Abstract
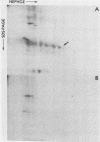
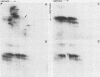
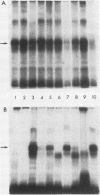
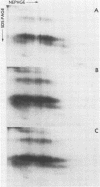
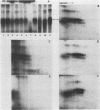
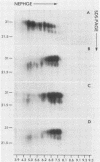
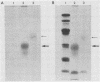
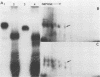
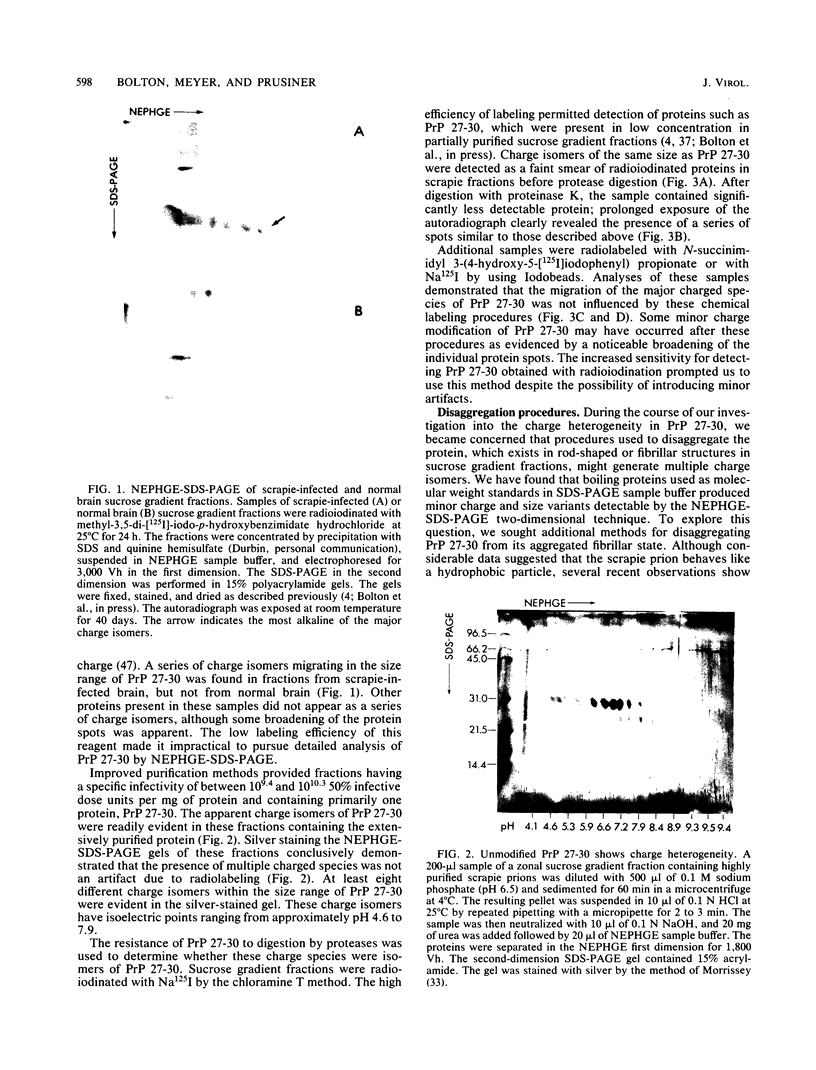
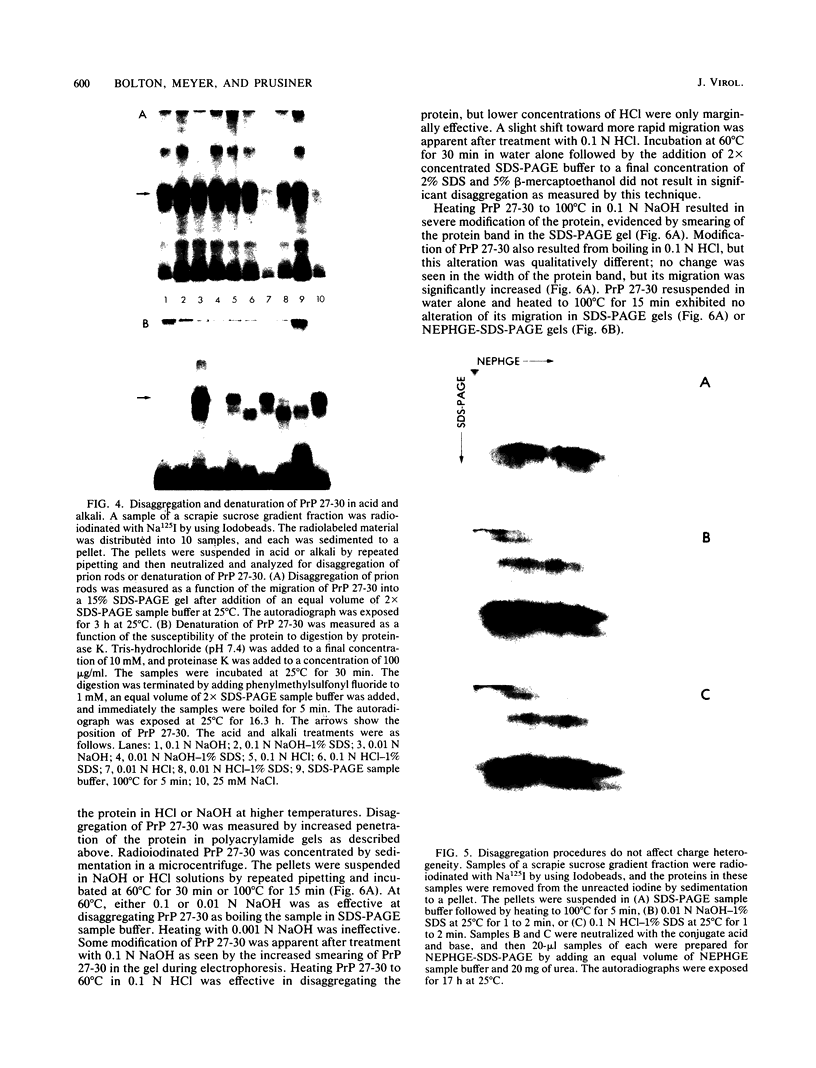
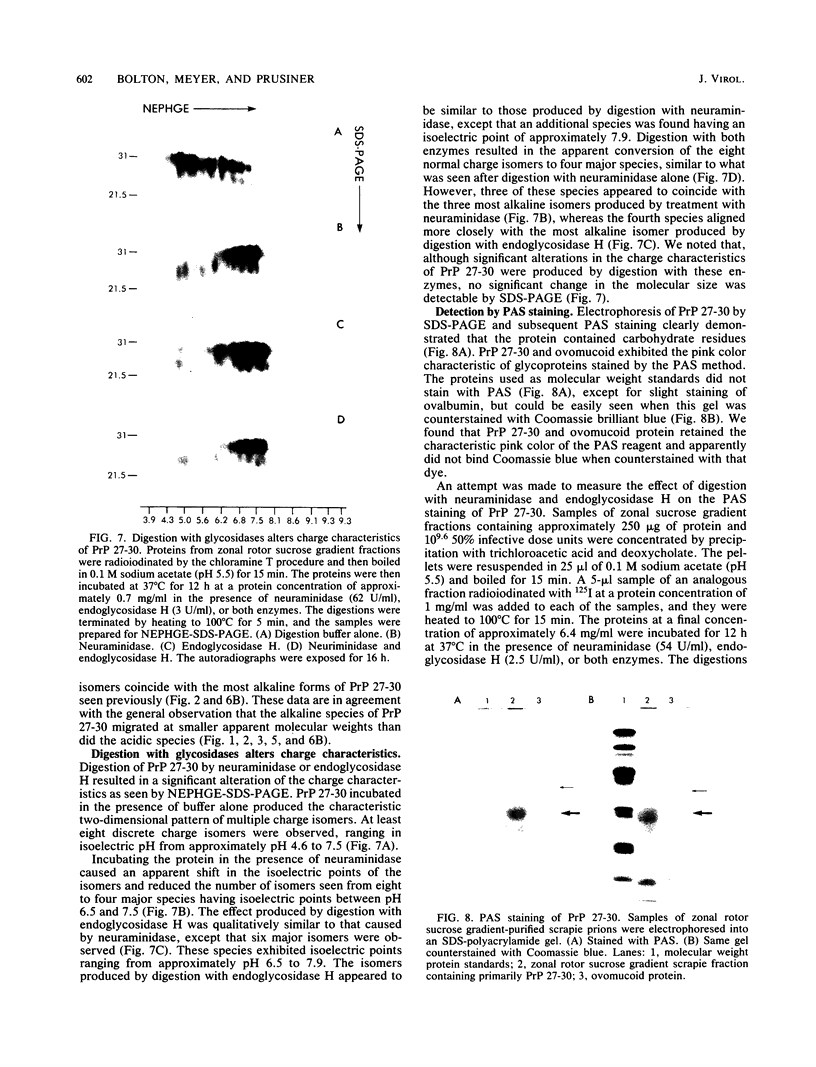
The major scrapie prion protein, designated PrP 27-30, exhibited both charge and size heterogeneity after purification from infected hamster brains. Eight or more discrete charge isomers of PrP 27-30 with isoelectric points ranging from approximately pH 4.6 to 7.9 were found by using non-equilibrium pH gradient electrophoresis in the first dimension followed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis in the second dimension. The charge isomers were detected by silver staining as well as by radioiodination. The procedures used to disaggregate PrP 27-30 before electrophoresis in the first dimension do not appear to be responsible for the charge heterogeneity. However, heating PrP 27-30 to 100 degrees C for 15 min in 0.1 N NaOH or 0.1 N HCl resulted in modification of the protein and alteration of its electrophoretic pattern. A PrP 27-30 fragment (molecular weight, 17,100 to 21,900) obtained by cyanogen bromide cleavage also exhibited charge and size heterogeneity. Periodic acid-Schiff staining of PrP 27-30 electrophoresed into sodium dodecyl sulfate-polyacrylamide gels demonstrated that carbohydrate residues are attached to the protein. Digestion of PrP 27-30 with neuraminidase and endo-beta-N-acetylglucosaminidase H resulted in significant changes in the isoelectric pH of PrP 27-30 isomers, whereas digestion with alkaline phosphatase had no effect. Our results demonstrate that PrP 27-30 is a sialoglycoprotein; this is consistent with several properties of this protein and of the scrapie prion.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bendheim P. E., Barry R. A., DeArmond S. J., Stites D. P., Prusiner S. B. Antibodies to a scrapie prion protein. Nature. 1984 Aug 2;310(5976):418–421. doi: 10.1038/310418a0. [DOI] [PubMed] [Google Scholar]
- Boersma D. P., Saleh F., Nakamura K., Compans R. W. Structure and glycosylation of Tacaribe viral glycoproteins. Virology. 1982 Dec;123(2):452–456. doi: 10.1016/0042-6822(82)90278-1. [DOI] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton D. C., McKinley M. P., Prusiner S. B. Identification of a protein that purifies with the scrapie prion. Science. 1982 Dec 24;218(4579):1309–1311. doi: 10.1126/science.6815801. [DOI] [PubMed] [Google Scholar]
- Cahnmann J. H., Arnon R., Sela M. Isolation and characterization of active fragments obtained by cleavage of immunoglobulin G with cyanogen bromide. J Biol Chem. 1966 Jul 25;241(14):3247–3255. [PubMed] [Google Scholar]
- Dietzschold B., Wiktor T. J., Macfarlan R., Varrichio A. Antigenic structure of rabies virus glycoprotein: ordering and immunological characterization of the large CNBr cleavage fragments. J Virol. 1982 Nov;44(2):595–602. doi: 10.1128/jvi.44.2.595-602.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser H. The pathology of a natural and experimental scrapie. Front Biol. 1976;44:267–305. [PubMed] [Google Scholar]
- GROSS E., WITKOP B. Nonenzymatic cleavage of peptide bonds: the methionine residues in bovine pancreatic ribonuclease. J Biol Chem. 1962 Jun;237:1856–1860. [PubMed] [Google Scholar]
- Gibbons R. A., Hunter G. D. Nature of the scrapie agent. Nature. 1967 Sep 2;215(5105):1041–1043. doi: 10.1038/2151041a0. [DOI] [PubMed] [Google Scholar]
- Glossmann H., Neville D. M., Jr Glycoproteins of cell surfaces. A comparative study of three different cell surfaces of the rat. J Biol Chem. 1971 Oct 25;246(20):6339–6346. [PubMed] [Google Scholar]
- Gullick W. J., Tzartos S., Lindstrom J. Monoclonal antibodies as probes of acetylcholine receptor structure. 1. Peptide mapping. Biochemistry. 1981 Apr 14;20(8):2173–2180. doi: 10.1021/bi00511a016. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hilmert H., Diringer H. A rapid and efficient method to enrich SAF-protein from scrapie brains of hamsters. Biosci Rep. 1984 Feb;4(2):165–170. doi: 10.1007/BF01120313. [DOI] [PubMed] [Google Scholar]
- Hsu C. H., Kingsbury D. W. Contribution of oligosaccharide sulfation to the charge heterogeneity of a viral glycoprotein. J Biol Chem. 1982 Aug 10;257(15):9035–9038. [PubMed] [Google Scholar]
- Hsu C. H., Kingsbury D. W. NS phosphoprotein of vesicular stomatitis virus: subspecies separated by electrophoresis and isoelectric focusing. J Virol. 1982 Apr;42(1):342–345. doi: 10.1128/jvi.42.1.342-345.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C. H., Kingsbury D. W. Topography of phosphate residues in Sendai virus proteins. Virology. 1982 Jul 15;120(1):225–234. doi: 10.1016/0042-6822(82)90020-4. [DOI] [PubMed] [Google Scholar]
- Huang K. P., Robinson J. C., Chou J. Y. Phosphoprotein-phosphatase activity associated with human placental alkaline phosphatase. Biochem Biophys Res Commun. 1976 May 3;70(1):186–192. doi: 10.1016/0006-291x(76)91126-8. [DOI] [PubMed] [Google Scholar]
- Hughes E. N., Colombatti A., August J. T. Murine cell surface glycoproteins. Purification of the polymorphic Pgp-1 antigen and analysis of its expression on macrophages and other myeloid cells. J Biol Chem. 1983 Jan 25;258(2):1014–1021. [PubMed] [Google Scholar]
- Hunter G. D., Gibbons R. A., Kimberlin R. H., Millson G. C. Further studies of the infectivity and stability of extracts and homogenates derived from scrapie affected mouse brains. J Comp Pathol. 1969 Jan;79(1):101–108. doi: 10.1016/0021-9975(69)90033-4. [DOI] [PubMed] [Google Scholar]
- Katoh I., Yoshinaka Y., Luftig R. B. Murine leukaemia virus p30 heterogeneity as revealed by two-dimensional gel electrophoresis is not an artefact of the technique. J Gen Virol. 1984 Apr;65(Pt 4):733–741. doi: 10.1099/0022-1317-65-4-733. [DOI] [PubMed] [Google Scholar]
- Kopaciewicz W., Regnier F. E. Nonideal size-exclusion chromatography of proteins: effects of pH at low ionic strength. Anal Biochem. 1982 Oct;126(1):8–16. doi: 10.1016/0003-2697(82)90102-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leach B. S., Collawn J. F., Jr, Fish W. W. Behavior of glycopolypeptides with empirical molecular weight estimation methods. 1. In sodium dodecyl sulfate. Biochemistry. 1980 Dec 9;19(25):5734–5741. doi: 10.1021/bi00566a011. [DOI] [PubMed] [Google Scholar]
- Markwell M. A. A new solid-state reagent to iodinate proteins. I. Conditions for the efficient labeling of antiserum. Anal Biochem. 1982 Sep 15;125(2):427–432. doi: 10.1016/0003-2697(82)90025-2. [DOI] [PubMed] [Google Scholar]
- Marsh R. F., Kimberlin R. H. Comparison of scrapie and transmissible mink encephalopathy in hamsters. II. Clinical signs, pathology, and pathogenesis. J Infect Dis. 1975 Feb;131(2):104–110. doi: 10.1093/infdis/131.2.104. [DOI] [PubMed] [Google Scholar]
- McFarlin D. E., Raff M. C., Simpson E., Nehlsen S. H. Scrapie in immunologically deficient mice. Nature. 1971 Oct 1;233(5318):336–336. doi: 10.1038/233336a0. [DOI] [PubMed] [Google Scholar]
- McKinley M. P., Bolton D. C., Prusiner S. B. A protease-resistant protein is a structural component of the scrapie prion. Cell. 1983 Nov;35(1):57–62. doi: 10.1016/0092-8674(83)90207-6. [DOI] [PubMed] [Google Scholar]
- Millson G. C., Hunter G. D., Kimberlin R. H. The physico-chemical nature of the scrapie agent. Front Biol. 1976;44:243–266. [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Poduslo J. F. Glycoprotein molecular-weight estimation using sodium dodecyl sulfate-pore gradient electrophoresis: comparison of tris-glycine and tris-borate-EDTA buffer systems. Anal Biochem. 1981 Jun;114(1):131–139. doi: 10.1016/0003-2697(81)90463-2. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Bolton D. C., Groth D. F., Bowman K. A., Cochran S. P., McKinley M. P. Further purification and characterization of scrapie prions. Biochemistry. 1982 Dec 21;21(26):6942–6950. doi: 10.1021/bi00269a050. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Groth D. F., Bolton D. C., Kent S. B., Hood L. E. Purification and structural studies of a major scrapie prion protein. Cell. 1984 Aug;38(1):127–134. doi: 10.1016/0092-8674(84)90533-6. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Groth D. F., Cochran S. P., Masiarz F. R., McKinley M. P., Martinez H. M. Molecular properties, partial purification, and assay by incubation period measurements of the hamster scrapie agent. Biochemistry. 1980 Oct 14;19(21):4883–4891. doi: 10.1021/bi00562a028. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., McKinley M. P., Bowman K. A., Bolton D. C., Bendheim P. E., Groth D. F., Glenner G. G. Scrapie prions aggregate to form amyloid-like birefringent rods. Cell. 1983 Dec;35(2 Pt 1):349–358. doi: 10.1016/0092-8674(83)90168-x. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B. Novel proteinaceous infectious particles cause scrapie. Science. 1982 Apr 9;216(4542):136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- Raghow R., Portner A., Hsu C. H., Clark S. B., Kingsbury D. W. Charge heterogeneity in polypeptides of negative strand RNA viruses. Virology. 1978 Oct 15;90(2):214–225. doi: 10.1016/0042-6822(78)90305-7. [DOI] [PubMed] [Google Scholar]
- Rohwer R. G. Scrapie infectious agent is virus-like in size and susceptibility to inactivation. Nature. 1984 Apr 12;308(5960):658–662. doi: 10.1038/308658a0. [DOI] [PubMed] [Google Scholar]
- Rohwer R. G. Virus like sensitivity of the scrapie agent to heat inactivation. Science. 1984 Feb 10;223(4636):600–602. doi: 10.1126/science.6420887. [DOI] [PubMed] [Google Scholar]
- Swarup G., Cohen S., Garbers D. L. Selective dephosphorylation of proteins containing phosphotyrosine by alkaline phosphatases. J Biol Chem. 1981 Aug 10;256(15):8197–8201. [PubMed] [Google Scholar]
- Tarentino A. L., Maley F. Purification and properties of an endo-beta-N-acetylglucosaminidase from Streptomyces griseus. J Biol Chem. 1974 Feb 10;249(3):811–817. [PubMed] [Google Scholar]
- Wood F. T., Wu M. M., Gerhart J. C. The radioactive labeling of proteins with an iodinated amidination reagent. Anal Biochem. 1975 Dec;69(2):339–349. doi: 10.1016/0003-2697(75)90136-0. [DOI] [PubMed] [Google Scholar]