Abstract
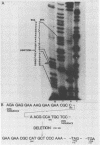
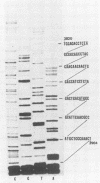
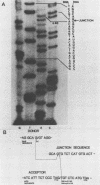
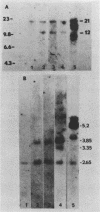
Our previous studies have argued persuasively that in murine sarcoma virus ts110 (MuSVts110) the gag and mos genes are fused out of frame due to a approximately 1.5-kilobase (kb) deletion of wild-type murine sarcoma virus 349 (MuSV-349) viral information. As a consequence of this deletion, infected cells grown at 39 degrees C appear morphologically normal, producing a 4-kb viral RNA and a truncated gag gene product, P58gag. At 33 degrees C, however, MuSVts110-infected cells appear transformed, producing two viral RNAs, about 4 and 3.5 kb in length, and two viral proteins, P58gag and P85gag-mos. Recent S1 nuclease analyses (Nash et al., J. Virol. 50:478-488, 1984) suggested strongly that at 33 degrees C about 430 bases surrounding the out-of-frame gag-mos junction and bounded by consensus splice donor and acceptor sites are excised from the 4-kb RNA to form the 3.5-kb RNA. As a result of this apparent splicing event, the gag and mos genes seemed to be fused in frame and allowed the translation of P85gag-mos. In the present study, DNA primers hybridizing to the MuSVts110 4- and 3.5-kb RNAs just downstream of the gag-mos junction points were used to sequence these junctions by the primer extension method. We observed that, relative to wild-type MuSV-349 5.2-kb RNA, the MuSVts110 4-kb RNA had suffered a 1,488-base deletion as a result of the fusion of wild-type gag gene nucleotide 2404 to wild-type mos gene nucleotide 3892. This gag-mos junction is out of frame, containing both TAG and TGA termination codons in the reading frame 42 and 50 bases downstream of the gag-mos junction, respectively. Thus, the MuSVts110 4-kb RNA can only be translated into a truncated gag precursor containing an additional C-terminal 14 amino acid residues derived from an alternate mos gene reading frame. Similar analyses of the MuSVts110 3.5-kb RNA showed a further loss of both gag and mos sequences over those deleted in the original 1,488-base deletion. In the MuSVts110 3.5-kb RNA, we found that gag nucleotide 2017 was fused to mos nucleotide 3936 (nucleotide 2449 in the MuSVts110 4-kb genome). This 431-base excised fragment is bounded exactly by in-frame consensus splice donor and acceptor sequences. As a consequence of this splice event, the TAG codon is excised and the restoration of the original mos gene reading frame allows the TGA codon to be bypassed.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball J., McCarter J. A., Sunderland S. M. Evidence for helper independent murine sarcoma virus. I. Segregation of replication-defective and transformation-defective viruses. Virology. 1973 Nov;56(1):268–284. doi: 10.1016/0042-6822(73)90305-x. [DOI] [PubMed] [Google Scholar]
- Blair D. G., Hull M. A., Finch E. A. The isolation and preliminary characterization of temperature-sensitive transformation mutants of Moloney sarcoma virus. Virology. 1979 Jun;95(2):303–316. doi: 10.1016/0042-6822(79)90486-0. [DOI] [PubMed] [Google Scholar]
- Brown R. L., Horn J. P., Wible L., Arlinghaus R. B., Brinkley B. R. Sequence of events in the transformation process in cells infected with a temperature-sensitive transformation mutant of Moloney murine sarcoma virus. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5593–5597. doi: 10.1073/pnas.78.9.5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer K., Reddy E. P., Aaronson S. A. Translational products of Moloney murine sarcoma virus RNA: identification of proteins encoded by the murine sarcoma virus src gene. J Virol. 1981 May;38(2):704–711. doi: 10.1128/jvi.38.2.704-711.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue D. J., Hunter T. A generalized method of subcloning DNA fragments by restriction site reconstruction: application to sequencing the amino-terminal coding region of the transforming gene of Gazdar murine sarcoma virus. Nucleic Acids Res. 1982 Apr 24;10(8):2549–2564. doi: 10.1093/nar/10.8.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamelin R., Brizzard B. L., Nash M. A., Murphy E. C., Jr, Arlinghaus R. B. Temperature-sensitive viral RNA expression in Moloney murine sarcoma virus ts110-infected cells. J Virol. 1985 Feb;53(2):616–623. doi: 10.1128/jvi.53.2.616-623.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn J. P., Wood T. G., Murphy E. C., Jr, Blair D. G., Arlinghaus R. B. A selective temperature-sensitive defect in viral RNA expression in cells infected with a ts transformation mutant of murine sarcoma virus. Cell. 1981 Jul;25(1):37–46. doi: 10.1016/0092-8674(81)90229-4. [DOI] [PubMed] [Google Scholar]
- Junghans R. P., Murphy E. C., Jr, Arlinghaus R. B. Electron microscopic analysis of ts1 10 Moloney mouse sarcoma virus, a variant of wild-type virus with two RNAs containing large deletions. J Mol Biol. 1982 Oct 25;161(2):229–250. doi: 10.1016/0022-2836(82)90150-4. [DOI] [PubMed] [Google Scholar]
- Lyons D. D., Murphy E. C., Jr, Mong S. M., Arlinghaus R. B. The translation products of Moloney murine sarcoma virus-124 RNA. Virology. 1980 Aug;105(1):60–70. doi: 10.1016/0042-6822(80)90156-7. [DOI] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E. C., Jr, Arlinghaus R. B. Comparative tryptic peptide analysis of candidate P85gag-mos of ts110 Moloney murine sarcoma virus and P38-P23 mos gene-related proteins of wild-type virus. Virology. 1982 Sep;121(2):372–383. doi: 10.1016/0042-6822(82)90175-1. [DOI] [PubMed] [Google Scholar]
- Nash M., Brown N. V., Wong J. L., Arlinghaus R. B., Murphy E. C., Jr S1 nuclease mapping of viral RNAs from a temperature-sensitive transformation mutant of murine sarcoma virus. J Virol. 1984 May;50(2):478–488. doi: 10.1128/jvi.50.2.478-488.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papkoff J., Lai M. H., Hunter T., Verma I. M. Analysis of transforming gene products from Moloney murine sarcoma virus. Cell. 1981 Nov;27(1 Pt 2):109–119. doi: 10.1016/0092-8674(81)90365-2. [DOI] [PubMed] [Google Scholar]
- Rao R. N., Rogers S. G. Plasmid pKC7: a vector containing ten restriction endonuclease sites suitable for cloning DNA segments. Gene. 1979 Sep;7(1):79–82. doi: 10.1016/0378-1119(79)90044-1. [DOI] [PubMed] [Google Scholar]
- Reddy E. P., Smith M. J., Aaronson S. A. Complete nucleotide sequence and organization of the Moloney murine sarcoma virus genome. Science. 1981 Oct 23;214(4519):445–450. doi: 10.1126/science.6170110. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stanker L. H., Horn J. P., Gallick G. E., Kloetzer W. S., Murphy E. C., Jr, Blair D. G., Arlinghaus R. B. Gag-mos Polyproteins encoded by variants of the Moloney strain of mouse sarcoma virus. Virology. 1983 Apr 15;126(1):336–347. doi: 10.1016/0042-6822(83)90483-x. [DOI] [PubMed] [Google Scholar]
- Van Beveren C., van Straaten F., Galleshaw J. A., Verma I. M. Nucleotide sequence of the genome of a murine sarcoma virus. Cell. 1981 Nov;27(1 Pt 2):97–108. doi: 10.1016/0092-8674(81)90364-0. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westin E. H., Wong-Staal F., Gelmann E. P., Dalla-Favera R., Papas T. S., Lautenberger J. A., Eva A., Reddy E. P., Tronick S. R., Aaronson S. A. Expression of cellular homologues of retroviral onc genes in human hematopoietic cells. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2490–2494. doi: 10.1073/pnas.79.8.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood T. G., Peltier-Horn J., Robey W. G., Blair D. G., Arlinghaus R. B. Characterization of virus-specified proteins present in NRK cells infected with a temperature-sensitive transformation mutant of Moloney murine sarcoma virus. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):747–754. doi: 10.1101/sqb.1980.044.01.080. [DOI] [PubMed] [Google Scholar]