Abstract
Nine Staphylococcus capitis isolates from blood cultures of newborns were examined for resistance to vancomycin. MICs were within the susceptible range, but population profiling revealed a resistant subpopulation. Only isolates with the largest subpopulation were identified as heteroresistant to vancomycin by Etest. This finding may have therapeutic implications.
Recent reports indicate the possible emergence of Staphylococcus capitis as a significant pathogen causing late-onset sepsis in very-low-birth-weight (VLBW) infants (<1,500 g) (6, 16, 18, 20). Reduced susceptibility to vancomycin has been reported in several species of coagulase-negative staphylococci (1, 2, 4, 9, 10, 17); however, there is very little information on the levels of such resistance in S. capitis. Reduced susceptibility to vancomycin occurring in methicillin-resistant staphylococci translates into limited treatment options, particularly in newborn infants.
Heteroresistant S. capitis isolates may escape detection because MICs of vancomycin of ≤4 μg/ml are generally interpreted as susceptible (5). Agar-based screening tests for detecting heteroresistance in staphylococci are simple to perform (14, 15, 19); however, the sensitivity and specificity of Etest strips are superior (22). Population analysis profiling (PAP) is the most reliable method for detecting heteroresistance, but it is time-consuming and fails to provide results in a clinically relevant time frame (19). Data on the prevalence and level of vancomycin resistance are essential for assessing clinical relevance and treatment options for infants infected with S. capitis. This study examines a collection of nine S. capitis isolates obtained from blood cultures of VLBW infants in the Neonatal Intensive Care Unit (NICU) at the Royal Women's Hospital, Melbourne, Australia between 1998 and 2002 (3). Pulsed-field gel electrophoresis combined with Southern blot analysis and probing with insertion element IS256 showed they were closely related yet unique, except for two isolates from the same infant (3). The reference strains used were S. capitis ATCC 27840 and Staphylococcus aureus Mu3 (ATCC 700698) and Mu50 (ATCC 700699) (11-13).
Isolates were screened for vancomycin heteroresistance on brain heart infusion agar (BHIA) (Oxoid Ltd., Hampshire, England) containing 4 μg/ml of vancomycin (VAN 4) (Sigma-Aldrich Pty. Ltd., Sydney, Australia) (12). MICs were determined by conventional methods and by vancomycin and teicoplanin Etest strips (AB Biodisk, Solna, Sweden) (8), taking care to include small colonies within the clear zone. The PAP profiles were interpreted by calculating area under the concentration-time curve for test and Mu3 (AUCtest/AUCMu3) ratios with the aid of GraphPad Prism 5 software (San Diego, CA) (19, 21). Since the AUC is affected by the size of the initial inoculum, CFUs were standardized to match the initial inoculum of Mu3 for each replicate. All tests were performed on at least three separate occasions.
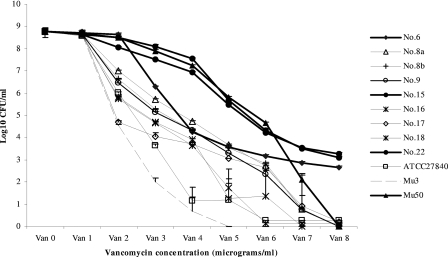
The various methods differed in their ability to detect resistant subpopulations of S. capitis: broth microdilution (CLSI) was the least sensitive method, detecting only one resistant isolate, and the Etest detected three resistant isolates, while VAN 4 screening and PAP analysis detected resistant subpopulations in all isolates (Table 1). Three isolates produced variable results on the VAN 4 screening plates, indicating instability of their heteroresistant phenotype. Visual examination of the PAP graphs showed heterogeneous resistance with strain-dependent differences in the sizes of the resistant subpopulation (Fig. 1). For the three most resistant strains (Mu50 and isolates 15 and 22), there was complete agreement between the results of PAP-AUC analysis, VAN 4 screens, and the Etest. Isolate 6 had a very high PAP-AUC value, produced variable screening results, and was interpreted as nonheteroresistant by the Etest. The discrepant PAP-AUC value could be explained by the unusual shape of the PAP graph, reflecting high colony counts on VAN 2 followed by a sharp drop on VAN 3 plates (Fig. 1). All other isolates and reference strains showed PAP-AUC values close to those of Mu3, but were not heteroresistant according to the Etest. Rankings of isolates according to the size of the resistant subpopulation were generally similar by PAP-AUC analysis and MICs generated by Etests.
TABLE 1.
Vancomycin resistance of S. capitis isolates
| Isolate or strain no. | Result bya:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| PAP-AUCb
|
Vancomycin screen (4 μg/ml)c
|
CLSI standard
|
Etest
|
||||||
| MIC (μg/ml)
|
Interpretation | ||||||||
| Mean (SE) AUCtest/Mu3 ratio | Interpretation | No. of colonies | Interpretation | MIC μg/ml | Interpretation | Vancomycin | Teicoplanin | ||
| S. capitis 6 | 1.7 (0.15) | I | 7, 86, CG | V | 2 | S | 6 | 2 | S |
| S. capitis 22 | 1.7 (0.12) | I | CG | R | 4 | S | 12 | 16 | H |
| S. capitis 15 | 1.3 (0.08) | I | CG | R | 4 | S | 12 | 16 | H |
| S. aureus Mu50 | 1.6 (0.05) | I | CG | R | 8 | R | 16 | 12 | H |
| S. capitis 17 | 1.2 (0.04) | H | 4 | H | 2 | S | 3 | 4 | S |
| S. capitis 8ad | 1.1 (0.03) | H | 2 | H | 1 | S | 4 | 3 | S |
| S. capitis 9 | 1.1 (0.03) | H | 0, 2, CG | V | 1 | S | 4 | 2 | S |
| S. capitis 16 | 1.1 (0.03) | H | 1 | H | 1 | S | 3 | 2 | S |
| S. capitis 8bd | 1.0 (0.04) | H | 12 | H | 1 | S | 8 | 3 | S |
| S. capitis 18 | 1.0 (0.05) | H | 0, 36, CG | V | 2 | S | 4 | 4 | S |
| S. capitis ATCC 27840 | 1.0 (0.01) | H | 1 | H | 1 | S | 2 | 0.2 | S |
| S. aureus Mu3 | 1 | H | 14 | H | 2 | 8 | 16 | H | |
I, intermediate; S, susceptible; H, possibly heteroresistant; R, potentially resistant; V, variable results; CG, confluent growth on at least three of four replicates.
Mean (standard error) AUC determined by PAP. An AUCtest/Mu3 ratio of ≤0.90 was interpreted as susceptible, a ratio of 0.90 to 1.3 was interpreted as heteroresistant, and a ratio of ≥1.3 was interpreted as intermediate.
Number of colonies on BHIA containing 4 μg/ml vancomycin after 48 h.
Isolates 8a and 8b were collected on different occasions from the same infant.
FIG. 1.
Population analysis profiling of S. capitis strains isolated from blood cultures of infants. Heavy lines, intermediate. The means of three separate investigations and standard errors are presented.
These results suggest that a vancomycin-heteroresistant subpopulation is present in all isolates of S. capitis. They confirm the unreliability of conventional MICs except for the most resistant isolates and show that the Etest and the VAN 4 screening tests detect only the most resistant isolates but fail to detect isolates with smaller resistant subpopulations, which could be enriched during vancomycin therapy.
This report is, to the best of our knowledge, only the second to describe a cluster of vancomycin-heteroresistant S. capitis strains among VLBW infants in an NICU. Van Der Zwet et al. (18) demonstrated variable proportions of resistant subpopulations in S. capitis isolates with closely related or identical genetic profiles. These data suggest that heteroresistant S. capitis strains, which are undetectable by standard MIC measurement, could be emerging pathogens in NICUs.
The origin of these nine closely related vancomycin-heteroresistant isolates, present in the NICU for at least 5 years, is enigmatic. It is possible that frequent vancomycin use in the unit led to enrichment of resistant cells present in a common ancestor, resulting in subpopulations of variable size. Although the outcome for S. capitis septicemia in VLBW infants is generally good in our unit, it is of concern that further increases in vancomycin resistance could occur. Our observation of a resistant subpopulation in all S. capitis strains examined, including ATCC 27840, which was deposited in 1975, suggests that heteroresistance to vancomycin could be an intrinsic property of S. capitis. Although more isolates of S. capitis need to be examined, given that the relationship between vancomycin heteroresistance and treatment failure is still uncertain (7), it would be wise to consider all isolates as potentially resistant and to monitor clinical responses to vancomycin very carefully, particularly with more deep-seated infections such as osteomyelitis or infections where antibiotic penetration is an issue, such as endocarditis and meningitis. There is an urgent need for more data on the clinical relevance of vancomycin heteroresistance in staphylococci, in particular S. capitis, and for the development of reliable, rapid, and inexpensive methods to detect such resistance (7).
Acknowledgments
This work was supported by internal funding from the School of Applied Sciences, RMIT University, Australia, and the Department of Microbiology and Infectious Diseases, Royal Children's and Royal Women's Hospitals, Australia.
Footnotes
Published ahead of print on 2 July 2008.
REFERENCES
- 1.Biavasco, F., C. Vignaroli, and P. E. Varaldo. 2000. Glycopeptide resistance in coagulase-negative staphylococci. Eur. J. Clin. Microbiol. Infect. Dis. 19403-417. [DOI] [PubMed] [Google Scholar]
- 2.Biavasco, F., C. Vignaroli, R. Lazzarini, and P. E. Varaldo. 2000. Glycopeptide susceptibility profiles of Staphylococcus haemolyticus bloodstream isolates. Antimicrob. Agents Chemother. 443122-3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradford, R., R. Abdul Manan, A. J. Daley, C. Pearce, A. Ramalingam, D. D'mello, Y. Mueller, W. Uahwatanasakul, Y. Qu, D. Grando, S. Garland, and M. Deighton. 2006. Coagulase-negative staphylococci in very low birth weight infants: genetic markers do not distinguish invasive strains from blood culture contaminants. Eur. J. Clin. Microbiol. Infect. Dis. 5283-290. [DOI] [PubMed] [Google Scholar]
- 4.Center, K. J., A. C. Reboli, R. Hubler, G. L. Rodgers, and S. S. Long. 2003. Decreased vancomycin susceptibility of coagulase-negative staphylococci in a neonatal intensive care unit: evidence of spread of Staphylococcus warneri. J. Clin. Microbiol. 414660-4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clinical Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: approved standard, 7th ed. Clinical Laboratory Standards Institute, Wayne, PA.
- 6.de Silva, G. D. I., M. Kantzanou, A. Justice, R. C. Massey, A. R. Wilkinson, N. P. J. Day, and S. J. Peacock. 2002. The ica operon and biofilm production in coagulase-negative staphylococci associated with carriage and disease in a neonatal intensive care unit. J. Clin. Microbiol. 40382-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falagas, M. E., G. C. Makris, G. Dimopoulos, and D. K. Matthaiou. 2008. Heteroresistance: a concern of increasing clinical significance? Clin. Microbiol. Infect. 14101-104. [DOI] [PubMed] [Google Scholar]
- 8.Fitzgibbon, M. M., A. S. Rossney, and B. O'Connell. 2007. Investigation of reduced susceptibility to glycopeptides among methicillin-resistant isolates from patients in Ireland and evaluation of agar screening methods for the detection of heterogeneously glycopeptide-intermediate S. aureus. J. Clin. Microbiol. 452554-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Froggatt, J. W., J. L. Johnston, D. W. Galetto, and G. L. Archer. 1989. Antimicrobial resistance in nosocomial isolates of Staphylococcus haemolyticus. Antimicrob. Agents Chemother. 33460-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garrett, D. O., E. Jochimsen, K. Murfitt, B. Hill, S. McAllister, P. Nelson, R. V. Spera, R. K. Sall, F. C. Tenover, J. Johnston, B. Zimmer, and W. R. Jarvis. 1999. The emergence of decreased susceptibility to vancomycin in Staphylococcus epidermidis. Infect. Control Hosp. Epidemiol. 20167-170. [DOI] [PubMed] [Google Scholar]
- 11.Hanaki, H., K. Kuwahara-Arai, S. Boyle-Vavra, R. S. Daum, H. Labischinski, and K. Hiramatsu. 1998. Activated cell wall synthesis is associated with vancomycin resistance in methicillin-resistant Staphylococcus aureus clinical strains Mu3 and Mu50. J. Antimicrob. Chemother. 42199-209. [DOI] [PubMed] [Google Scholar]
- 12.Hiramatsu, K., N. Aritaka, H. Hanaki, S. Kawasaki, Y. Hosoda, S. Hori, Y. Fukuchi, and I. Kobayashi. 1997. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet 3501670-1673. [DOI] [PubMed] [Google Scholar]
- 13.Hiramatsu, K., H. Hanaki, T. Ino, K. Yabuta, T. Oguri, and F. C. Tenover. 1997. Methicillin-resistant Staphylococcus aureus with reduced vancomycin susceptibility. J. Antimicrob. Chemother. 40135-146. [DOI] [PubMed] [Google Scholar]
- 14.Howden, B. P., P. B. Ward, P. D. R. Johnson, P. P. Charles, and M. L. Grayson. 2005. Low-level vancomycin resistance in Staphylococcus aureus—an Australian perspective. Eur. J. Clin. Microbiol. Infect. Dis. 24100-108. [DOI] [PubMed] [Google Scholar]
- 15.Liu, C., and H. F. Chambers. 2003. Staphylococcus aureus with heterogeneous resistance to vancomycin: epidemiology, clinical significance, and critical assessment of diagnostic methods. Antimicrob. Agents Chemother. 473040-3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ng, P. C., V. C. Y. Chow, C. H. Lee, J. M. L. Ling, H. L. Wong, and R. C. Y. Chan. 2006. Persistent Staphylococcus capitis septicaemia in a preterm infant. Pediatr. Infect. Dis. J. 25652-654. [DOI] [PubMed] [Google Scholar]
- 17.Sieradzki, K., P. Villari, and A. Tomasz. 1998. Decreased susceptibilities to teicoplanin and vancomycin among coagulase-negative methicillin-resistant clinical isolates of staphylococci. Antimicrob. Agents Chemother. 42100-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Der Zwet, W. C., Y. J. Debets-Ossenkopp, E. Reinders, M. Kapi, P. H. M. Savelkoul, R. M. Van Elburg, K. Hiramatsu, and C. M. J. E. Vandenbroucke-Grauls. 2002. Nosocomial spread of a Staphylococcus capitis strain with heteroresistance to vancomycin in a neonatal intensive care unit. J. Clin. Microbiol. 402520-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walsh, T. R., A. Bolmström, A. Qwärnström, P. Ho, M. Wootton, R. A. Howe, A. P. MacGowan, and D. Diekema. 2001. Evaluation of current methods for detection of staphylococci with reduced susceptibility to glycopeptides. J. Clin. Microbiol. 392439-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang, S.-M., C.-C. Liu, H.-W. Tseng, Y.-J. Yang, C.-H. Lin, A.-Y. Huang, and Y.-H. Wu. 1999. Staphylococcus capitis bacteremia of very low birth weight premature infants at neonatal intensive care units: clinical significance and antimicrobial susceptibility. J. Immunol. Infect. 3226-32. [PubMed] [Google Scholar]
- 21.Wooton, M., R. A. Howe, R. Hillman, T. R. Walsh, P. M. Bennett, and W. MacGowan. 2001. A modified population analysis profile (PAP) method to detect hetero-resistance to vancomycin in Staphylococcus aureus in a UK hospital. J. Antimicrob. Chemother. 47399-403. [DOI] [PubMed] [Google Scholar]
- 22.Wooton, M., A. P. MacGowan, T. R. Walsh, and R. A. Howe. 2007. A multicenter study evaluating the current strategies for isolating Staphylococcus aureus strains with reduced susceptibility to glycopeptides. J. Clin. Microbiol. 45329-332. [DOI] [PMC free article] [PubMed] [Google Scholar]