Abstract
We performed dengue virus (DENV) serology and quantitative real-time pan-DENV reverse transcription-PCR (RT-PCR) on 186 sera of 171 patients returning from the tropics. DENV loads significantly decreased with increasing times of disease and were higher in immunoglobulin M-negative samples. In the first week of disease, pan-DENV RT-PCR is the test of choice.
With increasing international travel and tourism to and from regions of endemicity, dengue virus (DENV) infections are more frequently encountered in nontropical regions (4), calling for efficient and timely diagnostic methods. Since DENV-specific antibodies used for routine diagnostics reach a detectable level only after the initial phase of disease (3), we evaluated the role of quantitative reverse transcription-PCR (RT-PCR) for early diagnosis of DENV infection.
Patient samples were tested for the presence of anti-DENV immunoglobulin M (IgM) and IgG antibodies by using a rapid serological assay (dengue duo cassette; Panbio, Sinnamon Park, Australia) according to the manufacturer's instructions. The physicians in charge were asked to fill out a questionnaire to collect information on the patients.
RNA was extracted using a QIAamp viral RNA mini kit (Qiagen AG, Hombrechtikon, Switzerland) according to the manufacturer's instructions. The real-time RT-PCR method for the detection of all DENV subtypes (panDV RT-PCR) was adapted from a published protocol (2) (Table 1).
TABLE 1.
Primers and probes
| Name | Sequence (5′ to 3′)a | PCR | Reference |
|---|---|---|---|
| DenSm1 | GGA GAG ACC AGA GAT CCT GCT GTb | panDV RT-PCR | 2 |
| DenAs1 | CAT TCC ATT TTC TGG CGT TC | panDV RT-PCR | 2 |
| DenAs2 | CAA TCC ATC TTG CGG CGC TC | panDV RT-PCR | 2 |
| DenP | FAM-CAG CAT CAT TCC AGG CAC AG-BHQ1 | panDV RT-PCR | 2 |
| Den4-P | FAM-CAA CAT CAA TCC AGG CAC AG-BHQ1c | panDV RT-PCR | 2 |
FAM, 6-carboxyfluorescein; BHQ1, black hole quencher 1.
The sequence of the published forward primer DenS was modified to take into account new sequences recently added to the NCBI database. The modified position (m1) is shown in bold.
To improve detection of DENV type IV, the additional probe Den4-P was used together with the original DenP probe (5 pmol each per reaction). The modified positions are shown in bold.
The panDV RT-PCRs were run with a total volume of 25 μl including 5 μl template RNA, using an iScript one-step RT-PCR kit for probes (Bio-Rad, Reinach, Switzerland). The thermal profile consisted of one RT step at 50°C for 10 min, a denaturation step at 95°C for 5 min, and 45 cycles of 95°C for 30 s and 60°C for 60 s. Quantification was performed using a standard curve generated by dilution of a reference plasmid containing the target region from DENV type I. All samples were tested in triplicate. An additional replicate was spiked with 1,000 copies of the reference plasmid to detect potential inhibitions. As an RT control, 1e5 copies of in vitro-transcribed DENV-1 RNA were tested in parallel.
Data analysis was performed using SPSS software (version 13.0.0 for Macintosh). P values of <0.05 were regarded as significant.
The study protocol was approved by the local scientific ethics committee (Ethikkommission beider Basel, Basel, Switzerland, no. 05/07).
We analyzed a total of 186 serum or plasma samples from 171 travelers returning from the tropics with clinical suspicion of DENV infection. Retrospective subset A consisted of 25 IgM-positive samples from 24 patients. The median age was 38 years (interquartile range [IQR], 28 to 53). The gender distribution was 7 women (29.2%) and 17 men (70.8%). Four patients of this subset tested positive by panDV RT-PCR (Table 2). DENV-specific IgG was found in four samples (four patients, all IgM positive): three were panDV RT-PCR negative, and one was panDV RT-PCR positive.
TABLE 2.
Results of the diagnostic tests
| Result | No. of samples/no. (%) of patients in:
|
||
|---|---|---|---|
| Retrospective subset A | Prospective subset B | Total | |
| DENV positive | 25/24 (30.6) | 51/45 (30.6) | 76/69 (49.3) |
| PCR positive, IgM positive | 4/4 (16.7) | 22/17 (11.6) | 26/21 (12.3) |
| PCR negative, IgM positive | 21/20 (8.3) | 19/18 (12.2) | 40/38 (22.2) |
| PCR positive, IgM negative | 10/10 (6.8)a | 10/10 (5.8) | |
| DENV negative | 110/102 (69.4) | 110/102 (59.6) | |
| Total | 25/24 | 161 (147) | 186 (171) |
Four patients (eight samples, all IgG negative and PCR positive) showed IgM seroconversion. They were accounted for as IgM negative and PCR positive.
Subset B consisted of 161 samples from 147 patients, collected prospectively between May 2006 and June 2007. The median age was 41 years (IQR, 29 to 51), and the gender distribution was 64 women (43.5%) and 82 men (55.7%). The gender of one patient was unknown. Overall, 32 samples (27 patients) tested positive by panDV RT-PCR. Out of those, 10 were IgM negative (Table 2). DENV-specific IgG was found in 16 samples (15 patients): 5 were panDV RT-PCR positive and had detectable IgM, 8 were panDV RT-PCR negative but had detectable IgM, and 3 were PCR and IgM negative.
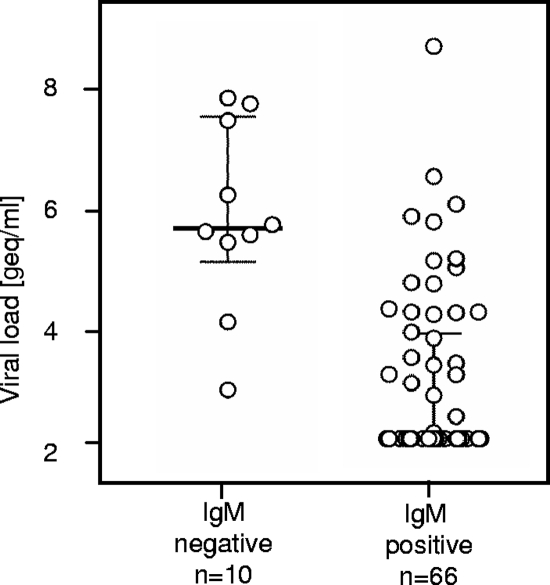
Median DENV loads were significantly higher in IgM-negative samples than in IgM-positive samples (5.47e5 versus 2e2 genome equivalents [geq]/ml, respectively) (Fig. 1). We found no significant difference in DENV loads when the 6 IgG-positive/panDV RT-PCR-positive samples were compared to the 30 IgG-negative/panDV RT-PCR-positive samples.
FIG. 1.
DENV loads of IgM-negative and IgM-positive samples. The viral loads of IgM-positive samples were significantly higher than those of IgM-negative samples (P < 0.00001; Mann-Whitney U test). The median is indicated by a solid bar. The whiskers show the IQR. Nondetectable viral loads are plotted at the detection limit of 200 geq/ml.
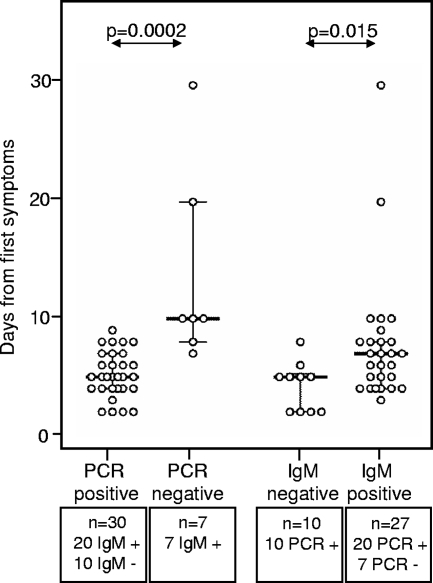
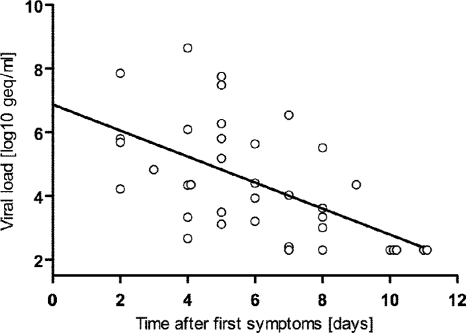
For 37 of 45 patients (82.2%) with DENV infection diagnosed as positive by panDV RT-PCR or IgM testing, the dates of the first reported symptoms and of the blood samplings were available. For the panDV RT-PCR-positive samples, the median time lapse between symptom start and testing was 5 days (IQR, 4 to 7 days). For the panDV RT-PCR-negative samples, the median time lapse was 10 days (IQR, 8 to 20 days). This difference was statistically significant (Fig. 2). The measured DENV loads significantly decreased with increasing times between first reported symptoms and samplings (Fig. 3). No viral load was detected after day 9.
FIG. 2.
Time from first symptoms to DENV test results. The times between the first symptoms and the blood samplings were known for 37 DENV-positive patients in prospective subset B. There was a significant difference between PCR-positive and PCR-negative samples (P = 0.0002; Mann-Whitney U test) as well as between IgM-negative and IgM-positive samples (P = 0.015; Mann-Whitney U test). The median is indicated by a solid bar. The whiskers show the IQR.
FIG. 3.
DENV loads and time from first symptoms. The viral load inversely correlated with the time between the start of the symptoms and the sampling (P = 0.00006; Spearman's rho test). A linear regression analysis was performed on the data set (slope, −0.41; y intercept, 6.88 log geq/ml; x intercept, 11.15 days; r2, 0.3368).
For IgM-positive samples, the median time lapse between symptom start and testing was 7 days (IQR, 4 to 8 days). For the IgM-negative and panDV RT-PCR-positive samples, the median time lapse was 5 days (IQR, 2 to 5.25). This difference was statistically significant (Fig. 2).
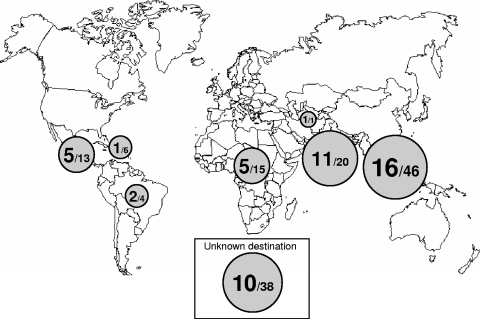
The travel destinations for 105 of 147 patients (71.4%) in prospective subset B were known (Fig. 4). Most patients returned from South East Asia (46 patients [43.8%]) and India (20 patients [19.0%]). The median duration of stay was 20.5 days (IQR, 15 to 31), and the durations for DENV-positive and -negative patients were not different (medians of 20 days versus 21 days, respectively). Fever and headache were the most frequently reported symptoms but were not associated with positive DENV test results. However, rash and thrombocytopenia correlated with positive DENV test results obtained by panDV RT-PCR or IgM testing (Table 3). Both signs are not specific for DV infection and can accompany a number of other diseases, particularly those of viral origin. Nevertheless, low platelet counts and rash in febrile patients returning from tropical regions should trigger diagnostic testing for DV.
FIG. 4.
Travel destinations of patients with suspected DENV infection. In prospective subset B, travel destination was known for 105 patients, of which 41 were DENV positive. The surface of the disk is proportional to the number of DENV-positive cases (larger number). The smaller number indicates the total number of patients from the respective region.
TABLE 3.
Signs and symptoms for the patients in prospective subset B
| Symptom | No. of patients (% of patients with valid results)
|
Pa | ||
|---|---|---|---|---|
| Total | DENV positive | DENV negative | ||
| Fever | 96 (93) | 38 (97) | 58 (91) | 0.189 |
| Headache | 77 (84) | 27 (79) | 50 (86) | 0.375 |
| Insect bite | 33 (83) | 10 (71) | 23 (88) | 0.613 |
| Myalgia | 53 (72) | 22 (73) | 31 (70) | 0.834 |
| Arthralgia | 32 (53) | 12 (60) | 20 (50) | 0.518 |
| Diarrhea | 36 (52) | 11 (50) | 25 (53) | 0.867 |
| Rash | 32 (45) | 21 (64) | 11 (29) | 0.005b |
| Thrombocytopenia | 22 (37) | 20 (71) | 2 (6) | <0.00001b |
Pearson chi-square test.
Significant correlation (P < 0.05).
A potential limitation of our study is false-positive IgM due to known cross-reactions between the rapid assay used and rheumatoid factors or other viruses in up to 4.8% of the cases (1). Moreover, although the primers and probes were adapted to the currently available DENV sequences, we cannot exclude that some virus variants might have been underquantified or not detected, due to mutations in the target regions. However, we had the opportunity to evaluate our assay with a quality control panel published by Lemmer et al. (5) and our data fully matched the expected results.
In conclusion, our study shows that DENV testing should be considered for febrile patients returning from tropical regions, in particular patients with rash and thrombocytopenia. If the symptoms last for 1 week, panDV RT-PCR should be requested, whereas after 1 week, IgM serology is the test of choice.
Acknowledgments
We thank the team at the Molecular Diagnostics laboratory of the Institute for Medical Microbiology of the University of Basel, the team at the Hematology laboratory of the Swiss Tropical Institute for skilled support of this study, E. Hunsperger (CDC, San Juan) for DENV strains, M. Niedrig (RKI, Berlin, Germany) for DENV quality control panel, and A. Egli for comments.
Footnotes
Published ahead of print on 23 July 2008.
REFERENCES
- 1.Blacksell, S. D., P. N. Newton, D. Bell, J. Kelley, M. P. Mammen, Jr., D. W. Vaughn, V. Wuthiekanun, A. Sungkakum, A. Nisalak, and N. P. Day. 2006. The comparative accuracy of 8 commercial rapid immunochromatographic assays for the diagnosis of acute dengue virus infection. Clin. Infect. Dis. 421127-1134. [DOI] [PubMed] [Google Scholar]
- 2.Drosten, C., S. Gottig, S. Schilling, M. Asper, M. Panning, H. Schmitz, and S. Gunther. 2002. Rapid detection and quantification of RNA of Ebola and Marburg viruses, Lassa virus, Crimean-Congo hemorrhagic fever virus, Rift Valley fever virus, dengue virus, and yellow fever virus by real-time reverse transcription-PCR. J. Clin. Microbiol. 402323-2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halstead, S. B. 2007. Dengue. Lancet 3701644-1652. [DOI] [PubMed] [Google Scholar]
- 4.Jelinek, T., N. Muhlberger, G. Harms, M. Corachan, M. P. Grobusch, J. Knobloch, U. Bronner, H. Laferl, A. Kapaun, Z. Bisoffi, J. Clerinx, S. Puente, G. Fry, M. Schulze, U. Hellgren, I. Gjorup, P. Chalupa, C. Hatz, A. Matteelli, M. Schmid, L. N. Nielsen, S. da Cunha, J. Atouguia, B. Myrvang, and K. Fleischer. 2002. Epidemiology and clinical features of imported dengue fever in Europe: sentinel surveillance data from TropNetEurop. Clin. Infect. Dis. 351047-1052. [DOI] [PubMed] [Google Scholar]
- 5.Lemmer, K., O. Donoso Mantke, H. G. Bae, J. Groen, C. Drosten, and M. Niedrig. 2004. External quality control assessment in PCR diagnostics of dengue virus infections. J. Clin. Virol. 30291-296. [DOI] [PubMed] [Google Scholar]