Abstract
RNase R is a 3′-5′ highly processive exoribonuclease that can digest RNAs with extensive secondary structure. We analyzed the global effect of eliminating RNase R on the Pseudomonas putida transcriptome and the expression of the rnr gene under diverse conditions. The absence of RNase R led to increased levels of many mRNAs, indicating that it plays an important role in mRNA turnover.
Attaining a proper balance between mRNA synthesis and degradation is essential for determining levels of gene expression. The degradation of mRNAs in eubacteria involves the actions of different RNases and usually starts with endonucleolytic cleavage of the RNA molecule, followed by exoribonucleolytic digestion of the resulting fragments to yield 5′ mononucleotides (21). RNA decay has been studied mainly in Escherichia coli, which has at least five endonucleases and eight exoribonucleases (21). Among the 3′-5′ exoribonucleases, RNase II and polynucleotide phosphorylase (PNPase) are responsible for most mRNA degradation (24). Some of these nucleases form part of the degradosome, a complex devoted to RNA turnover. In E. coli, PNPase forms part of the degradosome while RNase II does not (reference 37 and references therein). Both nucleases are significantly inhibited by RNA secondary structures; the action of PNPase is probably helped by RhlB RNA helicase, which is also part of the degradosome. RNase R is a 3′-5′ exoribonuclease that is very processive and can efficiently digest RNAs having extensive secondary structures, such as rRNA, RNAs containing repetitive extragenic palindromic (REP) sequences, or the tmRNA (transfer-messenger RNA) required for trans-translation (7, 8, 33). RNase R is also involved in the processing of 16S and 5S rRNA (30). The gene encoding RNase R has been named vacB or rnr. In E. coli, rnr gene mutant strains are viable under standard laboratory conditions, although a double mutant lacking both RNase R and PNPase is not viable (8, 9). This suggests that PNPase compensates for the absence of RNase R in the rnr mutant strains. The levels of RNase R increase in response to stress conditions, such as entry into stationary phase, cold shock, or growth under carbon, nitrogen, or phosphorus limitation (5, 6), suggesting that it performs an important role in vivo. Homologues of the E. coli rnr gene are found in most bacteria (22, 41), which supports the notion that RNase R plays an important role in the metabolism of at least some kinds of RNAs. In Shigella flexneri (38) and Aeromonas hydrophyla (15), inactivation of rnr reduces pathogenicity.
Although many of the conclusions about RNA decay obtained for E. coli are valid for other bacterial species, variations may exist related to the different lifestyles of the microorganisms. For example, RNase R forms part of the degradosome in Pseudomonas syringae, but not in E. coli (29). RNase R may become particularly important at low temperatures, since inactivation of the rnr gene inhibits growth of P. syringae (30) and Pseudomonas putida (32) at 4°C, but not at 22°C. At low temperature, P. syringae rnr mutants accumulate unprocessed 16S and 5S rRNA (30).
The importance of RNase R in mRNA turnover in vivo has been studied only for a few individual mRNAs (1, 28), for mRNAs containing REP sequences (7), or for the particular case of tmRNA (5, 18, 20, 33). To get a more global view of the role of RNase R in mRNA turnover, we analyzed the effect of inactivating the rnr gene on the cellular transcriptome of P. putida KT2440, as well as the expression of this gene under diverse conditions.
To inactivate the P. putida KT2440 (16) rnr gene (PP4880), a DNA fragment containing rnr was PCR amplified from chromosomal DNA using oligonucleotides that hybridize, respectively, 126 nucleotides upstream from the ATG start site or 23 nucleotides downstream from the stop codon. The PCR product obtained, 2,813 bp in size, was cloned into plasmid pGEM-T Easy, producing pRNR-G. A 694-bp SmaI fragment present within rnr was replaced by a tetracycline resistance determinant, obtained from mini-Tn5Tc (17) with SmaI, producing pRNR-GT. The rnr::tet gene was excised from pRNR-GT as a NotI DNA fragment and cloned at the NotI site of plasmid pKNG101, which is designed for marker exchange mutagenesis (19). The plasmid obtained, pRNR3, was transferred to P. putida KT2440 to inactivate the rnr gene by marker exchange, as described previously (19). The absence of a wild-type rnr gene and the presence of the rnr::tet allele were verified by PCR in several isolates (not shown). An isolate named KTRNR1 was selected for further work.
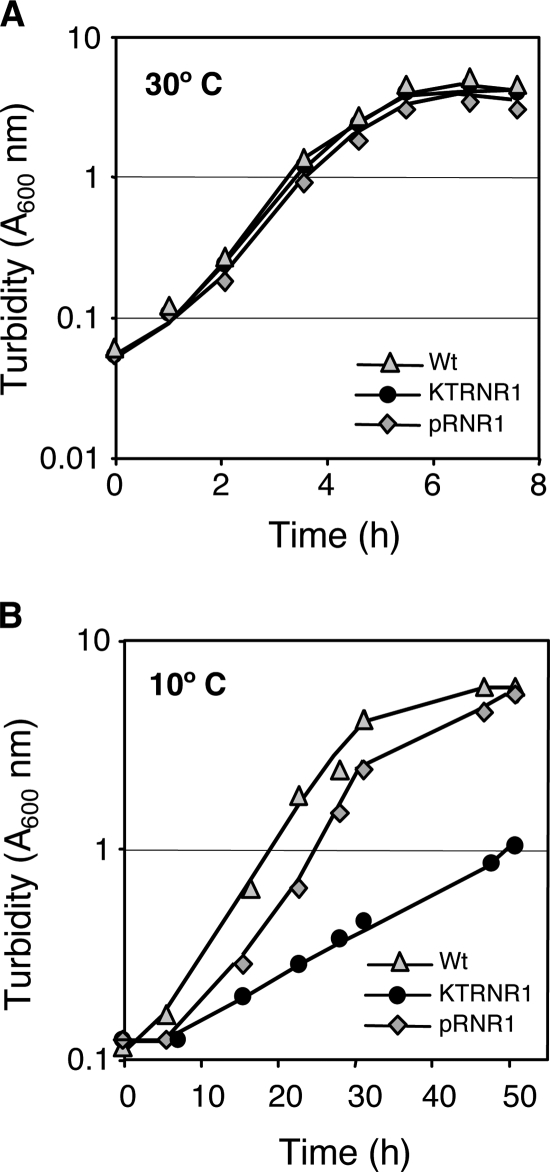
Inactivation of the P. putida KT2440 rnr gene impairs growth at 4°C, but not at 30°C (32). Growth at other temperatures was not reported. We found that inactivating the rnr gene in P. putida KT2440 had no effect on the growth rate in complete LB medium (34) at 30°C (Fig. 1A) but significantly reduced the growth rate at 10°C: the doubling time increased from 6 ± 0.2 h in the wild-type strain to 16.5 ± 0.5 h in the rnr mutant strain (Fig. 1B). Following cell growth at 10°C by counting viable cells, rather than by turbidity, yielded very similar growth rates; the doubling times were about 7 h for the wild-type strain and 15.5 h for the rnr mutant strain. The reduced growth rate of the rnr mutant strain at 10°C is consistent with the proposed role of RNase R in degrading the highly structured RNA, since secondary structures are probably more stable at low temperatures. Introduction of a plasmid containing a functional rnr gene into the rnr-deficient strain KTRNR1 restored the growth rate at 10°C to a level similar to that of the wild type strain, both when growth was followed by turbidity (Fig. 1B) and when it was followed by measuring viable cells (not shown). The plasmid used, pRNR1, contained a 3.4-kbp DNA fragment including rnr and PP4889, located immediately downstream, under the influence of the tacp promoter. This DNA segment was obtained from the P. putida KT2440 genome by PCR, cloned into pGEM-T Easy (Promega), excised as a BamHI-HindIII segment, and cloned between the same sites of the broad-host-range plasmid pVLT35 (14). The sequence of the amplified DNA fragment was determined to ensure the absence of undesired mutations.
FIG. 1.
Influence of RNase R on P. putida growth at different temperatures. P. putida strains KT2440 (Wt), KTRNR1 (an rnr derivative of KT2440), and KTRNR1 containing plasmid pRNR1 (which includes a wild-type copy of the rnr gene) were cultivated in flasks containing LB medium at either 30°C (A) or 10°C (B). Growth was followed by measuring the turbidity at 600 nm.
The apparent dispensability of RNase R at 30°C suggests that it may not play an important role at this temperature or that other nucleases can perform its role under these conditions. To gain deeper insight into this problem, the effect of inactivating the rnr gene in the mRNA pools of the cell was analyzed by comparing the transcriptome profiles of P. putida strains KT2440 (wild type) and KTRNR1 (an rnr derivative of KT2440) using a genome-wide oligonucleotide-based DNA microarray. This microarray, which was described previously (40; Array Express database accession number A-MEXP-313), includes spots for mRNAs, but not for rRNAs or tRNAs, and therefore, our analysis was focused only on mRNAs. The total-RNA preparations used, corresponding to cells grown in complete LB medium and collected at mid-exponential phase (A600, 0.6), were purified as described previously (25). The absence of DNA was checked by PCR using primers for rpoN, as described previously (25). cDNA was obtained from RNA preparations of the control and test strains (KT2440 and KTRNR1, respectively), fluorescently labeled with either Cy3 or Cy5, mixed, and used to hybridize the DNA microarray as reported previously (40). Five microarrays corresponding to five independent experiments (biological replicas) were used. The results for each replica were normalized and statistically analyzed as described previously (40), using the LIMMA software package (35). Differential expression was calculated using linear models and empirical Bayes-moderated t statistics (35, 36). The probability values obtained (P values) were adjusted for multiple testing to control the false-discovery rate (4). Genes whose mRNA levels increased or decreased more than twofold upon inactivation of the rnr gene, with a corrected P value lower than 0.05 and rendering a fluorescence signal higher than 300 units, are listed in Table S1 in the supplemental material. Using these cutoff values, the mRNA levels of 172 genes increased and those of 27 genes decreased upon inactivation of rnr.
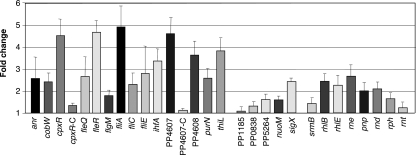
To validate the results obtained from the microarrays, the mRNA levels of 16 genes were compared in the wild-type and rnr strains by real-time reverse transcription (RT)-PCR. Three RNA samples corresponding to three independent experiments were analyzed, with each sample measured in triplicate. The reactions were performed as described previously (25), starting from 10 μg of total RNA and measuring the relative amounts of mRNA for each gene (experiment versus control), following the 2−ΔCt method (23). The oligonucleotides used for each gene are listed in Table S2 in the supplemental material. The absence of DNA in the RNA preparations was verified by real-time PCR using oligonucleotides for rpoN. The genes selected included 11 showing elevated mRNA levels in the rnr mutant strain (anr, cobW, cpxR, fleR, fliA, fliE, ihfA, PP4607, PP4608, purN, and thiL) and five that apparently remained unchanged (PP1185, PP0838, PP5264, nuoM, and sigX), as deduced from the microarray assays. The real-time RT-PCR assays confirmed that the 11 genes detected as upregulated in the microarrays indeed had elevated mRNA levels in the rnr mutant strain (Fig. 2). For two of these genes, PP4607 and cpxR, we also measured by real-time RT-PCR the effects of complementing the rnr mutation of strain KTRNR1 by the introduction of plasmid pRNR1, which contains a wild-type copy of the rnr gene; in both cases, the mRNA levels returned to wild-type values (Fig. 2, PP4607-C and cpxR-C). Among the five genes that appeared in the microarrays not to have been affected by the absence of RNase R, the real-time RT-PCR assays indicated changes in mRNA levels lower than 1.5-fold for four of them (PP1185, PP0838, PP5264, and nuoM) and an increase of 2.5-fold for sigX (Fig. 2). This suggests that the microarrays were probably underestimating the real number of genes that were affected by the absence of RNase R.
FIG. 2.
Effects of inactivating the rnr gene on the levels of selected mRNAs. Strains KT2440 (wild type) or KTRNR1 (an rnr derivative of KT2440) were cultivated in LB medium at 30°C. At a turbidity of 0.6, aliquots were taken and processed to obtain total RNA. The amounts of the mRNAs corresponding to the indicated genes were measured by real-time RT-PCR. The values indicate the ratios of the mRNA levels observed for strain KTRNR1 relative to those of the wild-type strain and correspond to the average of three independent assays; the standard error is shown.
Where there was an effect, the final consequence of the rnr mutation was in most cases an increase in mRNA levels. This is consistent with a role for RNase R in mRNA degradation and indicates that its absence cannot be fully compensated for by other RNases. In other words, RNase R seems to play a substantial role in mRNA turnover when cells grow at 30°C, although its absence did not lead to a decreased growth rate under the conditions analyzed.
E. coli RNase R is particularly active on mRNAs containing REP sequences and has been proposed to play an important role in the turnover of mRNAs containing such sequences (7). The P. putida KT2440 genome contains 938 REP sequences (2, 39) and 5,421 annotated genes (26), which means that, on average, 17% of the genes can be associated with a REP sequence. Among the 199 genes listed in Table S1 in the supplemental material, only 22 had REP sequences downstream of or within the coding sequence. This percentage (11%) is similar to or even lower than that of the complete genome. Therefore, among the genes affected by the lack of RNase R, those containing REP sequences are not overrepresented. This is consistent with the finding that, in E. coli, REP-containing RNA fragments accumulate to high levels when both RNase R and PNPase are absent, but not when RNase R is the only nuclease absent (7).
Intrinsic transcriptional terminators include a region of hyphenated inverted symmetry that can form a stem-loop structure (27). Although digestion of these terminator-associated secondary structures has not been related to RNase R, they may be particularly good substrates for the exonuclease. About 9% of the P. putida KT2440 genes include a predicted transcriptional terminator (http://www.tigr.org). Only 8.6% of the genes listed in Table S1 in the supplemental material have a predicted transcriptional terminator, which argues against an important, or at least an exclusive, role for RNase R in eliminating these secondary structures from mRNAs.
The genes affected by the absence of RNase R belonged to all of the different gene classes (see Table S3 in the supplemental material). However, those corresponding to the flagellar apparatus or to the biosynthesis of cofactors were overrepresented. The organization of flagellar genes in P. putida KT2440 is highly similar to that in Pseudomonas aeruginosa (13). In P. aeruginosa, the flagellar regulon consists of four classes of genes that are differentially regulated in a coordinated and hierarchical manner by the RpoN, FleQ, FleS, FleR, FliA, and FlgM regulatory proteins (12). Microarrays (see Table S1 in the supplemental material) and/or RT-PCR analyses (Fig. 2) indicated that the mRNA levels of rpoN, fleQ, fleR, and fliA were elevated in the rnr mutant strain, although those of flgM changed little. FleQ is the master regulator of the flagellar regulon (12); its levels are controlled by the Vfr regulator and its activity by the FleN antiactivator (10, 11). FleQ, together with the RpoN alternative sigma factor, activates the expression of the gene clusters flhF-fleN, fliEFGHIJ, fliLMNOPQR-flhB, flhA, and fleSR (12). Microarrays detected elevated mRNA levels for several of these genes (flhF, fliE, flhB, and fleR). The case of fleR was confirmed by real-time RT-PCR (Fig. 2). The elevated mRNA levels of the alternative sigma factor FliA, which were not paralleled by an increase in its antisigma, FlgM, led to increased expression of the FliA-dependent gene fliC, which codes for flagellin (Fig. 2).
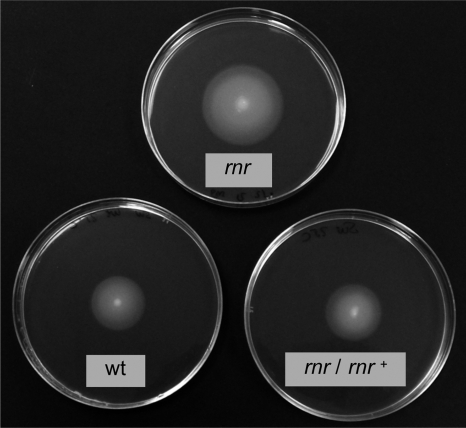
To analyze whether the altered expression of flagellar genes had an impact on cell physiology, the swimming abilities of P. putida strain KT2440, its rnr derivative KTRNR1, and the complemented strain KTRNR1/pRNR1 were analyzed in plates containing 10 g/liter tryptone and 5 g/liter NaCl solidified with 0.3% (wt/vol) agarose, as previously described (31). Polarly flagellated bacteria inoculated as a stabbed spot onto this kind of plate swim through water-filled channels and create concentric rings around the point of inoculation (31). Overnight cultures of each strain were adjusted to the same turbidity (A600), and 3 μl was stabbed into each plate. After overnight incubation at 25°C, the migration of bacteria through the agar from the point of inoculation was monitored. Cells of strain KT2440 generated a growth halo of 2.5 ± 0.1 cm, while the halo of the rnr mutant strain KTRNR1 was 3.6 ± 0.2 cm, which was a 46% increase (Fig. 3). The size of the halo reverted to wild-type values (2.4 ± 0.1 cm) in the complemented strain KTRNR1/pRNR1. Therefore, inactivation of RNase R leads to increased motility as determined in semisolid (0.3% [wt/vol]) agar plates.
FIG. 3.
Motilities of P. putida strains having a wild-type or an inactivated rnr allele. P. putida strains KT2440 (wt), KTRNR1 (an rnr derivative of KT2440), and KTRNR1 containing plasmid pRNR1 (which includes a wild-type copy of the rnr gene) were inoculated as stabbed spots in plates containing 0.3% (wt/vol) agarose. The halos were visualized after the plates were incubated overnight at 25°C.
The increase in the mRNA levels of a given gene in the rnr strain could reflect the fact that this mRNA is a direct substrate for RNase R, but it could also be an indirect effect, for example, if a change in the mRNA levels of a given gene led to an increase in the transcription, rather than in the mRNA stability, of another gene. To find mRNAs that could possibly be direct targets for RNase R, the decay rates of a subset of the mRNAs showing elevated levels in the rnr mutant strain were determined in the wild-type and the rnr strains. Both strains were cultivated in LB medium at 30°C, and at a turbidity of 0.6, rifampin was added to 100 μg/ml to inhibit transcription by RNA polymerase. At different time points, aliquots were taken and immediately frozen. Total RNA was purified, and the amounts of the mRNAs of the genes listed in Table 1 were measured by real-time RT-PCR (see Figure S1 in the supplemental material). The half-life was calculated from the mRNA decay rates observed in three independent assays. As shown in Table 1, inactivation of the rnr gene led to a significant increase in the half-lives of the mRNAs corresponding to cpxR, PP4607, fliA, fleR, PP4608, and cobW. The effects were more moderate for thiL and purN and slight or undetectable for ihfA, anr, and PP4980. Therefore, the turnover of at least some of the mRNAs depends directly on RNase R under the conditions tested. Among the genes analyzed, only PP4607 had a REP sequence at the 3′ end; none of the genes had a predicted transcriptional terminator.
TABLE 1.
Half-lives of the mRNAs corresponding to the indicated genes in the wild-type (KT2440) and rnr-derivative (KTRNR1) strainsa
| Gene | mRNA half-life (min)
|
Increase (n-fold) | |
|---|---|---|---|
| KT2440 (wild type) | KTRNR1 (rnr) | ||
| PP3372 (cpxR) | 5 | 54 | 11 |
| PP4607 | 5 | 40 | 8 |
| PP4341 (fliA) | 9 | >60 | >6 |
| PP4371 (fleR) | 9.5 | >60 | >6 |
| PP4608 | 5.5 | 27 | 5 |
| PP3508 (cobW) | 8 | 41 | 5 |
| PP0519 (thiL) | 7 | 30 | 4.3 |
| PP1664 (purN) | 13 | 40 | 3 |
| PP2471 (ihfA) | 5 | 11 | 2.2 |
| PP4265 (anr) | 4,5 | 9 | 2 |
| PP4980 | 4 | 6 | 1.5 |
The values were estimated from the mean decay rate for each mRNA after rifampin addition in cultures incubated at 30°C. The decay rates are shown in Fig. S1 in the supplemental material.
We also analyzed whether inactivation of P. putida rnr could generate a compensatory response leading to altered expression of other enzymes involved in RNA turnover. The absence of RNase R led to an increase in the mRNA levels of the RhlB (PP1295, or rhlB) and RhlE (PP4980, or rhlE) RNA helicases, although those of other RNA helicases, such as SrmB (PP4532, or srmB) were hardly affected (Fig. 2). Expression of RNase E (PP1905, or rne), PNPase (PP4708, or pnp), and RNase D (PP4591, or rnd) increased between 2- and 2.5-fold, while those of RNase H (PP5294, or rnh) and RNase T (PP1085, or rnt) were hardly affected. In summary, the absence of RNase R led to moderate increases in the mRNA levels of some RNases and RNA helicases, but other RNases and RNA helicases were not affected.
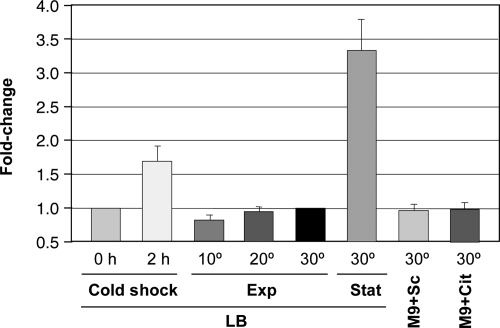
Expression of RNase R is cold inducible in E. coli (5, 6), but not in P. syringae (30). P. putida grows optimally at 30°C but can also grow at 4°C. The lower growth rate of the P. putida rnr mutant strain at 10°C indicates that RNase R becomes particularly important at low temperatures, when other nucleases presumably cannot compensate for its absence. The expression of the P. putida rnr gene increased less than twofold after a 2-hour cold shock in which the culture temperature was decreased from 30°C to 10°C (Fig. 4), a value that is at least an order of magnitude lower than that observed for E. coli (5). Expression of rnr differed little when cells grew exponentially in LB medium at a constant temperature of 10°C, 20°C, or 30°C (Fig. 4). E. coli RNase R is induced in response to stress conditions, such as entry into stationary phase or growth in a minimal salts medium containing succinate or citrate as the carbon source (6). In P. putida, rnr expression levels at 30°C were similar in a complete medium and in an M9 minimal salts medium (34) supplemented with trace elements (3) and 15 mM succinate or citrate as the carbon source (Fig. 4). Upon entry into stationary phase, however, rnr expression increased more than threefold (Fig. 4). We conclude that the expression pattern of the rnr gene in P. putida differs from that in E. coli.
FIG. 4.
Expression of the rnr gene under different conditions. Total RNA was obtained from cells grown under the indicated conditions, and the mRNA levels of the rnr gene were compared by real-time RT-PCR. The conditions compared were as follows: (i) 2 hours after a cold shock in LB medium (transfer from 30°C to 10°C) relative to the mRNA levels immediately before the cold shock (0 h); (ii) exponential growth in LB medium at 10°C, 20°C, or 30°C, with the mRNA levels at 30°C taken as a reference; (iii) entry into stationary phase (A600, 2.2 [Stat]) versus exponential phase (A600, 0.6 [Exp]) in LB medium at 30°C, with the latter taken as a reference; and (iv) exponential growth (A600, 0.6) at 30°C in a minimal salts medium containing succinate (M9+Sc) or citrate (M9+Cit) as the carbon source compared to LB medium under the same conditions. The relative amount of mRNA for each gene (experiment versus control) was determined following the 2−ΔΔCt method (23), using the mRNA levels of rpoN as an internal control in all cases except for the cold-shock assays, where rpoD was used. The error bars indicate standard errors.
In summary, our results indicate that P. putida RNase R plays an important role in mRNA turnover even under conditions in which its absence does not compromise growth. At 30°C, which is the optimum growth temperature for P. putida, the absence of RNase R led to increased levels of many mRNAs. In several cases, the effect was due to an increase in mRNA stability, suggesting that they could be direct targets for this enzyme and that other nucleases are less efficient than RNase R in their digestion. This, in turn, seemed to generate an indirect response in the expression of other genes. The absence of RNase R led to compensatory changes in the RNA decay machinery, changes that allowed apparently normal growth but that did not fully restore mRNA turnover. The elevated expression of RNA helicases and of PNPase (a 3′-5′ exoribonuclease that is less effective than RNase R on highly structured RNA) in the rnr mutant strain, together with the fact that the negative effect of the rnr mutation on the growth rate increased as the temperature decreased, suggests that mRNA secondary structures become more stable and difficult to digest as the growth temperature decreases, a situation in which RNase R becomes increasingly important. Finally, our results indicate that the expression pattern of the rnr gene in P. putida under some conditions is different from that in E. coli, probably reflecting the different lifestyles of the two bacterial species.
Microarray data accession number.
The results of the microarray experiments described in this article were deposited in the Array Express database (accession number E-MEXP-1440).
Supplementary Material
Acknowledgments
We are grateful to G. Morales for help with real-time PCR, to L. Yuste for excellent technical assistance, and to the Genomics Service at CNB for processing of microarrays.
P. Fonseca was the holder of an FPU fellowship from the Ministerio de Educación y Ciencia. This work was supported by grant BFU2006-00767/BMC from the Spanish Ministry of Education and Science.
Footnotes
Published ahead of print on 18 July 2008.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Andrade, J. M., F. Cairrao, and C. M. Arraiano. 2006. RNase R affects gene expression in stationary phase: regulation of ompA. Mol. Microbiol. 60219-228. [DOI] [PubMed] [Google Scholar]
- 2.Aranda-Olmedo, I., R. Tobes, M. Manzanera, J. L. Ramos, and S. Marqués. 2002. Species-specific repetitive extragenic palindromic (REP) sequences in Pseudomonas putida. Nucleic Acids Res. 301826-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauchop, T., and S. R. Eldsen. 1960. The growth of microorganisms in relation to their energy supply. J. Gen. Microbiol. 23457-569. [DOI] [PubMed] [Google Scholar]
- 4.Benjamini, Y., and Y. Hochberg. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57289-300. [Google Scholar]
- 5.Cairrao, F., A. Cruz, H. Mori, and C. M. Arraiano. 2003. Cold shock induction of RNase R and its role in the maturation of the quality control mediator SsrA/tmRNA. Mol. Microbiol. 501349-1360. [DOI] [PubMed] [Google Scholar]
- 6.Chen, C., and M. P. Deutscher. 2005. Elevation of RNase R in response to multiple stress conditions. J. Biol. Chem. 28034393-34396. [DOI] [PubMed] [Google Scholar]
- 7.Cheng, Z. F., and M. P. Deutscher. 2005. An important role for RNase R in mRNA decay. Mol. Cell 17313-318. [DOI] [PubMed] [Google Scholar]
- 8.Cheng, Z. F., and M. P. Deutscher. 2003. Quality control of ribosomal RNA mediated by polynucleotide phosphorylase and RNase R. Proc. Natl. Acad. Sci. USA 1006388-6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng, Z. F., Y. Zuo, Z. Li, K. E. Rudd, and M. P. Deutscher. 1998. The vacB gene required for virulence in Shigella flexneri and Escherichia coli encodes the exoribonuclease RNase R. J. Biol. Chem. 27314077-14080. [DOI] [PubMed] [Google Scholar]
- 10.Dasgupta, N., E. P. Ferrell, K. J. Kanack, S. E. West, and R. Ramphal. 2002. fleQ, the gene encoding the major flagellar regulator of Pseudomonas aeruginosa, is sigma-70 dependent and is downregulated by Vfr, a homolog of Escherichia coli cyclic AMP receptor protein. J. Bacteriol. 1845240-5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dasgupta, N., and R. Ramphal. 2001. Interaction of the antiactivator FleN with the transcriptional activator FleQ regulates flagellar number in Pseudomonas aeruginosa. J. Bacteriol. 1836636-6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dasgupta, N., M. C. Wolfgang, A. L. Goodman, S. K. Arora, J. Jyot, S. Lory, and R. Ramphal. 2003. A four-tiered transcriptional regulatory circuit controls flagellar biogenesis in Pseudomonas aeruginosa. Mol. Microbiol. 50809-824. [DOI] [PubMed] [Google Scholar]
- 13.Dasgupta, S., S. K. Arora, and R. Ramphal. 2004. The flagellar system of Pseudomonas aeruginosa, p. 675-698. In J. L. Ramos (ed.), Pseudomonas, vol. 1. Kluwer Academic, New York, NY. [Google Scholar]
- 14.de Lorenzo, V., L. Eltis, B. Kessler, and K. N. Timmis. 1993. Analysis of Pseudomonas gene products using lacIq/Ptrp-lac plasmids and transposons that confer conditional phenotypes. Gene 12317-24. [DOI] [PubMed] [Google Scholar]
- 15.Erova, T. E., V. G. Kosykh, A. A. Fadl, J. Sha, A. J. Horneman, and A. K. Chopra. 2008. Cold shock exoribonuclease R (VacB) is involved in Aeromonas hydrophila pathogenesis. J. Bacteriol. 1903467-3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franklin, F. C., M. Bagdasarian, M. M. Bagdasarian, and K. N. Timmis. 1981. Molecular and functional analysis of the TOL plasmid pWWO from Pseudomonas putida and cloning of genes for the entire regulated aromatic ring meta cleavage pathway. Proc. Natl. Acad. Sci. USA 787458-7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herrero, M., V. de Lorenzo, and K. N. Timmis. 1990. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J. Bacteriol. 1726557-6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong, S. J., Q. A. Tran, and K. C. Keiler. 2005. Cell cycle-regulated degradation of tmRNA is controlled by RNase R and SmpB. Mol. Microbiol. 57565-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaniga, K., I. Delor, and G. R. Cornelis. 1991. A wide-host-range suicide vector for improving reverse genetics in gram-negative bacteria: inactivation of the blaA gene of Yersinia enterocolitica. Gene 109137-141. [DOI] [PubMed] [Google Scholar]
- 20.Karzai, A. W., and R. T. Sauer. 2001. Protein factors associated with the SsrA.SmpB tagging and ribosome rescue complex. Proc. Natl. Acad. Sci. USA 983040-3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kushner, S. R. 2002. mRNA decay in Escherichia coli comes of age. J. Bacteriol. 1844658-4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li, Z., X. Gong, V. H. Joshi, and M. Li. 2005. Co-evolution of tRNA 3′ trailer sequences with 3′ processing enzymes in bacteria. RNA 11567-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 25402-408. [DOI] [PubMed] [Google Scholar]
- 24.Mohanty, B. K., and S. R. Kushner. 2003. Genomic analysis in Escherichia coli demonstrates differential roles for polynucleotide phosphorylase and RNase II in mRNA abundance and decay. Mol. Microbiol. 50645-658. [DOI] [PubMed] [Google Scholar]
- 25.Morales, G., A. Ugidos, and F. Rojo. 2006. Inactivation of the Pseudomonas putida cytochrome o ubiquinol oxidase leads to a significant change in the transcriptome and to increased expression of the CIO and cbb3-1 terminal oxidases. Environ. Microbiol. 81764-1774. [DOI] [PubMed] [Google Scholar]
- 26.Nelson, K. E., C. Weinel, I. T. Paulsen, R. J. Dodson, H. Hilbert, V. A. Martins dos Santos, D. E. Fouts, S. R. Gill, M. Pop, M. Holmes, L. Brinkac, M. Beanan, R. T. DeBoy, S. Daugherty, J. Kolonay, R. Madupu, W. Nelson, O. White, J. Peterson, H. Khouri, I. Hance, P. C. Lee, E. Holtzapple, D. Scanlan, K. Tran, A. Moazzez, T. Utterback, M. Rizzo, K. Lee, D. Kosack, D. Moestl, H. Wedler, J. Lauber, D. Stjepandic, J. Hoheisel, M. Straetz, S. Heim, C. Kiewitz, J. Eisen, K. N. Timmis, A. Dusterhoft, B. Tümmler, and C. M. Fraser. 2002. Complete genome sequence and comparative analysis of the metabolically versatile Pseudomonas putida KT2440. Environ. Microbiol. 4799-808. [DOI] [PubMed] [Google Scholar]
- 27.Nudler, E., and M. E. Gottesman. 2002. Transcription termination and anti-termination in E. coli. Genes Cells 7755-768. [DOI] [PubMed] [Google Scholar]
- 28.Oussenko, I. A., T. Abe, H. Ujiie, A. Muto, and D. H. Bechhofer. 2005. Participation of 3′-to-5′ exoribonucleases in the turnover of Bacillus subtilis mRNA. J. Bacteriol. 1872758-2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Purusharth, R. I., F. Klein, S. Sulthana, S. Jager, M. V. Jagannadham, E. Evguenieva-Hackenberg, M. K. Ray, and G. Klug. 2005. Exoribonuclease R interacts with endoribonuclease E and an RNA helicase in the psychrotrophic bacterium Pseudomonas syringae Lz4W. J. Biol. Chem. 28014572-14578. [DOI] [PubMed] [Google Scholar]
- 30.Purusharth, R. I., B. Madhuri, and M. K. Ray. 2007. Exoribonuclease R in Pseudomonas syringae is essential for growth at low temperature and plays a novel role in the 3′ end processing of 16 and 5 S ribosomal RNA. J. Biol. Chem. 28216267-16277. [DOI] [PubMed] [Google Scholar]
- 31.Rashid, M. H., and A. Kornberg. 2000. Inorganic polyphosphate is needed for swimming, swarming, and twitching motilities of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 974885-4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reva, O. N., C. Weinel, M. Weinel, K. Bohm, D. Stjepandic, J. D. Hoheisel, and B. Tümmler. 2006. Functional genomics of stress response in Pseudomonas putida KT2440. J. Bacteriol. 1884079-4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richards, J., P. Mehta, and A. W. Karzai. 2006. RNase R degrades non-stop mRNAs selectively in an SmpB-tmRNA-dependent manner. Mol. Microbiol. 621700-1712. [DOI] [PubMed] [Google Scholar]
- 34.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 35.Smyth, G. K. 2004. Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 3article 3. [DOI] [PubMed]
- 36.Smyth, G. K., and T. Speed. 2003. Normalization of cDNA microarray data. Methods 31265-273. [DOI] [PubMed] [Google Scholar]
- 37.Steege, D. A. 2000. Emerging features of mRNA decay in bacteria. RNA 61079-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tobe, T., C. Sasakawa, N. Okada, Y. Honma, and M. Yoshikawa. 1992. vacB, a novel chromosomal gene required for expression of virulence genes on the large plasmid of Shigella flexneri. J. Bacteriol. 1746359-6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tobes, R., and J. L. Ramos. 2005. REP code: defining bacterial identity in extragenic space. Environ. Microbiol. 7225-228. [DOI] [PubMed] [Google Scholar]
- 40.Yuste, L., A. B. Hervás, I. Canosa, R. Tobes, J. I. Jiménez, J. Nogales, M. M. Pérez-Pérez, E. Santero, E. Díaz, J. L. Ramos, V. de Lorenzo, and F. Rojo. 2006. Growth-phase dependent expression of the Pseudomonas putida KT2440 transcriptional machinery analyzed with a genome-wide DNA microarray. Environ. Microbiol. 8165-177. [DOI] [PubMed] [Google Scholar]
- 41.Zuo, Y., and M. P. Deutscher. 2001. Exoribonuclease superfamilies: structural analysis and phylogenetic distribution. Nucleic Acids Res. 291017-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.