrent with elevations in serum TG concentrations of 80–200% (13–15). Clearly, the overconsumption of fructose can raise lipogenesis when it is fed for as little as 6 d (13). Recently, Chong et al. (16) reported the effect of acute, eucaloric replacement of glucose with fructose on lipogenesis in healthy subjects consuming a liquid breakfast of mixed macronutrients [carbohydrate (CHO) and fat]. Compared with glucose, fructose consumed with the fat-containing liquid increased the 4-h appearance of the meal’s fatty acids in VLDL, supporting greater reesterification of breakfast fat in the liver. Given the natural diurnal pattern of blood TG, which rises throughout the day and peaks around midnight, we similarly hypothesized that a fructose-induced rise in lipogenesis in the morning would further increase TG concentrations after the next meal. We also sought to test 2 different levels of fructose to determine their lipogenic effects in healthy, relatively lean research subjects.
Materials and Methods
Human subjects
Written informed consent was obtained from all volunteers (University of Minnesota Institutional Review Board no. 0407M61925). Subject recruitment occurred via advertisement and initially, 15 volunteers were screened to identify the 6 subjects (4 males and 2 females) included in the study. Two screening visits were conducted at least 16 d apart and occurred at the General Clinical Research Center (GCRC) at Fairview-University Medical Center in Minneapolis, MN. On these occasions, fasting blood draws were used to verify hematocrit and normal levels of hemoglobin, glucose, TG, LDL cholesterol, HDL cholesterol, total cholesterol, alanine transaminase, bilirubin, insulin, and apolipoprotein (apo) B and A1 concentrations (all analyses performed using a Vitros Analyzer 950). Before all of the blood draws in this study, subjects fasted for 12 h, were well hydrated, and had abstained from alcohol for at least 48 h. Body composition was determined by dual energy X-ray absorptiometry (Lunar) during a screening visit. Recruitment criteria were age 18–45 y, nonsmoking, and stable body weight in men or premenopausal women. Subjects were excluded if they had a history of diabetes or any other metabolic disease or took medications known to affect lipid metabolism. Other exclusion criteria were pregnancy, use of oral contraceptives, vegan diet, excessive exercise (>5 h/wk), or an exceptionally low or high body weight [<80% or > 130% by Metropolitan Weight Tables (17)]. Subjects maintained consistent exercise and activity patterns for 3 mo prior to the metabolic studies. Ad libitum food consumption was assessed by 3-d food records and a FFQ. Time periods between the CHO tests were at least 28 d apart for men and at least 27 d apart for women who were studied during their luteal phase of the menstrual cycle.
Study design
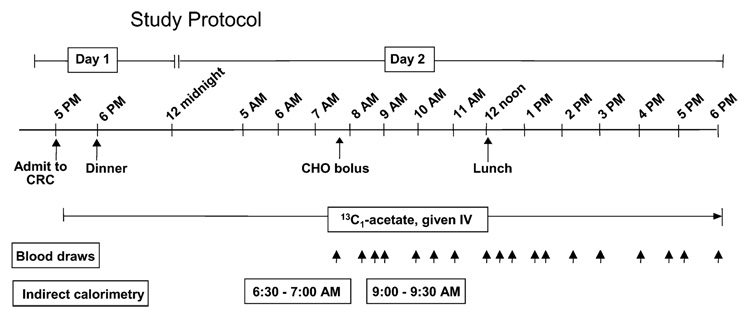
Three different CHO solutions were tested to determine the effect of sequential replacement of glucose by fructose on the stimulation of hepatic lipogenesis. These solutions included a control bolus of 100% glucose and 0% fructose (hereafter denoted 100:0), a weight-for-weight mixture of 50% glucose:50% fructose (50:50), and one of 25% glucose:75% fructose (25:75). Information regarding solution preparation and quantity of CHO fed in the tests is described in detail below. For 3 d prior to each metabolic test, subjects were instructed to consume a weight-maintaining diet of constant energy based on the Harris-Benedict equation (18) with comparison to 3-d food records of usual intake recorded during screening. Following directions from a registered dietitian, pretest meals were prepared by the subjects themselves and consisted of whole foods. Because the recent energy balance of the subject can significantly affect fatty acid metabolism (19), the goal of the pretest diet was to have it be of known composition and yet match the subject’s background diet, providing 53% of total energy from CHO, 32% from fat, and 15% from protein. The actual mean profile of the 3-d prestudy diet was 8277 ± 819 kJ/d with 52 ± 3% of total energy from CHO (23±6%of total energy from sugars), 31±1% from fat, and 17±3% from protein, and included 19±5 g of fiber and 1.1±0.5 g/d of (n-3) fatty acids. Except for the different CHO solutions, the protocol for each metabolic test was the same (Fig. 1).
FIGURE 1.
Study protocol. An i.v. line was placed on d 1 and 13C1-acetate infused for 25 h. The CHO solutions were fed between 0745 and 0800 and a standardized lunch was served at 1205 (4.33 h).
On d 1 of the inpatient study, the subject reported to the GCRC between 1630 and 1700. At 1730, an i.v. line was placed in an antecubital vein and an infusion of Na 13C1-acetate was started as described previously (20). Between 1800 and 1830, the subject consumed a dinner that met 40% of his/her daily energy needs, with the same macronutrient percentages as the background diet. The subject slept overnight at the GCRC with the i.v. running, and remained fasted until 0745 on d 2, at which time the CHO bolus was consumed, providing 14% of the subject’s daily energy needs. The lunch, consumed at 1205, was adjusted to provide 37% of the subject’s daily energy needs and consisted of standardized foods. An example from a single subject (Table 1) is: a turkey sandwich: whole wheat bread (60 g), turkey (60 g), Miracle Whip (15 g), lettuce (10 g), and tomato (50 g); Fritos (50 g);2% milk (240 g); canned pineapple (51 g); grape juice (100 g); and Oreos (32 g). For this quantity (688 g), the total content of sugars was 61.3 g (measured by Covance). The subject was given 15 min to consume the lunch so that by 1220 (time, 4.58 h), each subject had consumed ~51% of their total daily energy needs.
TABLE 1.
Composition of the different CHO boluses and the lunch1
| Amount and type of CHO in test |
|||
|---|---|---|---|
| Test name | Glucose | Fructose | |
| g | |||
| 100:0 | 85.3 ± 22.3 | ||
| 50:50 | 42.7 ± 11.1 | 42.7 ± 11.1 | |
| 25:75 | 21.3 ± 5.6 | 64.1 ± 16.7 | |
| Lunch components2 | Quantity | Energy | Proportion of total energy |
|---|---|---|---|
| g | kJ | % | |
| Energy | 3730 | ||
| Fat | 31.5 | 1187 | 31.8 |
| Protein | 39.1 | 654 | 17.5 |
| CHO | 112.8 | 1888 | 50.6 |
| Total sugars | 61.3 | 1026 | 27.5 |
| Fructose | 24.8 | 415 | 11.1 |
| Glucose | 22.7 | 380 | 10.2 |
| Lactose | 11.7 | 196 | 5.3 |
| Maltose | 2.1 | 35 | 0.9 |
| Fiber | 10.7 |
Values are means ± SD. Subjects received different amounts of sugars based on their body weight relative to a 70-kg man receiving 75 g and the mean weight of the subjects of 79.6 kg.
Each subject received an amount of lunch to provide 37% of their daily energy intake. The quantities (g) presented here are representative data from a single subject.
Composition and preparation of the CHO solutions
We considered a number of factors in designing the solutions of CHO administered in this study. First, when this study began, the acute effect of an increasing dose of fructose to immediately stimulate lipogenesis had, to our knowledge, never before been tested and the primary goal of this study was to quantitate the disposal of these CHO mixtures into the lipogenesis pathway. The quantity of CHO fed was partly chosen to allow comparison to previous studies of hepatic glucose disposal (21) and to the metabolism of glucose (75 g) occurring after an oral glucose tolerance test, a procedure commonly utilized in medicine. Second, the stimulation of lipogenesis would depend on the dose of CHO relative to the subject’s total energy needs (19). For example, a set dose fed to a small woman would more likely stimulate lipogenesis compared with that dose given to a man with a greater body weight. To avoid this confounder, each subject was given a dose of CHO based on their body weight relative to 70 kg. Third, fructose and glucose have different levels of sweetness and, thus, sensory differences could potentially affect hepatic lipogenesis by some unknown mechanism, or if the various mixtures were diluted with different amounts of water to match them for sweetness, the volume differences could affect the outcomes. To control for sensory properties, before we began the study, a pilot trial was performed to determine the concentrations of CHO mixtures that would be beyond the sweetness thresholds of all solutions. Sensory tests were performed under the direction of Dr. Zata Vickers at the University of Minnesota. Using a concentration of 35.7 g glucose/100 mL tap water as a standard, 20 volunteers were asked to compare the sweetness of several concentrations of the sweetener mixtures to a standard by placing a mark on a scale labeled ‘same as the standard’ at the center, ‘much less sweet’ at the left end of the scale, and ‘much more sweet’ at the right end of the scale. We used regression analysis to calculate the concentration of the sweetened mixture above which could not be distinguished as more sweet. Solutions of 35.7% (wt:wt) exceeded the sweetness thresholds for all mixtures. In this study, the different CHO tests were randomized with respect to the order of solutions administered and the subjects, staff administering the solution at the GCRC, and the technicians generating the data in the laboratory were all unaware of the study treatments.
To prepare for the protocol shown in Figure 1, on the morning of each test, the CHO and water were combined with unsweetened Kool-Aid lemonade mix and the solution was served ice cold to increase palatability. Subjects were given 15 min to consume the ~8 ounces of liquid. Indirect calorimetry was performed for 30 min between 0630 to 0730 (fasting state) and 0900 to 0930 (1 h post-CHO bolus) using a Deltatrac II Metabolic Cart (Sensor Medix) in the hooded mode. Subjects rested, watched TV, or read during the study. Nonenergy containing, non-caffeinated drinks were available upon request. Starting at 0740 and continuing until 1800, blood samples were drawn and serum separated immediately by centrifugation (3000 × g; 10 min, 20°C). Serum samples were kept on ice, a preservative cocktail was added (22), and samples were divided into aliquots for glucose, insulin, NEFA, serum TG analysis, and lipoprotein isolation.
Lipoprotein isolation
The CHO bolus was consumed at time 0 and the lunch was consumed at time 4.33 h. Total TG-rich lipoproteins (tTRL) were isolated from serum (23) at 12 time points throughout d 2 including: −0.08, 0.92, 2.25, 2.75, 3.25, 4.25, 4.58, 5.75, 7.25, 8.25, 9.42, and 10.25 h. In the morning, this lipoprotein fraction contained particles predominantly of hepatic origin (VLDL), because only sugars had been fed in the morning meal. By contrast, the lunch contained fat and, therefore, the postlunch tTRL fraction included both chylomicrons and VLDL. For 6 of the time points after lunch (4.25, 4.58, 5.75, 7.25, 8.25, and 9.42 h), an additional procedure (24) was used to isolate lipoproteins with a Svedberg flotation rate (Sf) of >400, which includes primarily chylomicrons with small quantities of very large VLDL and Sf 60–400, which includes large VLDL with a very short half-life (24). After lunch, the apoB100 concentrations in the fraction Sf 60–400 were determined by SDS-PAGE (22). The mol:mol ratio of TG:apoB100 was calculated using molecular weights of TG and apoB100 of 850 and 530,000, respectively.
Analysis of metabolic variables and GC-MS
Serum glucose concentrations were measured on a Vitros Analyzer 950 (Ortho-Clinical Diagnostics), whereas insulin was determined via chemiluminescent immunoassay (Diagnostic Products). Concentrations of NEFA (kit no. 994 79405E, Wako Chemicals) and serum TG in tTRL and VLDL fractions were determined via enzymatic assays (994–40491 Wako Chemicals) on a microtiter spectrophotometer (Model EL 340 Microplate, Bio-Tek Instruments). Serum leptin and adiponectin were measured using radioimmunoassay and gastric inhibitory polypeptide was measured using ELISA (Linco kits number no. HL-81HK, HADP-61HK, and EZHGIP-54K, respectively, Linco). To prepare for GC-MS, TG in tTRL and VLDL were separated and fatty acids derivatized to FAME as described previously (24). GC was performed to determine fatty acid composition on an Agilent Technologies 6890 GC fitted with a flame ionization detector with a split injection of 20:1 (25). tTRL- and VLDLTG FAME were separated on a SP-2560 column (Sigma-Aldrich), 100-m × 0.25-mm i.d. × 0.20-µm film thickness with helium as a carrier gas. GC-MS was performed using a DB-225, 30-m column (250-mm i.d. and 0.25-µm film thickness, J&W, Chromtech) in an Agilent 6890 GC with helium as the carrier gas. Selected ion monitoring was used for ions with mass:charge ratios of 270, 271, 272, which were analyzed with an Agilent 5975 mass spectrometer. Newly made fatty acids from de novo lipogenesis were calculated by mass isotopomer distribution analysis (MIDA) (26).
Calculations and statistical analysis
The 16-carbon fatty acid, palmitate, was used as the indicator of changes in lipogenesis because it represents the primary product of the fatty acid synthesis pathway (27). Lipogenesis data are presented as the fractional lipogenic rate, which is derived from the MIDA calculation, and also as the absolute lipogenic rate, which was calculated as follows. At each time point, the concentration of lipoprotein-TG fatty acids (in µmol/L) derived from an enzymatic TG assay was multiplied by the molar percentage of fatty acids that were palmitate in that TG, measured by GC analysis of TG-FAME. This quantity was then multiplied by the percentage of TG-FAME palmitate derived from de novo lipogenesis, as measured from GC-MS using the MIDA calculation (26).
The Sf > 400 lipogenesis data were analyzed as the absolute area under the curve (AUC), because these values were decaying throughout the postlunch phase. All other metabolic data (glucose, NEFA, insulin, serum TG, tTRL-TG, and VLDL-TG) were analyzed by incremental AUC (iAUC). Data are presented relative to the fasting value for all areas during the morning post-CHO ingestion, postlunch, and for the entire day (total iAUC = post-CHO + postlunch areas). In addition, to assess the isolated effect of lunch on changes in metabolite concentrations, the iAUC after lunch was also calculated relative to the lowest value occurring at the start of the lunch. In the tables, this iAUC is denoted as the value “reset at lunch” and serves to limit the effect of any carryover of elevated concentration as a result of the morning CHO feeding. Statistical analyses were performed using Statview for Windows (version 5.0.1, SAS Institute). Unless otherwise noted, values in the text are means ± SEM. A P-value of < 0.05 was considered significant. P < 0.07 are also noted, given the sample size of 6. In addition to AUC analysis, differences between treatments were analyzed using repeated-measures ANOVA. The Bonferroni/Dunn test was used for post hoc analysis.
Results
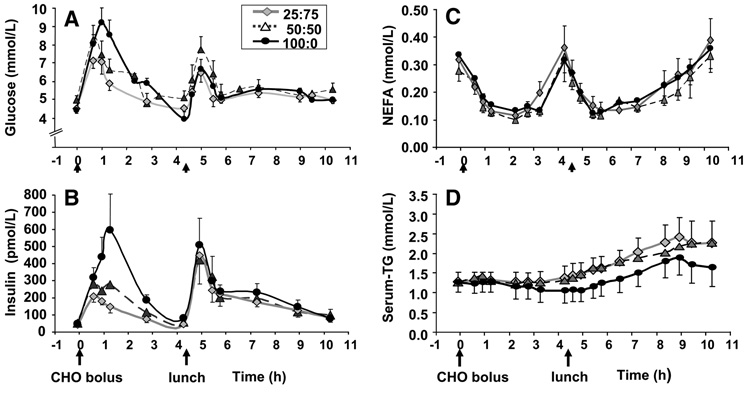
During screening, the group of 6 subjects were 28 ± 8 y (mean ± SD) (range, 19–40 y); had a BMI of 24.3 ± 2.8 kg/m2 (18.9–27.3 kg/m2); 79.6 ± 9.9 kg body weight (60.7–86.7 kg); body fat percent, 27.8 ± 6.8% (20.2–38.6%); lean mass percent, 67.9 ± 7.5% (55.1–76.8%); and fasting concentrations of TG, 1.03 ± 0.32 mmol/L (0.75–1.54 mmol/L); glucose, 4.5 ± 0.2 mmol/L (4.4–4.8 mmol/L); and insulin, 65 6 9 pmol/L (9–86 pmol/L). The doses of CHO fed during each of the tests reflect the subjects’ weight (Table 1). Compositional analysis of a representative lunch (Table 1) revealed that of the total CHO, roughly one-half was derived from mono- and disaccharides with equal quantities of fructose and glucose. Changes in the serum concentrations of glucose, insulin, NEFA, and TG are shown in Figure 2 and the iAUC data presented in Table 2. In general, for glucose and insulin concentrations, iAUC did not differ between 50:50 and 25:75; however, there were differences between these 2 tests and the 100:0 test. Specifically, glucose concentrations reached their highest levels after the 100:0, which was expected given the greater amount of glucose fed during this test. The iAUC relative to fasting for serum glucose after the standardized lunch did not differ between the treatments (P = 0.840). However, when the lunch values were reset to a prelunch nadir, the iAUC for 100:0 was greater than for either 50:50 or 25:75. This can be interpreted as differing responses of serum glucose after the lunch depending on the CHO solution administered that morning. Over the entire day, glucose iAUC was greater after the 100:0 treatment compared with the 25:75 (Table 2). Post-CHO insulin concentrations (Fig. 2B) were also higher after the 100:0 and as analyzed by iAUC were significantly greater than the fructose-containing tests. Postlunch 100:0 iAUC for insulin continued to be >25:75. However, this greater postlunch insulin iAUC was related to the morning response; when the baseline level was reset at lunch, the postlunch areas did not differ between the 3 tests. For all treatments, NEFA concentrations were suppressed 80% after the CHO boluses (Fig. 2C; Table 2) and suppressed again after lunch such that iAUC did not differ between any of the treatments for either time frame.
FIGURE 2.
Concentrations of serum glucose (A), insulin (B), NEFA (C), and TG (D) after consumption of CHO solutions and a lunch. Values are means ± SEM, n = 6. Time 0 denotes when the CHO bolus was fed. The solid food lunch was fed at 4.33 h (denoted by arrow).
TABLE 2.
AUC for serum concentrations of glucose, insulin, TG, and NEFA in subjects after 100:0, 50:50, and 25:75 glucose:fructose treatments1,2
| Treatments |
|||
|---|---|---|---|
| 100:0 | 50:50 | 25:75 | |
| Glucose, mmol/L·h | |||
| Post-CHO | 7.40 ± 1.37 | 4.37 ± 1.50* | 3.86 ± 1.27** |
| Postlunch | 5.13 ± 1.24 | 4.08 ± 1.91 | 4.31 ± 1.67 |
| Reset at lunch | 8.38 ± 1.69 | 3.44 ± 1.60** | 4.20 ± 1.24** |
| Total iAUC | 12.53 ± 2.42 | 8.46 ± 2.92 | 8.17 ± 2.91** |
| Insulin, pmol/L·h | |||
| Post-CHO | 896 ± 313 | 494 ± 185** | 279 ± 61** |
| Postlunch | 1068 ± 174 | 839 ± 197 | 860 ± 196** |
| Reset at lunch | 1245 ± 208 | 1057 ± 237 | 926 ± 213 |
| Total iAUC | 1964 ± 408 | 1333 ± 286** | 1139 ± 189** |
| NEFA, mmol/L·h | |||
| Post-CHO | −0.62 ± 0.19 | −0.49 ± 0.16 | −0.54 ± 0.09 |
| Postlunch | −0.71 ± 0.25 | −0.60 ± 0.26 | −0.62 ± 0.20 |
| Reset at lunch | −0.60 ± 0.33 | −0.57 ± 0.28 | −0.74 ± 0.36 |
| Total iAUC | −1.33 ± 0.43 | −1.09 ± 0.41 | −1.16 ± 0.27 |
| Serum TG, mmol/L·h | |||
| Post-CHO | −0.42 ± 0.27 | 0.19 ± 0.23** | −0.15 ± 0.36 |
| Postlunch | 1.23 ± 1.03 | 3.68 ± 1.08** | 4.11 ± 1.21** |
| Reset at lunch | 2.56 ± 0.60 | 3.33 ± 0.66 | 4.08 ± 0.90** |
| Total iAUC | 0.81 ± 1.27 | 3.87 ± 1.29** | 3.96 ± 1.38** |
Data are means ± SEM (n = 6) of the iAUC for all variables.
Asterisks indicate different from 100:0: P < 0.07
P < 0.05.
Areas were analyzed relative to the fasting value in the morning post-CHO ingestion, postlunch, and for the entire day (total iAUC = the post-CHO 1 postlunch areas). “Reset at lunch” denotes the iAUC after lunch relative to the lowest value occurring at the start of the lunch.
Serum TG concentrations decreased following the 100:0 test and remained constant when 50:50 and 25:75 were fed (Fig. 2D), resulting in a significantly greater morning iAUC after 50:50 compared with 100:0 (Table 2). Following lunch for all treatments, serum TG rose significantly and the iAUC for 50:50 and 25:75 were significantly >100:0. The greater iAUC observed for 50:50 after lunch may have been related to higher prelunch values, because resetting the baseline at lunch resulted in similar postlunch iAUC between 50:50 and 100:0. The postlunch area for 25:75 was also elevated and after resetting the baseline for 25:75, it remained >100:0 by 60%, supporting the concept that the elevated postlunch response was independent of the morning response. Over the entire day, consumption of 50:50 and 25:75 solutions resulted in significantly greater iAUC for serum TG compared with the 100:0.
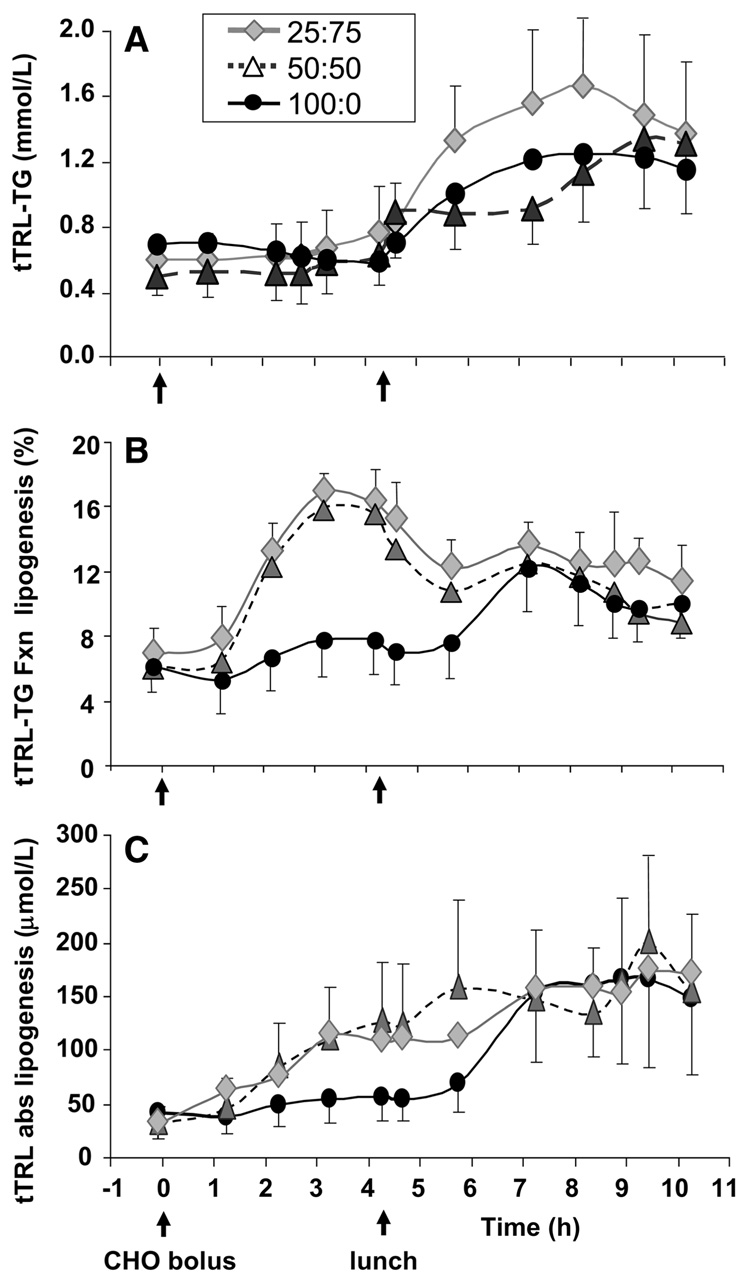
For tTRL-TG, the post-CHO concentration tended to be higher after 50:50 compared with 100:0 (Fig. 3A; Table 3). Similar to the serum TG concentration, tTRL-TG after 100:0 was associated with a negative iAUC. The postlunch tTRL-TG iAUC for 25:75 was >50:50 and both of these were greater than after 100:0. Resetting the baseline at lunch demonstrated that 25:75 remained significantly >50:50. For the total day, 25:75 had a significantly greater iAUC for tTRL-TG compared with 100:0. The percentage of tTRL-TG fatty acids that were palmitic acid (16:0, our marker of a lipogenic fatty acid) remained constant throughout the day (Supplemental Fig. 1). Palmitoleic acid (16:1) may be a marker of lipogenesis with prolonged feeding of high CHO diets, because it has been shown to be higher in serum TG during eucaloric CHO feeding for 25 d (28), during 4 d of overfeeding (27), and lower in blood TG following 12 wk of dietary CHO restriction (29). In this study, the percentage of palmitoleic did not change in the morning and decreased after consumption of the meal (Supplemental Fig. 1).
FIGURE 3.
tTRL-TG concentrations (A) and fractional (B) and absolute lipogenesis (C). Values are means ± SEM, n = 6. Time 0 denotes 0745 when the CHO bolus was fed. The solid food lunch was fed at 4.33 h. Abbreviations: abs, absolute; fxn, fractional.
TABLE 3.
AUC for serum concentrations of TG in tTRL, Sf > 400 lipoproteins, and VLDL and the TG derived from de novo lipogenesis in subjects after 100:0, 50:50, and 25:75 glucose:fructose treatments1,2
| Treatments |
|||
|---|---|---|---|
| 100:0 | 50:50 | 25:75 | |
| tTRL TG concentration, µmol/L·h | |||
| Post-CHO | −151 ± 118 | 103 ± 277* | 78 ± 303 |
| Postlunch | 1541 ± 689 | 2385 ± 882* | 3536 ± 1273**,† |
| Reset at lunch | 2098 ± 442 | 1679 ± 475 | 2654 ± 831†† |
| Total daylong iAUC | 1390 ± 975 | 2487 ± 1138* | 3614 ± 1504** |
| tTRL-TG concentration derived from de novo lipogenesis, µmol/L·h | |||
| Post-CHO | 33 ± 40 | 215 ± 102** | 230 ± 148 |
| Postlunch | 486 ± 238 | 704 ± 392* | 620 ± 337 |
| Reset at lunch | 333 ± 150 | 185 ± 136 | 178 ± 87 |
| Total daylong iAUC | 520 ± 273 | 919 ± 490* | 850 ± 485 |
| Sf > 400 Lipoprotein-TG concentration, µmol/L·h | |||
| Postlunch | 720 ± 188 | 617 ± 219 | 1211 ± 290 |
| Sf > 400-TG derived from de novo lipogenesis, Abs AUC µmol/L·h | |||
| Postlunch | 19 ± 13 | 19 ± 16 | 93 ± 84 |
| VLDL-TG concentration, µmol/L·h | |||
| Postlunch | 544 ± 165 | 456 ± 301 | 365 ± 112 |
| VLDL-TG derived from de novo lipogenesis, µmol/L·h | |||
| Postlunch | 125 ± 60 | 91 ± 64 | 70 ± 19 |
Data are means ± SEM (n = 6) and are displayed in Figure 3 and Figure 4. Data are iAUC for all variables relative to the fasting value except for Sf > 400-TG derived from de novo lipogenesis, which is presented as the absolute AUC.
3Asterisks indicate different from 100:0: P < 0.07
P < 0.05.
Daggers indicate different from 50:50: P < 0.07
P < 0.05 compared with 50:50.
Values were obtained during the morning post-CHO ingestion, postlunch, and for the entire day (total iAUC = the post-CHO + postlunch areas). “Reset at lunch” denotes the iAUC after lunch relative to the lowest value occurring at the start of the lunch.
The fraction of tTRL-TG palmitate derived from de novo lipogenesis (Fig. 3B) demonstrated a significant stimulation in the morning following 50:50 and 25:75. This fraction, when multiplied by the quantity of palmitate in tTRL-TG, gives the measure of absolute lipogenesis (Fig. 3C). The stimulation of tTRL-TG lipogenesis demonstrated by a greater post-CHO iAUC after the 50:50 solution (Table 3) was more variable after 25:75. Postlunch, the 100:0 lipogenic rate increased appreciably to match that of the other solutions (Fig. 3B,C); the 50:50 solution still tended to have a higher tTRL-TG lipogenesis compared with after 100:0 postlunch and for the entire day. When the absolute lipogenesis was reset at lunch, the iAUC for 100:0 did not differ from the other 2 tests (P = 0.441).
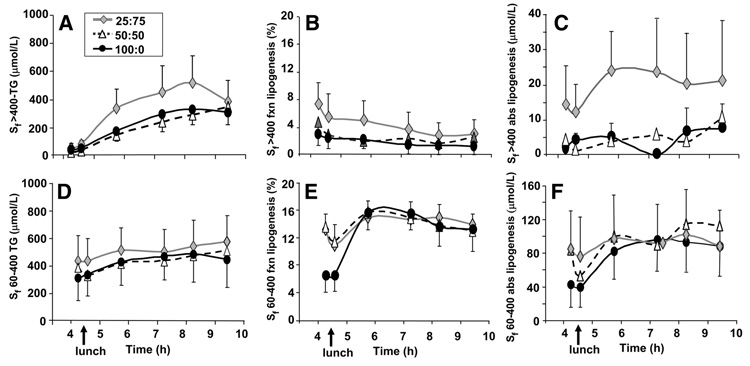
Because the meal fed at noon contained 32% of energy from fat, chylomicrons would be found in the tTRL fraction after lunch and dilute the apparent lipogenic rate. To determine the de novo production of fatty acids from the liver, lipoproteins in the Sf > 400 were separated from Sf 60–400 in the tTRL fraction at 6 time points after lunch. The percentage of de novo fatty acids evident in the Sf > 400 fraction (Fig. 4B) at 4 h averaged 3–8% and decreased throughout the afternoon, resulting in a small, absolute quantity of de novo fatty acids in Sf > 400 lipoproteins. Early after lunch for all of the treatments, the fractional hepatic lipogenic rate (Fig. 4E) decreased after the morning stimulation and increased again postlunch after a short delay, resulting in similar postlunch absolute lipogenesis rates in VLDL between the treatments (Fig. 4F; Table 3). After lunch, the apoB100 content in the Sf 60–400 lipoprotein fraction did not differ between the tests (21 ± 11, 22 ± 7, and 56 ± 35 nmol/L for 100:0, 50:50, and 25:75, respectively; P < 0.161), nor did the amount of TG per particle in these fractions differ (60,516 ± 26,245, 33,831 ± 6340, and 33,986 ± 165,61 mol TG/mol apoB, respectively; P < 0.225).
FIGURE 4.
Sf > 400 and Sf 60–400 lipoprotein-TG concentrations (A and D), and fractional (B and E) and absolute lipogenesis (C and F). Values are means ± SEM, n = 6. Time 0 denotes 0745 when the CHO bolus was fed. The solid food lunch was fed at 4.33 h.
Fractional lipogenesis values from each individual’s 3 tests were analyzed to assess the repeatability of this measurement within a single subject (Supplemental Table 1). Among the subjects, the fasting lipogenesis varied from 0.0 to 15.3%; however, the within-subject SD was low (0.9%). The results of respiratory gas measurements revealed that for all treatments, compared with fasting, post-CHO respiratory quotients were elevated, rising from 0.77 ± 0.02 to 0.88 ± 0.03 1 h after the CHO bolus (P < 0.03; data not shown). CHO oxidation increased (P < 0.02) and fat oxidation decreased (P < 0.04) to the same extent after all treatments. Serum concentrations of the hormones, gastric-inhibitory peptide, leptin, and adiponectin did not differ between the treatments (Supplemental Fig. 2), likely as a result of the limited number of subjects.
Discussion
In health, the liver possesses a remarkable flexibility to change metabolism between the fasted and fed states (21). For example, we have recently found a 65% change in the sources of fatty acids used for hepatic VLDL-TG synthesis when research subjects went from fasting to the postprandial state (20,30,31), with significant increases in de novo lipogenesis after meals containing glucose as the CHO source (32). Having been somewhat surprised by the magnitude of the lipogenic effect of glucose-containing liquid meals in our previous work, the question arose as to how these results might be modified by the presence of fructose, a CHO known to stimulate lipogenesis in animals (33) and humans (5,13). We hypothesized that the replacement of even a small amount of the glucose with fructose would increase lipogenic rates.
In this study, after the 100:0 glucose bolus, fractional lipogenesis rose to only 8%, which is quite low compared with the 23% observed previously (32). After consumption of the 50:50 and 25:75 solutions used here, the peak in lipogenesis was 17%. These “meals” were liquid in nature and the timing of the serum glucose excursion curves in the present research suggests that the solutions were all readily absorbed. The lower values of de novo lipogenesis observed here with 85 g glucose compared with our previous study’s 112-g dose resulted from the lower amount of CHO fed and from the absence of fat and protein in the solutions used herein (i.e. less total energy in the meal). Thus, one conclusion from these studies is that lipogenesis is only slightly increased after a glucose bolus similar to that routinely used for a medical oral glucose tolerance test. Second, the timing of the morning peak in fractional lipogenesis (Fig. 2, 3–4 h across all treatments) was consistent with past literature (32,34) and shows that this delay occurs whether fat is fed in the meal or not, as in the present study. Third, fructose significantly stimulated lipogenesis. By contrast, after lunch the quantities of newly made fatty acids were similar between the treatments, consistent with a set amount of fatty acids being made from the constant amount of CHO fed in this meal.
A 4th observation made here was constant serum and tTRL-TG concentrations during the first 4 h after fructose at either dose, compared with a slight reduction in TG following 100:0 (Fig. 2D and Fig. 3A). Whether this difference was due to increased clearance of blood TG following 100:0 and/or maintenance of TG secretion with fructose cannot be definitively evaluated by the present study design. However, the greater fractional lipogenic rates in the morning after fructose suggest that elevated secretion of TG was the mechanism. Focusing on the postlunch metabolism, when a meal was fed after the 100:0, the fractional lipogenic rate rose (Fig. 3B) to yield equivalent absolute de novo fatty acids postlunch compared with the other treatments (Fig. 3C). Yet, this stimulation was not sufficient to bring the postlunch serum TG and tTRL-TG concentrations up to the level of the 25:75 test (Fig. 2D and Fig. 3A). The data show that fructose acutely and significantly increased lipogenesis, which served to maintain TG concentrations in the morning and also led to greater TG concentrations after lunch. Further, the effect after lunch was greater than could be accounted for by the slight elevation in the morning. This suggests that the morning rise in de novo lipogenesis led to increased use of other fatty acid sources (plasma NEFA, dietary or stored TG) for TG synthesis after lunch. In rodent liver perfusate experiments, fructose increased fatty acid esterification (33) and a recent study by Chong et al. (16) provided direct support of this hypothesis in humans. In this latter report, the authors did not show a significant increase in absolute fatty acid production after the bolus of fructose. However, the lipogenic pathway was labeled only during breakfast consumption via the addition of labeled fructose added to a sweet drink. This bolus-labeling scheme would not have allowed enough time for isotopic labeling of fatty acids used for subsequent VLDL-TG synthesis. It can take anywhere from 12 to 36 h of labeling to be able to accurately identify newly made fatty acids in VLDL, because de novo fatty acids enter a delay pool before being used for VLDL-TG synthesis (34). Furthermore, it appears that the consumption of a meal facilitates the movement of these fatty acids through this cytosolic delay pool (Supplemental Fig. 3).
The observed significant increase in fractional lipogenesis during the morning after fructose is not likely a mathematical result of a reduction in the liver’s use of fatty acids derived from the plasma NEFA pool. This is because the concentration of tTRL-TG remained unchanged in the morning (iAUC were close to 0) and NEFA concentrations decreased after all 3 treatments, whereas the pattern of change in lipogenesis varied among the tests. In previous studies, we demonstrated that the reduction in use of plasma NEFA for VLDL-TG synthesis occurs extremely fast and on a different timeline than the rise in de novo lipogenesis (Supplemental Fig. 3). Furthermore, late in the postprandial period (4 h), the reappearance of plasma NEFA in VLDL-TG takes place at the same time as the rise in lipogenesis (i.e. the 2 are not reciprocal). The early postprandial reduction in the use of plasma NEFA for VLDL-TG synthesis coincides with an appearance of unlabeled fatty acids in the particle (20,32), which could originate from a liver TG storage pool. We speculate that the liver calls upon stored TG to support and maintain VLDL-TG secretion postprandially when a significant decrease in the flow of plasma NEFA to liver has occurred.
It appears that de novo fatty acid synthesis does not result in the addition of excess TG fatty acid that are added to VLDL. Rather, their presence may prevent apoB degradation, resulting in more particles being secreted that are smaller in size, as suggested by the apoB data. Given the constant tTRL-TG concentrations across treatments, it is possible that a fructose-induced increase in lipogenesis displaces the use of stored TG for VLDL synthesis, or, more likely, that the stimulation of lipogenesis represents an intracellular signal for liver to esterify fatty acids from any source into TG. This effect may also depend on the sensitivity of the liver to insulin. If fructose does increase hepatic TG storage, fructose feeding could lead to fatty liver. Using magnetic resonance spectrometry, Le et al. (10) found no increase in hepatic TG stores in healthy men eucalorically consuming a high-fructose diet for 4 wk. Interestingly, in this article, the greater the serum TG concentration, the lower the level of hepatic TG post-treatment and the authors hypothesized that “exportation of newly formed TG as VLDL-TG is a key element to prevent liver-TG stores from expanding” (10). Our data support this concept.
The large increase in de novo lipogenesis postlunch after 100:0, which had barely stimulated lipogenesis in the morning, resulted in postlunch lipogenic rates for all treatments being similar. This observation suggests that under healthy, well-regulated metabolism, there may be a maximum rate of lipogenesis that can be achieved given the quantity of the CHO fed or a maximum rate depending on the time of day. This robust increase in de novo lipogenesis postprandially in healthy subjects, in contrast to the lack of an observed increase in obese patients with fatty liver (35), suggests that a significant increase in fatty acid synthesis after CHO consumption can be used as one characteristic to test for metabolic flexibility (36,37).
In an overfeeding study, Lammert et al. (14) noted that within their group of research subjects, the same individuals repeatedly had the highest de novo lipogenesis values, no matter which diet was fed chronically (eucaloric, or excess energy from fat or CHO). Here, we were also able to demonstrate good reproducibility in the fasting lipogenic rate of individual subjects during the repeated tests and we think this was due to the relatively tight control of the subjects’ food and alcohol intakes and activity levels before the tests. This fact will support future studies focusing on environmental and genetic factors that predict elevated fatty acid synthesis postprandially so as to investigate how lipogenesis may contribute to atherogenic risk during positive energy balance in obese and insulin-resistant subjects.
Strengths of this study include the blinded and random order of the treatments and adjustment for the quantity of CHO fed depending on the subjects’ total daily energy needs. The key limitation of the study is a small sample size. However, the repeated measures design supports the notion that the differences observed among these 18 metabolic tests were real and would be reproducible. Another limitation may lie in the fact that the subjects consumed the CHO solutions in the fasted state, which would underestimate lipogenesis for 3 reasons. First, as described earlier, to achieve maximal labeling of lipogenesis a full day of acetate infusion may be needed (34,38); second, palmitate was used as the marker of lipogenesis when other fatty acids (16:1, 18:1) may have been made de novo but were not specifically tested; and third, at this time of day, much of the CHO consumed would be burned as energy and/or go to refill hepatic glycogen stores (6,33). Serving the beverages in the morning in the fasted state, did, however, isolate the effect of the CHO from the effects of excess energy intake, a confounder in overfeeding studies. Further, the consumption of a sweetened beverage in lieu of breakfast is not unheard of in real life.
In summary, these data show a significant immediate lipogenic effect of fructose in healthy, lean research subjects that was associated with greater serum TG concentrations in the morning and after a subsequent meal. The data suggest that an early stimulation of lipogenesis after fructose, consumed in the fasted state (even as a small amount in a mixture of sugars) augments subsequent postprandial lipemia. The additional postlunch elevation in TG was only partially due to a carry over from the morning, suggesting that fructose consumption creates a metabolic milieu that alters subsequent handling of nutrients flowing to the liver. This study, together with the strong, recent data presented by Chong et al. (16), provide compelling evidence that fructose causes hyperlipidemia postprandially both directly, through the synthesis of fatty acids, and indirectly, by increasing liver reesterification of fatty acids from all sources.
Supplementary Material
Acknowledgments
We thank Dr. Zata Vickers, University of Minnesota, for expert guidance on the prestudy sensory tests, and Dr. David D’Alessio, University of Cincinnati, for assistance with the measurements of gastric inhibitory peptide, leptin, and adiponectin.
Footnotes
Supported by grants from the NRC Resources/NIH General Clinical Research Center Program (M01-RR00400) at the University of Minnesota, and unrestricted research funds from the Cargill Higher Education Fund and the Sugar Association.
Author disclosures: E. J. Parks, L. E. Skokan, M. T. Timlin, and C. S. Dingfelder, no conflicts of interest.
Supplemental Table 1 and Supplemental Figures 1–3 are available with the online posting of this paper at jn.nutrition.org.
Abbreviations used: AUC, area under the curve; CHO, carbohydrate; iAUC, incremental area under the curve; GCRC, General Clinical Research Center; MIDA, mass isotopomer distribution analysis; Sf, Svedberg flotation rate; TG, triacylglycerol; tTRL, total triacylglycerol-rich lipoprotein; 100:0: a mixture of 100% glucose and 0% fructose wt:wt; 75:25: 75:25 glucose: fructose wt:wt; 50:50: 50:50 glucose:fructose wt:wt.
Literature Cited
- 1.Nikkila EA, Pelkonen R. Enhancement of alimentary hyperglyceridemia by fructose and glycerol in man. Proc Soc Exp Biol Med. 1966;123:91–94. doi: 10.3181/00379727-123-31411. [DOI] [PubMed] [Google Scholar]
- 2.Crapo PA, Reaven GM, Olefsky J. Plasma glucose and insulin responses to orally administered simple and complex carbohydrates. Diabetes. 1976;25:741–747. [PubMed] [Google Scholar]
- 3.Hallfrisch J, Reiser S, Prather ES. Blood lipid distribution of hyperinsulinemic men consuming three levels of fructose. Am J Clin Nutr. 1983;37:740–748. doi: 10.1093/ajcn/37.5.740. [DOI] [PubMed] [Google Scholar]
- 4.Schwarz J-M, Schutz Y, Froidevaux F, Acheson KJ, Jeanpretre N, Schneider H, Felber J-P, Jequier E. Thermogenesis in men and women induced by fructose vs glucose added to a meal. Am J Clin Nutr. 1989;49:667–674. doi: 10.1093/ajcn/49.4.667. [DOI] [PubMed] [Google Scholar]
- 5.Schwarz JM, Neese RA, Shackelton CHL, Hellerstein MK. De novo lipogenesis (DNL) during fasting and oral fructose in lean and obese hyperinsulinemic subjects. Diabetes. 1993;42 Suppl:39A. [Google Scholar]
- 6.Tounian P, Schneiter P, Henry S, Jequier E, Tappy L. Effects of infused fructose on endogenous glucose production, gluconeogenesis, and glycogen metabolism in healthy humans. Am J Physiol. 1994;267:E710–E714. doi: 10.1152/ajpendo.1994.267.5.E710. [DOI] [PubMed] [Google Scholar]
- 7.Jeppesen J, Chen YDI, Zhou MY, Schaaf P, Coulston A, Reaven GM. Postprandial triglyceride and retinyl ester responses to oral fat: effects of fructose. Am J Clin Nutr. 1995;61:787–791. doi: 10.1093/ajcn/61.4.787. [DOI] [PubMed] [Google Scholar]
- 8.Lee BM, Wolever TM. Effect of glucose, sucrose and fructose on plasma glucose and insulin responses in normal humans: comparison with white bread. Eur J Clin Nutr. 1998;52:924–928. doi: 10.1038/sj.ejcn.1600666. [DOI] [PubMed] [Google Scholar]
- 9.Bantle JP, Raatz SK, Thomas W, Georgopoulos A. Effects of dietary fructose on plasma lipids in healthy subjects. Am J Clin Nutr. 2000;72:1128–1134. doi: 10.1093/ajcn/72.5.1128. [DOI] [PubMed] [Google Scholar]
- 10.Le KA, Faeh D, Stettler R, Ith M, Kreis R, Vermathen P, Boesch C, Ravussin E, Tappy L. A 4-wk high-fructose diet alters lipid metabolism without affecting insulin sensitivity or ectopic lipids in healthy humans1–4. Am J Clin Nutr. 2006;84:1374–1379. doi: 10.1093/ajcn/84.6.1374. [DOI] [PubMed] [Google Scholar]
- 11.Teff K, Elliott S, Tschop M, Kieffer T, Rader D, Heiman M, Townsend R, Keim N, D’Alessio D, Havel P, et al. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J Clin Endocrinol Metab. 2004;89:2963–2972. doi: 10.1210/jc.2003-031855. [DOI] [PubMed] [Google Scholar]
- 12.Hollenbeck C. Dietary fructose effects on lipoprotein metabolism and risk for coronary artery disease. Am J Clin Nutr. 1993;58 Suppl:S800–S809. doi: 10.1093/ajcn/58.5.800S. [DOI] [PubMed] [Google Scholar]
- 13.Faeh D, Minehira K, Schwarz J, Periasami R, Seongus P, Tappy L. Effect of fructose overfeeding and fish oil administration on hepatic de novo lipogenesis and insulin sensitivity in healthy males. Diabetes. 2005;54:1907–1913. doi: 10.2337/diabetes.54.7.1907. [DOI] [PubMed] [Google Scholar]
- 14.Lammert O, Grunnet N, Faber P, Bjornsbo KS, Dich J, Larsen LO, Neese RA, Hellerstein MK, Quistorff B. Effects of isoenergetic overfeeding of either carbohydrate or fat in young men. Br J Nutr. 2000;84:233–245. [PubMed] [Google Scholar]
- 15.McDevitt RM, Bott SJ, Harding M, Coward WA, Bluck LJ, Prentice AM. De novo lipogenesis during controlled overfeeding with sucrose or glucose in lean and obese women. Am J Clin Nutr. 2001;74:737–746. doi: 10.1093/ajcn/74.6.737. [DOI] [PubMed] [Google Scholar]
- 16.Chong MF, Fielding BA, Frayn KN. Mechanisms for the acute effect of fructose on postprandial lipemia. Am J Clin Nutr. 2007;85:1511–1520. doi: 10.1093/ajcn/85.6.1511. [DOI] [PubMed] [Google Scholar]
- 17.Metropolitan Life Insurance Company. Metropolitan height and weight tables. 1. Vol. 64. New York: Metropolitan Life Insurance Company; 1983. pp. 3–9. [PubMed] [Google Scholar]
- 18.Harris JA, Benedict FG. Washington DC: Carnegie Institute; Biometric study of basal metabolism in man. 1919 Report No.: Publication no. 279.
- 19.Schwarz JM, Neese RA, Turner S, Dare D, Hellerstein MK. Short-term alterations in carbohydrate energy intake in humans: striking effects on hepatic glucose production, de novo lipogenesis, lipolysis, and whole-body fuel selection. J Clin Invest. 1995;96:2735–2743. doi: 10.1172/JCI118342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barrows BR, Parks EJ. Contributions of different fatty acid sources to VLDL-triacylglycerol in the fasted and fed-states. J Clin Endocrinol Metab. 2006;91:1446–1452. doi: 10.1210/jc.2005-1709. [DOI] [PubMed] [Google Scholar]
- 21.Meyer C, Dostou JM, Welle SL, Gerich JE. Role of human liver, kidney, and skeletal muscle in postprandial glucose homeostasis. Am J Physiol. 2002;282:E419–E427. doi: 10.1152/ajpendo.00032.2001. [DOI] [PubMed] [Google Scholar]
- 22.Kotite L, Bergeron N, Havel RJ. Quantification of apolipoproteins B-100, B-48, and E in human triglyceride-rich lipoproteins. J Lipid Res. 1995;36:890–900. [PubMed] [Google Scholar]
- 23.Havel RJ, Eder HA, Bragdon JH. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955;34:1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parks EJ, Krauss RM, Christiansen MP, Neese RA, Hellerstein MK. Effects of a low-fat, high-carbohydrate diet on VLDL-triglyceride assembly, production and clearance. J Clin Invest. 1999;104:1087–1096. doi: 10.1172/JCI6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parks EJ, German JB, Davis PA, Frankel EN, Kappagoda CT, Rutledge JC, Hyson DA, Schneeman BO. Reduced susceptibility of LDL from patients participating in an intensive atherosclerosis treatment program. Am J Clin Nutr. 1998;68:778–785. doi: 10.1093/ajcn/68.4.778. [DOI] [PubMed] [Google Scholar]
- 26.Hellerstein MK, Neese RA. Mass isotopomer distribution analysis at eight years: theoretical, analytic, and experimental considerations. Am J Physiol. 1999;276:E1146–E1170. doi: 10.1152/ajpendo.1999.276.6.E1146. [DOI] [PubMed] [Google Scholar]
- 27.Aarsland A, Wolfe RR. Hepatic secretion of VLDL fatty acids during stimulated lipogenesis in men. J Lipid Res. 1998;39:1280–1286. [PubMed] [Google Scholar]
- 28.Hudgins LC, Hellerstein M, Selfman C, Neese R, Diakun J, Hirsch J. Human fatty acid synthesis is stimulated by a eucaloric low fat, high carbohydrate diet. J Clin Invest. 1996;97:2081–2091. doi: 10.1172/JCI118645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forsythe CE, Phinney SD, Fernandez ML, Quann EE, Wood RJ, Bibus DM, Kraemer WJ, Feinman RD, Volek JS. Comparison of low fat and low carbohydrate diets on circulating fatty acid composition and markers of inflammation. Lipids. 2008;43:65–77. doi: 10.1007/s11745-007-3132-7. [DOI] [PubMed] [Google Scholar]
- 30.Barrows BR, Timlin MT, Parks EJ. Spillover of dietary fatty acids and use of serum nonesterified fatty acids for the synthesis of VLDL-triacylglycerol under two different feeding regimens. Diabetes. 2005;54:2668–2673. doi: 10.2337/diabetes.54.9.2668. [DOI] [PubMed] [Google Scholar]
- 31.Timlin MT, Barrows BR, Parks EJ. Increased dietary substrate delivery alters hepatic fatty acid recycling in healthy men. Diabetes. 2005;54:2694–2701. doi: 10.2337/diabetes.54.9.2694. [DOI] [PubMed] [Google Scholar]
- 32.Timlin MT, Parks EJ. The temporal pattern of de novo lipogenesis in the postprandial state. Am J Clin Nutr. 2005;81:35–42. doi: 10.1093/ajcn/81.1.35. [DOI] [PubMed] [Google Scholar]
- 33.Mayes PA. Intermediary metabolism of fructose. Am J Clin Nutr. 1993;58:S754–S765. doi: 10.1093/ajcn/58.5.754S. [DOI] [PubMed] [Google Scholar]
- 34.Vedala A, Wang W, Neese RA, Christiansen MP, Hellerstein MK. Delayed secretory pathway contributions to VLDL-triglycerides from plasma NEFA, diet, and de novo lipogenesis in humans. J Lipid Res. 2006;47:2562–2574. doi: 10.1194/jlr.M600200-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.Donnelly KL, Smith CI, Schwarzenberg SJ, Jesserun J, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Storlien L, Oakes ND, Kelly DE. Metabolic inflexibility. Proc Nutr Soc. 2004;63:363–368. doi: 10.1079/PNS2004349. [DOI] [PubMed] [Google Scholar]
- 37.Ukropcova B, McNeil M, Sereda O, de Jonge L, Xie H, Bray GA, Smith SR. Dynamic changes in fat oxidation in human primary myocytes mirror metabolic characteristics of the donor. J Clin Invest. 2005;115:1934–1941. doi: 10.1172/JCI24332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hudgins LC, Hellerstein MK, Seidman CE, Neese RA, Tremaroli JD, Hirsch J. Relationship between carbohydrate-induced hypertriglyceridemia and fatty acid synthesis in lean and obese subjects. J Lipid Res. 2000;41:595–604. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.