Abstract
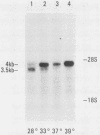
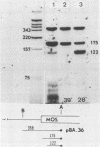
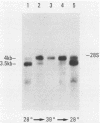
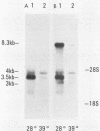
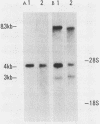
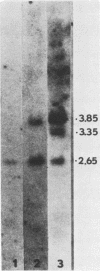
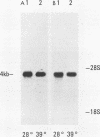
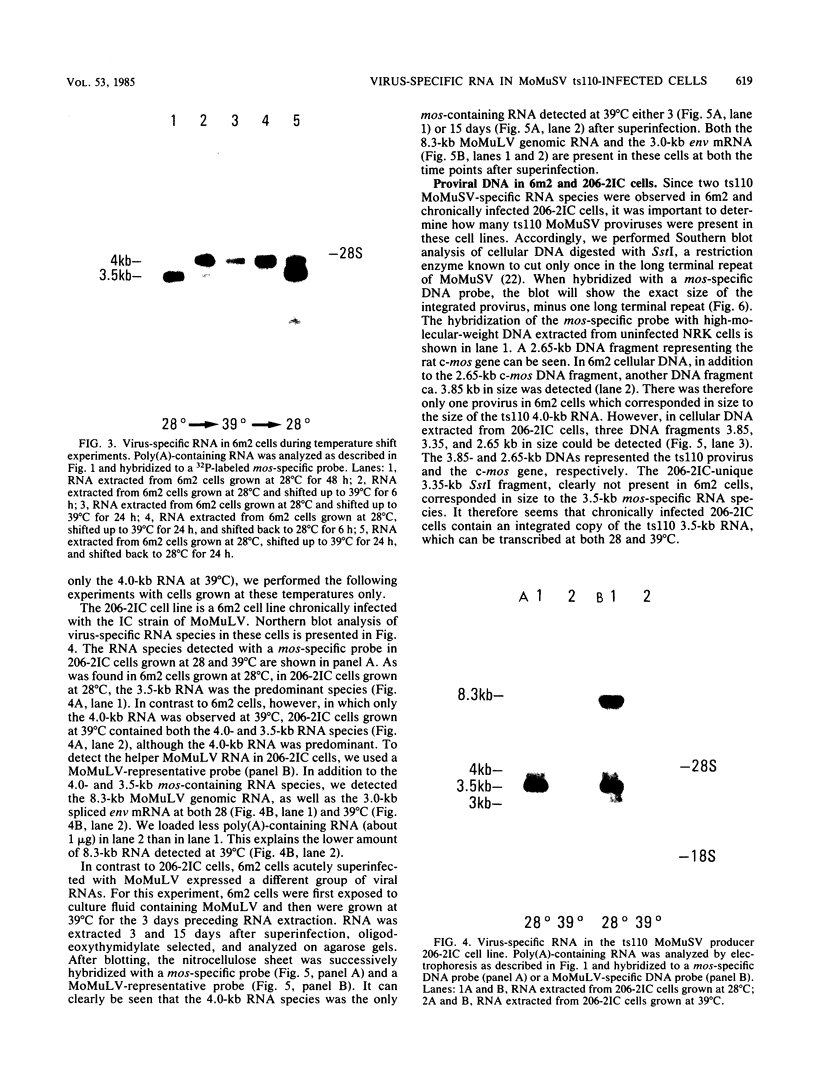
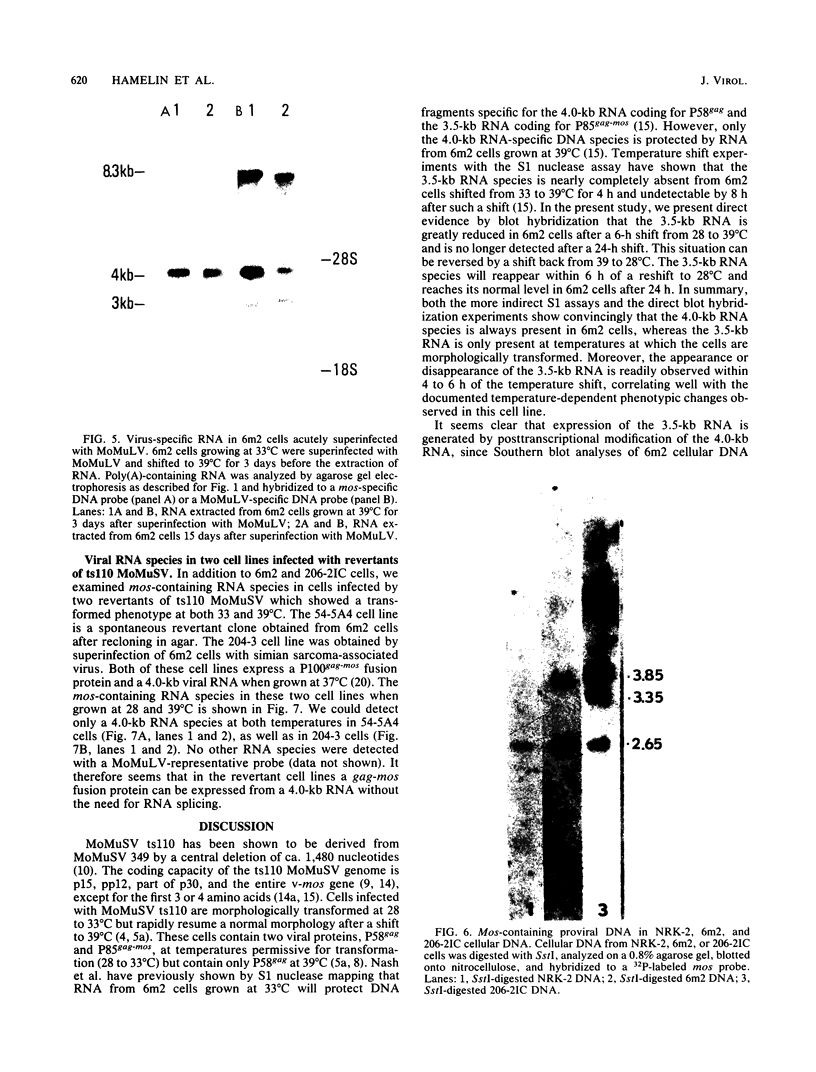
We examined the mos-specific intracellular RNA species in 6m2 cells, an NRK cell line nonproductively infected with the ts110 mutant of Moloney murine sarcoma virus. These cells present a normal phenotype at 39 degrees C and a transformed phenotype at 28 or 33 degrees C, expressing two viral proteins, termed P85gag-mos and P58gag, at 28 to 33 degrees C, whereas only P58gag is expressed at 39 degrees C. It has been previously shown that 6m2 cells contain two virus-specific RNA species, a 4.0-kilobase (kb) RNA coding for P58gag and a 3.5-kb RNA coding for P85gag-mos. Using both Northern blot and S1 nuclease analyses, we show here that the 3.5-kb RNA is the predominant viral RNA species in 6m2 cells grown at 28 degrees C, whereas only the 4.0-kb RNA is detected at 39 degrees C. During temperature shift experiments, the 3.5-kb RNA species disappears after a shift from 28 to 39 degrees C and is detected again after a shift back from 39 to 28 degrees C. By Southern blot analysis, we have detected only one ts110 proviral DNA in the 6m2 genome. This observation, as well as previously published heteroduplex and S1 nuclease analyses which showed that the 3.5-kb RNA species lacks about 430 bases found at the gag gene-mos gene junction in the 4.0-kb RNA, suggests that the 3.5-kb RNA is a splicing product of the 4.0-kb RNA. The absence of the 3.5-kb RNA when 6m2 cells are grown at 39 degrees C indicates that the splicing reaction is thermosensitive. The splicing defect of the ts110 Moloney murine sarcoma virus viral RNA in 6m2 cells cannot be complemented by acute Moloney murine leukemia virus superinfection, since no 3.5-kb ts110 RNA was detected in acutely superinfected 6m2 cells maintained at 39 degrees C. The spliced Moloney murine leukemia virus env mRNA, however, is found in acutely infected cells maintained at 39 degrees C, suggesting that the lack of ts110 viral RNA splicing at 39 degrees C is not due to an obvious host defect. In sharp contrast, however, 6m2 cells chronically superinfected with Moloney murine leukemia virus produce a 3.5-kb RNA species at 39 degrees C as well as at 28 degrees C and contain proviral DNAs corresponding to the two viral RNA species.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair D. G., Hull M. A., Finch E. A. The isolation and preliminary characterization of temperature-sensitive transformation mutants of Moloney sarcoma virus. Virology. 1979 Jun;95(2):303–316. doi: 10.1016/0042-6822(79)90486-0. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Brown R. L., Horn J. P., Wible L., Arlinghaus R. B., Brinkley B. R. Sequence of events in the transformation process in cells infected with a temperature-sensitive transformation mutant of Moloney murine sarcoma virus. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5593–5597. doi: 10.1073/pnas.78.9.5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischinger P. J., Moore C. O., O'Connor T. E. Isolation and identification of a helper virus found in the Moloney sarcoma-leukemia virus complex. J Natl Cancer Inst. 1969 Apr;42(4):605–622. [PubMed] [Google Scholar]
- Gallick G. E., Hamelin R., Maxwell S., Duyka D., Arlinghaus R. B. The gag-mos hybrid protein of ts110 Moloney murine sarcoma virus: variation of gene expression with temperature. Virology. 1984 Dec;139(2):366–374. doi: 10.1016/0042-6822(84)90382-9. [DOI] [PubMed] [Google Scholar]
- Hamelin R., Devaux J., Honoré N., Auger-Buendia M. A., Tavitian A. Characterization of viral RNA in cells transformed by various isolates of Moloney murine sarcoma virus. J Gen Virol. 1983 Sep;64(Pt 9):2057–2062. doi: 10.1099/0022-1317-64-9-2057. [DOI] [PubMed] [Google Scholar]
- Hamelin R., Larsen C. J., Tavitian A. Effects of actinomycin D, toyocamycin and cycloheximide on the synthesis of low-molecular-weight nuclear RNAs in HeLa cells. Eur J Biochem. 1973 Jun;35(2):350–356. doi: 10.1111/j.1432-1033.1973.tb02846.x. [DOI] [PubMed] [Google Scholar]
- Horn J. P., Wood T. G., Blair D. G., Arlinghaus R. B. Partial characterization of a moloney murine sarcoma virus 85,000-dalton polypeptide whose expression correlates with the transformed phenotype in cells infected with a temperature-sensitive mutant virus. Virology. 1980 Sep;105(2):516–525. doi: 10.1016/0042-6822(80)90052-5. [DOI] [PubMed] [Google Scholar]
- Horn J. P., Wood T. G., Murphy E. C., Jr, Blair D. G., Arlinghaus R. B. A selective temperature-sensitive defect in viral RNA expression in cells infected with a ts transformation mutant of murine sarcoma virus. Cell. 1981 Jul;25(1):37–46. doi: 10.1016/0092-8674(81)90229-4. [DOI] [PubMed] [Google Scholar]
- Junghans R. P., Murphy E. C., Jr, Arlinghaus R. B. Electron microscopic analysis of ts1 10 Moloney mouse sarcoma virus, a variant of wild-type virus with two RNAs containing large deletions. J Mol Biol. 1982 Oct 25;161(2):229–250. doi: 10.1016/0022-2836(82)90150-4. [DOI] [PubMed] [Google Scholar]
- Kloetzer W. S., Maxwell S. A., Arlinghaus R. B. Further characterization of the P85gag-mos -associated protein kinase activity. Virology. 1984 Oct 15;138(1):143–155. doi: 10.1016/0042-6822(84)90154-5. [DOI] [PubMed] [Google Scholar]
- Kloetzer W. S., Maxwell S. A., Arlinghaus R. B. P85gag-mos encoded by ts110 Moloney murine sarcoma virus has an associated protein kinase activity. Proc Natl Acad Sci U S A. 1983 Jan;80(2):412–416. doi: 10.1073/pnas.80.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E. C., Jr, Arlinghaus R. B. Comparative tryptic peptide analysis of candidate P85gag-mos of ts110 Moloney murine sarcoma virus and P38-P23 mos gene-related proteins of wild-type virus. Virology. 1982 Sep;121(2):372–383. doi: 10.1016/0042-6822(82)90175-1. [DOI] [PubMed] [Google Scholar]
- Nash M. A., Brizzard B. L., Wong J. L., Murphy E. C., Jr Murine sarcoma virus ts110 RNA transcripts: origin from a single proviral DNA and sequence of the gag-mos junctions in both the precursor and spliced viral RNAs. J Virol. 1985 Feb;53(2):624–633. doi: 10.1128/jvi.53.2.624-633.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash M., Brown N. V., Wong J. L., Arlinghaus R. B., Murphy E. C., Jr S1 nuclease mapping of viral RNAs from a temperature-sensitive transformation mutant of murine sarcoma virus. J Virol. 1984 May;50(2):478–488. doi: 10.1128/jvi.50.2.478-488.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sharp P. A. Speculations on RNA splicing. Cell. 1981 Mar;23(3):643–646. doi: 10.1016/0092-8674(81)90425-6. [DOI] [PubMed] [Google Scholar]
- Stanker L. H., Gallick G. E., Horn J. P., Arlinghaus R. B. P85gag-mos encoded by ts110 Moloney murine sarcoma virus: rapid at the restrictive temperature. J Gen Virol. 1983 Oct;64(Pt 10):2203–2211. doi: 10.1099/0022-1317-64-10-2203. [DOI] [PubMed] [Google Scholar]
- Stanker L. H., Horn J. P., Gallick G. E., Kloetzer W. S., Murphy E. C., Jr, Blair D. G., Arlinghaus R. B. Gag-mos Polyproteins encoded by variants of the Moloney strain of mouse sarcoma virus. Virology. 1983 Apr 15;126(1):336–347. doi: 10.1016/0042-6822(83)90483-x. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beveren C., van Straaten F., Galleshaw J. A., Verma I. M. Nucleotide sequence of the genome of a murine sarcoma virus. Cell. 1981 Nov;27(1 Pt 2):97–108. doi: 10.1016/0092-8674(81)90364-0. [DOI] [PubMed] [Google Scholar]
- Wood T. G., Peltier-Horn J., Robey W. G., Blair D. G., Arlinghaus R. B. Characterization of virus-specified proteins present in NRK cells infected with a temperature-sensitive transformation mutant of Moloney murine sarcoma virus. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):747–754. doi: 10.1101/sqb.1980.044.01.080. [DOI] [PubMed] [Google Scholar]