Abstract
Group C rotaviruses are important human enteric pathogens that have also been detected in a variety of mammalian species, including pigs. Group C rotaviruses have been identified in piglets with diarrhea, but their ecology remains to be elucidated. By screening of 292 fecal samples collected from 4- to 5-week-old asymptomatic pigs from four herds in Ireland between 2005 and 2007, 13 (4.4%) samples tested positive by reverse transcription-PCR for group C rotavirus. Group A rotaviruses were also detected in 19 samples but not in conjunction with group C viruses. The gene encoding the major group C neutralization antigen, the outer capsid protein VP7, was sequenced. The majority of the strains were very closely related to each other (>99% amino acid [aa] identity) and were characterized as genogroup G1 since they were genetically related to the prototype porcine strain Cowden (92.6% aa identity). Conversely, two strains (1GA/05/Cork/Ire and 281/07/Dublin/Ire) were characterized as genogroup G6 since they displayed the highest identity (89.2 to 94.0% aa) to porcine G6 strains (43/06-22-like). Unexpectedly, one such G6 strain, 1GA/05/Cork/Ire, lacked the 4-aa insertion in the VP7 variable region VR8 found in all the other G6 group C rotaviruses. This study provides evidence that porcine group C rotavirus may be detected not infrequently in asymptomatic piglets. In addition, it provides evidence that, unlike the human viruses, porcine group C rotaviruses display broad genetic heterogeneity, which may pose a challenge for the development of prophylactic tools.
Rotaviruses are classified into seven antigenically distinct groups (A to G) on the basis of a common group antigen, the inner capsid protein (VP6). Groups A, B, and C are associated with acute gastroenteritis in humans and animals, while groups D, E, F, and G have been detected only in animals (9, 20).
Group A rotaviruses (GARVs) are a major cause of acute gastroenteritis in humans and in a variety of mammalian and avian species. In humans, GARVs are estimated to cause 138 million cases of gastroenteritis annually, resulting in approximately 870,000 deaths, mostly in developing countries (13). The rotavirus genome consists of 11 double-stranded RNA (dsRNA) segments enclosed in a triple-layered capsid. The outer capsid proteins, VP7 and VP4, are important for immune protection and for vaccine development (19) and provide the basis for a dual classification system into G (VP7) and P (VP4) types (9).
Group C rotaviruses (GCRVs) were first identified in swine in the 1980s and were subsequently also found in humans (3, 35, 37). Human infection by GCRV has been associated with both sporadic episodes and large outbreaks of gastroenteritis in all age groups and appears to be globally distributed (1, 2, 5, 6, 18, 24, 25, 34, 38, 41, 43). Large-scale epidemiological studies have revealed that the prevalence may range from 0.6% to 6.8% (2, 24), and GCRVs are regarded as emerging human pathogens.
Limited seroepidemiologic surveys suggest that group GCRVs are widespread and presumably enzootic in pig herds (38, 44, 46). Outbreaks of diarrhea associated with GCRVs have been documented in nursing, weaning, and postweaning pigs (22, 30, 37, 38, 42), either alone or in mixed infections with other enteric pathogens. The signs include profuse diarrhea and, occasionally, vomiting (22, 30). A large-scale study in Italy (27) revealed that GCRVs may be detected in as many as 31.3% of fecal samples of 1- to 3-month-old pigs, with enteritis chiefly in coinfection with GARVs and enteric caliciviruses.
Antigenic analysis of some isolates by cross-neutralization and sequence analysis of the outer capsid proteins VP7 and VP4 of human and animal GCRVs have revealed that these viruses, similarly to GARVs, are antigenically and genetically heterogeneous, and at least six distinct VP7 genogroups (G type) and three VP4 genogroups (P type) have been distinguished. The porcine GCRVs exhibit a variety of VP7 genotypes (G1, G3, G5, and G6), while the bovine strains identified to date are G2 and the human strains are G4 (22, 28, 29, 33, 34, 47, 48).
Based on the seroprevalence rates of GCRVs in human populations in rural settings (17), a potential zoonotic role of animal GCRVs has been proposed. However, molecular analysis of human GCRV strains would not seem to support this theory, as all of the human GCRVs form a closely related group, suggesting a strong host-species restriction (2, 34). These inconsistencies may be a result of the limited amount of sequence data available for animal strains. Collection of epidemiological and molecular data on GCRVs in animals is crucial to better understand the ecology of GCRVs and to investigate their genetic/antigenic diversity and zoonotic potential. Evidence for the zoonotic potential of porcine GCRVs has been revealed by analyses of archival fecal samples of Brazilian children who were infected with porcine-like GCRVs (10).
In this study, we investigated the occurrence of infections by GCRVs in swine herds in Ireland, between 2005 and 2007. Several collections of samples were screened by using GCRV-specific primers based on the VP6 gene. The VP7 gene sequences of the identified GCRV strains were determined.
MATERIALS AND METHODS
Sample collection and preparation.
A total of 292 fecal samples were obtained from 4- to 5-week-old asymptomatic pigs from porcine herds in Ireland over 3 years, from 2005 to 2007. The samples were collected from three different herds in County Cork. Eighty samples were collected in May 2005 (farm 1), 80 samples were collected in September 2006 (farm 2), and 102 samples were collected in May 2007 (farm 3). A collection of 30 samples (farm 4) was also obtained in March 2007, in County Dublin, from animals which were 8 to 9 weeks old.
dsRNA preparation.
Total nucleic acids were extracted from the samples by a standard phenol-chloroform method with ethanol precipitation. The extracted nucleic acids were resuspended in 100 μl of sterile diethyl pyrocarbonate H2O and stored at −80°C prior to use.
Detection of GARVs and GCRVs by RT-PCR.
The dsRNA extracted from the fecal samples was denatured in dimethyl sulfoxide 50% at 97°C for 5 min and immediately cooled in ice. Thereafter, the samples were tested for the presence of GARVs and GCRVs by reverse transcription-PCR (RT-PCR) on an MJ Research PTC 200 Thermocycler (GMI Inc., MN). For the detection of GARVs, the VP4-specific oligonucleotide primers and thermal conditions described previously by Gentsch et al. were used (11) (Table 1). For the detection of GCRVs, a primer pair targeting the VP6 gene described by Sánchez-Fauquier et al., which amplifies a 320-bp fragment (nucleotide [nt] 1014 to nt 1334), was used (40) (Table 1). The thermal conditions were as follows: 42°C for 90 min and 94°C for 5 min, followed by 40 cycles of 94°C for 1 min, 55°C for 30 s, and 72°C for 1 min, plus a final extension of 72°C for 10 min. The amplicons were analyzed in 1.5% agarose gels following ethidium bromide staining and UV light transillumination.
TABLE 1.
Primers used for the detection of porcine GARVs and porcine GCRVs
| Targeta | Primer (sense) | Sequence (5′-3′) |
|---|---|---|
| VP4 of GARV | Con3 (+) | TGG CTT CGC CAT TTT ATA GAC A |
| Con2 (−) | ATT TCG GAC CAT TTA TAA CC | |
| VP7 of GCRV | GrpC-Vp7-20 (+) | GCT GTC TGA CAA ACT GGT C |
| GrpC-Vp7-1062 (−) | GCC ACA TGA TCT TGT TTA CGC | |
| VP6 of GCRV | F(BMJ45) (+) | AGC CAC ATA GTT CAC ATT TC |
| R(BMJ44) (−) | AGT CCG TTC TAT GTG ATT C |
RT-PCR amplification of the VP7 gene of GCRVs.
A one-step RT-PCR procedure (Qiagen OneStep RT-PCR kit) was utilized to amplify the eighth genome segment, which encodes the VP7, using the oligonucleotide primers GrC-VP7-20 and GrC-VP7-1062 (34) (Table 1). The primer pair amplifies a 1,040-bp-long fragment spanning nt 20 to 1062 of the VP7 genome segment. The RT-PCR thermal file was as follows: 50°C for 30 min and 95°C for 15 min, followed by 40 cycles of 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min, plus a final extension of 72°C for 10 min.
Sequence analysis of the VP7 gene of GCRVs.
All the samples identified as positive by the GCRV-specific PCR were subjected to sequencing. The PCR products were purified using a QIAquick PCR purification kit (Qiagen Ltd., West Sussex, England) and sequenced commercially at MWG Biotech (MWG Biotech, Ebersberg, Germany).
The sequences were assembled, edited, and analyzed using the BioEdit software package version 2.1 (14). Preliminary analysis was accomplished by comparing the sequences available in the database using the Web-based programs BLAST (http://www.ncbi.nlm.nih.gov/BLAST) and FASTA (http://www.ebi.ac.uk/fasta33). Phylogenetic and molecular evolutionary analyses were conducted using MEGA version 2.1 (23). A phylogenetic tree based on the VP7 sequences was elaborated with both the parsimony and distance methods, supplying a statistical support with bootstrapping over 100 replicates.
Nucleotide sequence accession numbers.
The VP7 gene sequences of strains 1GA/05/Cork, 281/07/Dublin, and 45/06/Cork were entered in the GenBank/EMBL/DDBJ databases under accession numbers EU624403, EU624404, and EU624405, respectively.
RESULTS
RT-PCR screening for GARVs and GCRVs.
A total of 13 out of 292 samples (4.4%) were found to contain GCRV, while 19 samples (6.5%) contained GARV. None of the GCRV-positive samples were found to contain GARV. The detection of GCRVs was carried out by RT-PCR using a primer pair based on the VP6 gene, described by Sánchez-Fauquier et al. (40). The GCRV-positive samples were detected in a scattered fashion over the entire study period (2005 to 2007) and in all of the herds surveyed. GCRVs were detected in 3/80 samples of the 2005 collection, 1/80 samples from 2006, and 8/102 samples from 2007, all of which had been collected in County Cork. In addition, GCRV infection was diagnosed in 1/30 samples from the 2007 collection made in County Dublin.
Sequence analysis of the gene encoding the outer capsid protein VP7.
The VP7 gene sequences of 10/13 GCRV samples were obtained, while the PCR amplicons of three samples were not exploitable for sequence analyses. Preliminary sequence analysis by BLAST and FASTA revealed that eight strains were more similar to Cowden-like (86 to 89% nt identity) G1 porcine GCRV strains and that two strains were more similar to 43/06-22-like (82 to 87% nt) G6 porcine strains.
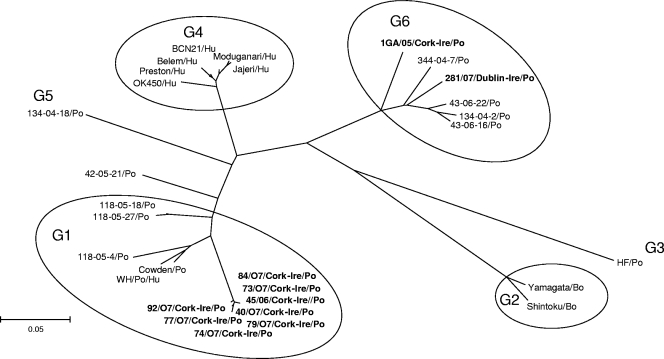
Using VP7 sequences of a selection of human and animal GCRV strains (Table 2), a phylogenetic tree was generated (Fig. 1). Six genetic lineages were clearly resolved, each one corresponding to a G genotype. Eight of the Irish GCRV strains were grouped along with the G1 porcine strains Cowden, WH, 118-05-4, 118-05-18, and 118-05-27. Two Irish strains, 1GA/05/Cork-Ire and 281/07/Dublin-Ire, clustered with the G6 porcine strains 344/04-7, 43/06-22, 134/04-2, and 43/06-16. Strain 1GA/05/Cork-Ire was detected in the south of Ireland in 2005, while strain 281/07/Dublin-Ire was identified in County Dublin, on the east coast of Ireland, in 2007.
TABLE 2.
List of reference animal and human strains with genotype/serotype designation and accession no.
| Strain | G genotype | Origin | Accession no. |
|---|---|---|---|
| po/Cowden | 1 | United States | M61101 |
| po/WH | 1 | United States | U31749 |
| po/118/05-18 | 1 | Italy | EF464648 |
| po/118/05-4 | 1 | Italy | EF464652 |
| po/118/05-27 | 1 | Italy | EF464649 |
| bo/Yamagata | 2 | Japan | AB108681 |
| bo/Shintoku | 2 | Japan | U31750 |
| po/HF | 3 | United States | U31748 |
| hu/Preston | 4 | United Kingdom | X77258 |
| hu/Belem | 4 | United Kingdom | X77256 |
| hu/OK450 | 4 | Japan | D87544 |
| hu/BCN21 | 4 | Spain | AM118023 |
| hu/Moduganari | 4 | Japan | AF325806 |
| Hu/Jajeri | 4 | Japan | AF325805 |
| po/134/04-18 | 5 | Italy | EF464653 |
| po/42/05-21 | 1/4 | Italy | EF464650 |
| po/43/06-22 | 6 | Italy | EF464657 |
| po/134/04-2 | 6 | Italy | EF464655 |
| po/43/06-16 | 6 | Italy | EF464656 |
FIG. 1.
Neighbor-joining unrooted tree based on the VP7 amino acid sequences of the following porcine GCRVs described in this study and reference strains: the porcine (Po) strains Cowden, WH, 118-05-4, 118-05-27, and 118-05-18; the bovine (Bo) strains Shintoku and Yamagata; the porcine strain HF; the human (Hu) strains OK450, Preston, Belem, BCN21, Moduganari, and Jajeri; the porcine strain 134-04-18; and the porcine strains 43-06-16, 134-04-2, 43-06-22, and 344-04-7.
A matrix of pairwise comparison based on the VP7 amino acid (aa) sequence between human and animal GCRV strains was elaborated (Table 3). The VP7 sequences of the Irish strains, 1GA/05/Cork-Ire and 281/07/Dublin-Ire, showed 92.2% aa identity to each other and 89.2 to 94.0% aa identity to the Italian G6 strains 134-04-2 and 43/06-22, while identity to the other strains was 59.4% to 80.5%. The porcine strains 45/06/Cork-Ire and 92/07/Cork-Ire showed 99.6% aa identity to each other and 91.5 to 92.6% aa identity with the prototype porcine strains Cowden and WH, while identity to the other strains was 67.9 to 83.8%.
TABLE 3.
VP7 comparison (% amino acid identity) between the porcine GCRVs detected in this study and other human and animal GCRVsa
| Strain | Accession no. | % Amino acid identity
|
||
|---|---|---|---|---|
| 45/06/Cork Ire/G1 | 281/07/Dublin Ire/G6 | 1GA/05/Cork Ire/G6 | ||
| Cowden/Po/G1 | M61101 | 92.7 | 75.4 | 76.4 |
| WH/Po/G1 | U31749 | 91.5 | 73.4 | 74.3 |
| 118-05-1/Po/G1 | EF464651 | 89.5 | 75.1 | 75.4 |
| 45/06/Cork-Ire/Po/G1 | 75.1 | 76.4 | ||
| 42-05-21/Po/G1/G4 | EF464650 | 87.8 | 78.1 | 78.0 |
| 134-04-2/Po/G6 | EF464655 | 75.4 | 92.6 | 89.2 |
| 43-06-22/Po/G6 | EF464657 | 75.7 | 94.0 | 91.9 |
| 281/07/Dublin-Ire/Po/G6 | 75.1 | 92.2 | ||
| 1GA/05/Cork-Ire/Po/G6 | 76.4 | 92.3 | ||
| 134-04-18/Po/G5 | EF464653 | 82.1 | 75.4 | 77.1 |
| HF/Po/G3 | U31748 | 67.9 | 59.4 | 68.6 |
| Yamagata/Bo/G2 | AB108681 | 72.3 | 76.4 | 76.0 |
| Shintoku/Bo/G2 | U31750 | 71.1 | 75.4 | 74.4 |
| Preston/Hu/G4 | X77258 | 83.8 | 80.5 | 80.4 |
| Belem/Hu/G4 | X77256 | 82.8 | 80.2 | 79.4 |
| OK450/Hu/G4 | D87544 | 82.4 | 79.5 | 79.4 |
Values were calculated using 296 residues, following the removal of the gaps. The highest identity values for each strain to reference viruses are bold. Po, porcine; Hu, human; Bo, bovine.
The VP7 sequences of the G6 strains 1GA/05/Cork and 281/07/Dublin and the VP7 sequences of the G1 strains 45/06/Cork and 40/07/Cork were analyzed in detail. The sequence of strain 1GA/05/Cork was 951 nt in length, corresponding to a fragment of the protein of 315 aa (nt 1 to 951). The sequence of strain 281/07/Dublin was 933 nt in length, corresponding to a 306-aa fragment of the VP7 protein (nt 16 to 933). The sequence of strain 45/06/Cork was 978 nt in length and corresponded to a 332-aa fragment (nt 7 to 978) of the outer capsid protein, while the sequence of strain 40/07/Cork was 990 nt long and encompassed a 329-aa fragment of the VP7 (nt 3 to 990).
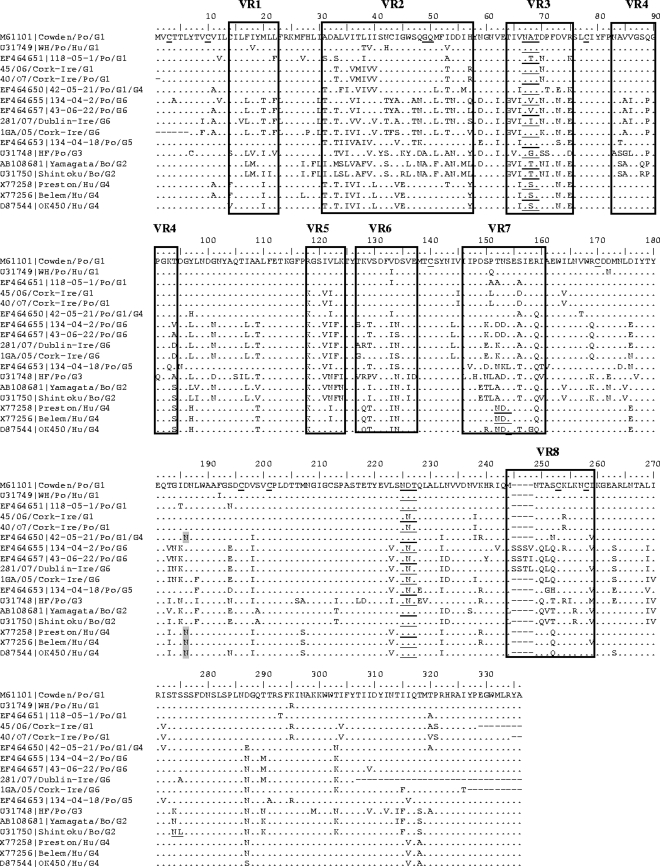
A comparison of the deduced amino acid VP7 sequences of Irish GCRV strains 1GA/05/Cork, 281/07/Dublin, 45/06/Cork, and 40/07/Cork with the amino acid VP7 sequences from animal and human GCRVs was carried out (Fig. 2). Similarly to the Italian G6 strains, the VP7 gene of strain 281/07/Dublin-Ire possessed a 4-aa insertion in the VR8 at the carboxy terminus of the protein, but this was SSTL instead of SSSV or SSTI found in the Italian strains. Unexpectedly, the G6 strain 1GA/05/Cork-Ire did not possess this unique insertion.
FIG. 2.
Comparison of the deduced amino acid VP7 sequences from animal and human GCRVs. The genotypes of GCRVs are also given. Identical amino acids are indicated by dots, and gaps are shown by dashes. The variable regions VR-1 to VR-8 (48) are boxed. Potential N-linked glycosylation sites are underlined. Also, the conserved cysteine residues and the putative signal cleavage site GQ are underlined in the consensus sequence (strain po/Cowden).
The potential N-linked glycosylation sites (Asn-X-Ser/Thr) located at aa 67 to 69 and 225 to 227 were conserved in the Irish porcine GCRV strains. The putative signal cleavage site at residues 49 to 50 (AGQ) and the cysteines at residues 3, 10, 78, 140, 170, 196, 212, 253, and 258 (residues 249 and 254 of strain Cowden) were also highly conserved.
DISCUSSION
GCRVs are an important cause of acute gastroenteritis in humans and animals. In this study, evidence for the occurrence of GCRV in asymptomatic piglets in Irish porcine farms and for genetic heterogeneity among the identified strains was collected. By using RT-PCR, GCRVs were detected in 13/292 fecal specimens (4.4%) from 4/4 swine herds over the 3 years of the study, suggesting the continual circulation of GCRVs in the piggeries. Interestingly, evidence for the circulation of porcine GARVs in the same herds was also obtained, since GARVs were also detected in 19 samples but not in conjunction with GCRVs, although mixed infection by GCRV and GARV was not detected (Table 4).
TABLE 4.
Relative distribution of two enteric viruses (GCRV and GARV) in fecal specimens of asymptomatic pigsa
| Origin (no. of specimens) | Year of collection | No. of positive samplesb
|
|
|---|---|---|---|
| GARV | GCRV | ||
| Cork (80) | 2005 | 6 | 3 |
| Cork (80) | 2006 | 4 | 1 |
| Dublin (30) | 2007 | 0 | 1 |
| Cork (102) | 2007 | 9 | 8 |
| Total | 19 | 13 | |
For the detection of GCRVs, RT-PCR was carried out for all the samples using a VP6-based RT-PCR (40). GCRV-positive samples were obtained from four herds in two cities in Ireland, Cork and Dublin.
Of a total of 292 samples, 6.50% were positive for GARV and 4.4% were positive for GCRV.
The presence of GARVs and GCRVs in asymptomatic animals is not unexpected, since asymptomatic infections by rotaviruses have been identified in previous studies (26, 32). Several mechanisms could account for the occurrence of asymptomatic infections in those animals, such as lingering maternally derived immunity (15) or the circulation of naturally attenuated rotavirus strains (7, 8, 16, 31). Interestingly, the prevalence of GARV and GCRV infection in the asymptomatic animals was low compared to that reported in studies of symptomatic animals (21, 27, 39). Paired evaluation of the distribution of the GCRVs in symptomatic and asymptomatic animals is consistent with a pathogenic role of GCRVs in piglets. Also, experimental infections in animals have clearly demonstrated the pathogenic properties of GCRVs in animals, either alone or in synergism with other pathogens (7, 12, 45).
The VP7 genes of the detected GCRVs were subjected to sequence analysis, and the majority of the strains were characterized as having a G1 genotype, since they were similar to the prototype porcine strain Cowden. Interestingly, two strains were found to be distantly related to G1 GCRVs and to be more similar to unusual porcine GCRVs that were recently identified in Italy and proposed as having a novel G6 genotype (28). In addition, one of the G6 Irish strains (1GA/05/Cork-Ire) lacked a 4-aa insertion in the highly variable region VR8, which is conserved in all the other G6 strains (28). The presence of amino acid insertions/deletions in the rotavirus outer capsid protein VP7 has already been described. A single amino acid insertion, Asn-76, has been identified in the VP7 of some G4 human GARVs, and the increased frequency of G4 human GARVs in Argentina from 1997 to 1998 has been related to the emergence of this variant (4). Accordingly, our data suggest that even larger amino acid stretches may be inserted/deleted in the VP7 without altering the viability of the virus.
Analysis of porcine GCRV strains has revealed broad genetic diversity. Strains Cowden, HF, 134/04-18, and 43/06-22 are each prototypes of different G types, G1, G3, G5, and G6, respectively (28, 47, 48). The antigenic relationships among the various G types have not been investigated in detail, and only limited information is available. Antigenic diversity has been observed among the porcine strain Cowden (G1), the bovine strain Shintoku (G2), and the porcine strain HF (G3), which share 69.9 to 74.7% aa identity (47, 48). The reasons for and the effects of this antigenic diversification are unclear. For GARVs, there is evidence that the immune response to rotavirus infection is primarily serotype specific (9). Taking into account the antigenic heterogeneity of human GARVs, a polyvalent vaccine, which contains VP7 genes derived from human G1, G2, G3, and G4 strains and a VP4 gene derived from a human P[8] virus (RotaTeq; Merck), has been developed for the prevention of rotavirus-induced diarrhea in children. Also, a monovalent G1P[8] human attenuated vaccine has been licensed (Rotarix; GlaxoSmithKline), since most human infections are due to G1P[8] viruses (36, 49).
It is unclear whether the antigenic/genetic diversity observed among porcine GCRVs may pose a challenge for future prophylaxis programs for the prevention of enteritis in suckling and weaning piglets. The 2006 Irish Regional Veterinary Laboratories Surveillance Report demonstrated that pneumonia, postweaning multisystemic wasting syndrome, and enteritis were the most frequently detected causes of mortality in pigs (Department of Agriculture, Fisheries and Food; http://www.agriculture.gov.ie/areasofi/cvrl/VetSurveillance2006.pdf). It will be crucial to increase the surveillance for porcine enteric viruses, including GCRVs, and to gather information on the genetic diversity of GARVs and GCRVs in order to plan adequate measures of control of enteritis in piglets.
In conclusion, this study provides evidence that porcine GCRV may be detected in asymptomatic piglets but much less frequently than in symptomatic animals. In addition, it provides evidence that, unlike the human viruses, porcine GCRVs display broad genetic heterogeneity. In order to increase the understanding of GCRV epidemiology and ecology and to definitively assess their role in piglet enteritis, it would be opportune to include GCRVs in the diagnostic algorithms for porcine enteric infections.
Acknowledgments
We thank Bill Cashman for stimulating discussions and for assistance with the collection of specimens.
This work was funded by Technological Research Sector Strand I (PO 6176) and FIRM research grants (05/R&D/CIT/365) awarded to Helen O'Shea.
Footnotes
Published ahead of print on 16 July 2008.
REFERENCES
- 1.Arista, S., L. Giovannelli, D. Pistoia, A. Cascio, M. Parea, and G. Gerna. 1990. Electropherotypes, subgroups and serotypes of human rotavirus strains causing gastroenteritis in infants and young children in Palermo, Italy from 1985 to 1989. Res. Virol. 141435-448. [DOI] [PubMed] [Google Scholar]
- 2.Bányai, K., B. Jiang, A. Bogdán, B. Horváth, F. Jakab, E. Meleg, V. Martella, L. Magyari, B. Melegh, and G. Szucs. 2006. Prevalence and molecular characterization of human group C rotaviruses in Hungary. J. Clin. Virol. 37317-322. [DOI] [PubMed] [Google Scholar]
- 3.Bohl, E. H., L. J. Saif, K. W. A. Theil, G. Agnes, and R. F. Cross. 1982. Porcine pararotavirus: detection, differentiation from rotavirus, and pathogenesis in gnotobiotic pigs. J. Clin. Microbiol. 15312-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bok, K., D. O. Matson, and J. A. Gomez. 2002. Genetic variation of capsid protein VP7 in genotype G4 human rotavirus strains: simultaneous emergence and spread of different lineages in Argentina. J. Clin. Microbiol. 402016-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bridger, J. C., S. Pedley, and M. A. McCrae. 1986. Group C rotaviruses in humans. J. Clin. Microbiol. 23760-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caul, E. O., C. R. Ashley, J. M. Darville, and J. C. Bridger. 1990. Group C rotavirus associated with fatal enteritis in a family outbreak. J. Med. Virol. 30201-205. [DOI] [PubMed] [Google Scholar]
- 7.Chang, K. O., P. R. Nielsen, L. A. Ward, and L. J. Saif. 1999. Dual infection of gnotobiotic calves with bovine strains of group A and porcine-like group C rotaviruses influences pathogenesis of the group C rotavirus. J. Virol. 739284-9293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang, K. O., O. H. Vandal, L. Yuan, D. C. Hodgins, and L. J. Saif. 2001. Antibody-secreting cell responses to rotavirus proteins in gnotobiotic pigs inoculated with attenuated or virulent human rotavirus. J. Clin. Microbiol. 392807-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciarlet, M., and M. K. Estes. 2002. Rotaviruses: basic biology, epidemiology and methodologies, p. 2753-2773. In G. Bitton (ed.), Encyclopedia of environmental microbiology. John Wiley & Sons, New York, NY.
- 10.Gabbay, Y. B., A. A. Borges, C. R. M. Barardi, A. C. Linhares, J. D. P. Mascarenhas, J. D. P. Simoe, R. I. Glass, and B. Jiang. 2006. Excretion profiles of porcine and human group C rotaviruses in children from Belem, Para, Brazil: evidence for interspecies transmission and sequential infections, p. 109. Proceedings of the 9th Double Stranded RNA Viruses Meeting, Cape Town, South Africa.
- 11.Gentsch, J. R., R. I. Glass, P. Woods, V. Gouvea, M. Gorziglia, J. Flores, B. K. Das, and M. K. Bhan. 1992. Identification of group A rotavirus gene 4 types by polymerase chain reaction. J. Clin. Microbiol. 301365-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gouet, P., M. Contrepois, H. C. Dubourguier, Y. Riou, R. Scherrer, J. Laporte, J. F. Vautherot, J. Cohen, and R. L'Haridon. 1978. The experimental production of diarrhoea in colostrum deprived axenic and gnotoxenic calves with enteropathogenic Escherichia coli, rotavirus, coronavirus, and in a combined infection of rotavirus and E. coli. Ann. Rech. Vet. 9433-440. [PubMed] [Google Scholar]
- 13.Gressener, B. D., A. Sutanto, M. Linehan, and I. G. Djelantik. 2005. Hopes and fears for rotavirus vaccines. Lancet 365190.. [DOI] [PubMed] [Google Scholar]
- 14.Hall, T. A. 1999. BioEdit: a user friendly biological sequence alignment and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 4195-98. [Google Scholar]
- 15.Hodgins, D. C., S. Y. Kang, L. deArriba, V. Parreño, L. A. Ward, L. Yuan, T. To, and L. J. Saif. 1999. Effects of maternal antibodies on protection and development of antibody responses to human rotavirus in gnotobiotic pigs. J. Virol. 73186-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iosef, C., T. Van Nguyen, K. Jeong, K. Bengtsson, B. Morein, Y. Kim, K. O. Chang, M. S. Azevedo, L. Yuan, P. Neilsen, and L. J. Saif. 2002. Systemic and intestinal antibody secreting cell responses and protection in gnotobiotic pigs immunized orally with attenuated Wa human rotavirus and Wa 2/6-rotavirus-like-particles associated with immunostimulating complexes. Vaccine 201741-1753. [DOI] [PubMed] [Google Scholar]
- 17.Iturriza-Gómara, M., I. Clarke, U. Desselberger, D. Brown, D. Thomas, and J. Gray. 2004. Seroepidemiology of group C rotavirus infection in England and Wales. Eur. J. Epidemiol. 19589-595. [DOI] [PubMed] [Google Scholar]
- 18.Jiang, B. M., P. H. Dennehy, S. Spangenberger, J. R. Gentsch, and R. I. Glass. 1995. First detection of group C rotavirus in fecal specimens of children with diarrhea in the United States. J. Infect. Dis. 17245-50. [DOI] [PubMed] [Google Scholar]
- 19.Kapikian, A. Z., and R. M. Chanock. 1996. Rotaviruses, p. 1787-1825. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 3rd ed., vol 2. Lippincott Williams and Wilkins, Philadelphia, PA.
- 20.Kapikian, A. Z., Y. Hoshino, and R. M. Chanock. 2001. Rotaviruses, p. 1787-1833. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams and Wilkins, Philadelphia, PA.
- 21.Katsuda, K., M. Kohmoto, K. Kawashima, and H. Tsunemitsu. 2006. Frequency of enteropathogen detection in suckling and weaned pigs with diarrhea in Japan. J. Vet. Diagn. Investig 18350-354. [DOI] [PubMed] [Google Scholar]
- 22.Kim, Y., K. O. Chang, B. Straw, and L. J. Saif. 1999. Characterization of group C rotaviruses associated with diarrhea outbreaks in feeder pigs. J. Clin. Microbiol. 371484-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar, S., K. Tamura, I. B. Jakobsen, and M. Nei. 2001. MEGA2: molecular evolutionary genetics analysis software. Bioinformatics 17244-245. [DOI] [PubMed] [Google Scholar]
- 24.Kuzuya, M., R. Fujii, M. Hamano, M. Yamada, K. Shinozaki, A. Sasagawa, S. Hasegawa, H. Kawamoto, K. Matsumoto, A. Kawamoto, A. Itagaki, S. Funatsumaru, and S. Urasawa. 1998. Survey of human group C rotaviruses in Japan during the winter of 1992 to 1993. J. Clin. Microbiol. 366-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuzuya, M., M. Hamano, M. Nishijima, R. Fujii, H. Ogura, M. Tanaka, A. Oda, S. Kusaka, and M. Naitou. 2005. An outbreak of acute gastroenteritis caused by human group C rotavirus in a welfare institution in Okayama prefecture. Jpn. J. Infect. Dis. 58255-257. [PubMed] [Google Scholar]
- 26.Lecce, J. G., and M. W. King. 1978. Role of rotavirus (reo-like) in weanling diarrhea of pigs. J. Clin. Microbiol. 8454-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martella, V., K. Bányai, E. Lorusso, A. Bellacicco, N. Decaro, M. Camero, G. Bozzo, P. Moschidou, S. Arista, G. Pezzotti, A. Lavazza, and C. Buonavoglia. 2007. Prevalence of group C rotaviruses in weaning and post-weaning pigs with enteritis. Vet. Microbiol. 12326-33. [DOI] [PubMed] [Google Scholar]
- 28.Martella, V., K. Bányai, E. Lorusso, N. Decaro, A. Bellacicco, C. Desario, M. Corrente, G. Greco, P. Moschidou, M. Tempesta, S. Arista, M. Ciarlet, A. Lavazza, and C. Buonavoglia. 2007. Genetic heterogeneity in the VP7 of group C rotaviruses. Virology 367358-366. [DOI] [PubMed] [Google Scholar]
- 29.Mawatari, T., A. Taneichi, T. Kawagoe, M. Hosokawa, K. Togashi, and H. Tsunemitsu. 2004. Detection of a bovine group C rotavirus from adult cows with diarrhea and reduced milk production. J. Vet. Med. Sci. 66887-890. [DOI] [PubMed] [Google Scholar]
- 30.Morin, M., R. Magar, and Y. Robinson. 1990. Porcine group C rotavirus as a cause of neonatal diarrhea in a Quebec swine herd. Can. J. Vet. Res. 54385-389. [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen, T. V., C. Iosef, K. Jeong, Y. Kim, K. O. Chang, K. Lovgren-Bengtsson, B. Morein, M. S. Azevedo, P. Lewis, P. Nielsen, L. Yuan, and L. J. Saif. 2003. Protection and antibody responses to oral priming by attenuated human rotavirus followed by oral boosting with 2/6-rotavirus-like particles with immunostimulating complexes in gnotobiotic pigs. Vaccine 214059-4070. [DOI] [PubMed] [Google Scholar]
- 32.Parra, G. I., G. Vidales, J. A. Gomez, F. M. Fernandez, V. Parreño, and K. Bok. 2008. Phylogenetic analysis of porcine rotavirus in Argentina: increasing diversity of G4 strains and evidence of interspecies transmission. Vet. Microbiol. 126243-250. [DOI] [PubMed] [Google Scholar]
- 33.Qian, Y. A., B. M. Jiang, L. J. Saif, S. Y. Kang, Y. Ishimaru, Y. Yamashita, M. Oseto, and K. Y. Green. 1991. Sequence conservation of gene 8 between human and porcine group C rotaviruses and its relationship to the VP7 gene of group A rotaviruses. Virology 182562-569. [DOI] [PubMed] [Google Scholar]
- 34.Rahman, M., S. Banik, A. S. Faruque, K. Taniguchi, D. A. Sack, M. Van Ranst, and T. Azim. 2005. Detection and characterization of human group C rotaviruses in Bangladesh. J. Clin. Microbiol. 434460-4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodger, S. M., R. F. Bishop, and I. H. Holmes. 1982. Detection of a rotavirus-like agent associated with diarrhea in an infant. J. Clin. Microbiol. 16724-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruiz-Palacios, G. M., I. Pérez-Schael, F. R. Velázquez, H. Abate, T. Breuer, S. C. Clemens, et al. 2006. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N. Engl. J. Med. 35411-22. [DOI] [PubMed] [Google Scholar]
- 37.Saif, L. J., E. H. Bohl, K. W. Theil, R. F. Cross, and J. A. House. 1980. Rotavirus-like, calicivirus-like, and 23-nm virus-like particles associated with diarrhea in young pigs. J. Clin. Microbiol. 12105-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saif, L. J., and B. Jiang. 1994. Nongroup A rotaviruses of humans and animals. Curr. Top. Microbiol. Immunol. 185339-371. [DOI] [PubMed] [Google Scholar]
- 39.Saif, L. J., B. Rosen, and A. Parwani. 1994. Animal rotaviruses, p. 279-367. In A. Kapikian (ed.), Viral infections of the gastrointestinal tract. Marcel Dekker, Inc., New York, NY.
- 40.Sánchez-Fauquier, A., E. Roman, J. Colomina, I. Wilhelmi, R. I. Glass, and B. Jiang. 2003. First detection of group C rotavirus in children with acute diarrhea in Spain. Arch. Virol. 148399-404. [DOI] [PubMed] [Google Scholar]
- 41.Schnagl, R. D., K. Boniface, P. Cardwell, D. McCarthy, C. Ondracek, B. Coulson, J. Erlich, and F. Morey. 2004. Incidence of group C human rotavirus in central Australia and sequence variation of the VP7 and VP4 genes. J. Clin. Microbiol. 422127-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sigolo de San Juan, C., R. C. Bellinzoni, N. Mattion, J. La Torre, and E. A. Scodeller. 1986. Incidence of group A and atypical rotaviruses in Brazilian pig herds. Res. Vet. Sci. 41270-272. [PubMed] [Google Scholar]
- 43.Szucs, G., M. Kende, and M. Uj. 1987. Atypical human rotaviruses in Hungary. Ann. Inst. Pasteur Virol. 138391-395. [Google Scholar]
- 44.Terrett, L. A., L. J. Saif, K. W. Theil, and E. M. Kohler. 1987. Physicochemical characterization of porcine pararotavirus and detection of virus and viral antibodies using cell culture immunofluorescence. J. Clin. Microbiol. 25268-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thouless, M. E., R. F. DiGiacomo, and B. J. Deeb. 1996. The effect of combined rotavirus and Escherichia coli infections in rabbits. Lab. Anim. Sci. 46381-385. [PubMed] [Google Scholar]
- 46.Tsunemitsu, H., B. Jiang, and L. J. Saif. 1992. Detection of group C rotavirus antigens and antibodies in animals and humans by enzyme-linked immunosorbent assays. J. Clin. Microbiol. 302129-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsunemitsu, H., B. Jiang, Y. Yamashita, M. Oseto, H. Ushijima, and L. J. Saif. 1992. Evidence of serologic diversity within group C rotaviruses. J. Clin. Microbiol. 303009-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsunemitsu, H., B. Jiang, and L. J. Saif. 1996. Sequence comparison of the VP7 gene encoding the outer capsid glycoprotein among animal and human group C rotaviruses. Arch. Virol. 141705-713. [DOI] [PubMed] [Google Scholar]
- 49.Vesikari, T., D. O. Matson, P. Dennehy, P. Van Damme, M. Santosham, and Z. Rodriguez. 2006. Safety and efficacy of a pentavalent human-bovine (WC3) reassortment rotavirus vaccine. N. Engl. J. Med. 35423-33. [DOI] [PubMed] [Google Scholar]