Abstract
Pseudomonas aeruginosa and Bordetella pertussis produce lipopolysaccharide (LPS) that contains 2,3-diacetamido-2,3-dideoxy-d-mannuronic acid (d-ManNAc3NAcA). A five-enzyme biosynthetic pathway that requires WbpA, WbpB, WbpE, WbpD, and WbpI has been proposed for the production of this sugar in P. aeruginosa, based on analysis of genes present in the B-band LPS biosynthesis cluster. In the analogous B. pertussis cluster, homologs of wbpB to wbpI were present, but a putative dehydrogenase gene was missing; therefore, the biosynthetic mechanism for UDP-d-ManNAc3NAcA was unclear. Nonpolar knockout mutants of each P. aeruginosa gene were constructed. Complementation analysis of the mutants demonstrated that B-band LPS production was restored to P. aeruginosa knockout mutants when the relevant B. pertussis genes were supplied in trans. Thus, the genes that encode the putative oxidase, transaminase, N-acetyltransferase, and epimerase enzymes in B. pertussis are functional homologs of those in P. aeruginosa. Two candidate dehydrogenase genes were located by searching the B. pertussis genome; these have 80% identity to P. aeruginosa wbpO (serotype O6) and 32% identity to wbpA (serotype O5). These genes, wbpO1629 and wbpO3150, were shown to complement a wbpA knockout of P. aeruginosa. Capillary electrophoresis was used to characterize the enzymatic activities of purified WbpO1629 and WbpO3150, and mass spectrometry analysis confirmed that the two enzymes are dehydrogenases capable of converting UDP-d-GlcNAc, UDP-d-GalNAc, to a lesser extent, and UDP-d-Glc, to a much lesser extent. Together, these results suggest that B. pertussis produces UDP-d-ManNAc3NAcA through the same pathway proposed for P. aeruginosa, despite differences in the genomic context of the genes involved.
Lipopolysaccharide (LPS) is the major component of the outer membranes of gram-negative bacteria. Conceptually, it can be divided into three parts, namely, lipid A, core oligosaccharide, and the O antigen. In Pseudomonas aeruginosa, the following two forms of O antigen are produced: A-band O antigen, which is common to most strains (10, 27); and B-band O antigen, which is variable and forms the basis of the International Antigenic Typing Scheme (28). The B-band O antigen of P. aeruginosa PAO1 (serotype O5) contains a repeating trisaccharide of 2-acetamido-3-acetamidino-2,3-dideoxy-d-mannuronic acid (d-ManNAc3NAmA), 2,3-diacetamido-2,3-dideoxy-d-mannuronic acid (d-ManNAc3NAcA), and 2-acetamido-2,6-dideoxy-d-galactose (N-acetyl-d-fucosamine [d-FucNAc]) (23). P. aeruginosa serotypes O2, O16, O18, and O20 have very similar repeat units; for this reason, these serotypes are collectively called P. aeruginosa serogroup O2. In contrast, the B-band O antigen of P. aeruginosa serotype O6 is made of repeating tetramer units of l-rhamnose (l-Rha), N-acetyl-d-galactosaminuronic acid (d-GalNAcA), 2-deoxy-2-formamido-d-galacturonic acid (d-GalNFoA), and 2-acetamido-2,6-dideoxy-d-glucose (d-QuiNAc) (22).
Nomenclature for the conceptual division of LPS differs between different organisms. In Bordetella pertussis, an obligate human pathogen and the causative agent of whooping cough, the core oligosaccharide is linked to a structure called the band A trisaccharide. The band A trisaccharide from B. pertussis 1414 is composed of N-acetyl-d-glucosamine (d-GlcNAc), 2,3-diacetamido-2,3-dideoxy-d-mannuronic acid (d-ManNAc3NAcA), and 2-acetamido-4-methylaminofucose (FucNAc4NMe) (6). The related species Bordetella bronchiseptica, a pathogen of animals, and Bordetella parapertussis, a pathogen of both humans and animals, also have d-ManNAc3NAcA present in the LPS (33). B. bronchiseptica and B. parapertussis LPS also contains a repeating polysaccharide known as the O antigen (12). The O antigen contains 2,3-diacetamido-2,3-dideoxy-l-galactosamine (l-GalNAc3NAcA) (33), which is thought to be synthesized from UDP-d-ManNAc3NAcA by the enzymes of the wbm gene cluster (21). In B. pertussis, the LPS is not capped with the O antigen due to the deletion of the wbm cluster (33).
It is intriguing that the rare di-N-acetylated mannuronic acid sugar residue d-ManNAc3NAcA is present in both P. aeruginosa serogroup O2 and in the LPS of B. pertussis. LPS is an important virulence factor of both P. aeruginosa and B. pertussis: in P. aeruginosa, the O antigen has been shown to be involved in protection from phagocytosis (13) and serum-mediated killing (11), and it also plays a role in eliciting a high level of immune response in the host (9). In addition, mutants of P. aeruginosa that lack the O antigen have a 50% lethal dose that is 1,000-fold higher than that of the wild-type organism in an animal model (9). In B. pertussis, the band A trisaccharide prevents clearance of the organism by host surfactant protein A (38) and confers protection to the bacterium from complement-mediated cell lysis (18). Mutant strains of B. pertussis that lack the band A trisaccharide were shown to be defective in colonization of the mouse trachea and nasal cavity (18). Mutants of B. bronchiseptica lacking wild-type LPS showed reduced resistance to oxidative stress and antimicrobial peptides (2, 44).
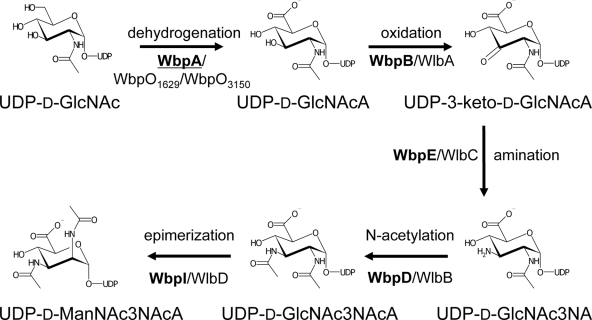
The biosynthesis of UDP-d-ManNAc3NAcA in P. aeruginosa has been studied by use of genetic and biochemical techniques. A five-step biosynthesis pathway involving the sequential catalytic activities of WbpA, WbpB, WbpE, WbpD, and WbpI has been proposed (Fig. 1). Genetic evidence has already been given that the initial enzyme and the last enzymes (encoded by wbpA, wbpD, and wbpI) are essential for B-band O antigen biosynthesis (5, 43). The initial enzyme of the pathway, WbpA, is a 6-dehydrogenase that converts UDP-d-GlcNAc to UDP-N-acetylglucosaminuronic acid (UDP-d-GlcNAcA) (31). Another 6-dehydrogenase, WbpO of P. aeruginosa serotype O6, has 53% similarity to WbpA and has been shown to convert UDP-d-GalNAc to UDP-d-GalNAcA for use in the O antigen but is also capable of converting UDP-d-GlcNAc to UDP-d-GlcNAcA (30, 48). In P. aeruginosa PAK (serotype O6), the WbpO enzyme is required for both O antigen biosynthesis and flagellin glycosylation (30). The B-band O antigen biosynthesis cluster of P. aeruginosa serotype O6 contains wbpO followed by wbpP (3), which encodes a 4-epimerase that can catalyze the reversible conversion of UDP-d-GlcNAc to UDP-d-GalNAc or UDP-d-GlcNAcA to UDP-d-GalNAcA (8, 30). Despite both enzymes being bifunctional, data from kinetic analysis of WbpO and equilibrium analysis of WbpP suggested a preference in vivo for WbpO to work first, converting UDP-d-GlcNAc to UDP-d-GlcNAcA, followed by WbpP, converting UDP-d-GlcNAcA to UDP-d-GalNAcA (30). Thus, homologs of either WbpA or WbpO are theoretically capable of providing the required 6-dehydrogenation of UDP-d-GlcNAc to initiate the UDP-d-ManNAc3NAcA biosynthesis pathway.
FIG. 1.
Proposed biosynthetic pathway for UDP-d-ManNAc3NAcA in P. aeruginosa serogroup O2 and B. pertussis. Protein names are indicated in bold for P. aeruginosa and in normal text for B. pertussis. WbpA is underlined to show that it lacks a B. pertussis homolog within the band A trisaccharide biosynthesis cluster.
The intermediate three steps in the UDP-d-ManNAc3NAcA biosynthesis pathway have been proposed, but functional evidence for the role of the enzymes has yet to be provided. UDP-d-GlcNAcA is thought to be used in an oxidation reaction catalyzed by WbpB, forming UDP-2-acetamido-2-deoxy-d-ribo-hex-3-uluronic acid (UDP-3-keto-d-GlcNAcA), which in turn is the substrate for transamination by WbpE, yielding UDP-2-acetamido-3-amino-2,3-dideoxy-d-glucuronic acid (UDP-d-GlcNAc3NA). This residue is thought to be the substrate for WbpD, a putative N-acetyltransferase, which synthesizes UDP-2,3-diacetamido-2,3-dideoxy-d-glucuronic acid (UDP-d-GlcNAc3NAcA), utilizing acetyl-coenzyme A as a cofactor (43). WbpI is the final enzyme in the pathway and is a known to be a UDP-d-GlcNAc3NAcA 2-epimerase that produces UDP-d-ManNAc3NAcA for incorporation into B-band LPS (46). Each of the required enzymes is encoded in the B-band O-antigen biosynthesis cluster (37).
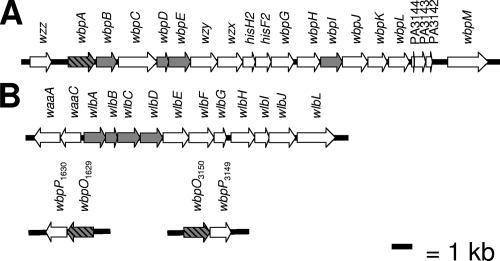
The analogous band A trisaccharide biosynthesis cluster in B. pertussis contains 12 genes, which include homologs of the second to fifth genes encoding enzymes involved in UDP-d-ManNAc3NAcA synthesis in P. aeruginosa (Fig. 2). The missing gene in this cluster is an open reading frame (ORF) encoding a putative UDP-d-GlcNAc 6-dehydrogenase, required for the first step in the pathway. Thus, it was unclear whether synthesis of UDP-d-ManNAc3NAcA in B. pertussis could follow the same pathway as that in P. aeruginosa. Further analysis of the B. pertussis genome sequence led to the identification of two putative dehydrogenases, WbpO1629 and WbpO3150, which were named based on the existing annotation and genomic positions. In this study, we used genetic and biochemical approaches to determine if either or both of the identified wbpO homologs may participate in UDP-d-ManNAc3NAcA biosynthesis in Bordetella pertussis.
FIG. 2.
Comparison of LPS biosynthetic loci from P. aeruginosa and B. pertussis (32, 42). (A) B-band O antigen gene cluster from P. aeruginosa PAO1 (serotype O5). (B) Band A trisaccharide gene cluster from B. pertussis Tohama I, also known as the wlb locus. Initial proposed functions for the genes in these clusters were provided from sequence and mutational analysis of P. aeruginosa and B. pertussis, respectively (4, 35). Genes known or predicted to be involved in the biosynthesis of UDP-d-ManNAc3NAcA are shaded gray; genes known or predicted to encode dehydrogenases are shaded gray with black hatching.
This report documents the complementation of knockout mutants of wbpA (encoding a 6-dehydrogenase), wbpB (encoding a putative oxidase), wbpE (encoding a putative transaminase), wbpD (encoding a putative N-acetyltransferase), and wbpI (encoding a 2-epimerase) derived from P. aeruginosa PAO1 with wbpO1629 and wbpO3150, wlbA, wlbC, wlbB, and wlbD, respectively, of B. pertussis BP536. Each gene was able to restore B-band LPS production to the respective knockout mutant, indicating that each pair has the same function in vivo. The B. pertussis enzymes WbpO1629 and WbpO3150 have not been studied previously, so both were overexpressed and purified for use in reactions with a panel of possible substrates. Both WbpO1629 and WbpO3150 were shown to be 6-dehydrogenases that utilize UDP-d-GlcNAc and UDP-d-GalNAc, like the 6-dehydrogenase of P. aeruginosa serotype O6 (WbpO), but the enzymes of B. pertussis were shown to also have a novel ability to use UDP-d-Glc. Collectively, these results suggest that B. pertussis produces UDP-d-ManNAc3NAcA through the same biochemical pathway proposed for P. aeruginosa, despite differences in the genomic context of the genes involved.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. Bacterial strains were routinely propagated at 37°C in Luria-Bertani (LB) broth (Invitrogen Canada Inc., Burlington, Ontario, Canada). Pseudomonas isolation agar (Difco Becton, Sparks, MD) was used for selecting transconjugants from the mating experiments. The antibiotics in the selection media were used at the following concentrations for Escherichia coli strains: ampicillin, 100 μg/ml; kanamycin, 50 μg/ml; chloramphenicol, 34 μg/ml; gentamicin (Gm), 15 μg/ml; and tetracycline, 15 μg/ml. For P. aeruginosa strains, the following antibiotics were used: carbenicillin, 500 μg/ml; Gm, 300 μg/ml; and tetracycline, 60 μg/ml. Isopropyl-β-d-thiogalactopyranoside (IPTG) was used at 0.4 mM for expression studies. Antibiotics and IPTG were obtained from Sigma-Aldrich (Oakville, Ontario, Canada).
TABLE 1.
Strains and plasmids used for this study
| Strain or plasmid | Relevant characteristics | Reference or source |
|---|---|---|
| Strains | ||
| E. coli strains | ||
| JM109 | General use cloning strain | 47 |
| DH5α | General use cloning strain | Life Technologies, Inc. |
| SM10 | Mating donation strain | 41 |
| B. pertussis strains | ||
| BP536 | Smr derivative of Tohama I | 36 |
| P. aeruginosa strains | ||
| PAO1 | Serotype O5; P. aeruginosa type strain | 17 |
| KEP-A3 | wbpA knockout in PAO1; Gmr | 5 |
| ELW-B1 | wbpB knockout in PAO1; Gmr | This study |
| ELW-E100 | wbpE knockout in PAO1; Gmr | This study |
| CQW47 | wbpD knockout in PAO1; Gmr | 43 |
| KEP-I9 | wbpI knockout in PAO1; Gmr | 5 |
| Plasmids | ||
| pPS856 | FRT cassette vector including Gmr determinant | 20 |
| pUCP26 | pUC18-derived broad-host-range cloning vector | 45 |
| pUCP27 | pUC19-derived broad-host-range cloning vector (opposite multiple cloning site) | 45 |
| pCR-Blunt II-TOPO | Efficient one-step ligase-free cloning vector | Invitrogen, Inc. |
| pCQW14 | His6-WbpA in pET28a | 31 |
| p27-His6-WbpA | His6-WbpA in pUCP26 (XbaI/XhoI fragment from pCQW14 subcloned into XbaI/SalI sites of pUCP27) | This study |
| pET-His6-WbpB | His6-WbpB in pET28a (BamHI/EcoRI fragment from PCR) | This study |
| pTOPO-WbpB-His6 | wbpB without stop codon, cloned into pCR-Blunt II-TOPO after PCR with NcoI/XhoI sites in primers | This study |
| pET-WbpB-His6 | wbpB without stop codon, NcoI/XhoI fragment from TOPO-WbpB-His6 subcloned into pET28a | This study |
| p27-His6-WbpB | His6-WbpB in pUCP27 (XbaI/HindIII fragment from pET-His6-WbpB) | This study |
| p27-WbpB-His6 | WbpB-His6 in pUCP27 (PCR on pET-WbpB-His6 with XbaI/HindIII sites in primers, product ligated to XbaI/SalI sites of pUCP27) | This study |
| pWMJL085 | His6-WbpE in pET23dr (NcoI/BamHI fragment from PCR) | Wayne L. Miller (unpublished work) |
| p26-His6-WbpE | His6-WbpE in pUCP26 (XbaI/BamHI fragment from pWMJL085) | This study |
| pCQW13 | His6-WbpD in pUCP26 | 43 |
| pWMJL072 | His6-WbpI in pET23dr (AflIII/BamHI fragment from PCR ligated to NcoI/BamHI sites of pET23dr) | 46 |
| p26-His6-WbpI | His6-WbpI in pUCP26 (XbaI/BamHI fragment from pET23dr-wbpI) | This study |
| p27-WlbC | wlbC in pUCP27 (BamHI/EcoRI fragment from PCR) | This study |
| p26-WlbB | wlbB in pUCP26 (EcoRI/BamHI fragment from PCR) | This study |
| pEWJL201 | His6-WlbD in pET23dr (NcoI/BamHI fragment from PCR) | 46 |
| p26-His6-WlbD | His6-WlbD in pUCP26 (XbaI/BamHI fragment from pEWJL201) | This study |
| BbLPS1 | Bordetella bronchiseptica cosmid containing wlb cluster | 33 |
| p26-WlbA | wlbA in pUCP26 (EcoRI/BamHI fragment from PCR on BBLPS1) | This study |
| pET-His6-WbpO1629 | His6-WbpO1629 in pET28a (NdeI/EcoRI fragment from PCR) | This study |
| p27-His6-WbpO1629 | His6-WbpO1629 in pUCP27 (XbaI/EcoRI fragment from pET-His6-WbpO1629) | This study |
| pET-His6-WbpO3150 | His6-WbpO3150 in pET28a (NdeI/EcoRI fragment from PCR) | This study |
| p27-His6-WbpO3150 | His6-WbpO3150 in pUCP27 (XbaI/EcoRI fragment from pET-His6-WbpO3150) | This study |
General DNA methods.
Oligonucleotide primers were prepared by the Laboratory Services Division of the University of Guelph (Guelph, Ontario, Canada) or by Sigma-Genosys Canada (Oakville, Ontario, Canada). Sequences of all primers used and a listing of PCR cycling conditions are available on request. Restriction enzymes and T4 DNA ligase were from Invitrogen (Burlington, Ontario, Canada), except for XbaI from New England Biolabs (Pickering, Ontario, Canada). All enzymes were used according to the manufacturers' instructions. After ligation, plasmids were transformed into E. coli JM109 or DH5α by chemical transformation. For complementation, plasmid DNA was introduced into P. aeruginosa by chemical transformation according to the benchtop method (7). All constructs were verified by restriction digestion and/or nucleotide sequencing at the Laboratory Services Division of the University of Guelph.
Generation of nonpolar knockout mutants.
Null mutants of wbpA, wbpD, and wbpI in the P. aeruginosa PAO1 background were previously constructed by our group (5, 43). Knockout mutants of wbpB and wbpE were constructed using the allelic replacement method described by Schweizer and Hoang (40). Briefly, knockout constructs of wbpB and wbpE were generated by insertional mutagenesis with a Gm resistance cassette. For the wbpB knockout, the gene was amplified from P. aeruginosa PAO1 genomic DNA, using primers that incorporate a BamHI site and an EcoRI site, and the resultant product was ligated into pET-28a (Novagen, Mississauga, Ontario, Canada). The wbpB gene was disrupted by insertion of the Gmr cassette from PstI-digested pPS856 into the two internal PstI sites. The wbpB::Gm region was PCR amplified using KOD polymerase according to the manufacturer's directions (Novagen), using primers that introduced HindIII sites at each end. After PCR, the product was purified using a HighPure PCR purification kit (Roche) and digested with HindIII. The HindIII-digested wbpB::Gm construct was ligated into HindIII-digested pEX18Ap (20). Clones were confirmed by restriction digestion and then transformed into E. coli SM10 for introduction into P. aeruginosa PAO1 by conjugal transfer. Conjugal transfer and phenotypic selection were carried out according to the methods described by Wenzel et al. (43). For the wbpE knockout, wbpE was first cloned into an expression vector and then subcloned into a suicide vector for use in knockout construction. The wbpE gene from P. aeruginosa PAO1 was amplified from genomic DNA by PCR, using Pwo polymerase according to the method supplied by the manufacturer (Roche Diagnostics, Laval, Quebec, Canada). Both the wbpE PCR product and pET-23dr were digested with NcoI and BamHI and ligated overnight at 15°C, using T4 DNA ligase (NEB). The resulting expression construct, pWMJL085, was used as a template for a PCR using primers BADE5 and BADE3. The PCR product was then ligated into pBAD24 via XbaI and SphI sites. The Gmr cassette from pUCGm was removed via SmaI and blunt end ligated into the MscI site of wbpE, generating pBAD24-wbpE::Gm. The disrupted wbpE::Gm construct was excised through digestion by XbaI and HindIII and ligated to XbaI/HindIII-digested pEX18Ap. The final construct, pEX18Ap-wbpE::Gm, was introduced into P. aeruginosa PAO1 by conjugal transfer and selection as previously described for the wbpB knockout. Successful knockouts of each gene were confirmed by a Gmr Cbs phenotype, correct PCR fragment size, silver staining of LPS, and Western blotting with the anti-B-band monoclonal antibody (MAb) MF15-4.
Assignment of B. pertussis gene functions.
The amino acid sequences of the five enzymes of P. aeruginosa proposed to be involved in UDP-d-ManNAc3NAcA biosynthesis were used to query the Sanger database of predicted B. pertussis proteins. The top-scoring hits were considered in combination with the locations of the genes, with preference given to those in the band A trisaccharide biosynthesis cluster. Assignment of homologous pairs of enzymes from P. aeruginosa and B. pertussis, using the NCBI BLAST 2 Sequences tool (http://blast.ncbi.nlm.nih.gov/bl2seq/wblast2.cgi), formed the hypotheses to be tested by cross-complementation analysis. Homologs of all genes selected for analysis from B. pertussis are also present in B. bronchiseptica and B. parapertussis.
Construction of complementation vectors.
The complementation vectors used were pUCP26 and pUCP27, which differ only in the orientation of the multiple cloning site. If available, the P. aeruginosa genes were subcloned from existing pET-28a or pET-23dr expression constructs.
(i) Dehydrogenases.
Plasmid pCQW14 (31) was used to subclone His6-WbpA via XbaI-XhoI ligated to XbaI-SalI of pUCP27 to create p27-His6-WbpA. The WbpA gene homologs from B. pertussis, wbpO3150 and wbpO1629, were amplified from a boiled lysate of B. pertussis BP536 by use of KOD polymerase according to the manufacturer's directions (Novagen). wbpO PCR products purified by gel excision were digested and ligated via NdeI-EcoRI into pET-28a to yield the expression constructs. 28a-His6-WbpO3150 was then digested with XbaI and EcoRI to liberate the His6-WbpO3150 fragment, which was ligated into XbaI-EcoRI-digested pUCP27. 28a-His6-WbpO1629 was digested with XbaI and HindIII to liberate the His6-WbpO1629 fragment, which was ligated into XbaI-HindIII-digested pUCP26.
(ii) Oxidases.
The wbpB gene was PCR amplified from PAO1 chromosomal DNA by using primers that incorporated BamHI and EcoRI restriction sites, using Pwo polymerase according to the manufacturer's instructions (Invitrogen). The wbpB PCR product was cleaned and ligated into pET-28a via BamHI-EcoRI sites to yield pET-His6-WbpB, an expression vector for His6-WbpB. For complementation, pET-His6-WbpB was digested with XbaI and HindIII, and the liberated fragment was ligated into pUCP27. A C-terminally polyhistidine-tagged construct was also generated by amplification of wbpB without a stop codon from chromosomal DNA, using KOD polymerase according to the manufacturer's directions (Novagen). The PCR product was then TOPO cloned with a pCR-Blunt-II-TOPO kit (Invitrogen) according to the manufacturer's protocol. wbpB was then subcloned via NcoI and XhoI sites in the primers and ligated into pET-28a, generating pET-WbpB-His6. For the complementation construct, PCR was performed on pET-WbpB-His6, with XbaI and HindIII sites in the primers. KOD polymerase was used according to the manufacturer's directions (Novagen). The digested PCR product was ligated to XbaI-SalI-digested pUCP27. The WbpB gene homolog from B. pertussis, wlbA, was amplified from the cosmid BbLPS1, which contains the band A trisaccharide biosynthesis cluster from B. bronchiseptica (32, 33). Use of BbLPS1 allowed chromosomal PCR problems to be resolved, and the sequence of wlbA from B. pertussis was identical to that from B. bronchiseptica. KOD polymerase was used according to the manufacturer's directions (Novagen), and after PCR, the wlbA band was purified by gel excision, using a HighPure PCR purification kit (Roche). The purified wlbA PCR product was digested using the EcoRI and BamHI restriction sites on the primers and then ligated into pUCP26. Clones were confirmed by DNA sequencing.
(iii) Transaminases.
pWMJL085 was digested with XbaI and BamHI to liberate the His6-WbpE insert; this was then ligated into pUCP26. The WbpE gene homolog from B. pertussis, wlbC, was amplified from a boiled lysate of B. pertussis BP536 by use of KOD polymerase according to the manufacturer's directions (Novagen). After PCR, the wlbC band was purified by gel excision, and the purified wlbC PCR product was ligated into pUCP27 via BamHI and EcoRI sites.
(iv) N-Acetyltransferases.
pCQW13, which encodes His6-WbpD in pUCP26, has been described previously (43). The homologous gene wlbB was amplified from a boiled lysate of B. pertussis BP536 by use of KOD polymerase according to the manufacturer's directions (Novagen). Using the EcoRI and BamHI restriction sites on the primers, wlbB was digested and ligated into pUCP26.
(v) Epimerases.
p26-His6-WbpI and p26-His6-WlbD were subcloned from previously described expression constructs in pET-23dr (46). The affinity-tagged genes were liberated, ligated to pUCP26 via XbaI and BamHI sites, and verified by restriction digestion.
Preparation and analysis of LPS.
Small-scale LPS preparation was performed by the method of Hitchcock and Brown (19). LPS was separated by using 12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (25) or 4 to 12% Bis-Tris NuPage gels (Invitrogen) and was visualized by an ultrafast silver staining method (14). Western immunoblotting was performed according to a standard procedure used in our laboratory, and the blots were probed using MAb MF15-4, specific for B-band O antigen (26). The secondary antibody was alkaline phosphatase-conjugated goat anti-mouse immunoglobulin G (Jackson Immunoresearch). Blots were developed according to standard protocols for colorimetric detection (Qiagen, Mississauga, Ontario, Canada).
Protein expression and purification.
Expression clones were transformed into E. coli BL21(DE3) and induced using 1 mM IPTG for 3 h at 37°C. Cultures (250 ml) were harvested by centrifugation at 5,000 × g for 10 min in an Avanti J25I centrifuge (Beckman Coulter Canada, Mississauga, Ontario, Canada), and purification was carried out according to the protocol established for His6-WbpA (31). Proteins separated by SDS-PAGE were visualized by staining with Coomassie brilliant blue R-250 (Sigma-Aldrich). Proteins were quantitated by spectrophotometric assay, using the formula A280 = ɛCl, where ɛ is 37,820 M−1cm−1, as predicted by ProtParam software (15), and Cl is molar concentration and path length (in cm).
Dehydrogenase reactions and CE analyses.
Dehydrogenase reactions were similar to those used for His6-WbpA (31), with mixtures consisting of 2.5 mM NAD+, 100 mM Tris-HCl buffer, pH 7.5, 100 mM ammonium sulfate, 1 mM substrate, and 10.5 μg of enzyme in a 35-μl volume. UDP-d-GlcNAc, UDP-d-GalNAc, UDP-d-Glc, and UDP-d-Gal were obtained from Sigma-Aldrich and tested individually as potential substrates. TDP-d-Glc was produced from TTP and glucose-1-phosphate through reaction with RmlA and RmlB and then verified by mass spectrometry (MS) analysis (unpublished data). Reactions were allowed to proceed for 4 h at 37°C and were then stopped by freezing at −20°C until capillary electrophoresis (CE) analysis. For CE analysis, 35 μl of water was added to each reaction mix, and the samples were centrifuged for 5 min at 12,000 × g at room temperature before 60-μl aliquots were loaded into a P/ACE MDQ glycoprotein system (Beckman Coulter). The capillary was bare silica (75 μm by 60 cm, with a detector at 50 cm), and the running buffer was 25 mM sodium tetraborate, pH 9.5. The capillary was conditioned at the start of each run with 1 M sodium hydroxide for 2 min, followed by running buffer for 2 min. Samples were introduced by pressure injection for 16 s, and separation was performed at 22 kV. Detection was done by measuring UV absorbance, monitored at 254 nm. Electropherograms were analyzed using Beckman 32 Karat software and SigmaPlot. Reactions were performed in triplicate, and results were averaged to calculate the percent conversion for each enzyme with each substrate. For comparison to theoretical reaction products, known standards were obtained from Sigma-Aldrich (UDP-d-GlcA) or through enzymatic synthesis using His6-WbpA or His6-WbpO according to established methods (UDP-d-GlcNAcA and UDP-d-GalNAcA) (30).
MS.
For MS, unpurified dehydrogenase reaction mixtures were mixed directly with matrix solution (2 mg of 3,4-dihydroxybenzoic acid in 20% ethanol) at an analyte-to-matrix ratio of 1:2 (vol/vol), and 1 μl was spotted on the matrix-assisted laser desorption ionization (MALDI) sample target and allowed to dry at room temperature. In some cases, good crystal formation was not achieved and the spots on the MALDI plate looked shiny; in these cases, the sample spots were covered by an additional 1 μl of matrix solution. After the samples were dried at room temperature, crystals were forming on the edges of the sample spots. Proteins were present in the reaction mixtures with UDP-d-Glc and UDP-d-GlcNAc but were removed by filtration using a Microcon YM-10 centrifugal filter device (Millipore Corporation, Bedford, MA) for the UDP-d-GalNAc sample. Analysis of the total reaction mixtures was performed using a MALDI-time-of-flight MS instrument (model Reflex III; Bruker, Germany) equipped with a 337-nm nitrogen laser (Biological Mass Spectrometry Facility, University of Guelph). Samples were analyzed in reflectron and negative-ion modes, scanning from 0 to 1,000 m/z and using ion suppression of up to 150 m/z. For all experiments, ion sources 1 and 2 were held at 20 kV and 16.35 kV, respectively, and guiding lens voltage was held at 9.75 kV. The reflector detection gain was set up at 5.3, with pulsed ion extraction at 200 ns. The nitrogen laser power was set to the minimum level necessary to generate a reasonable signal and to avoid possible degradation of analytes. Typically, 15 to 25% of laser energy was used. Two-point external calibration was performed, using the [M − H]− (223.06 Da) and [2M − H]− (447.12 Da) peaks of sinapinic acid (SA; Sigma-Aldrich) prepared in acetonitrile-water solution (1 mg SA in 100 μl of H2O-CH3CN [1:1]). The mass accuracy with external calibration using SA was estimated to be below 5 ppm.
RESULTS
Functional prediction of B. pertussis genes required for UDP-d-ManNAc3NAcA biosynthesis.
Based on the similarities of the predicted amino acid sequences to those of P. aeruginosa sequences, six ORFs were assigned putative functions. The wlb locus, known to be involved in band A trisaccharide biosynthesis in Bordetella (1), encodes the putative oxidase (wlbA), N-acetyltransferase (wlbB), and transaminase (wlbC) as well as the previously characterized UDP-d-GlcNAc3NAcA 2-epimerase (wlbD) (46). WlbA has 40% similarity to WbpB of P. aeruginosa, WlbB shares 85% similarity with WbpD, WlbC has 74% similarity to WbpE, and WlbD shares 70% similarity with WbpI. ORFs encoding proteins with similarity to WbpO and WbpA dehydrogenases from P. aeruginosa were not found inside the band A trisaccharide biosynthesis cluster but, rather, elsewhere in the chromosome (Fig. 2). Two ORFs, BP1629 and BP3150, were found in separate loci that may be involved in polysaccharide biosynthesis; BP1629 is in a putative capsule locus, while BP3150 is part of a cryptic locus. Both ORFs are adjacent to genes encoding putative homologs of WbpP, the 4-epimerase that works with the WbpO dehydrogenase in P. aeruginosa serotype O6. BP1630 and BP3149, the putative wbpP genes from B. pertussis, encode proteins that are 100% identical to each other and 80% similar to WbpP of P. aeruginosa serotype O6. The putative dehydrogenases, BP1629 and BP3150, share 90% similarity to WbpO of P. aeruginosa (WbpOO6) and 54% similarity to WbpA, contain conserved domains of UDP-glucose/GDP-mannose dehydrogenase families, and are 99% identical to each other. For convenience, the genes were therefore called wbpO1629 and wbpO3150. The sequence differences between WbpO1629 and WbpO3150 are restricted to the N terminus of the proteins: WbpO1629 has five additional amino acids relative to WbpO3150, but after the initial 63 bp, the remaining sequences were identical even at the DNA level. Although the predicted Rossmann fold for predicted NAD(P) binding is close to the N terminus, the GXGXXG motif is located at amino acids 17 to 23 of WbpO1629 and 12 to 17 of WbpO3150, so the differences between the two proteins do not seem to affect any structural features. Both WbpO1629 and WbpO3150 were targeted for further characterization to demonstrate that they are dehydrogenases and to investigate if their functional properties are more like those of WbpO of P. aeruginosa serotype O6 or WbpA of P. aeruginosa serotype O5.
Complementation of P. aeruginosa knockout constructs with corresponding wild-type genes.
Knockouts of wbpB and wbpE were transformed with plasmids encoding His6-WbpB, WbpB-His6, or His6-WbpE. B-band O antigen was detected in each strain after transformation, but the empty vector alone could not restore B-band production (data not shown). A wbpA knockout strain was previously complemented by wbpA (5) and was complemented here with plasmid-encoded His6-WbpA (Fig. 3). Complementation of wbpD and wbpI knockouts with wbpD and wbpI was also previously reported (5, 43, 46), and these strains can also be complemented with N-terminally histidine-tagged fusions (data not shown).
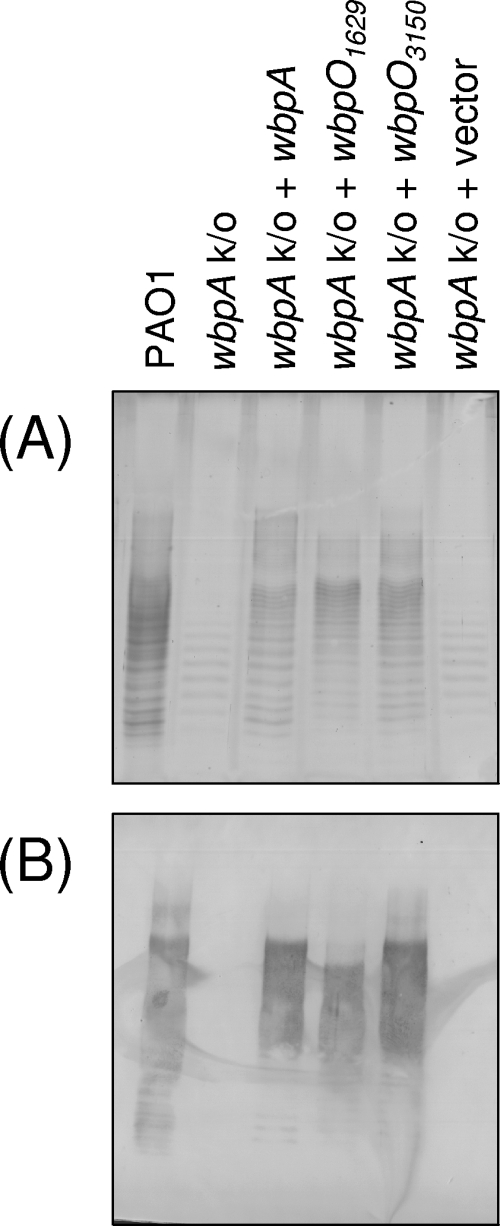
FIG. 3.
Cross-complementation of wbpA knockouts in P. aeruginosa PAO1 with plasmid-encoded His6-WbpA or either His6-WbpO1629 or His6-WbpO3150 from B. pertussis. (A) Silver-stained SDS-PAGE gel; (B) Western blot with anti-B-band O antigen MAbs. B-band O antigen was not detected in the wbpA knockout strain or the knockout transformed with empty vector; B-band O antigen was detected when the knockout strains were complemented with plasmid-encoded His6-WbpA, His6-WbpO1629, or His6-WbpO3150.
Cross-complementation of putative B. pertussis UDP-d-GlcNAc dehydrogenases.
SDS-PAGE was used to separate the LPS and was allowed to run for an extended period to improve the resolution of the B-band O antigen bands. The control P. aeruginosa PAO1 LPS revealed a ladder-like banding pattern that is known to represent individual LPS species with different numbers of O antigen repeats (Fig. 3). Because of the extended running time for SDS-PAGE, the LPS core bands are not visible on the gel. The wbpA knockout of P. aeruginosa PAO1 is devoid of B-band O antigen but still produces A-band O antigen in a variety of lengths. A-band O antigen bands were present on the silver-stained SDS-PAGE gel but were not reactive with the B-band-specific MAb MF15-4 in a Western blot (Fig. 3). B-band O antigen was restored when His6-WbpA was expressed in trans, as tested by LPS silver staining and Western blotting with MAb MF15-4 (Fig. 3). No differences were detected in the modality of the A-band or B-band O antigens. The hexahistidine tag did not interfere with the in vivo function of His6-WbpO1629 or His6-WbpO3150, since the plasmid-carried His-tagged genes were capable of restoring B-band O antigen production in the wbpA mutant (Fig. 3). As expected, the empty vector control was not able to complement the B-band O antigen deficiency in the wbpA mutant (Fig. 3).
Cross-complementation of B. pertussis band A biosynthesis cluster members.
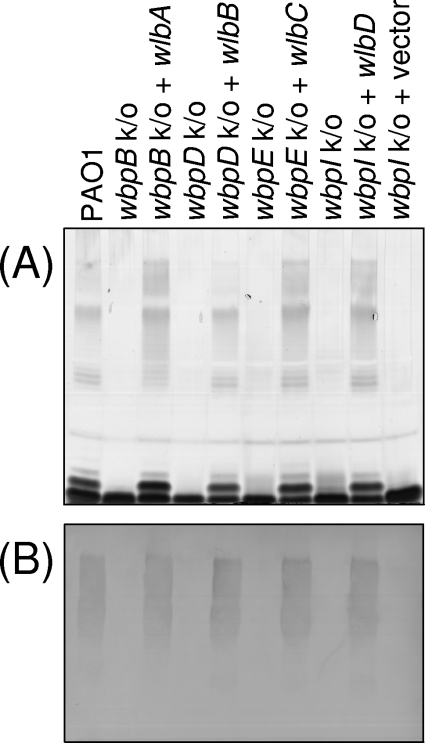
Silver-stained SDS-PAGE analysis of wild-type PAO1 LPS showed two dark bands of low molecular weight (Fig. 4). These are known as the core and core-plus-one O-antigen repeat unit, based on previous findings of Western blotting with anti-core and anti-core-plus-one MAbs (data not shown). In addition, a range of high-molecular-weight molecules are present, which are the LPS bands containing A- or B-band O antigen polymers. Knockouts of the P. aeruginosa PAO1 genes wbpB, wbpE, wbpD, and wbpI were expected to abrogate the functions of the oxidase, transaminase, N-acetyltransferase, and epimerase, respectively. In comparison to strain PAO1, each individual knockout construct lacked B-band O antigen, as determined by the lack of reactivity to MAb MF15-4 (B band specific) in the Western immunoblot (Fig. 4). In addition, the core-plus-one O antigen band was absent in the LPS of each of the knockout strains (Fig. 4). As expected, an empty vector control did not alter the LPS profile (Fig. 4). When copies of the putative oxidase gene (wlbA), putative transaminase gene (wlbC), putative N-acetyltransferase gene (wlbB), and established 2-epimerase gene (wlbD) from B. pertussis were individually provided on plasmids to P. aeruginosa oxidase (wbpB), transaminase (wbpE), N-acetyltransferase (wbpD), and epimerase (wbpI) knockout strains, respectively, the production of B-band O antigen was restored (Fig. 4).
FIG. 4.
Cross-complementation of various wbp knockouts in P. aeruginosa PAO1 by homologs from the wlb locus of B. pertussis. (A) Silver-stained SDS-PAGE gel; (B) Western blot with anti-B-band O antigen MAbs. B-band O antigen was not detected in the knockout strains or knockout strains transformed with an empty vector; B-band O antigen was detected when the knockout strains were complemented with the relevant B. pertussis gene.
Purification of recombinant dehydrogenases.
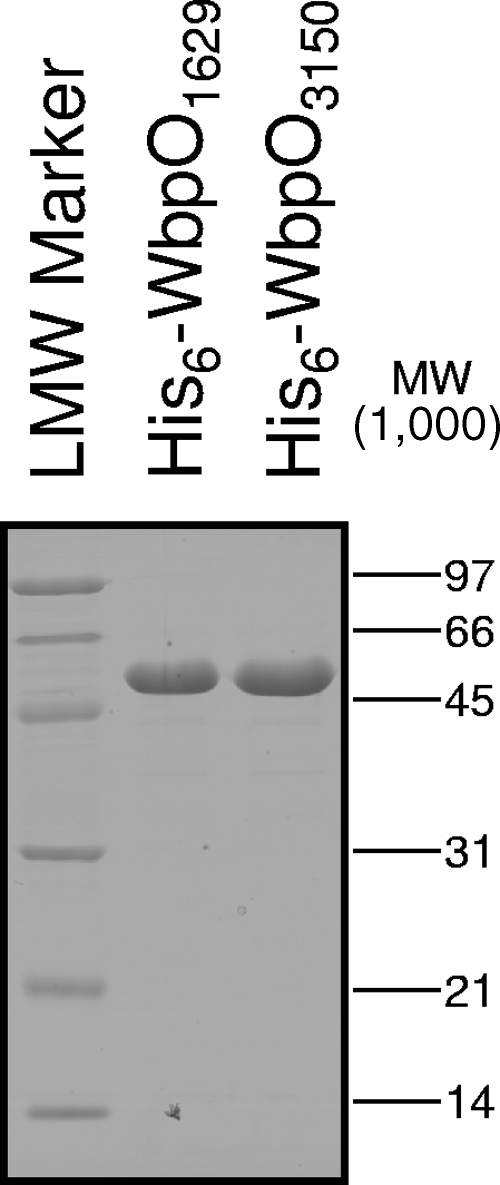
Milligram quantities of His6-WbpO1629 and His6-WbpO3150 were obtained after affinity chromatography and buffer exchange, at >98% purity, as judged by SDS-PAGE and densitometry analysis (Fig. 5). His6-WbpO1629 and His6-WbpO3150 are predicted to have molecular weights of 49,388 and 49,007, respectively, and the apparent molecular weights are in agreement with these estimates (Fig. 5).
FIG. 5.
SDS-PAGE analysis of purified dehydrogenase proteins after Ni+-affinity chromatography and buffer exchange according to the work of Miller et al. (31). Proteins were expressed with a hexahistidine tag from pET-28a. All proteins were free of contamination.
CE of dehydrogenase-substrate reactions.
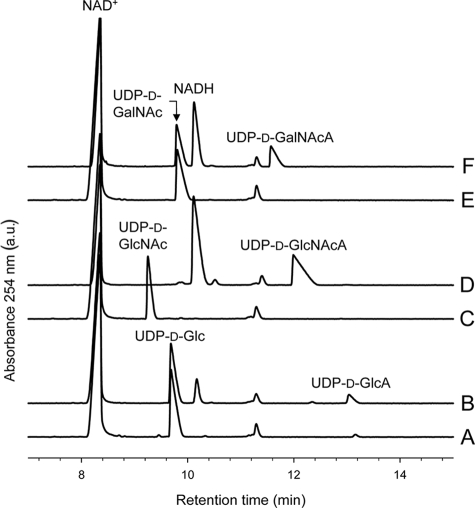
Control reaction mixtures lacking enzyme resolved three peaks in each case; the two peaks which were consistent among all traces were identified as NAD+ and a minor contaminant present with NAD+, while the third peak was identified as the sugar-nucleotide substrate based on the use of reagent standards (Fig. 6). Reaction mixtures containing WbpO1629 or WbpO3150 and UDP-d-Glc, UDP-d-GlcNAc, or UDP-d-GalNAc showed two new large peaks that were not present in the controls. One of these peaks was found for the reactions with all of the substrates and was identified as NADH (Fig. 6). The remaining unique peaks found in reactions using UDP-d-Glc, UDP-d-GlcNAc, or UDP-d-GalNAc were identified as UDP-d-GlcA, UDP-d-GlcNAcA, or UDP-d-GalNAcA, respectively, after addition of the relevant UDP-hexuronic acid standard. The percent conversion of UDP-d-GlcNAc or UDP-d-GalNAc to UDP-d-GlcNAcA or UDP-d-GalNAcA, respectively, was not significantly different between WbpO1629 and WbpO3150 (t test; α = 0.05). Conversely, the percent conversion of UDP-d-Glc to UDP-d-GlcA was significantly lower for WbpO1629 than for WbpO3150 (t test; α = 0.05). No reaction product peak could be discerned when either TDP-d-Glc or UDP-d-Gal was used as the substrate for reaction with WbpO1629 or WbpO3150 (Table 2).
FIG. 6.
Electropherograms from CE of dehydrogenase reactions. (A) Control reaction mix (no enzyme) with NAD+ and UDP-d-Glc. (B) Reaction with His6-WbpO3150, NAD+, and UDP-d-Glc yielded UDP-d-GlcA. (C) Control reaction mix with NAD+ and UDP-d-GlcNAc. (D) Reaction with His6-WbpO3150, NAD+, and UDP-d-GlcNAc yielded UDP-d-GlcNAcA. The same reaction is catalyzed by His6-WbpA, as previously shown by Miller et al. (31). (E) Control reaction mix with NAD+ and UDP-d-GalNAc. (F) Reaction with His6-WbpO3150, NAD+, and UDP-d-GalNAc yielded UDP-d-GalNAcA. The same reaction is catalyzed by P. aeruginosa His6-WbpO, as previously shown by Miller et al. (30).
TABLE 2.
Percent conversion from substrate to product for WbpO1629 and WbpO3150 reactions analyzed by CE
| Substrate | Enzyme | % Conversiona |
|---|---|---|
| UDP-d-Glc | WbpO1629 | 5.4 ± 1.8 |
| WbpO3150 | 13.1 ± 2.1 | |
| TDP-d-Glc | WbpO1629 | ND |
| WbpO3150 | ND | |
| UDP-d-GlcNAc | WbpO1629 | 100 |
| WbpO3150 | 95.8 ± 7.3 | |
| UDP-d-Gal | WbpO1629 | ND |
| WbpO3150 | ND | |
| UDP-d-GalNAc | WbpO1629 | 28.7 ± 2.2 |
| WbpO3150 | 37.4 ± 6.0 |
ND, none detected. Data are means ± standard deviations.
MS of dehydrogenase reactions.
Reactions used for CE analysis were further analyzed by MS. Control reaction mixtures lacking enzyme were found to contain ions of either 565.39 m/z or 606.40 m/z (in negative-ion mode), consistent with the expected mass of UDP-d-Glc or UDP-d-GlcNAc/UDP-d-GalNAc, respectively (the epimers UDP-d-GlcNAc and UDP-d-GalNAc have the same expected mass). Reactions with His6-WbpO3150 and UDP-d-Glc present showed the presence of ions consistent with both UDP-d-Glc (665.50 m/z) and UDP-d-GlcA (579.48 m/z). Reactions with His6-WbpO3150 and UDP-d-GlcNAc or UDP-d-GalNAc present showed the presence of ions consistent with the expected mass of UDP-d-GlcNAcA or UDP-d-GalNAcA (620.35 m/z), but unreacted substrate (606.39 m/z) only was detected in reactions with UDP-d-GalNAc. This indicates that the reaction with UDP-d-GlcNAc was the only one to go to completion and that His6-WbpO3150 catalyzes the production of UDP-d-GlcA, UDP-d-GlcNAcA, or UDP-d-GalNAcA.
DISCUSSION
In P. aeruginosa, five genes are predicted to be required for the production of UDP-d-ManNAc3NAcA, including wbpA, wbpB, wbpE, wbpD, and wbpI. The evidence presented here demonstrates that B-band O antigen is not produced when wbpB or wbpE is knocked out in P. aeruginosa (Fig. 4), consistent with a deficiency in UDP-d-ManNAc3NAcA synthesis. Thus, together with prior studies, genetic evidence has been obtained to show that all five members of the proposed pathway are essential for the production of UDP-d-ManNAc3NAcA in P. aeruginosa PAO1. Complementation of wbpB and wbpE knockouts with His-tagged versions of the wbpB and wbpE gene products have established that His6-WbpB and His6-WbpE are functional (data not shown) and can be used for further biochemical studies.
By constructing a complete set of knockouts in the genes that encode enzymes for the UDP-d-ManNAc3NAcA biosynthetic pathway, we made a unique set of tools whereby the function of the corresponding homologs from B. pertussis could be tested through pairwise cross-complementation. Initial assignments of B. pertussis genes to particular pathway steps, which was based on alignment comparison of the gene products with those encoded by the wbp cluster of P. aeruginosa PAO1, were supported by the ability of each B. pertussis gene to cross-complement the relevant wbp knockout (Fig. 3 and 4). This genetic evidence suggests that wbpO1629 and wbpO3150 encode UDP-d-GlcNAc 6-dehydrogenases, wlbA encodes a UDP-d-GlcNAcA 3-oxidase, wlbC encodes a UDP-d-3-keto-GlcNAcA 3-aminotransferase, wlbB encodes a UDP-d-GlcNAc3NA 3-N-acetyltransferase, and wlbD encodes a UDP-d-GlcNAc3NAcA 2-epimerase. The discovery of functionally identical B. pertussis homologs to the P. aeruginosa UDP-d-ManNAc3NAcA biosynthesis enzymes suggests that UDP-d-ManNAc3NAcA production occurs through the same enzymatic steps in B. pertussis.
The presence of functional homologs encoding putative UDP-d-ManNAc3NAcA biosynthesis enzymes in P. aeruginosa and B. pertussis (Fig. 2) raises questions about the evolutionary conservation of the proposed pathway. P. aeruginosa and B. pertussis are members of the Proteobacteria, belonging to the Gammaproteobacteria and Betaproteobacteria, respectively. Homologs of each of these five genes can also be identified in several other proteobacteria, including Marinomonas sp. (Gammaproteobacteria), Polaromonas naphthalenivorans (Betaproteobacteria), and Wolinella succinogenes (Epsilonproteobacteria). A subgroup contains homologs to wbpABED but not wbpI (which encodes a UDP-d-GlcNAc3NAcA 2-epimerase), suggesting that UDP-d-GlcNAc3NAcA may be produced; these include Roseovarius nubinhibens (Alphaproteobacteria) and Ralstonia eutropha (Betaproteobacteria). In many cases, the genes homologous to those of the UDP-d-ManNAc3NAcA biosynthesis cluster from P. aeruginosa PAO1 are also found in clusters. Unfortunately, little is known about the composition of the LPS in these gram-negative bacteria at this time, although evidence has been given for the presence of mannose-derived residues in the O antigens of W. succinogenes and Wolinella recta (16, 24). Interestingly, evidence has been given for the presence of ManNAc3NAcA in the gram-positive bacterium Geobacillus stearothermophilus (29, 39), which belongs to the Firmicutes. Sequencing of Geobacillus stearothermophilus is in progress at the University of Oklahoma, and the use of tBLASTn searches (http://www.genome.ou.edu/blast/bstearo_blastall.html) indicates that homologs of all five UDP-d-ManNAc3NAcA genes are present [B. Roe, S. Lewis, B. Perry, F. Najar, and R. Morales-Diaz, Bacillus (Geobacillus) stearothermophilus genome sequencing project]. Although further work is required, we suggest that the proposed pathway for UDP-d-ManNAc3NAcA biosynthesis may be highly conserved among this broad range of bacteria. In future studies, the findings of this study can be exploited to predict the functions of the genes required for UDP-d-ManNAc3NAcA biosynthesis in organisms known to produce ManNAc3NAcA.
CE analysis showed that WbpO1629 and WbpO3150 are promiscuous 6-dehydrogenases, capable of utilizing UDP-d-GlcNAc, UDP-d-GalNAc, and UDP-d-Glc as substrates, but not TDP-d-Glc or UDP-d-Gal (Fig. 3 and Table 2). MS analysis of reactions confirmed the production of UDP-d-GlcNAcA, UDP-d-GalNAcA, and UDP-d-GlcA by use of His6-WbpO3150, but MS was not used to analyze His6-WbpO1629 reactions, since CE had demonstrated the products of both dehydrogenases to be identical. The two WbpO enzymes from Bordetella have higher similarity at the amino acid level to the UDP-d-GlcNAc/UDP-d-GalNAc 6-dehydrogenase WbpO of P. aeruginosa serotype O6 than to the UDP-d-GlcNAc 6-dehydrogenase WbpA of P. aeruginosa serotype O5; the ability of the B. pertussis enzymes to use both UDP-d-GlcNAc and UDP-d-GalNAc further supports the annotation with the gene name wbpO. WbpA acts as the first enzyme in the UDP-d-ManNAc3NAcA pathway that we have presented, and it is clear that the B. pertussis WbpO enzymes can provide the same activity, since they complement the wbpA knockout and can catalyze UDP-d-GlcNAc 6-dehydrogenation in vitro. It is interesting that WbpO1629 and WbpO3150 can use UDP-d-Glc as a substrate, because neither of the homologs from P. aeruginosa, WbpA and WbpO, has this ability. This is a novel finding because although other UDP-d-Glc 6-dehydrogenases are known, to our knowledge these enzymes are the first that have been shown to utilize UDP-d-GlcNAc, UDP-d-GalNAc, and UDP-d-Glc as substrates. Although the percent conversion of UDP-d-Glc to UDP-d-GlcA was significantly lower for WbpO1629 than for WbpO3150, both values were <15% conversion, so the difference may not be physiologically relevant. Also of biochemical interest is the fact that TDP-d-Glc was not utilized, suggesting that both enzymes can discriminate between nucleotides, despite their somewhat loose specificity for different sugars.
A remaining question surrounds the presence of the two copies of wbpO in B. pertussis. This study has provided evidence that the products of the two wbpO copies can convert the same substrates. However, the distinct locations in separate putative polysaccharide biosynthesis loci suggest that each wbpO gene might be involved in the production of different polysaccharide molecules. Two identically functioning copies of wbpO might be important to meet the many demands for 6-dehydrogenation that the cell requires.
The presence of neighboring wbpO and wbpP homologs in two separate loci is conserved in the related species B. bronchiseptica and B. parapertussis. In B. pertussis, UDP-d-ManNAc3NAcA is used for the production of the band A trisaccharide, but in B. bronchiseptica and B. parapertussis, ManNAc3NAcA is also found in the O antigen linker (34). The O antigen linker also contains ManNAc3NAcAN, a uronamide derivative of ManNAc3NAcA, and GlcNAc3NAcAN, a uronamide derivative of an intermediate from the ManNAc3NAcA pathway (34). The Bordetella O antigen produced by B. bronchiseptica and B. parapertussis contains l-GalNAc3NAcA and l-GalNAc3NAcAN, believed to be derivatives of UDP-d-ManNAc3NAcA synthesized by enzymes of the wbm locus (21, 34). Based on our hypothesis, an active copy of either of the wbpO genes should be essential for the production of all these uronic acid sugars.
In conclusion, P. aeruginosa and B. pertussis produce LPS that contain the rare sugar d-ManNAc3NAcA. Cross-complementation analysis was used to demonstrate that B-band LPS production was restored to P. aeruginosa knockout mutants when genes encoding B. pertussis oxidase, transaminase, N-acetyltransferase, and epimerase enzymes were supplied in trans. Two dehydrogenases, WbpO1629 and WbpO3150, were identified in B. pertussis and were shown to cross-complement a UDP-d-GlcNAc 6-dehydrogenase knockout of P. aeruginosa. Biochemical analysis showed that both WbpO proteins have the novel ability to catalyze 6-dehydrogenation of UDP-d-GlcNAc, UDP-d-GalNAc, and UDP-d-Glc in vitro. Thus, these results suggest that B. pertussis and P. aeruginosa use the same biosynthetic pathway for the production of UDP-d-ManNAc3NAcA but that the first step in the pathway in B. pertussis is catalyzed by a novel promiscuous 6-dehydrogenase that is biochemically distinct from its homolog in P. aeruginosa PAO1.
Acknowledgments
This work was supported by a grant from the Canadian Institutes of Health Research (CIHR) to J.S.L. (MOP 14687) and by a grant from the Natural Science and Engineering Research Council of Canada to A.P. E.L.W. holds a CIHR Canada graduate scholarship (doctoral), and J.S.L. holds a Canada Research Chair in Cystic Fibrosis and Microbial Glycobiology funded by the Canadian Foundation for Innovation.
We thank Aaron Rothstein for technical assistance, Wayne Miller for the construction of pWMJL085 and initial work on ELW-E100, Dana Kocincova for the synthesis of TDP-d-glucose, and Jerry King for the synthesis of UDP-d-GalNAcA. Thanks also to Dyanne Brewer and Armen Charchoglyan of the Biological Mass Spectrometry Facility at the University of Guelph for assistance with MALDI-TOF analysis.
Footnotes
Published ahead of print on 11 July 2008.
REFERENCES
- 1.Allen, A., and D. Maskell. 1996. The identification, cloning and mutagenesis of a genetic locus required for lipopolysaccharide biosynthesis in Bordetella pertussis. Mol. Microbiol. 1937-52. [DOI] [PubMed] [Google Scholar]
- 2.Banemann, A., H. Deppisch, and R. Gross. 1998. The lipopolysaccharide of Bordetella bronchiseptica acts as a protective shield against antimicrobial peptides. Infect. Immun. 665607-5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bélanger, M., L. L. Burrows, and J. S. Lam. 1999. Functional analysis of genes responsible for the synthesis of the B-band O antigen of Pseudomonas aeruginosa serotype O6 lipopolysaccharide. Microbiology 1453505-3521. [DOI] [PubMed] [Google Scholar]
- 4.Burrows, L. L., D. F. Charter, and J. S. Lam. 1996. Molecular characterization of the Pseudomonas aeruginosa serotype O5 (PAO1) B-band lipopolysaccharide gene cluster. Mol. Microbiol. 22481-495. [DOI] [PubMed] [Google Scholar]
- 5.Burrows, L. L., K. E. Pigeon, and J. S. Lam. 2000. Pseudomonas aeruginosa B-band lipopolysaccharide genes wbpA and wbpI and their Escherichia coli homologues wecC and wecB are not functionally interchangeable. FEMS Microbiol. Lett. 189135-141. [DOI] [PubMed] [Google Scholar]
- 6.Caroff, M., J. Brisson, A. Martin, and D. Karibian. 2000. Structure of the Bordetella pertussis 1414 endotoxin. FEBS Lett. 4778-14. [DOI] [PubMed] [Google Scholar]
- 7.Chuanchuen, R., C. T. Narasaki, and H. P. Schweizer. 2002. Benchtop and microcentrifuge preparation of Pseudomonas aeruginosa competent cells. BioTechniques 33760-763. [DOI] [PubMed] [Google Scholar]
- 8.Creuzenet, C., M. Bélanger, W. W. Wakarchuk, and J. S. Lam. 2000. Expression, purification, and biochemical characterization of WbpP, a new UDP-GlcNAc C4 epimerase from Pseudomonas aeruginosa serotype O6. J. Biol. Chem. 27519060-19067. [DOI] [PubMed] [Google Scholar]
- 9.Cryz, S. J., Jr., T. L. Pitt, E. Furer, and R. Germanier. 1984. Role of lipopolysaccharide in virulence of Pseudomonas aeruginosa. Infect. Immun. 44508-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Currie, H. L., J. Lightfoot, and J. S. Lam. 1995. Prevalence of gca, a gene involved in synthesis of A-band common antigen polysaccharide in Pseudomonas aeruginosa. Clin. Diagn. Lab. Immunol. 2554-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dasgupta, T., T. R. de Kievit, H. Masoud, E. Altman, J. C. Richards, I. Sadovskaya, D. P. Speert, and J. S. Lam. 1994. Characterization of lipopolysaccharide-deficient mutants of Pseudomonas aeruginosa derived from serotypes O3, O5, and O6. Infect. Immun. 62809-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Fabio, J. L., M. Caroff, D. Karibian, J. C. Richards, and M. B. Perry. 1992. Characterization of the common antigenic lipopolysaccharide O-chains produced by Bordetella bronchiseptica and Bordetella parapertussis. FEMS Microbiol. Lett. 76275-281. [DOI] [PubMed] [Google Scholar]
- 13.Engels, W., J. Endert, M. A. Kamps, and C. P. van Boven. 1985. Role of lipopolysaccharide in opsonization and phagocytosis of Pseudomonas aeruginosa. Infect. Immun. 49182-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fomsgaard, A., N. Høiby, G. H. Shand, R. S. Conrad, and C. Galanos. 1988. Longitudinal study of antibody response to lipopolysaccharides during chronic Pseudomonas aeruginosa lung infection in cystic fibrosis. Infect. Immun. 562270-2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gasteiger, E., C. Hoogland, A. Gattiker, S. Duvaud, M. R. Wilkins, R. D. Appel, and A. Bairoch. 2005. Protein identification and analysis tools on the ExPASy server, p. 571-607. In J. M. Walker (ed.), The proteomics protocols handbook. Humana Press, Totowa, NJ.
- 16.Gillespie, J., S. T. Weintraub, G. G. Wong, and S. C. Holt. 1988. Chemical and biological characterization of the lipopolysaccharide of the oral pathogen Wolinella recta ATCC 33238. Infect. Immun. 562028-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hancock, R. E., and A. M. Carey. 1979. Outer membrane of Pseudomonas aeruginosa: heat- 2-mercaptoethanol-modifiable proteins. J. Bacteriol. 140902-910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harvill, E. T., A. Preston, P. A. Cotter, A. G. Allen, D. J. Maskell, and J. F. Miller. 2000. Multiple roles for Bordetella lipopolysaccharide molecules during respiratory tract infection. Infect. Immun. 686720-6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hitchcock, P. J., and T. M. Brown. 1983. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J. Bacteriol. 154269-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoang, T. T., R. R. Karkhoff-Schweizer, A. J. Kutchma, and H. P. Schweizer. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 21277-86. [DOI] [PubMed] [Google Scholar]
- 21.King, J. D., N. J. Harmer, A. Preston, C. M. Palmer, M. Rejzek, R. A. Field, T. L. Blundell, and D. J. Maskell. 2007. Predicting protein function from structure—the roles of short-chain dehydrogenase/reductase enzymes in Bordetella O-antigen biosynthesis. J. Mol. Biol. 374749-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knirel, Y. A., O. V. Bystrova, N. A. Kocharova, U. Zahringer, and G. B. Pier. 2006. Conserved and variable structural features in the lipopolysaccharide of Pseudomonas aeruginosa. J. Endotoxin Res. 12324-336. [DOI] [PubMed] [Google Scholar]
- 23.Knirel, Y. A., and N. K. Kochetkov. 1994. The structure of lipopolysaccharides of gram-negative bacteria. III. The structure of O-antigens: a review. Biochemistry 591325-1382. [Google Scholar]
- 24.Kokeguchi, S., O. Tsutsui, K. Kato, and T. Matsumura. 1991. Comparative study of lipopolysaccharides from Wolinella recta, W. curva, W. succinogenes and Campylobacter sputorum ssp. sputorum. FEMS Microbiol. Lett. 65291-297. [DOI] [PubMed] [Google Scholar]
- 25.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227680-685. [DOI] [PubMed] [Google Scholar]
- 26.Lam, J. S., L. A. MacDonald, M. Y. Lam, L. G. Duchesne, and G. G. Southam. 1987. Production and characterization of monoclonal antibodies against serotype strains of Pseudomonas aeruginosa. Infect. Immun. 551051-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lam, M. Y., E. J. McGroarty, A. M. Kropinski, L. A. MacDonald, S. S. Pedersen, N. Høiby, and J. S. Lam. 1989. Occurrence of a common lipopolysaccharide antigen in standard and clinical strains of Pseudomonas aeruginosa. J. Clin. Microbiol. 27962-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu, P. V., and S. Wang. 1990. Three new major somatic antigens of Pseudomonas aeruginosa. J. Clin. Microbiol. 28922-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Messner, P., U. B. Sleytr, R. Christian, G. Schulz, and F. M. Unger. 1987. Isolation and structure determination of a diacetamidodideoxyuronic acid-containing glycan chain from the S-layer glycoprotein of Bacillus stearothermophilus NRS 2004/3a. Carbohydr. Res. 168211-218. [DOI] [PubMed] [Google Scholar]
- 30.Miller, W. L., M. J. Matewish, D. J. McNally, N. Ishiyama, E. M. Anderson, D. Brewer, J. R. Brisson, A. M. Berghuis, and J. S. Lam. 2008. Flagellin glycosylation in Pseudomonas aeruginosa PAK requires the O-antigen biosynthesis enzyme WbpO. J. Biol. Chem. 2833507-3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller, W. L., C. Q. Wenzel, C. Daniels, S. Larocque, J. R. Brisson, and J. S. Lam. 2004. Biochemical characterization of WbpA, a UDP-N-acetyl-d-glucosamine 6-dehydrogenase involved in O-antigen biosynthesis in Pseudomonas aeruginosa PAO1. J. Biol. Chem. 27937551-37558. [DOI] [PubMed] [Google Scholar]
- 32.Parkhill, J., M. Sebaihia, A. Preston, L. D. Murphy, N. Thomson, D. E. Harris, M. T. Holden, C. M. Churcher, S. D. Bentley, K. L. Mungall, A. M. Cerdeno-Tarraga, L. Temple, K. James, B. Harris, M. A. Quail, M. Achtman, R. Atkin, S. Baker, D. Basham, N. Bason, I. Cherevach, T. Chillingworth, M. Collins, A. Cronin, P. Davis, J. Doggett, T. Feltwell, A. Goble, N. Hamlin, H. Hauser, S. Holroyd, K. Jagels, S. Leather, S. Moule, H. Norberczak, S. O'Neil, D. Ormond, C. Price, E. Rabbinowitsch, S. Rutter, M. Sanders, D. Saunders, K. Seeger, S. Sharp, M. Simmonds, J. Skelton, R. Squares, S. Squares, K. Stevens, L. Unwin, S. Whitehead, B. G. Barrell, and D. J. Maskell. 2003. Comparative analysis of the genome sequences of Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. Nat. Genet. 3532-40. [DOI] [PubMed] [Google Scholar]
- 33.Preston, A., A. G. Allen, J. Cadisch, R. Thomas, K. Stevens, C. M. Churcher, K. L. Badcock, J. Parkhill, B. Barrell, and D. J. Maskell. 1999. Genetic basis for lipopolysaccharide O-antigen biosynthesis in bordetellae. Infect. Immun. 673763-3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Preston, A., B. O. Petersen, J. O. Duus, J. Kubler-Kielb, G. Ben-Menachem, J. Li, and E. Vinogradov. 2006. Complete structures of Bordetella bronchiseptica and Bordetella parapertussis lipopolysaccharides. J. Biol. Chem. 28118135-18144. [DOI] [PubMed] [Google Scholar]
- 35.Preston, A., R. Thomas, and D. J. Maskell. 2002. Mutational analysis of the Bordetella pertussis wlb LPS biosynthesis locus. Microb. Pathog. 3391-95. [DOI] [PubMed] [Google Scholar]
- 36.Relman, D., E. Tuomanen, S. Falkow, D. T. Golenbock, K. Saukkonen, and S. D. Wright. 1990. Recognition of a bacterial adhesion by an integrin: macrophage CR3 (alpha M beta 2, CD11b/CD18) binds filamentous hemagglutinin of Bordetella pertussis. Cell 611375-1382. [DOI] [PubMed] [Google Scholar]
- 37.Rocchetta, H. L., L. L. Burrows, and J. S. Lam. 1999. Genetics of O-antigen biosynthesis in Pseudomonas aeruginosa. Microbiol. Mol. Biol. Rev. 63523-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schaeffer, L. M., F. X. McCormack, H. Wu, and A. A. Weiss. 2004. Bordetella pertussis lipopolysaccharide resists the bactericidal effects of pulmonary surfactant protein A. J. Immunol. 1731959-1965. [DOI] [PubMed] [Google Scholar]
- 39.Schaffer, C., H. Kahlig, R. Christian, G. Schulz, S. Zayni, and P. Messner. 1999. The diacetamidodideoxyuronic-acid-containing glycan chain of Bacillus stearothermophilus NRS 2004/3a represents the secondary cell-wall polymer of wild-type B. stearothermophilus strains. Microbiology 1451575-1583. [DOI] [PubMed] [Google Scholar]
- 40.Schweizer, H. P., and T. T. Hoang. 1995. An improved system for gene replacement and xylE fusion analysis in Pseudomonas aeruginosa. Gene 15815-22. [DOI] [PubMed] [Google Scholar]
- 41.Simon, R., U. Priefer, and A. Pühler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Bio/Technology 1784-791. [Google Scholar]
- 42.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. Lim, K. Smith, D. Spencer, G. K. Wong, Z. Wu, I. T. Paulsen, J. Reizer, M. H. Saier, R. E. Hancock, S. Lory, and M. V. Olson. 2000. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406959-964. [DOI] [PubMed] [Google Scholar]
- 43.Wenzel, C. Q., C. Daniels, R. A. Keates, D. Brewer, and J. S. Lam. 2005. Evidence that WbpD is an N-acetyltransferase belonging to the hexapeptide acyltransferase superfamily and an important protein for O-antigen biosynthesis in Pseudomonas aeruginosa PAO1. Mol. Microbiol. 571288-1303. [DOI] [PubMed] [Google Scholar]
- 44.West, N. P., H. Jungnitz, J. T. Fitter, J. D. McArthur, C. A. Guzman, and M. J. Walker. 2000. Role of phosphoglucomutase of Bordetella bronchiseptica in lipopolysaccharide biosynthesis and virulence. Infect. Immun. 684673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.West, S. E., H. P. Schweizer, C. Dall, A. K. Sample, and L. J. Runyen-Janecky. 1994. Construction of improved Escherichia-Pseudomonas shuttle vectors derived from pUC18/19 and sequence of the region required for their replication in Pseudomonas aeruginosa. Gene 14881-86. [DOI] [PubMed] [Google Scholar]
- 46.Westman, E. L., D. J. McNally, M. Rejzek, W. L. Miller, V. S. Kannathasan, A. Preston, D. J. Maskell, R. A. Field, J. R. Brisson, and J. S. Lam. 2007. Identification and biochemical characterization of two novel UDP-2,3-diacetamido-2,3-dideoxy-alpha-d-glucuronic acid 2-epimerases from respiratory pathogens. Biochem. J. 405123-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33103-119. [DOI] [PubMed] [Google Scholar]
- 48.Zhao, X., C. Creuzenet, M. Bélanger, E. Egbosimba, J. Li, and J. S. Lam. 2000. WbpO, a UDP-N-acetyl-d-galactosamine dehydrogenase from Pseudomonas aeruginosa serotype O6. J. Biol. Chem. 27533252-33259. [DOI] [PubMed] [Google Scholar]