Abstract
We screened for heteroresistant, vancomycin-intermediate Staphylococcus aureus (hVISA) among clinical isolates of methicillin-resistant S. aureus collected from three hospitals (two urban teaching hospitals and one community hospital) in the Detroit metropolitan area over a 22-year period. The Macro Etest method was used to screen all available isolates. Confirmation of hVISA-positive screens were confirmed by population-area under the concentration-time curve (AUC) analysis. A total of 1,499 isolates revealed hVISA/VISA rates of 2.2/0.4% (n = 225; 1986 to 1993), 7.6/2.3% (n = 356; 1994 to 2002), and 8.3/0.3% (n = 917; 2003 to 2007). Population-AUC analysis confirmed 92.6% of the hVISA-positive strains determined by the Macro Etest method. For the isolates with known sources (1,208), the predominant source of hVISA was blood (60%), followed by lung (21%), skin and wound infections (14%), abscess (1%), and other (4%). The percentage of hVISA-positive strains appeared to increase as a function of the vancomycin MIC. Staphylococcal cassette chromosome mec (SCCmec) typing revealed that the majority (56.9%) of the hVISA strains were SCCmec type II and 39.4% were type IV; the majority of these strains were collected from 2000 to 2007. Our data indicate that the prevalence of hVISA may be increasing. Based on the association of vancomycin treatment failure in patients with hVISA, surveillance of hVISA strains is warranted.
The prevalence of methicillin-resistant Staphylococcus aureus (MRSA) has continued to increase at a dramatic rate in recent years (20, 21). This organism is associated with higher rates of morbidity and mortality than methicillin-susceptible strains (16). Vancomycin is the primary treatment for this pathogen; however, the increasing use of this agent for a variety of gram-positive organisms, including MRSA, contributes to the growing burden of nonsusceptible strains in both the community and hospital settings (17). Unfortunately, vancomycin-intermediate S. aureus (VISA) and heteroresistant VISA (hVISA) strains have now been reported and are associated with vancomycin treatment failures (7, 10, 27). Although the presence of VISA is routinely screened for on the basis of the vancomycin MIC exceeding the breakpoint for susceptibility (27, 33), screening for hVISA is not routinely performed (15, 33, 37). The reasons for this discrepancy include the fact that the vancomycin MIC for these strains determined by routine testing is reported to be in the susceptible range. Screening for hVISA requires additional testing to reveal its heterovariant phenotype, and these methods are more labor-intensive and costly then routine susceptibility testing. In addition, the methods used for evaluating hVISA are not standardized. Despite these shortcomings, there is a growing need to begin screening for this organism on the basis of an increasing number of reports of vancomycin treatment failure for patients (2, 4, 12, 13, 19, 24).
The objectives of the present study were to screen and characterize clinical isolates of MRSA demonstrating heteroresistance to vancomycin from a collection of isolates spanning a 22-year period.
(This report was presented in part at the 17th European Congress of Clinical Microbiology and Infectious Diseases, Munich, Germany, March 2007 [abstr. 302].)
MATERIALS AND METHODS
Bacterial isolates.
MRSA isolates from 1986 to 2007 in the Detroit metropolitan area were collected from Detroit Receiving Hospital and Henry Ford Hospital, Detroit, MI, and William Beaumont Hospital, Royal Oak, MI, and from the SENTRY Antimicrobial Surveillance Database on the basis of oxacillin resistance and year (as available). These isolates were part of collections that had been previously used for surveillance studies by investigators in the area or by the SENTRY Surveillance Program. Mu3 (archetype hVISA) (11) was used as the control strain for population analysis profile (PAP)-area under the concentration-time curve (AUC) ratio determination. The infection source of the derived organisms was identified when available. All susceptibility testing and hVISA, VISA, and molecular testing were completed by the Anti-Infective Research Laboratory for the purpose of this investigation during a 1-year time period.
Antimicrobial agents and susceptibility testing.
Vancomycin analytical powder was obtained from Sigma Chemical Company, St. Louis, MO. Teicoplanin analytical powder was provided by Sanofi-Aventis. Stock solutions of each antibiotic were prepared fresh at the beginning of each week and kept frozen at −4°C. MICs and minimum bactericidal concentrations (MBCs) were determined for each isolate in duplicate by microdilution techniques with an inoculum of 5 × 105 CFU/ml according to the Clinical and Laboratory Standards Institute guidelines. Aliquots (5 μl) from clear wells were plated onto tryptic soy agar plates for the determination of the MBC, and all samples were incubated at 35°C for 24 h. Isolates for which the vancomycin MICs were 4 to 8 μg/ml were considered VISA according to the Clinical and Laboratory Standards Institute guidelines (5).
MET and modified PAP-AUC ratio.
The Macro Etest (MET) was performed on all strains according to the manufacturer's manual (EAS 003; AB Biodisk, Solna, Sweden) with brain heart infusion (BHI) agar. Briefly, several colonies were picked and suspended in normal saline to obtain a 2-McFarland-standard bacterial density. One hundred microliters of this suspension was evenly spread onto a 90-mm BHI (Difco, Detroit, MI) agar plate and allowed to dry. Vancomycin and teicoplanin Etest strips (AB Biodisk, Solna, Sweden) were applied to the surface of the BHI agar (Difco, Detroit, MI) in parallel but in opposite directions, and the plates were incubated at 35°C for 48 h. Zones were read at complete inhibition while carefully observing for visual hazy growth and microcolonies. A strain was considered positive for hVISA if microcolonies were detected at ≥8 μg/ml for both vancomycin and teicoplanin or at ≥12 μg/ml for teicoplanin alone (37). All strains positive for hVISA from this method were then further evaluated via PAP-AUC analysis. The modified PAPs were determined as previously described (34, 37). Briefly, organisms were cultured in supplemented Mueller-Hinton broth from an overnight growth, adjusted to a 108-CFU/ml density, and spiral plated (Don Whitley Scientific Limited, West Yorkshire, England) onto BHI agar (Difco, Detroit, MI) plates containing 0, 0.5, 1, 1.5, 2, 3, 4, and 8 μg/ml vancomycin. Colonies were counted after incubation for 48 h at 35°C. The resulting numbers of CFU per milliliter were plotted against the vancomycin concentration. The AUC for each strain was determined by the trapezoidal rule with SigmaPlot 9.0 (Systat Software, Inc., Richmond, CA). Each strain was run in conjunction with Mu3 as the control hVISA strain. A ratio was then calculated by dividing the AUC of the test strain by the AUC of Mu3. The PAP-AUC criteria for determination of hVISA have been previously described and are based on ratios of ≥0.90 for hVISA and ≥1.3 for VISA (36). The performance of the MET method for detecting hVISA was compared to the PAP-AUC ratio results.
SCCmec, Panton-Valentine leukocidin, and accessory gene regulator (agr) group and function.
The staphylococcal cassette chromosome mec (SCCmec) type was determined for all hVISA strains as previously described (38). Detection of the lukS-PV and lukF-PV genes was performed as previously described (18). The accessory gene regulator group and function were also determined for all hVISA strains. The agr group was determined by multiplex PCR as described by Peacock et al. (26). The expression of the agr gene cluster was determined by delta hemolysin production as described by Sakoulas et al. (29).
Statistical analysis.
Vancomycin MIC trends over the 22-year period were assessed by linear regression and nonparametric correlation (Spearman's ρ). Statistical significance was defined as a P of <0.05.
RESULTS
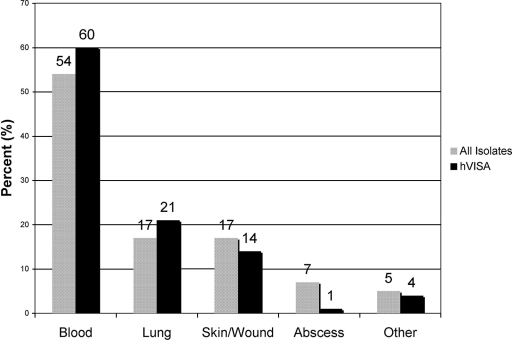
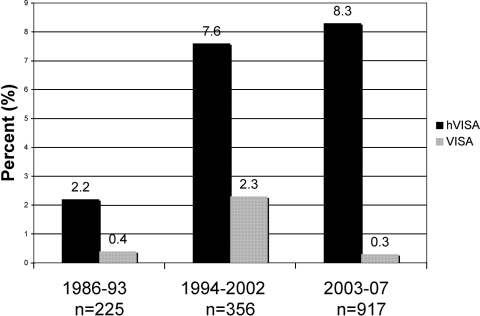
A total of 1,499 nonduplicate clinical isolates were collected over a 22-year period. The sources of 1,208 of these clinical isolates were identified and were similar between hVISA (n = 86) and non-hVISA strains (n = 1,102). Although blood was the most common source of hVISA, the isolates identified as hVISA were proportionally distributed among the various sources (Fig. 1). Of the 1,499 strains, 112 hVISA strains were identified by MET and confirmed by PAP-AUC ratio methods. The percentages of hVISA and VISA strains from 1986 to 2007 ranged from 2.2 to 8.3% and from 0.3 to 2.3%, respectively (Fig. 2). The PAP-AUC ratio analysis method confirmed 92.1% of the hVISA strains identified by the MET screening method. Molecular typing of the strains revealed that 56.9% of the hVISA strains identified were SCCmec type II, of which 53.2% belonged to agr group II. Of interest, 38.4% were SCCmec type IV (37.2% Panton-Valentine leukocidin positive), with 63.2% belonging to agr group I, indicating that some hVISA strains potentially came from the community. The majority (40/43) of hVISA strains that were SCCmec type IV were identified between the years 2000 and 2007. The agr function was absent or severely diminished in 21.2% of hVISA strains identified. There were a total of 14 strains identified as VISA on the basis of a vancomycin MIC of 4 to 8 μg/ml (MIC = 4 μg/ml; n = 13). Eleven were determined as hVISA on the basis of the PAP-AUC ratios, which ranged from 0.9 to 1.25. Two VISA strains were identified on the basis of their PAP-AUC ratios (1.42 [MIC = 4 μg/ml] and 1.54 [MIC = 8 μg/ml], respectively). The primary source of the VISA strains (on the basis of MIC definition) was blood (12/14).
FIG. 1.
Known infection sources of the isolates in this study (all isolates, n = 1,208; hVISA isolates, n = 86).
FIG. 2.
Percentages of S. aureus isolates tested in the Detroit area over 22 years that were either hVISA or VISA.
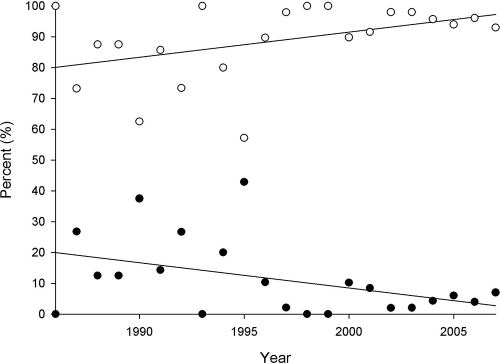
Vancomycin and teicoplanin susceptibilities are shown in Table 1. The vancomycin and teicoplanin MICs for 50 and 90% of the strains tested and the MIC ranges were 1, 2, and 0.06 to 8 μg/ml and 0.5, 2, and 0.03 to 64 μg/ml, respectively. The vancomycin MIC for 28.2% of the MRSA strains tested was 2 μg/ml. Overall, vancomycin susceptibility decreased over the 22-year period. The percentage of isolates for which the MIC was 0.5 μg/ml or less decreased from 19.4% between 1986 and 1989 to 6.6% during the years 2002 to 2007, while that of isolates for which the MIC was ≥1 μg/ml increased from 80.7 to 93.4% during the same time periods (Spearman's correlation, r = 0.97, P ≤ 0.01; regression analysis, r2 = 0.56 and 0.98, respectively; P = 0.04; Fig. 3). Of interest, the percentage of hVISA strains increased in conjunction with increasing MICs of both vancomycin and teicoplanin (Table 1). Vancomycin and teicoplanin MBCs did not appear to change over time. The vancomycin and teicoplanin MBCs for 50 and 90% of the strains tested were 1, 2, and 0.06 to 128 μg/ml and 1, 2, and 0.06 to 64 μg/ml, respectively. Two hVISA isolates were tolerant (MBC/MIC ratio, ≥32) to vancomycin, and three non-hVISA strains were tolerant to teicoplanin. All of the tolerant isolates were from blood and were obtained from different institutions.
TABLE 1.
Percentages of hVISA by PAP-AUC at different MICs of vancomycin and teicoplanina
| Drug and MIC (μg/ml) | No. of hVISA isolates | Total no. of isolates | % hVISA |
|---|---|---|---|
| Vancomycin | |||
| <0.5 | 0 | 4 | 0 |
| 0.5 | 3 | 94 | 3.2 |
| 1 | 52 | 858 | 6.1 |
| 2 | 44 | 381 | 11.6 |
| 4 | 11 | 13 | 84.6 |
| >4 | 0 | 1 | 0 |
| Teicoplanin | |||
| <0.25 | 0 | 14 | 0 |
| 0.25 | 2 | 271 | 0.7 |
| 0.5 | 9 | 578 | 1.6 |
| 1 | 31 | 322 | 9.6 |
| 2 | 38 | 123 | 30.9 |
| 4 | 7 | 16 | 43.8 |
| >4 | 13 | 23 | 56.5 |
For both agents, as the MIC increases, the percentage of hVISA increases.
FIG. 3.
Vancomycin susceptibilities determined by microtiter methods over time. The percentage of isolates for which the MIC was 0.5 μg/ml or less (filled circles) decreased from 19.4% between 1986 and 1989 to 6.6% from 2002 to 2007, while the percentage of isolates for which the MIC was 1 mg/liter or greater (open circles) increased from 80.6 to 93.4% during the same time period (r2 = 0.56 and 0.98, respectively; P = 0.04).
DISCUSSION
Infections involving hVISA are a particular problem since these strains are reported by clinical laboratories to be susceptible on the basis of current recommended breakpoints for vancomycin and teicoplanin (6, 33). Screening for heteroresistance to vancomycin or teicoplanin is not routinely performed, and therefore the true prevalence of hVISA is unknown. However, on the basis of the limited studies available, the range is 1.67 to 27% (3, 4, 8, 19). Of interest, 11 out of 14 strains that were considered VISA by MIC (MICs of 4 to 8 μg/ml) displayed heterogeneous characteristics. The MIC for the majority (13/14) of these strains was 4 μg/ml. We have previously described similar strains displaying this trait (1, 28). Although our present investigation does not represent a true prevalence study, the data derived from isolates sampled over the last 22 years may imply that the prevalence of hVISA is increasing. There are several limitations that should be mentioned regarding our study. First, we had limited access to information regarding clinical data and therefore could not evaluate patient antimicrobial exposure or clinical outcome. In addition, although the MET procedure has been found to have very reliable specificity (89.1%), it is possible that we missed some hVISA stains (37). The three time periods selected to evaluate the percentage of hVISA strains were selected on the basis of our ability to cluster the largest numbers of isolates from those periods where fewer isolates were available (1986 to 1993 and 1994 to 2002). Despite our efforts to control for this problem, more isolates were available to us during the later years (2003 to 2007), so some bias may exist secondary to the larger sample size of organisms tested during these years. Lastly, although all hVISA strains were nonduplicate and had molecular testing performed to identify the SCCmec type and agr group, we did not determine if clonality existed among our hVISA strains. Therefore, the above points should be considered when evaluating our data.
The evaluation of hVISA prevalence is extremely important since vancomycin therapeutic failures have been routinely reported in patients with hVISA infections. Howden et al. evaluated the outcomes of 25 patients with hVISA infections that included bacteremia, endocarditis, osteomyelitis, and septic arthritis (14). Vancomycin therapy was deemed a failure in 19 (76%) of the patients, of whom 15 had detectable S. aureus bacteremia after 7 days of therapy and 4 had S. aureus isolated from a sterile site 21 days after vancomycin therapy. Charles et al., in another investigation, evaluated the outcomes of patients with MRSA bacteremia over a 12-month period (4). The presence of hVISA bacteremia was identified in five episodes (3 patients; prevalence of 9.4%), and the outcomes of these patients were compared to those of 48 episodes (47 patients) of non-hVISA MRSA bacteremia. Interestingly, patients with hVISA bacteremia were significantly more likely to have persistent fever and bacteremia beyond 7 days, high-bacterial-load infections, and initially low serum vancomycin concentrations (<10 μg/ml). It was noted that patients failed to improve even after a vancomycin dosage adjustment was performed to ensure appropriate serum vancomycin concentrations.
The fact that patients did not improve despite receiving adjusted vancomycin dosages is important since it was previously reported that up to 74% of hVISA strains and 15% of wild-type MRSA strains are tolerant to vancomycin on the basis of the MBC/MIC ratio (15). Although there was a wide range of MBCs of both vancomycin (two isolates for which the MBC/MIC ratio was ≥32, both of which were hVISA) and teicoplanin (three non-hVISA isolates for which the MBC/MIC ratio was >32) in our data, the MBCs of both antimicrobials for 50 and 90% of the strains tested were 1 and 2 μg/ml and we did not observe a trend of an increasing MBC of either drug over time. We found that the majority (56.9%) of the hVISA strains were SCCmec type II and agr group II. The SCCmec type II gene has been commonly associated with traditional hospital-derived strains, whereas SCCmec type IV has been associated with community-associated MRSA. Of interest, 39.4% of the hVISA strains were SCCmec type IV, which, to our knowledge, has not been previously reported. In addition, Sakoulas et al. (30) previously reported that the majority of the hVISA and VISA strains tested to date are from agr group II. Patients with MRSA isolates belonging to agr group II have been associated with vancomycin failure, although an association with hVISA or VISA was not reported. Loss of agr function in S. aureus strains has also been associated with vancomycin failure or the emergence of resistance under suboptimal vancomycin therapy.
Two recent reports evaluating S. aureus susceptibility to vancomycin over 5-year increments of time have both demonstrated significant diminished susceptibility (32, 35). Similar to these reports, we observed a decreasing susceptibility to vancomycin in MRSA strains collected over a 22-year period. In addition, for 26.8% of the strains we tested, the vancomycin MIC was 2 μg/ml. There are a number of recent reports of patients with MRSA bacteremia treated with vancomycin that have demonstrated prolonged bacteremia, lower eradication rates, and higher mortality rates associated with vancomycin MICs of ≥1 μg/ml (9, 23, 30, 31). Mohr and Murray (22) recently reported that for as much as 30% of the MRSA blood isolates in a 1-year prevalence study at the Texas Medical Center, the vancomycin MIC was 2 μg/ml. Data from a national survey examining the susceptibility of 231,000 S. aureus strains up to 2005 demonstrated that for 16.2% of the strains tested, the vancomycin MIC was 2 μg/ml (33). Although there is evidence of vancomycin failure and MICs of ≥1 μg/ml, there have been no attempts to determine if these failures might be related to the presence of hVISA. On the basis of our finding of an increasing prevalence of hVISA, continued surveillance and evaluation of outcomes for patients with S. aureus infections as they relate to the vancomycin MIC and the presence of hVISA seem warranted.
Acknowledgments
This project received no outside funding support. M.J.R. has received grant support from, has served as a consultant to, or has participated as a speaker for Astellas, Cubist, Theravance, Targanta, Cerexa, Forrest, Johnson & Johnson, Pfizer, and Wyeth Pharmaceuticals.
Footnotes
Published ahead of print on 16 July 2008.
REFERENCES
- 1.Aeschlimann, J. R., E. Hershberger, and M. J. Rybak. 1999. Analysis of vancomycin population susceptibility profiles, killing activity, and postantibiotic effect against vancomycin-intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 431914-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ariza, J., M. Pujol, J. Cabo, C. Pena, N. Fernandez, J. Linares, J. Ayats, and F. Gudiol. 1999. Vancomycin in surgical infections due to methicillin-resistant Staphylococcus aureus with heterogeneous resistance to vancomycin. Lancet 3531587-1588. [DOI] [PubMed] [Google Scholar]
- 3.Bert, F., J. Clarissou, F. Durand, D. Delefosse, C. Chauvet, P. Lefebvre, N. Lambert, and C. Branger. 2003. Prevalence, molecular epidemiology, and clinical significance of heterogeneous glycopeptide-intermediate Staphylococcus aureus in liver transplant recipients. J. Clin. Microbiol. 415147-5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charles, P. G., P. B. Ward, P. D. Johnson, B. P. Howden, and M. L. Grayson. 2004. Clinical features associated with bacteremia due to heterogeneous vancomycin-intermediate Staphylococcus aureus. Clin. Infect. Dis. 38448-451. [DOI] [PubMed] [Google Scholar]
- 5.Clinical and Laboratory Standards Institute. 2006. M7-A7, methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—seventh edition. Clinical and Laboratory Standards Institute, Wayne, PA.
- 6.Clinical and Laboratory Standards Institute. 2008. M100-S18, performance standards for antimicrobial susceptibility testing, 18th informational supplement. Clinical and Laboratory Standards Institute, Wayne, PA.
- 7.Fridkin, S. K. 2001. Vancomycin-intermediate and -resistant Staphylococcus aureus: what the infectious disease specialist needs to know. Clin. Infect. Dis. 32108-115. [DOI] [PubMed] [Google Scholar]
- 8.Garnier, F., D. Chainier, T. Walsh, A. Karlsson, A. Bolmstrom, C. Grelaud, M. Mounier, F. Denis, and M. C. Ploy. 2006. A 1 year surveillance study of glycopeptide-intermediate Staphylococcus aureus strains in a French hospital. J. Antimicrob. Chemother. 57146-149. [DOI] [PubMed] [Google Scholar]
- 9.Hidayat, L. K., D. I. Hsu, R. Quist, K. A. Shriner, and A. Wong-Beringer. 2006. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicity. Arch. Intern. Med. 1662138-2144. [DOI] [PubMed] [Google Scholar]
- 10.Hiramatsu, K. 1998. The emergence of Staphylococcus aureus with reduced susceptibility to vancomycin in Japan. Am. J. Med. 1047S-10S. [DOI] [PubMed] [Google Scholar]
- 11.Hiramatsu, K., H. Hanaki, T. Ino, K. Yabuta, T. Oguri, and F. C. Tenover. 1997. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J. Antimicrob. Chemother. 40135-136. [DOI] [PubMed] [Google Scholar]
- 12.Howden, B. P. 2005. Recognition and management of infections caused by vancomycin-intermediate Staphylococcus aureus (VISA) and heterogenous VISA (hVISA). Intern. Med. J. 35(Suppl. 2)S136-S140. [DOI] [PubMed] [Google Scholar]
- 13.Howden, B. P., P. D. Johnson, P. B. Ward, T. P. Stinear, and J. K. Davies. 2006. Isolates with low-level vancomycin resistance associated with persistent methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob. Agents Chemother. 503039-3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howden, B. P., P. B. Ward, P. G. Charles, T. M. Korman, A. Fuller, P. du Cros, E. A. Grabsch, S. A. Roberts, J. Robson, K. Read, N. Bak, J. Hurley, P. D. Johnson, A. J. Morris, B. C. Mayall, and M. L. Grayson. 2004. Treatment outcomes for serious infections caused by methicillin-resistant Staphylococcus aureus with reduced vancomycin susceptibility. Clin. Infect. Dis. 38521-528. [DOI] [PubMed] [Google Scholar]
- 15.Jones, R. N. 2006. Microbiological features of vancomycin in the 21st century: minimum inhibitory concentration creep, bactericidal/static activity, and applied breakpoints to predict clinical outcomes or detect resistant strains. Clin. Infect. Dis. 42(Suppl. 1)S13-S24. [DOI] [PubMed] [Google Scholar]
- 16.Kaye, K. S., J. J. Engemann, E. Mozaffari, and Y. Carmeli. 2004. Reference group choice and antibiotic resistance outcomes. Emerg. Infect. Dis. 101125-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirst, H. A., D. G. Thompson, and T. I. Nicas. 1998. Historical yearly usage of vancomycin. Antimicrob. Agents Chemother. 421303-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lina, G., Y. Piemont, F. Godail-Gamot, M. Bes, M. O. Peter, V. Gauduchon, F. Vandenesch, and J. Etienne. 1999. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin. Infect. Dis. 291128-1132. [DOI] [PubMed] [Google Scholar]
- 19.Liu, C., and H. F. Chambers. 2003. Staphylococcus aureus with heterogeneous resistance to vancomycin: epidemiology, clinical significance, and critical assessment of diagnostic methods. Antimicrob. Agents Chemother. 473040-3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maree, C. L., R. S. Daum, S. Boyle-Vavra, K. Matayoshi, and L. G. Miller. 2007. Community-associated methicillin-resistant Staphylococcus aureus isolates causing healthcare-associated infections. Emerg. Infect. Dis. 13236-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDonald, L. C. 2006. Trends in antimicrobial resistance in health care-associated pathogens and effect on treatment. Clin. Infect. Dis. 42(Suppl. 2)S65-S71. [DOI] [PubMed] [Google Scholar]
- 22.Mohr, J. F., and B. E. Murray. 2007. Point: vancomycin is not obsolete for the treatment of infection caused by methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 441536-1542. [DOI] [PubMed] [Google Scholar]
- 23.Moise, P. A., G. Sakoulas, A. Forrest, and J. J. Schentag. 2007. Vancomycin in vitro bactericidal activity and its relationship to efficacy in clearance of methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob. Agents Chemother. 512582-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore, M. R., F. Perdreau-Remington, and H. F. Chambers. 2003. Vancomycin treatment failure associated with heterogeneous vancomycin-intermediate Staphylococcus aureus in a patient with endocarditis and in the rabbit model of endocarditis. Antimicrob. Agents Chemother. 471262-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reference deleted.
- 26.Peacock, S. J., C. E. Moore, A. Justice, M. Kantzanou, L. Story, K. Mackie, G. O'Neill, and N. P. Day. 2002. Virulent combinations of adhesin and toxin genes in natural populations of Staphylococcus aureus. Infect. Immun. 704987-4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rybak, M. J., and R. L. Akins. 2001. Emergence of methicillin-resistant Staphylococcus aureus with intermediate glycopeptide resistance: clinical significance and treatment options. Drugs 611-7. [DOI] [PubMed] [Google Scholar]
- 28.Rybak, M. J., R. Cha, C. M. Cheung, V. G. Meka, and G. W. Kaatz. 2005. Clinical isolates of Staphylococcus aureus from 1987 and 1989 demonstrating heterogeneous resistance to vancomycin and teicoplanin. Diagn. Microbiol. Infect. Dis. 51119-125. [DOI] [PubMed] [Google Scholar]
- 29.Sakoulas, G., G. M. Eliopoulos, R. C. Moellering, Jr., C. Wennersten, L. Venkataraman, R. P. Novick, and H. S. Gold. 2002. Accessory gene regulator (agr) locus in geographically diverse Staphylococcus aureus isolates with reduced susceptibility to vancomycin. Antimicrob. Agents Chemother. 461492-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakoulas, G., P. A. Moise-Broder, J. Schentag, A. Forrest, R. C. Moellering, Jr., and G. M. Eliopoulos. 2004. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J. Clin. Microbiol. 422398-2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soriano, A., F. Marco, J. A. Martinez, E. Pisos, M. Almela, V. P. Dimova, D. Alamo, M. Ortega, J. Lopez, and J. Mensa. 2008. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin. Infect. Dis. 46193-200. [DOI] [PubMed] [Google Scholar]
- 32.Steinkraus, G., R. White, and L. Friedrich. 2007. Vancomycin MIC creep in non-vancomycin-intermediate Staphylococcus aureus (VISA), vancomycin-susceptible clinical methicillin-resistant S. aureus (MRSA) blood isolates from 2001-05. J. Antimicrob. Chemother. 60788-794. [DOI] [PubMed] [Google Scholar]
- 33.Tenover, F. C., and R. C. Moellering, Jr. 2007. The rationale for revising the Clinical and Laboratory Standards Institute vancomycin minimal inhibitory concentration interpretive criteria for Staphylococcus aureus. Clin. Infect. Dis. 441208-1215. [DOI] [PubMed] [Google Scholar]
- 34.Walsh, T. R., A. Bolmstrom, A. Qwarnstrom, P. Ho, M. Wootton, R. A. Howe, A. P. MacGowan, and D. Diekema. 2001. Evaluation of current methods for detection of staphylococci with reduced susceptibility to glycopeptides. J. Clin. Microbiol. 392439-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang, G., J. F. Hindler, K. W. Ward, and D. A. Bruckner. 2006. Increased vancomycin MICs for Staphylococcus aureus clinical isolates from a university hospital during a 5-year period. J. Clin. Microbiol. 443883-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wootton, M., R. A. Howe, R. Hillman, T. R. Walsh, P. M. Bennett, and A. P. MacGowan. 2001. A modified population analysis profile (PAP) method to detect hetero-resistance to vancomycin in Staphylococcus aureus in a UK hospital. J. Antimicrob. Chemother. 47399-403. [DOI] [PubMed] [Google Scholar]
- 37.Wootton, M., A. P. MacGowan, T. R. Walsh, and R. A. Howe. 2007. A multicenter study evaluating the current strategies for isolating Staphylococcus aureus strains with reduced susceptibility to glycopeptides. J. Clin. Microbiol. 45329-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang, K., J.-A. McClure, S. Elsayed, T. Louie, and J. M. Conly. 2005. Novel multiplex PCR assay for characterization and concomitant subtyping of staphylococcal cassette chromosome mec types I to V in methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 435026-5033. [DOI] [PMC free article] [PubMed] [Google Scholar]