Abstract
Infection with human parechovirus 3 (HPeV3) was described for the first time in Japan in 2004 and reportedly is more often associated with severe disease than infection with HPeV1 or HPeV2. In 2004, infections with HPeV3 were observed for the first time in The Netherlands. Genetic analysis showed several different lineages, suggesting endemic circulation. We analyzed 163 cell culture isolates from the same number of patients tested in routine virological laboratories as part of the national enterovirus surveillance program. Isolates were collected between 2000 and 2007 and could not be characterized by routine methods. In total, 155 isolates (95%) were found positive for HPeV by a reverse transcription-PCR assay targeting the 5′ untranslated region, explaining the majority of the diagnostic deficit in enterovirus surveillance for these years. Typing of the isolates by use of partial genome sequencing of the VP1/2A region revealed the presence of 55 HPeV1, 2 HPeV2, 89 HPeV3, 1 HPeV4, and 8 HPeV5 isolates. We compared isolation dates, age groups affected, and clinical pictures, which were reported as part of the routine surveillance. Clear differences in epidemiology were observed, with HPeV3 occurring at intervals of 2 years and in the spring-summer season, whereas HPeV1 was observed in small numbers throughout each year, with a low in the summer months. HPeV3 infection affected younger children than HPeV1 infection and was significantly more often associated with fever, meningitis, and viremia.
Human parechovirus 1 (HPeV1) and HPeV2, initially known as echovirus 22 (EV22) and EV23, were first isolated in the summer of 1956 in Japan from stool specimens of children suffering from diarrhea (9). From that time on, HPeV infections have been detected all over the world. Common symptoms caused by these viruses are gastroenteritis, respiratory infections, and, in a smaller proportion of cases (usually in children <1 year old), encephalitis and flaccid paralysis (12, 20). Based on these disease symptoms and viral physicochemical properties (stability at a pH of <3 and in organic solvents), EV22 and -23 were initially grouped within the genus Enterovirus of the family Picornaviridae (18, 19). Subsequent molecular characterization showed that the viruses were quite distant from the enteroviruses: EV22 and EV23 showed no more than 30% amino acid identity with other picornaviruses, and in contrast to most other human viruses in this family, they do not shut down the host cell protein translation machinery (9, 18). For these reasons, EV22 and -23 were grouped into a new genus of the Picornaviridae, Parechovirus, and were renamed HPeV1 and HPeV2. With this new genus, nine picornavirus genera are known at present: Enterovirus, Rhinovirus, Cardiovirus, Aphthovirus, Hepatovirus, Erbovirus, Kobuvirus, Teschovirus, and Parechovirus (19).
In 2004 a new serotype of parechovirus (HPeV3), which reportedly was associated with more-severe disease than HPeV serotype 1 and 2 viruses (4, 5, 10), was described in Japan. In 2006 a fourth HPeV serotype was isolated from a neonate with fever (3). This isolate could not be neutralized by antibodies against serotypes 1 to 3. Together with a recently reported close relative (T92-15), HPeV2 CT86-6760 has been reclassified as a fifth genotype (HPeV5), since it is genetically distinct from the HPeV2 isolates (1). In 2007 a sixth serotype was reported, which could not be neutralized by antibodies against serotypes 1, 2, and 3 and which had an amino acid sequence distinct from those of types 1 to 5 (22). Recently a second HPeV6 isolate was recovered, which seemed to be a recombinant of HPeV6, -5, and -1 (2). Infection with HPeV5 and HPeV6 in The Netherlands was described for the first time in December 2007 (7).
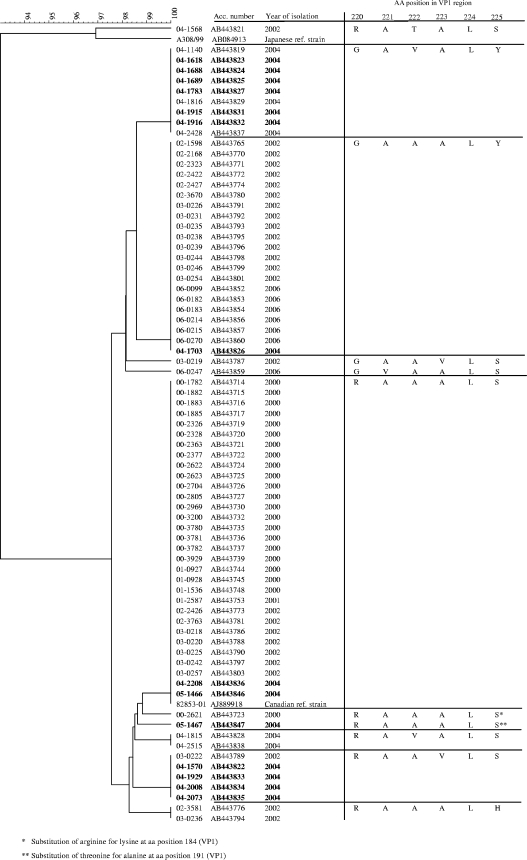
In the summer of 2004, HPeV3 was observed for the first time in The Netherlands (Arnhem). An outbreak was suspected when 14 children (aged 2 to 8 weeks) were admitted to the same hospital within a short time with clinical manifestations ranging from aseptic meningitis to a sepsis-like syndrome (21). Genetic analysis of the viral isolates, however, revealed that the infections were caused by several different lineages of HPeV3, which suggested endemic circulation of HPeV3 in The Netherlands (see Fig. 3). This observation triggered the present study on the prevalence and genetic diversity of parechoviruses in The Netherlands.
FIG. 3.
Clustering of the Dutch HPeV3 isolates from 2000 to 2007 and reference strains. The tree is based on multiple alignment of the amino acid sequences of the VP1/2A region (48 amino acids) by use of cluster analysis with the unweighted-pair group method using average linkages. The isolates associated with the 14 HPeV3 cases in Arnhem in 2004 are boldfaced.
MATERIALS AND METHODS
Clinical samples.
Primary diagnosis of enterovirus infections in The Netherlands is performed by 21 virological laboratories, which participate in the Weekly Sentinel Surveillance System of the Dutch Working Group on Clinical Virology. Fecal samples and throat swabs from children with systemic viral infections, with manifestations ranging from meningitis to gastrointestinal disorders, are cultured on combinations of enterovirus-sensitive cell lines: RD, tertiary monkey kidney (tMK), LLC-MK2, Vero, HEp-2, and various human fibroblast cell lines. Viral isolates with a cytopathic effect (CPE) characteristic of enteroviruses are confirmed as enteroviruses by a specific PCR assay (15, 17) or by an immunofluorescence test with broadly reactive monoclonal antibodies (Dako, CA). Cerebrospinal fluid samples are usually tested directly (without cell culture) by an enterovirus PCR assay for the presence of enteroviruses. Enterovirus PCR-positive isolates are typed by 15 of 21 virological laboratories by use of serum neutralization tests with polyclonal typing pools (provided by the National Institute for Public Health and the Environment [RIVM], Bilthoven, The Netherlands) (23). The RIVM receives a subset of all cell culture isolates from various types of specimens and from patients with different types of enterovirus infection. Isolates submitted to the RIVM either were negative by the locally performed enterovirus PCR or immunofluorescence test or were not typed by the local laboratory. Isolates that are typed at the local level are not sent to the RIVM. Positive diagnostic results are reported through the Weekly Sentinel Surveillance System of the Dutch Working Group on Clinical Virology.
Characterization of isolates for enterovirus surveillance.
Isolates submitted to our institute (RIVM) are routinely cultured on transgenic cells with the human poliovirus receptor (L20B) to exclude the presence of poliovirus in the isolates (24). In addition, the isolates are cultured on a cell line most similar to the cell line used by the supplying laboratory and are tested by an enterovirus PCR assay (15, 17). From 2000 to 2007, a total of 1,344 cell culture isolates were submitted to the RIVM, of which 1,023 isolates (76%) were confirmed as enterovirus positive by an enterovirus PCR assay and 248 isolates (19%) were either nontypeable or not typed. The remaining 73 isolates either were negative by cell culture at the RIVM or were proven to be positive for adenovirus, reovirus, or rhinovirus. Background data on the age of each patient, the year and month of isolation, and clinical symptoms were extracted from the enterovirus surveillance database at the RIVM.
Criteria for inclusion in the study.
Human parechoviruses are reported to grow selectively on cell cultures of simian origin (Vero, tMK, or LLC-MK2), with a CPE resembling that of enteroviruses, but to test negative by the enterovirus PCR assay (3, 7). By using these criteria for screening of the biobank of the 248 nontyped and nontypeable isolates, 163 cell culture isolates from individual patients were selected. These isolates were screened for the presence of HPeV by an in-house HPeV confirmatory PCR assay.
RNA extraction.
Viral RNA was extracted from cell cultures using the MagNA Pure LC total-nucleic-acid isolation kit with a MagNA Pure LC instrument (Roche Diagnostics, Almere, The Netherlands). External lysis was done by adding 200 μl cell culture to 300 μl lysis binding buffer (provided in the kit). Extraction was performed according to the manufacturer's instructions. Viral RNA was eluted in 50 μl elution buffer.
HPeV confirmatory PCR assay.
Primers were designed on the basis of a genome alignment of the reference strains HPeV1 Harris, HPeV2 Gregory, HPeV3 A308/99, HPeV3 Can82853-01, and HPeV5 CT86-6760 by using the BioEdit sequence alignment editor, version 7.0.0 (8). For the detection of HPeV, forward primer PE5F (5′-CCACGCTYGTGGAYCTTATG-3′) and reverse primer PE5R (5′-GGCCTTACAACTAGTGTTTGC-3′) were designed, targeting the 5′ untranslated region (5′ UTR) (nucleotides 292 to 553). For serotype classification, a PCR assay was designed using forward primer PEVF (5′-CAGCIGGTGARCAGATGAC-3′) and reverse primer PEVR (5′-ATCTAATTCACACTCTTCYTC-3′), targeting the VP1/2A region (nucleotides 2790 to 3238). In case PCR amplification with this primer set failed, primers Sab5F (5′-CTTCAGCTCAAGATGATGG-3′) and Sab5BR (5′-GAAACCTCTATCTAAATAWG-3′) were used. Reverse transcriptions were performed to convert the viral RNA to cDNA. For reverse transcription, 2.5 μl of the isolated viral RNA was incubated at 94°C for 2 min together with 1.0 μl of 50 μM antisense primer and 5.5 μl H2O. The samples were subsequently chilled on ice for 2 min and incubated at 42°C for 60 min together with 1.5 μl of 10× PCR buffer (pH 8.3) (100 mM Tris-HCl, 500 mM KCl), 1.8 μl of 25 mM MgCl2, 1.5 μl of 10 mM deoxynucleoside triphosphates, 0.5 μl of 10-U/μl avian myeloblastosis virus reverse transcriptase (Promega, Leiden, The Netherlands), and 0.7 μl of H2O. The reaction was finished by incubation of the samples at 94°C for 2 min, followed by incubation on ice.
PCR amplification of the 5′ UTR and VP1/2A region was performed on a LightCycler instrument. The 20-μl PCR mixture, containing 2 μl of cDNA from the reverse transcription (RT) reaction, 10 pmol of each primer, 4.5 mM MgCl2, 0.16 μl TaqStart antibody solution (BD Biosciences, Breda, The Netherlands), and 2 μl LightCycler DNA Master Sybr green I (Roche Diagnostics, Almere, The Netherlands), was added to a capillary tube and loaded into the LightCycler instrument. The 5′ UTR was amplified in the presence of 2 μl of 5× Q-solution (Qiagen, Venlo, The Netherlands). Samples were denatured for 1 min at 95°C and subjected to 45 cycles at 96°C for 3 s, 40°C for 4 s, and 72°C for 15 s. Amplicon size analysis was performed using gel electrophoresis. The PCR products were purified according to the manufacturer's protocol for the QIAquick PCR purification kit (Qiagen, Venlo, The Netherlands). Positive samples were typed by sequencing of the VP1/2A PCR product. The RT-PCR products were sequenced with the ABI Prism BigDye terminator cycle sequencing ready reaction kit, version 3.2 (Applied Biosystems, Foster City, CA) on an automated sequencer (model 3700; Applied Biosystems) using primer PEVF. Sequence data were edited using SeqMan software (DNAStar Inc., Madison, WI). The strains were genetically classified by using Bionumerics software (Applied Maths BVBA, Sint-Martens-Latem, Belgium).
Validation of the HPeV confirmatory PCR assay.
To evaluate the specificity of the HPeV confirmatory PCR assay, 86 cell culture isolates that tested positive by the enterovirus PCR assay were included in this test. To determine the detection limit of the test, RNA was extracted from 100 to 10−8 dilutions of HPeV1 Harris-positive cell culture supernatants (106.6 50% tissue culture infective doses [TCID50]/ml) and HPeV2 Gregory-positive cell culture supernatants (106.2 TCID50/ml) by using the MagNA Pure LC total-nucleic-acid isolation kit (Roche Diagnostics, Almere, The Netherlands) and was amplified by the RT-PCR assay. HPeV-positive isolates were typed by sequencing of the VP1/2A region. To test the sensitivity of this PCR assay, 15 HPeV1 isolates cultured between 1960 and 1967 were included in the test. These isolates had previously been characterized as HPeV1 by a serum neutralization assay.
Nucleotide sequence accession numbers.
The nucleotide sequence data reported in this paper will appear in the DDBJ/EMBL/GenBank nucleotide sequence databases with accession numbers AB443710 to AB443864.
RESULTS
Validation of the HPeV confirmatory PCR assay.
To evaluate the specificity of the HPeV confirmatory PCR assay, 86 enterovirus-positive isolates were included in this test. In total, 84 of these 86 isolates (98%) did not react in our HPeV PCR assay. Two isolates had a positive result. The presence of HPeV in these cell culture isolates was confirmed by sequencing of the VP1/2A region, indicating mixed infections of enterovirus (EV18 and coxsackievirus A20) and HPeV. To determine the detection limit of the confirmation test, RNA was extracted from 100 to 10−8 dilutions of HPeV1 Harris cell supernatants (106.6 TCID50/ml) and HPeV2 Gregory cell supernatants (106.2 TCID50/ml) and was amplified by the RT-PCR assay. For HPeV1 Harris, PCR-positive signals could be observed for dilutions from 100 to 10−2, representing ≥53 infectious virus particles in the reaction mixture. For HPeV2 Gregory, dilutions of 100 to 10−1 gave PCR-positive results, representing ≥210 infectious virus particles per reaction mixture.
Identification of HPeV.
In total, 163 unique patient isolates collected during enterovirus surveillance from 2000 to 2007 fit the selection criteria for the study in that they showed CPE only on cells of simian origin (Vero, tMK, and LLc-MK2) and tested negative for enteroviruses by PCR. The 5′ UTR PCR assay found 155 isolates (95%) to be HPeV, while 8 isolates (5%) were negative by both the enterovirus PCR assay and the HPeV PCR assay.
The 155 HPeV-positive isolates and the 2 isolates positive by both the enterovirus PCR assay and the HPeV PCR assay were further characterized on the basis of the VP1/2A sequence data. For reference, 15 HPeV1 isolates from our historic collection (1960 to 1967) were tested; all of them gave positive reactions. On the basis of similarity comparisons, 55 of the 155 isolates (35%) were characterized as HPeV1-like, 2 (1.3%) as HPeV2-like, 89 (57%) as HPeV3-like, 1 (0.6%) as HPeV4-like, and 8 (5%) as HPeV5-like. The HPeV4 isolate was the isolate that has been reported by Benschop et al. in 2006 (3). The two samples from cases of mixed infections with HPeV and enterovirus contained HPeV3-like isolates.
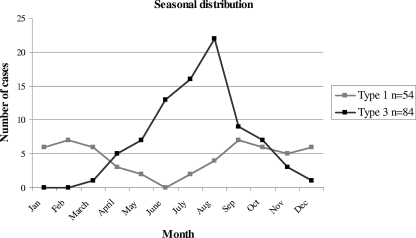
Seasonality of HPeV.
The month of sampling was known for 54 HPeV1 and 84 HPeV3 isolates. HPeV3 was isolated mainly during the spring and summer seasons, with a peak in July and August. This is in contrast with the seasonal distribution of HPeV1. A low level of HPeV1 circulation was observed throughout the year, with a low in the summer months (Fig. 1). For HPeV2, HPeV4, and HPeV5 isolates, no seasonal distribution could be described, possibly because of the low numbers.
FIG. 1.
Seasonal distribution of HPeV1 and HPeV3 isolated by routine virological laboratories in The Netherlands, 2000-2007.
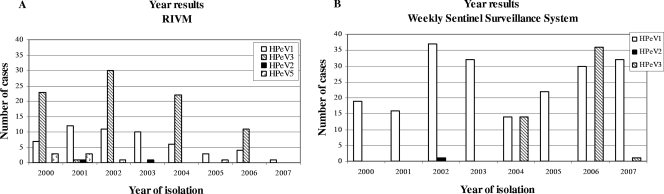
A periodicity could be seen in the occurrence of HPeV3 during the years 2000 to 2007. HPeV3 infections with more than 20 cases occurred at intervals of 2 years (Fig. 2A). In 2006 a decrease in the number of HPeV3 isolates (n = 11) in the RIVM collection was observed as a result of the implementation of HPeV3 diagnostics in routine laboratories (Fig. 2B). The 14 cases in 2004 (Fig. 2B) represent the cases in Arnhem where HPeV3 infections were observed for the first time in The Netherlands.
FIG. 2.
Distribution of HPeV during 2000 to 2007. (A) HPeV isolated at the RIVM; (B) HPeV reported by 21 diagnostic laboratories participating in the Weekly Sentinel System of the Dutch Working Group for Clinical Virology.
Clustering of the viral isolates on the basis of the VP1/2A sequence data showed that the seasonal peaks (at 2-year intervals) were caused by viruses belonging to more than one lineage (two to six lineages per season), although a major variant was found in each season (Fig. 3). The viruses isolated from the patients in 2004 belonged to four lineages. In 2006 the majority of the cases were caused by an HPeV3 lineage that also was common in 2002. All HPeV3 isolates, except for one in 2002, were most closely related to the Canadian HPeV3 reference strain CAN82853-01. Variability between these different lineages was observed in the C-terminal region of VP1 in the surface-exposed loop (amino acid positions 219 to 225 in VP1). Only two isolates (00-2621 and 05-1467) showed an additional substitution in the VP1/2A region (residues 184 and 191 of VP1, respectively). Only one isolate was most closely related to the Japanese HPeV3 reference strain A308/99.
Typing of HPeV1 and HPeV2 by serum neutralization tests with pooled or monotypic antisera has been performed routinely by 15 of 21 laboratories since the early 1990s. The HPeV1 and -2 isolates detected in this study were isolates that were not typed by other laboratories and were sent to the RIVM to exclude poliovirus infection. The total number of HPeV1 cases in The Netherlands reported by all members of the Weekly Sentinel Surveillance System of the Dutch Working Group on Clinical Virology confirms the annual occurrence of HPeV1 (Fig. 2B). The data available for 2007 indicate isolation of a mean of 3 HPeV isolates per week (32 were typed as HPeV1 and only 1 as HPeV3, confirming the biannual periodicity).
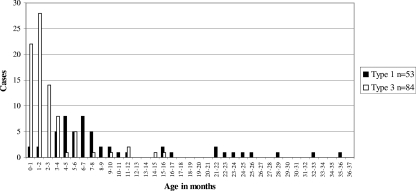
Age.
HPeV3 is reported to infect significantly younger children than HPeV1 (4). For 53 cases of HPeV1 infection and 84 cases of HPeV3 infection, the age of the patient was known (Fig. 4). HPeV1 was isolated from children with a mean age of 9.2 months (range, 0 to 35 months). HPeV3 infected children with a mean age of 1.9 months (range, 0 to 15 months). The ratio of male to female patients was 1.12 for HPeV1 and 1.26 for HPeV3.
FIG. 4.
Ages of patients with HPeV1 or HPeV3 infections.
Clinical symptoms.
Clinical symptoms were known for 33 children infected with HPeV1 and 34 children infected with HPeV3 (Table 1). HPeV3 infection was significantly more often associated with fever (P = 0.013), meningitis, and viremia (P = 0.001) than HPeV1 infection. Gastrointestinal symptoms such as diarrhea and vomiting were more often associated with HPeV1 infection (P = 0.006). Respiratory or other symptoms could not be associated with a specific serotype. Gastrointestinal symptoms, fevers, joint aches, and rashes were reported for children infected with HPeV5 (n = 3). No symptoms were documented for the three patients from whom HPeV2 (n = 2) and HPeV4 (n = 1) were isolated.
TABLE 1.
Clinical symptoms of children infected with HPeV1 and HPeV3
| Symptom type | No. of children infected with:
|
Pa | |
|---|---|---|---|
| HPeV1 (n = 33) | HPeV3 (n = 34) | ||
| Gastrointestinal | 19 | 8 | 0.006 |
| Respiratory | 6 | 2 | 0.15 |
| Meningitis/viremia | 3 | 16 | 0.001 |
| Fever | 8 | 19 | 0.013 |
| Other | 6 | 8 | 0.765 |
Calculated by Fisher's two-tailed test.
DISCUSSION
This study clarifies a large number of cell cultures with unexplained CPE collected during enterovirus surveillance from 2000 to 2007. In total, 163 of these isolates showed CPE only on cells of simian origin and were negative by the enterovirus PCR assay. Testing of these 163 isolates by an HPeV-specific PCR assay revealed the presence of 155 HPeV-positive isolates, explaining the majority of the diagnostic deficit in enterovirus surveillance for these years. Our HPeV confirmatory PCR assay had a detection limit sufficient for use as a confirmatory test of cell culture isolation. For direct detection of HPeV in clinical samples, it remains to be seen if a more sensitive diagnostic PCR assay is needed, because the concentration of virus in these samples is unknown.
Each year approximately 3.5% of all isolates reported by the 21 diagnostic laboratories as part of the enterovirus surveillance is represented by HPeV1. HPeV3 infections occurred at intervals of 2 years and appeared to represent 3.5% of all infections diagnosed for enterovirus surveillance in these years. These numbers emphasize the prevalence of HPeV3 and HPeV1 in The Netherlands, which thus has been underestimated for many years.
This study clearly shows the difference in seasonal distribution between HPeV1 and HPeV3 that was indicated by previous studies based on lower numbers of HPeV1 and HPeV3 isolates (2, 4). In addition, HPeV3 showed a clear periodicity in causing infections, with seasonal peaks in 2000, 2002, 2004, and 2006. In each of these years, the outbreaks were caused by viruses belonging to more than one lineage, indicating endemic circulation of HPeV3. Analysis of the VP1/2A sequence data showed that divergence between the different lineages occurred in the C-terminal variable loop of VP1, which is expected to be highly immunogenic (5). This suggests antigenic drift. Occurrence of variability in this region, however, can be expected. Variability among picornaviruses is reported to occur mainly in the surface-exposed, flexible loops of the capsid proteins, which connect the highly conserved β-sheets (11). It is not clear why HPeV3 infections occur at intervals of 2 years. The periodicity might be linked to a periodical occurrence of the source of HPeV3, to the minimal size of the susceptible population required for a seasonal epidemic, or to antigenic drift. Given the very young age of patients, antigenic variation may be necessary to escape protection from maternal antibodies. Periodical occurrence seems not to be a feature unique to HPeV3 infections. Genotypes 3, 4, and 5 of coxsackievirus B, diagnosis of which forms part of the national enterovirus surveillance system, are other examples of viruses that appear to show independent periodicity in causing infections (data not shown). It might be of interest to analyze the capsid regions of these viruses to determine whether antigenic drift occurs or not.
The HPeV3 isolates detected during our study were isolated from patients with a mean age of 1.9 months and were significantly more associated with fever, meningitis, and viremia than HPeV1 infection. The children infected with HPeV1 were clearly older, with a mean age of 9.2 months. Gastrointestinal symptoms such as diarrhea and vomiting were more often associated with HPeV1 infection. These findings and the size of our study group strengthen the suggestions of Benschop et al. (2006) that children infected with HPeV3 are significantly younger and more often show sepsis-like illness than children infected with HPeV1 (4). The difference in behavior between HPeV1 and HPeV3 might be explained by the difference in receptor usage between HPeV1 and HPeV3. An RGD (arginine-glycine-aspartic acid) motif is present in the C-terminal loop of VP1 of HPeV1, -2, -4, -5, and -6 and is responsible for the attachment of the parechovirus to the αvβ1 and αvβ3 (fibronectin) receptors present at the cell surface (6, 19). HPeV3 does not have an RGD motif (5, 10) and therefore uses another, RGD-independent route of cell entrance, which could be associated with a difference in tissue tropism and a different pathogenesis from that of HPeV1 infection.
The results of a previous study suggested that HPeV3 infections were especially but not only found in boys (male/female ratio, 9:1) (4). Our study, however, showed that the distribution of HPeV3 infections (male/female ratio, 1.26:1) does not clearly differ from that of enterovirus infections. Reports of enterovirus isolations are consistently more common from males than from females (13, 14).
This study shows that HPeV has been endemic in The Netherlands for the past 8 years and explains the majority of the diagnostic deficit in the enterovirus surveillance of 2000 to 2007. Routine implementation of an HPeV diagnostic PCR test in enterovirus surveillance is therefore recommended and is actually already in place in The Netherlands, since 2006 (16). This is the first time that HPeV3 infections have been reported to occur at intervals of 2 years. This study includes a large number of HPeV isolates, strengthening our observations and the suggestions from previous studies, such as the suggestion of Benschop et al. (2006) that HPeV3 is more often associated with sepsis-like illness in very young children (especially 0 to 4 months) (4).
Acknowledgments
We thank all the virologists participating in the Weekly Sentinel System of the Dutch Working Group for Clinical Virology for collecting and providing weekly positive diagnostic results. For participating in the enterovirus surveillance activities, we acknowledge the contribution of the laboratory staff of the RIVM (Ron Altena, Edin Jusic, Gökhan Uslu, and Bas van der Veer).
Footnotes
Published ahead of print on 9 July 2008.
REFERENCES
- 1.Al-Sunaidi, M., C. H. Williams, P. J. Hughes, D. P. Schnurr, and G. Stanway. 2007. Analysis of a new human parechovirus allows the definition of parechovirus types and the identification of RNA structural domains. J. Virol. 811013-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumgarte, S., L. K. de Souza Luna, K. Grywna, M. Panning, J. F. Drexler, C. Karsten, H. I. Huppertz, and C. Drosten. 2008. Prevalence, types, and RNA concentrations of human parechoviruses in patients with acute enteritis, including a sixth parechovirus type. J. Clin. Microbiol. 46242-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benschop, K. S., J. Schinkel, M. E. Luken, P. J. van den Broek, M. F. Beersma, N. Menelik, H. W. van Eijk, H. L. Zaaijer, C. M. VandenBroucke-Grauls, M. G. Beld, and K. C. Wolthers. 2006. Fourth human parechovirus serotype. Emerg. Infect. Dis. 121572-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benschop, K. S., J. Schinkel, R. P. Minnaar, D. Pajkrt, L. Spanjerberg, H. C. Kraakman, B. Berkhout, H. L. Zaaijer, M. G. Beld, and K. C. Wolthers. 2006. Human parechovirus infections in Dutch children and the association between serotype and disease severity. Clin. Infect. Dis. 42204-210. [DOI] [PubMed] [Google Scholar]
- 5.Boivin, G., Y. Abed, and F. D. Boucher. 2005. Human parechovirus 3 and neonatal infections. Emerg. Infect. Dis. 11103-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boonyakiat, Y., P. J. Hughes, F. Ghazi, and G. Stanway. 2001. Arginine-glycine-aspartic acid motif is critical for human parechovirus 1 entry. J. Virol. 7510000-10004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Vries, M., K. Pyrc, R. Berkhout, W. Vermeulen-Oost, R. Dijkman, M. F. Jebbink, S. Bruisten, B. Berkhout, and L. van der Hoek. 2008. Human parechovirus type 1, 3, 4, 5, and 6 detection in picornavirus cultures. J. Clin. Microbiol. 46759-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall, T. A. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 4195-98. [Google Scholar]
- 9.Hyypia, T., C. Horsnell, M. Maaronen, M. Khan, N. Kalkkinen, P. Auvinen, L. Kinnunen, and G. Stanway. 1992. A distinct picornavirus group identified by sequence analysis. Proc. Natl. Acad. Sci. USA 898847-8851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ito, M., T. Yamashita, H. Tsuzuki, N. Takeda, and K. Sakae. 2004. Isolation and identification of a novel human parechovirus. J. Gen. Virol. 85391-398. [DOI] [PubMed] [Google Scholar]
- 11.Joki-Korpela, P., M. Roivainen, H. Lankinen, T. Poyry, and T. Hyypia. 2000. Antigenic properties of human parechovirus 1. J. Gen. Virol. 811709-1718. [DOI] [PubMed] [Google Scholar]
- 12.Legay, V., J. J. Chomel, E. Fernandez, B. Lina, M. Aymard, and S. Khalfan. 2002. Encephalomyelitis due to human parechovirus type 1. J. Clin. Virol. 25193-195. [DOI] [PubMed] [Google Scholar]
- 13.Moore, M. 1982. Centers for Disease Control. Enteroviral disease in the United States, 1970-1979. J. Infect. Dis. 146103-108. [DOI] [PubMed] [Google Scholar]
- 14.Morens, D. M., and M. A. Pallansch. 1995. Epidemiology, p. 3-23. In H. A. Rotbart (ed.), Human enterovirus infections. American Society for Microbiology, Washington, DC.
- 15.Nix, W. A., M. S. Oberste, and M. A. Pallansch. 2006. Sensitive, seminested PCR amplification of VP1 sequences for direct identification of all enterovirus serotypes from original clinical specimens. J. Clin. Microbiol. 442698-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noordhoek, G. T., J. F. L. Weel, E. Poelstra, M. Hooghiemstra, and A. H. Brandenburg. 19 November 2007, posting date. Clinical validation of a new real-time PCR assay for detection of enteroviruses and parechoviruses, and implications for diagnostic procedures. J. Clin. Virol. doi: 10.1016/j.jcv.2007.09.011. [DOI] [PubMed]
- 17.Oberste, M. S., K. Maher, D. R. Kilpatrick, M. R. Flemister, B. A. Brown, and M. A. Pallansch. 1999. Typing of human enteroviruses by partial sequencing of VP1. J. Clin. Microbiol. 371288-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oberste, M. S., K. Maher, and M. A. Pallansch. 1998. Complete sequence of echovirus 23 and its relationship to echovirus 22 and other human enteroviruses. Virus Res. 56217-223. [DOI] [PubMed] [Google Scholar]
- 19.Racaniello, V. R. 2001. Picornaviridae: the viruses and their replication, p. 685-722. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed., vol. 1. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 20.Stanway, G., P. Joki-Korpela, and T. Hyypia. 2000. Human parechoviruses—biology and clinical significance. Rev. Med. Virol. 1057-69. [DOI] [PubMed] [Google Scholar]
- 21.Swanink, C. M. A., H. Vennema, H. G. Van der Avoort, and M. P. G. Koopmans. 2005. A newly identified parechovirus causing sepsis-like syndrome in neonates. Ned. Tijdschr. Med. Microbiol. 2005(Suppl.)S46. [Google Scholar]
- 22.Watanabe, K., M. Oie, M. Higuchi, M. Nishikawa, and M. Fujii. 2007. Isolation and characterization of novel human parechovirus from clinical samples. Emerg. Infect. Dis. 13889-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WHO. 2004. Polio laboratory manual, 4th ed., p.98-100. WHO, Geneva, Switzerland.
- 24.Wood, D. J., and B. Hull. 1999. L20B cells simplify culture of polioviruses from clinical samples. J. Med. Virol. 58188-192. [PubMed] [Google Scholar]