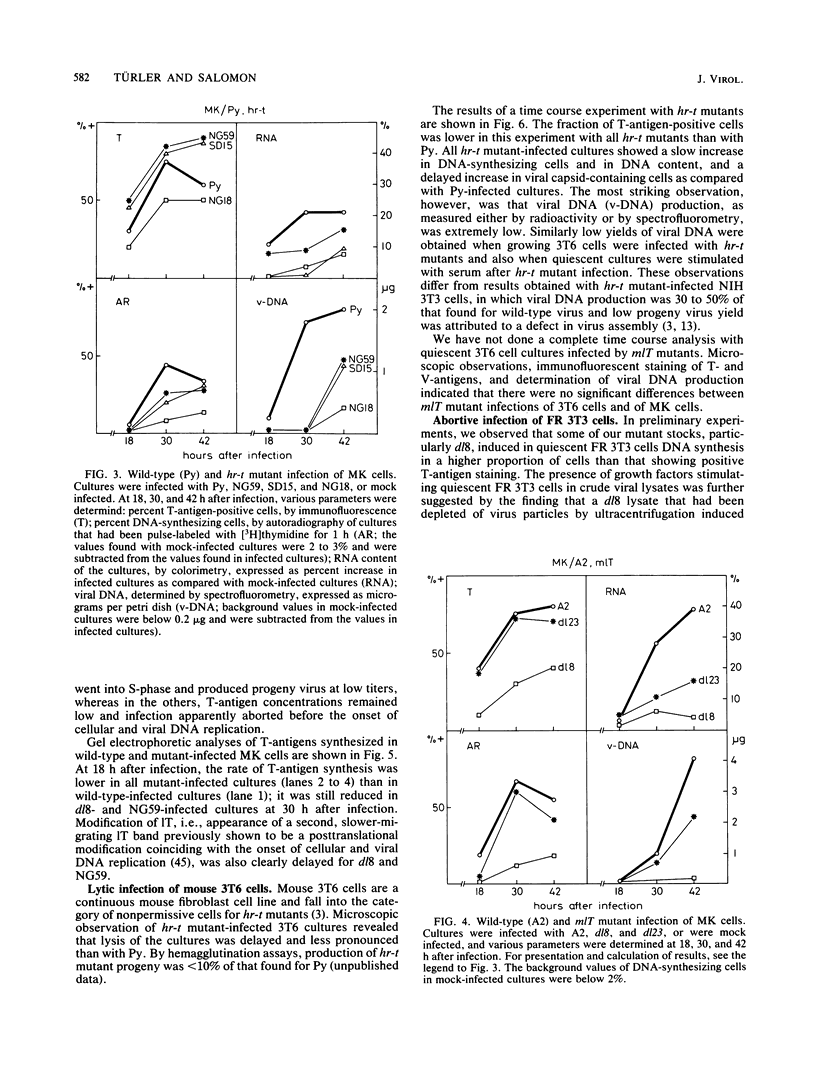
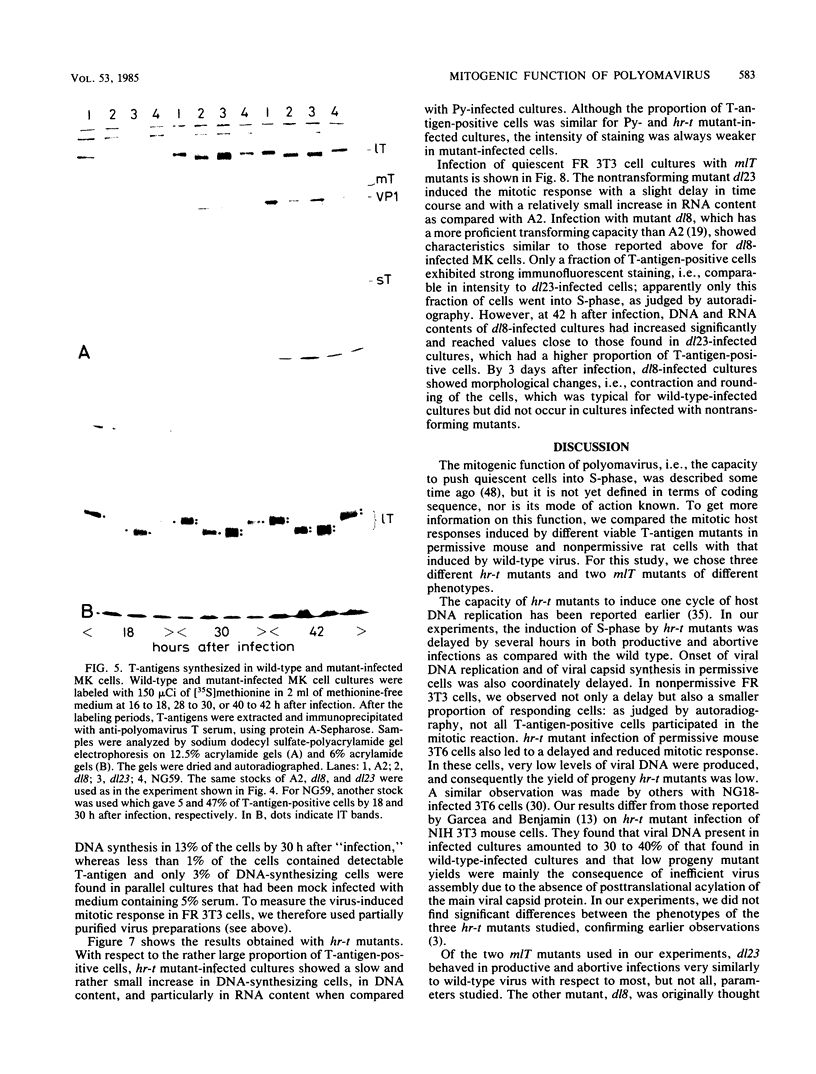
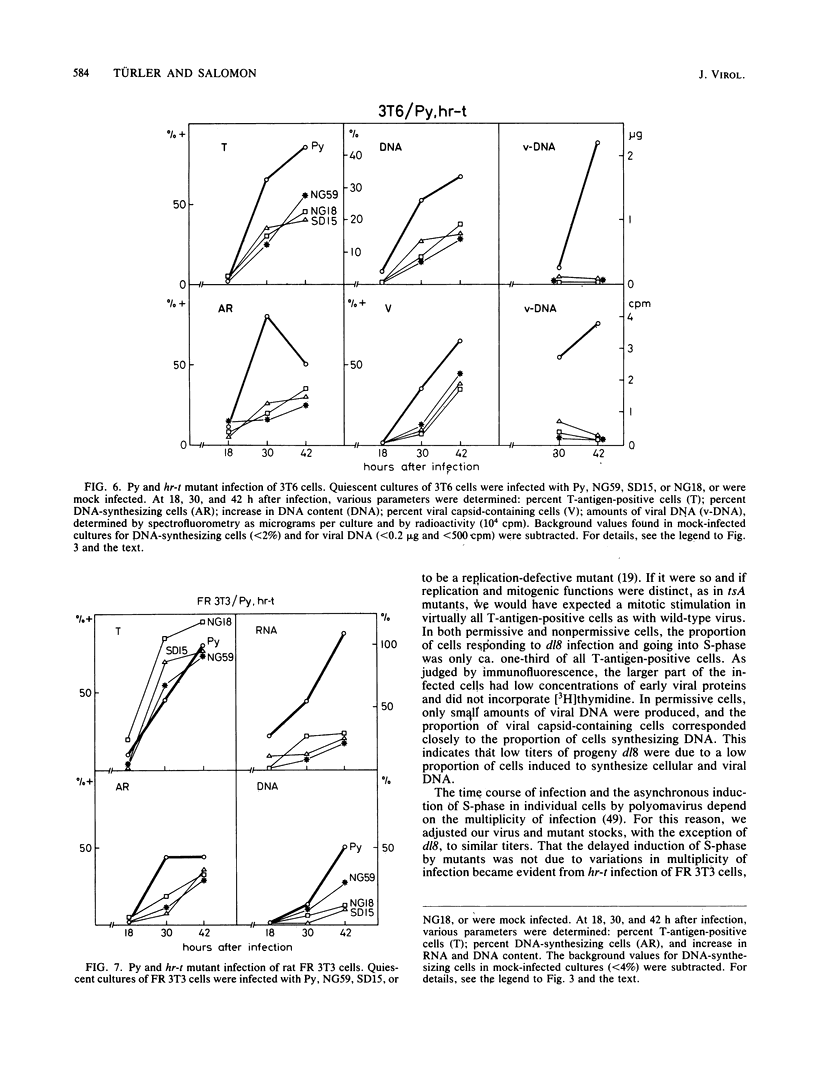
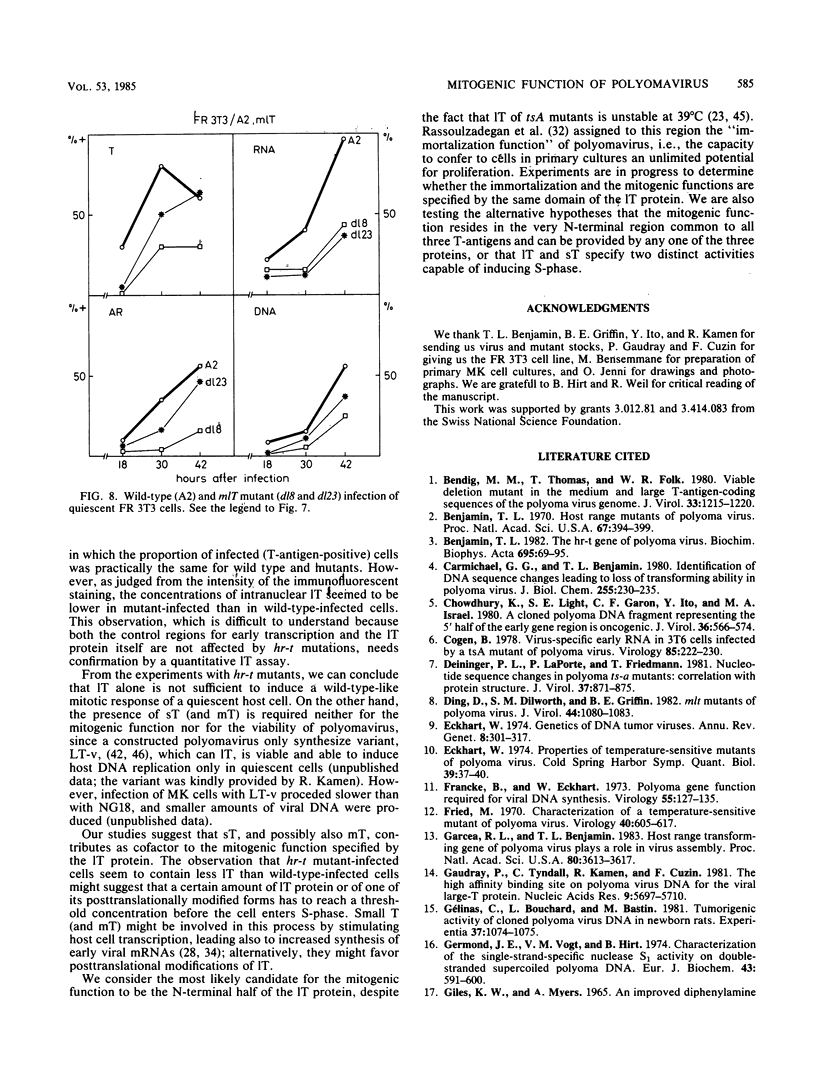
Abstract
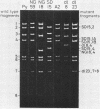
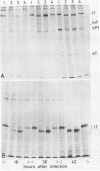
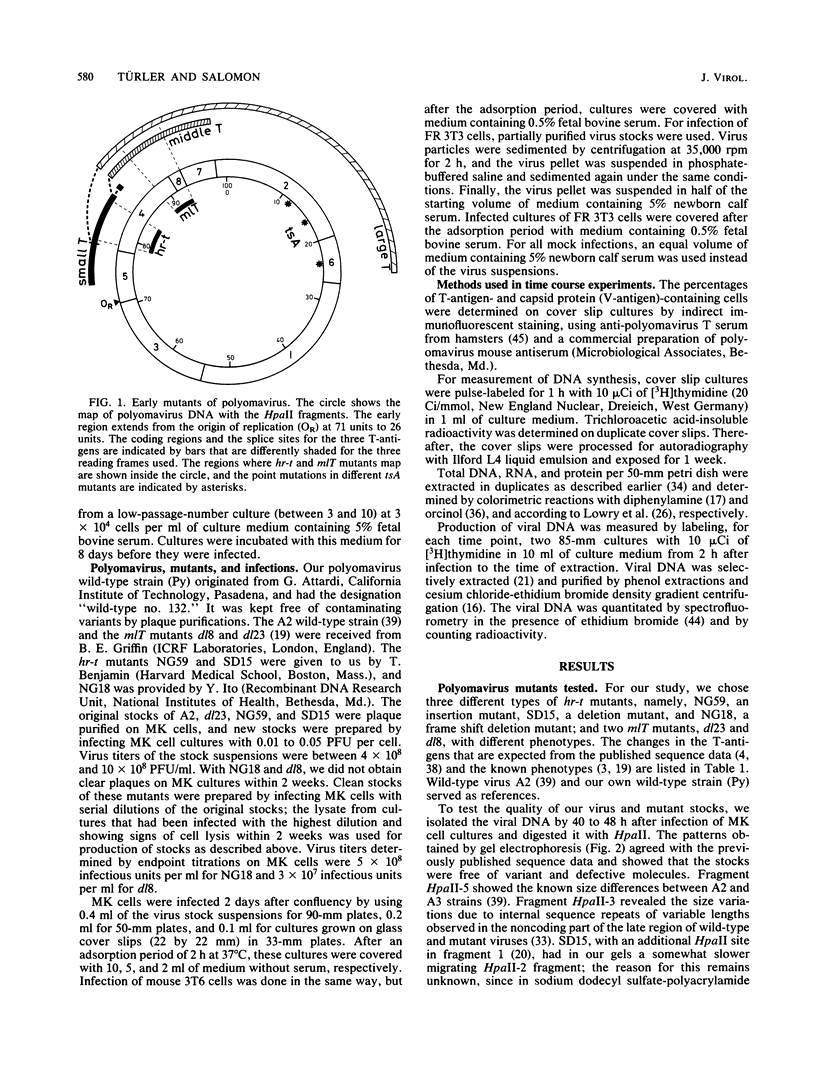
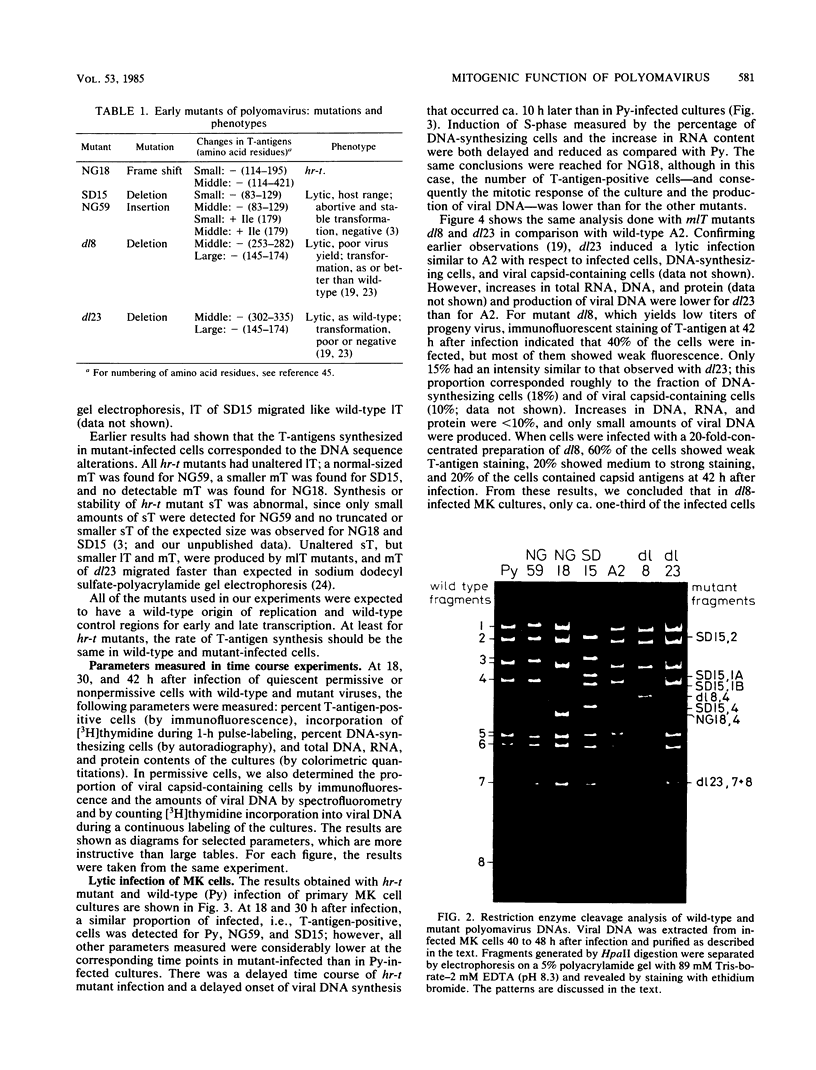
Using three different polyomavirus hr-t mutants and two polyomavirus mlT mutants, we studied induction of S-phase by mutants and wild-type virus in quiescent mouse kidney cells, mouse 3T6 cells, and FR 3T3 cells. At different times after infection, we measured the proportion of T-antigen-positive cells, the incorporation of [3H]thymidine, the proportion of DNA-synthesizing cells, and the increase in total DNA, RNA, and protein content of the cultures. In permissive mouse cells, we also determined the amount of viral DNA and the proportion of viral capsid-producing cells. In polyomavirus hr-t mutant-infected cultures, onset of host DNA replication was delayed by several hours, and a smaller proportion of T-antigen-positive cells entered S-phase than in wild-type-infected cultures. Of the two polyomavirus mlT mutants studied, dl-23 behaved similarly to wild-type virus in many, but not all, parameters tested. The poorly replicating but well-transforming mutant dl-8 was able to induce S-phase, and (in permissive cells) progeny virus production, in only about one-third of the T-antigen-positive cells. From our experiments, we conclude that mutations affecting small and middle T-antigen cause a reduction in the proportion of cells responding to virus infection and a prolongation of the early phase, i.e., the period before cells enter S-phase. In hr-t mutant-infected mouse 3T6 cells, production of viral DNA was less than 10% of that in wild-type-infected cultures; low hr-t progeny production in 3T6 cells was therefore largely due to poor viral DNA replication.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bendig M. M., Thomas T., Folk W. R. Viable deletion mutant in the medium and large T-antigen-coding sequences of the polyoma virus genome. J Virol. 1980 Mar;33(3):1215–1220. doi: 10.1128/jvi.33.3.1215-1220.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin T. L. Host range mutants of polyoma virus. Proc Natl Acad Sci U S A. 1970 Sep;67(1):394–399. doi: 10.1073/pnas.67.1.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin T. L. The hr-t gene of polyoma virus. Biochim Biophys Acta. 1982 Dec 21;695(2):69–95. doi: 10.1016/0304-419x(82)90018-x. [DOI] [PubMed] [Google Scholar]
- Carmichael G. G., Benjamin T. L. Identification of DNA sequence changes leading to loss of transforming ability in polyoma virus. J Biol Chem. 1980 Jan 10;255(1):230–235. [PubMed] [Google Scholar]
- Chowdhury K., Light S. E., Garon C. F., Ito Y., Israel M. A. A cloned polyoma DNA fragment representing the 5' half of the early gene region is oncogenic. J Virol. 1980 Nov;36(2):566–574. doi: 10.1128/jvi.36.2.566-574.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogen B. Virus-specific early RNA in 3T6 cells infected by a tsA mutant of polyoma virus. Virology. 1978 Mar;85(1):222–230. doi: 10.1016/0042-6822(78)90426-9. [DOI] [PubMed] [Google Scholar]
- Deininger P. L., LaPorte P., Friedmann T. Nucleotide sequence changes in polyoma ts-a mutants: correlation with protein structure. J Virol. 1981 Mar;37(3):871–875. doi: 10.1128/jvi.37.3.871-875.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D., Dilworth S. M., Griffin B. E. mlt Mutants of polyoma virus. J Virol. 1982 Dec;44(3):1080–1083. doi: 10.1128/jvi.44.3.1080-1083.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echkart W. Genetics of DNA tumor viruses. Annu Rev Genet. 1974;8:301–317. doi: 10.1146/annurev.ge.08.120174.001505. [DOI] [PubMed] [Google Scholar]
- Eckhart W. Properties of temperature-sensitive mutants of polyoma virus. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):37–40. doi: 10.1101/sqb.1974.039.01.007. [DOI] [PubMed] [Google Scholar]
- Francke B., Eckhart W. Polyoma gene function required for viral DNA synthesis. Virology. 1973 Sep;55(1):127–135. doi: 10.1016/s0042-6822(73)81014-1. [DOI] [PubMed] [Google Scholar]
- Fried M. Characterization of a temperature-sensitive mutant of polyoma virus. Virology. 1970 Mar;40(3):605–617. doi: 10.1016/0042-6822(70)90205-9. [DOI] [PubMed] [Google Scholar]
- Garcea R. L., Benjamin T. L. Host range transforming gene of polyoma virus plays a role in virus assembly. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3613–3617. doi: 10.1073/pnas.80.12.3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudray P., Tyndall C., Kamen R., Cuzin F. The high affinity binding site on polyoma virus DNA for the viral large-T protein. Nucleic Acids Res. 1981 Nov 11;9(21):5697–5710. doi: 10.1093/nar/9.21.5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germond J. E., Vogt V. M., Hirt B. Characterization of the single-strand-specific nuclease S1 activity on double-stranded supercoiled polyoma DNA. Eur J Biochem. 1974 Apr 16;43(3):591–600. doi: 10.1111/j.1432-1033.1974.tb03446.x. [DOI] [PubMed] [Google Scholar]
- Griffin B. E., Dilworth S. M. Polyomavirus: an overview of its unique properties. Adv Cancer Res. 1983;39:183–268. doi: 10.1016/s0065-230x(08)61036-2. [DOI] [PubMed] [Google Scholar]
- Griffin B. E., Maddock C. New classes of viable deletion mutants in the early region of polyoma virus. J Virol. 1979 Sep;31(3):645–656. doi: 10.1128/jvi.31.3.645-656.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gélinas C., Bouchard L., Bastin M. Tumorigenic activity of cloned polyoma virus DNA in newborn rats. Experientia. 1981 Oct 15;37(10):1074–1075. doi: 10.1007/BF02085017. [DOI] [PubMed] [Google Scholar]
- Hattori J., Carmichael G. G., Benjamin T. L. DNA sequence alterations in Hr-t deletion mutants of polyoma virus. Cell. 1979 Mar;16(3):505–513. doi: 10.1016/0092-8674(79)90025-4. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Ito Y., Spurr N., Dulbecco R. Characterization of polyoma virus T antigen. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1259–1263. doi: 10.1073/pnas.74.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Spurr N., Griffin B. E. Middle T antigen as primary inducer of full expression of the phenotype of transformation by polyoma virus. J Virol. 1980 Jul;35(1):219–232. doi: 10.1128/jvi.35.1.219-232.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandjian E. W., Matter J. M., Léonard N., Weil R. Simian virus 40 and polyoma virus stimulate overall cellular RNA and protein synthesis. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1476–1480. doi: 10.1073/pnas.77.3.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Magnusson G., Nilsson M. G., Dilworth S. M., Smolar N. Characterization of polyoma mutants with altered middle and large T-antigens. J Virol. 1981 Sep;39(3):673–683. doi: 10.1128/jvi.39.3.673-683.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter J. M., Tiercy J. M., Weil R. Sequential stimulation of cellular RNA synthesis in polyoma-infected mouse kidney cell cultures. Nucleic Acids Res. 1983 Oct 11;11(19):6611–6629. doi: 10.1093/nar/11.19.6611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mes A. M., Hassell J. A. Polyoma viral middle T-antigen is required for transformation. J Virol. 1982 May;42(2):621–629. doi: 10.1128/jvi.42.2.621-629.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson S. V., Magnusson G. T-antigen expression by polyoma mutants with modified RNA splicing. EMBO J. 1983;2(12):2095–2101. doi: 10.1002/j.1460-2075.1983.tb01708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak U., Dilworth S. M., Griffin B. E. Coding capacity of a 35 percent fragment of the polyoma virus genome is sufficient to initiate and maintain cellular transformation. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3278–3282. doi: 10.1073/pnas.77.6.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassoulzadegan M., Naghashfar Z., Cowie A., Carr A., Grisoni M., Kamen R., Cuzin F. Expression of the large T protein of polyoma virus promotes the establishment in culture of "normal" rodent fibroblast cell lines. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4354–4358. doi: 10.1073/pnas.80.14.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruley H. E., Fried M. Sequence repeats in a polyoma virus DNA region important for gene expression. J Virol. 1983 Jul;47(1):233–237. doi: 10.1128/jvi.47.1.233-237.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon C., Türler H., Weil R. Polyoma-induced stimulation of cellular RNA synthesis is paralleled by changed expression of the viral genome. Nucleic Acids Res. 1977;4(5):1483–1503. doi: 10.1093/nar/4.5.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel R., Benjamin T. L. Cellular alterations dependent upon the polyoma virus Hr-t function: separation of mitogenic from transforming capacities. Cell. 1978 Jul;14(3):587–599. doi: 10.1016/0092-8674(78)90244-1. [DOI] [PubMed] [Google Scholar]
- Seif R., Cuzin F. Temperature-sensitive growth regulation in one type of transformed rat cells induced by the tsa mutant of polyoma virus. J Virol. 1977 Dec;24(3):721–728. doi: 10.1128/jvi.24.3.721-728.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolar N., Griffin B. E. DNA sequences of polyoma virus early deletion mutants. J Virol. 1981 Jun;38(3):958–967. doi: 10.1128/jvi.38.3.958-967.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeda E., Arrand J. R., Smolar N., Walsh J. E., Griffin B. E. Coding potential and regulatory signals of the polyoma virus genome. Nature. 1980 Jan 31;283(5746):445–453. doi: 10.1038/283445a0. [DOI] [PubMed] [Google Scholar]
- Thomas T., Vollmer P., Folk W. R. Nucleotide sequence changes in polyoma virus A gene mutants. J Virol. 1981 Mar;37(3):1094–1098. doi: 10.1128/jvi.37.3.1094-1098.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman R., Cowie A., Favaloro J., Jat P., Kamen R. The structures of the spliced mRNAs encoding polyoma virus early region proteins. J Mol Appl Genet. 1981;1(2):83–92. [PubMed] [Google Scholar]
- Treisman R., Novak U., Favaloro J., Kamen R. Transformation of rat cells by an altered polyoma virus genome expressing only the middle-T protein. Nature. 1981 Aug 13;292(5824):595–600. doi: 10.1038/292595a0. [DOI] [PubMed] [Google Scholar]
- Tyndall C., La Mantia G., Thacker C. M., Favaloro J., Kamen R. A region of the polyoma virus genome between the replication origin and late protein coding sequences is required in cis for both early gene expression and viral DNA replication. Nucleic Acids Res. 1981 Dec 11;9(23):6231–6250. doi: 10.1093/nar/9.23.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Türler H. Interactions of Polyoma and Mouse DNAs III. Mechanism of Polyoma Pseudovirion Formation. J Virol. 1975 May;15(5):1158–1167. doi: 10.1128/jvi.15.5.1158-1167.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Türler H. The tumor antigens and the early functions of polyoma virus. Mol Cell Biochem. 1980 Sep 15;32(2):63–93. doi: 10.1007/BF00227801. [DOI] [PubMed] [Google Scholar]
- WINOCOUR E. Purification of polyoma virus. Virology. 1963 Feb;19:158–168. doi: 10.1016/0042-6822(63)90005-9. [DOI] [PubMed] [Google Scholar]
- Weil R., Kára J. Polyoma "tumor antigen": an activator of chromosome replication? Proc Natl Acad Sci U S A. 1970 Oct;67(2):1011–1017. doi: 10.1073/pnas.67.2.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil R. Viral 'tumor antigens': A novel type of mammalian regulator protein. Biochim Biophys Acta. 1978 Nov 17;516(3):301–388. doi: 10.1016/0304-419x(78)90012-4. [DOI] [PubMed] [Google Scholar]