Abstract
We found that Escherichia coli tolC mutants showed increased sensitivity to 5-aminolevulinic acid (ALA), a precursor of porphyrins. The tolC mutant cells grown in the presence of ALA showed a reddish brown color under visible light and a strong red fluorescence under near-UV irradiation. Fluorescence spectrometry and high-performance liquid chromatography analysis showed that the tolC mutant cells grown in the presence of ALA accumulated a large amount of coproporphyrin(ogen) intracellularly. In contrast, the wild-type cells produced coproporphyrin extracellularly. The tolC mutant cells grown in the presence of ALA, which were capable of surviving in the dark, were killed by near-UV irradiation, suggesting that the intracellular coproporphyrin(ogen) renders these cells photosensitive. These results suggest that the TolC-dependent efflux system is involved in the exclusion of porphyrin(ogen)s in E. coli.
The antibiotic alaremycin was discovered during the process of screening for inhibitors of bacterial chromosome partitioning using the anucleate cell blue assay in our laboratory (3). The anucleate cell blue assay is designed for detecting anucleate cells resulting from the inhibition of Escherichia coli chromosome partitioning. Alaremycin is structurally related to a precursor of porphyrins, namely 5-aminolevulinic acid (ALA), and therefore has been named alaremycin (for ALA-related antibiotic produced by Streptomyces sp.). In the course of studying its mechanism of action, we found that tolC mutants showed increased sensitivity to alaremycin (data not shown).
The E. coli outer membrane channel-tunnel protein TolC is involved in the exclusion of harmful substances such as antibiotics, dyes, organic solvents, and detergents (1, 20, 23, 33, 34). The crystal structure of the TolC protein recently has been determined (17). The TolC protein is composed of a transmembrane domain and a periplasmic domain and forms a homotrimer. The periplasmic barrel structure of TolC is connected to drug efflux pump proteins such as AcrB and AcrE, which are located on the inner membrane. Clamp proteins such as AcrA and ArcF link TolC and pump proteins in the periplasmic space (37, 39). Pump proteins seem to transport toxic cytoplasmic or periplasmic substances into the extracellular space across the outer membrane via the TolC channel.
The TolC-dependent efflux system is not limited to merely being a participant in the exclusion of exogenous harmful substances; it also is involved in the export of intracellular metabolites such as indole (16) and siderophores (5). Although indole, a tryptophan metabolite, is a common compound in E. coli cells, high concentrations of indole are harmful to the E. coli cells. The E. coli cells must, therefore, defend themselves from the toxic action of indole. It has been reported that the TolC-AcrEF efflux pump functions as an indole exporter (16). The TolC-dependent efflux system also is involved in the export of a catecholate siderophore, i.e., enterobactin. It also has been reported that enterobactin excretion is a two-step process involving the major facilitator EntS and the TolC-dependent efflux system (5).
ALA, which is structurally related to alaremycin, is a heme precursor. In E. coli, ALA is formed from glutamyl-tRNA by the action of glutamyl-tRNA reductase, which is encoded by hemA, and glutamate-1-semialdehyde aminotransferase, which is encoded by hemL (2, 13). ALA is an essential precursor for heme biosynthesis; however, increased concentrations of it induce oxidative stress in the presence of Fe2+ ions (4, 11, 28). The second step of heme biosynthesis is catalyzed by porphobilinogen synthase, which is encoded by hemB (18). HemB condenses two molecules of ALA to yield porphobilinogen. Further, four molecules of porphobilinogen are condensed to form uroporphyrinogen III by the action of porphobilinogen deaminase, which is encoded by hemC, and uroporphyrinogen III synthase, which is encoded by hemD (29, 35). Uroporphyrinogen III then is converted to protoporphyrin IX by successive decarboxylation and oxidation by HemE, HemF or HemN, and HemG (9, 14, 24, 26, 30, 40, 41). Finally, ferochelatase, encoded by hemH, converts protoporphyrin IX to heme by inserting Fe2+ into protoporphyrin IX (12).
In E. coli, heme is used as a cofactor by cytochromes in the electron transfer system (36) and by a hydrogen peroxide-scavenging enzyme named catalase (19). E. coli cannot utilize exogenous heme or porphyrins, and no heme/porphyrin uptake systems have been identified thus far. Therefore, heme is synthesized de novo by the above-mentioned heme biosynthesis pathway. It is reported that heme is incorporated into apo cytochromes in the periplasmic space probably by the action of the cytochrome c maturation system CcmABCDEFGH (31). However, the manner in which porphyrin molecules are transported from the cytoplasm to the periplasmic space across the inner membrane remains unknown (7).
During our study of the mechanism of action of alaremycin, we found that E. coli tolC mutants showed increased sensitivity to exogenous ALA. tolC mutant cells grown in the presence of ALA exhibited a strong red fluorescence under near-UV irradiation. Moreover, tolC mutant cells grown in the presence of ALA accumulated a large amount of porphyrin(ogen)s intracellularly, in contrast to the wild-type cells, which produced porphyrins extracellularly. These results suggest that the TolC-dependent efflux system is involved in porphyrin(ogen) exclusion in E. coli.
MATERIALS AND METHODS
Strains, plasmid, and growth medium.
The E. coli K-12 strain JA300 (F− thr leuB6 trpC1117 thi rpsL20 hsdS), its tolC derivative JA300T (JA300 but with tolC), and the tolC plasmid pMX, carrying the wild-type tolC gene on the low-copy-number vector pMW119 (Nippon Gene Co. Ltd., Tokyo, Japan), were used in this study (1). P1 phage-mediated transduction was used to construct fur::Tn5 derivatives of JA300 and JA300T by using BN904 (F− gltA6 fur::Tn5 galK30 pyrD36 relA1 rpsL129 thi-1 supE44 λ−) as the donor. The cells were grown in P medium containing 1% polypepton, 0.5% NaCl, and 0.1% glucose (pH 7.2).
Determination of the MIC.
The MIC of ALA was determined on P agar plates using conventional dilution methods. A total of 104 cells were spotted on a test agar plate, followed by incubation at 30°C for 24 h in the dark. The lowest concentration of ALA that completely inhibited colony formation was determined as the MIC.
Photosensitivity of the cells grown in the presence of ALA.
Each bacterial strain was grown at 30°C in P medium in the presence or absence of 10 μg/ml of ALA for 24 h. The cells were harvested by centrifugation, quickly washed with saline, and suspended in the same solution. The cells were suspended in a glass plate by being stirred and then were exposed to 366-nm near-UV light for 5 to 15 min. A 15-W UV lamp with a 366-nm filter placed at a distance of 5 cm from the plate was used for this purpose. After dilution, the irradiated cells were spread on P plates. The plates were incubated at 30°C for 24 h in the dark, and the number of viable cells was counted.
Extraction and measurement of intracellular ALA.
ALA was extracted using the method of Kanjo et al. (15). Briefly, each bacterial strain was grown at 30°C in P medium until an optical density at 660 nm of 0.9 was attained. Cells were harvested from 90 ml of the cultures by centrifugation at 5,800 × g for 10 min and then were suspended in 3 ml of fresh P medium. ALA then was added to the cell suspension at a final concentration of 100 or 300 μg/ml. The cells were incubated at 30°C for the indicated time periods. Further, 1 ml of the cell suspension was centrifuged and quickly washed twice with ice-cold water, and then the cells were suspended in 200 μl of 10% trichloroacetic acid, followed by the addition of 200 μl of a 1 M sodium acetate solution and 40 μl of methyl acetoacetate to the cell suspension. The mixture was boiled for 10 min at 100°C. After the mixture had cooled down to room temperature, 600 μl of ethyl acetate was added to the mixture. After centrifugation at 15,300 × g for 1 min at 4°C, the organic phase was used for the chromogenic reaction. Ehrlich's reagent (200 μl) was added to 400 μl of the organic phase, and the A553 of the aqueous phase was measured after 10 min.
Extraction and fractionation of porphyrins.
Total porphyrins were extracted using the method of Cox and Charles (8). Each bacterial strain was grown at 30°C in P medium until an optical density at 660 nm of 0.9 was attained, and then ALA was added to the broth at a final concentration of 10 μg/ml. The cell culture (20 ml) was centrifuged at 5,800 × g for 5 min to separate the cells from the medium. The cells were suspended in 20 ml of ice-cold water. Ethyl acetate-glacial acetic acid (3:1, vol/vol; 10 ml) was added to the medium and the cell suspension. The mixtures then were centrifuged at 5,800 × g for 10 min, and then the organic layer was recovered. The ethyl acetate extracts were washed with 1 ml of ice-cold water. Porphyrins were extracted from the ethyl acetate fractions using 3 ml of 3 M hydrochloric acid solution twice. The fluorescence spectra of the resultant hydrochloric acid solutions were collected using an F-2000 fluorescence spectrophotometer (Hitachi Co. Ltd., Tokyo, Japan) with an excitation wavelength of 405 nm. The fluorescence intensity at 596 nm was determined as an index of the total porphyrin content.
The porphyrin composition was analyzed according to the method of Nitzan et al. (25) with a minor modification. Hydrochloric acid solutions containing total porphyrins were separated using a high-performance liquid chromatography (HPLC) system equipped with a photodiode array detector (Waters Co., Chicago, IL) and an octadecyl silica gel column (ODS-100V; 5-μm particle size; 150 by 4.6 mm; Tosoh Co. Ltd., Tokyo, Japan). Porphyrins were eluted for 20 min at a flow rate of 1 ml/min with a linear gradient from 100% solvent A (10% acetonitrile in 1 M ammonium acetate; pH 5.1) to 100% solvent B (methanol-acetic acid, 10:1 [vol/vol]), followed by elution again with 100% solvent B.
Isolation of the periplasmic cell fraction.
The cells that were grown in the presence of 10 μg/ml ALA were suspended in an ice-cold solution of 0.5 M sucrose, 0.1 M Tris-HCl, and 1 mM Na-EDTA (pH 8.0). After a 10-min incubation on an ice bath, lysozyme was added to the solution at a final concentration of 16 μg/ml, and immediately thereafter an equal volume of ice-cold water was added. The suspension was again incubated on an ice bath for 5 min, and then MgSO4 was added at a final concentration of 18 mM. The suspension was centrifuged at 5,800 × g for 10 min. The clear supernatant was considered to contain the periplasmic cell fraction. The degree of contamination of this fraction with cytoplasmic material was estimated by comparing the malate dehydrogenase activity in this fraction to that in the total cell lysate. Malate dehydrogenase activity was assayed by determining the initial rate of NAD+ reduction at 25°C. The reaction mixture contained 50 mM Tris-HCl (pH 7.5), 10 mM MgCl2, 0.5 mM NAD+, the cytoplasmic cell fraction, and 40 mM l-malate. The reaction was initiated by the addition of l-malate, and the increase in the levels at A340 was monitored using a spectrophotometer (U-2800; Hitachi).
RESULTS
ALA sensitivity of tolC mutants.
During the study of the mechanism of action of alaremycin, we found that the tolC mutants showed increased sensitivity to alaremycin (data not shown). We presumed that the TolC-dependent efflux system was involved in alaremycin efflux. Since the structure of alaremycin is similar to that of ALA, we also examined the ALA sensitivity of the tolC mutants. As expected, the tolC mutants showed increased sensitivity to ALA. The MIC of ALA was 1.25 mg/ml and 2.5 mg/ml for the tolC mutant strain JA300T and the wild-type strain JA300, respectively. The ALA sensitivity of the tolC mutant was restored to that of the wild-type strain by the complementation of the former with a plasmid carrying the tolC gene.
Similar intracellular accumulation of ALA by wild-type and tolC mutant cells.
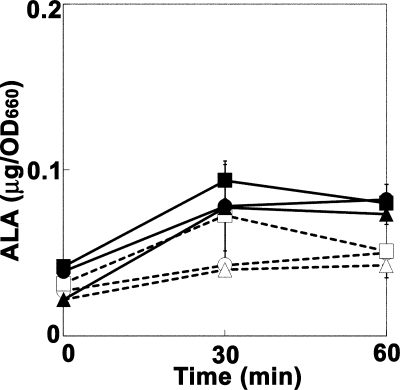
We suspected that the TolC-dependent efflux system was involved in ALA efflux and that the tolC mutants had accumulated a larger amount of ALA intracellularly than the wild-type cells. As a precursor of heme biosynthesis, ALA is an essential compound for E. coli cell growth. An increase in intracellular ALA concentration was, however, shown to be harmful to the E. coli cells, because ALA induces oxidative stress in the presence of ferric ions (4, 11, 28). Thus, the effect of the fur mutation, which causes the increased uptake of iron ions, on the ALA sensitivity of the E. coli cells was examined. Even in the presence of 1 mM FeCl3, the introduction of the fur::Tn5 mutation resulted in only a twofold increase in the ALA sensitivity in both the wild-type and the tolC mutant strains. This suggested that the intracellular ALA concentration was not markedly increased even if cells were exposed to high concentrations of ALA. We therefore examined the intracellular ALA concentrations in the wild-type and tolC mutant cells after they had been exposed to high concentrations of ALA. As shown in Fig. 1, the intracellular ALA concentration increased slightly in the first 30 min and reached a plateau thereafter when the cells were exposed to 100 or 300 μg/ml of ALA. No significant difference in the intracellular ALA concentration was observed between the wild-type and tolC mutant cells, suggesting that ALA was quickly excluded by the TolC-independent efflux system or that the incorporated ALA was rapidly metabolized to porphobilinogen by porphobilinogen synthase, which is encoded by the hemB gene, and to other downstream metabolites, i.e., porphyrin(ogen)s.
FIG. 1.
Accumulation of ALA in the wild-type and tolC mutant cells exposed to exogenous ALA. The cells were exposed to 100 (open symbols) or 300 (closed symbols) μg/ml ALA at 30°C. After the indicated time periods, ALA was extracted from the cells and was quantified as described in Materials and Methods. Circles, JA300 (wild-type strain); triangles, JA300T (containing tolC); squares, JA300T/pMX. The means (symbols) and standard deviations (error bars) are shown (n = 3). OD660, optical density at 660 nm.
tolC mutant cells showed red fluorescence under near-UV irradiation.
During the ALA accumulation experiments, we noticed that the tolC mutant cells grown in the presence of 10 μg/ml of ALA, a concentration at which the growth of the tolC mutants was not inhibited, showed a reddish brown color under visible light (Fig. 2, upper). Since heme and porphyrin(ogen)s, which are synthesized from ALA, are pigments that yield a red fluorescence, we suspected that the tolC mutant cells accumulated porphyrin(ogen)s intracellularly. As expected, the tolC mutant cells showed a strong red fluorescence under near-UV irradiation of 366 nm (Fig. 2, lower), which was a characteristic of porphyrin(ogen)s. In contrast, the wild-type cells showed a bluish white color under near-UV irradiation of 366 nm. This suggested that the tolC mutant cells accumulated a large amount of porphyrin(ogen)s intracellularly, while the wild-type cells did not.
FIG. 2.
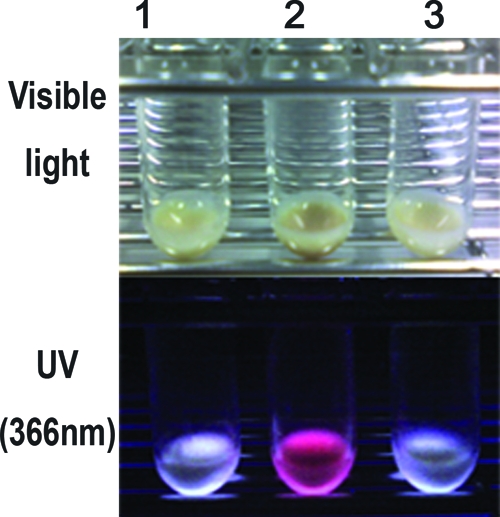
Red fluorescent pigments accumulated in the tolC mutant cells grown in the presence of ALA. Cells grown at 30°C for 24 h in the presence of 10 μg/ml ALA were harvested by centrifugation and were photographed under visible light (upper) or near-UV irradiation of 366 nm (lower). Lanes: 1, JA300 (wild-type strain); 2, JA300T (containing tolC); 3, JA300T/pMX.
Accumulation of porphyrin(ogen)s in tolC mutant cells.
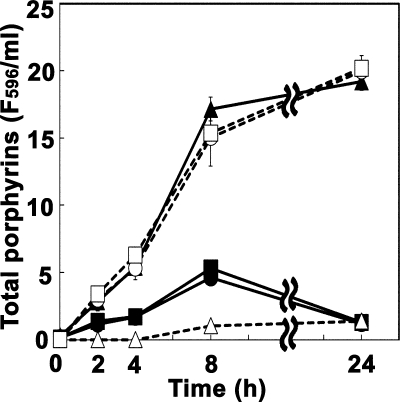
We extracted intracellular porphyrin(ogen)s from the wild-type and tolC mutant cells grown in the presence of 10 μg/ml ALA. Porphyrinogens, if present, would have been oxidized during the extraction procedures; therefore, the extracted samples should have contained porphyrins (10, 27). The total porphyrin concentrations were determined by fluorescence spectrometry with an excitation wavelength of 405 nm. As shown in Fig. 3, the tolC mutant cells accumulated a large amount of porphyrin(ogen)s intracellularly. On the other hand, the wild-type cells accumulated a smaller amount of porphyrins. We speculated that the TolC-dependent efflux system is involved in porphyrin(ogen) efflux. If our speculation was accurate, the wild-type cells would excrete porphyrin(ogen)s into the medium. As expected, the wild-type cells released a large amount of porphyrins in the medium, an amount that was almost comparable to the intracellular porphyrin(ogen) levels in the tolC mutant cells. The total amounts of porphyrin(ogen)s produced by the wild-type and tolC mutant cells were almost identical. We also examined if heme synthesis was stimulated in the tolC mutant cells grown in the presence of ALA. However, notable heme accumulation was not observed even in the presence of the fur mutation, which caused an increase in the iron uptake (data not shown).
FIG. 3.
Intracellular and extracellular porphyrin(ogen)s produced by the wild-type and tolC mutant cells grown in the presence of ALA. The cells were grown at 30°C in the presence of 10 μg/ml ALA for the indicated time periods. The cells were separated from the growth medium by centrifugation. Porphyrin(ogen)s were extracted from the cells as well as from the medium and were quantified as described in Materials and Methods. Circles, JA300 (wild-type strain); triangles, JA300T (containing tolC); squares, JA300T/pMX; open symbols, medium fraction; closed symbols, cell fraction. The means (symbols) and standard deviations (error bars) are shown (n = 3). F596, fluorescence intensity at 596 nm with an excitation wavelength of 405 nm.
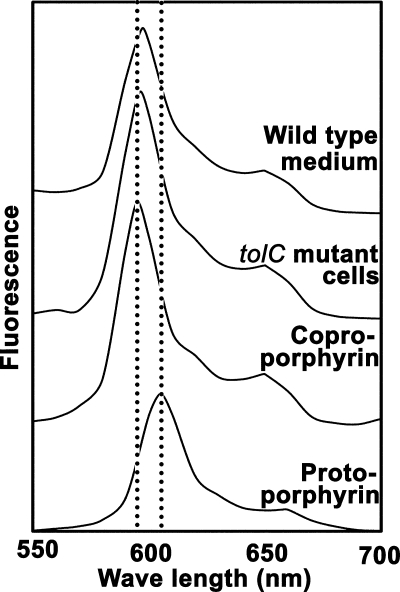
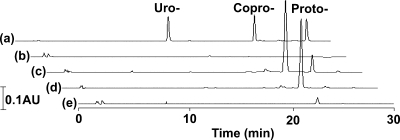
ALA is metabolized to protoporphyrinogen IX via uroporphyrinogen III and coproporphyrinogen III. Uroporphyrinogen III, coproporphyrinogen III, and protoporphyrinogen IX are converted to uroporphyrin III, coproporphyrin III, and protoporphyrin IX, respectively, by auto- or enzymatic oxidation. Finally, ferochelatase, which is encoded by hemH, converts protoporphyrin IX to heme by inserting Fe2+ into protoporphyrin IX. Porphyrins produced by the wild-type and tolC mutant strains grown in the presence of ALA were analyzed by fluorescence spectrometry. The fluorescence spectra of the total porphyrin fractions extracted from the wild-type medium and that extracted from the tolC mutant cells matched well with that of coproporphyrin (Fig. 4). This was confirmed by HPLC analysis. As shown in Fig. 5, the wild-type and tolC mutant strains grown in the presence of ALA produced mainly coproporphyrin. Coproporphyrin accounted for 94% of the total porphyrins extracted from the wild-type medium and 97% of the total porphyrins extracted from the tolC mutant cells. Since it is considered that porphyrinogens are oxidized to porphyrins during export or extraction procedures, it is possible that the tolC mutant cells accumulated coproporphyrinogen intracellularly. On the other hand, the extracellularly accumulated molecules in the wild-type medium might have been coproporphyrin itself.
FIG. 4.
Fluorescence spectra of porphyrins produced by the wild-type and tolC mutant cells. The fluorescence spectra of the total porphyrin fractions extracted from the wild-type medium and the tolC mutant cells at 24 h, as shown in Fig. 3, were collected at an excitation wavelength of 405 nm. The spectra of authentic coproporphyrin and protoporphyrin also are shown.
FIG. 5.
Composition of porphyrins. The total porphyrin fractions at 24 h, as shown in Fig. 3, were separated by HPLC as described in Materials and Methods. (a) Authentic samples. Uro-, uroporphyrin; Copro-, coproporphyrin; Proto-, protoporphyrin. (b) Wild-type cells. (c) Wild-type medium. (d) tolC mutant cells. (e) tolC mutant medium. The vertical scale shown on the left represents 0.1 absorbance unit (AU) at 393 nm.
It has been reported that enterobactin excretion is a two-step process involving the major facilitator EntS and the TolC-dependent efflux system (5). It also is possible that porphyrin(ogen) exclusion also is a two-step process, i.e., porphyrin(ogen)s are transported to the periplasm by a TolC-independent mechanism and then transported across the outer membrane by the TolC-dependent efflux system. We therefore examined the intracellular localization of accumulated porphyrin(ogen)s in the cells grown in the presence of ALA. It was revealed that a substantial proportion of porphyrins (97.4% ± 0.44% in the tolC mutant cells and 67.5% ± 0.45% in the wild-type cells) was recovered from the periplasmic cell fraction. The degree of contamination of the periplasmic cell fraction with cytoplasm was 11.3 and 21.3% for the tolC mutant and wild-type cells, respectively; this was determined using malate dehydrogenase as a marker enzyme for the cytoplasm. This finding indicates that TolC is involved in the transport of porphyrins from the periplasm into the medium. Since the periplasmic environment is more oxidative than that of the cytoplasm, the tolC mutant cells might accumulate coproporhyrin rather than coproporphyrinogen.
Photosensitivity of tolC mutants grown in the presence of ALA.
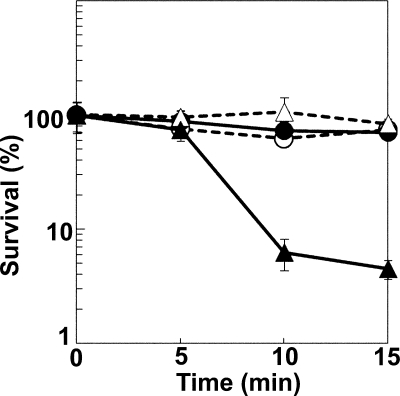
It is known that porphyrin(ogen)s function as photosensitizers. Previous studies reported that the hemH mutant strain, which accumulated protoporphyrin(ogen) due to a defect in ferochelatase, showed photosensitivity (21, 22, 45). We therefore examined the photosensitivity of the tolC mutant strain, which accumulated coproporphyrin(ogen). The wild-type and tolC mutant cells were grown in the presence of 10 μg/ml ALA for 24 h in the dark. The cells then were exposed to near-UV light at 366 nm, following which the number of viable cells was counted. As shown in Fig. 6, the tolC mutant cells grown in the presence of ALA exhibited decreased survival following the near-UV irradiation compared to the survival of wild-type cells grown under identical conditions. The tolC mutant cells grown in the absence of ALA did not show such photosensitivity. These results showed that coproporphyrin(ogen) synthesized from exogenous ALA functions as a photosensitizer in the tolC mutant cells.
FIG. 6.
Photosensitivity of the tolC mutant strain grown in the presence of ALA. Cells grown at 30°C for 24 h in the presence (closed symbols) or absence (open symbols) of 10 μg/ml ALA were exposed to near-UV light at 366 nm, and the number of viable cells was counted as described in Materials and Methods. Circles, JA300 (wild-type strain); triangles, JA300T (containing tolC). The means (symbols) and standard deviations (error bars) are shown (n = 3).
DISCUSSION
There are many genes in the E. coli chromosomal DNA that encode proteins associated with drug efflux. In particular, the outer membrane channel-tunnel protein, TolC, plays an important role in drug efflux. The TolC protein effluxes many harmful compounds in partnership with several inner membrane pump proteins, such as AcrAB and AcrEF. The TolC-dependent efflux system mostly exports periplasmic compounds to the extracellular space across the outer membrane (17, 20, 23).
The TolC-dependent efflux system also plays a role in the efflux of endogenous compounds; for example, the TolC and AcrEF pump is involved in indole efflux. Although indole, a tryptophan metabolite, is a common compound in the E. coli cells, high concentrations of indole are harmful to E. coli cells (16). TolC is involved in the export of useful compounds as well. Enterobactin, involved in the acquisition of extracellular Fe3+, is transported by TolC (5).
E. coli can utilize both de novo-synthesized and exogenous ALA. ALA is synthesized from glutamyl-tRNA by the action of glutamyl-tRNA reductase, which is encoded by hemA, and glutamate-1-semialdehyde aminotransferase, which is encoded by hemL (2, 13). On the other hand, extracellular ALA is incorporated into the cells via the dipeptide transporter system (43). Although ALA is a heme precursor, increased intracellular ALA concentrations cause oxidative stress in the presence of Fe2+ ions (4, 11, 28). Since ALA-induced reactive oxygen acts on the guanine base of DNA, ALA can be a mutagenic compound. Therefore, intracellular ALA concentrations are strictly regulated (42, 44). In this study, we examined the intracellular ALA concentrations in the wild-type and tolC mutant cells grown in the presence of ALA; however, no significant differences in the intracellular ALA concentration were noted between the wild-type and tolC mutant cells.
In the 1970s, Doss and Philippe-Dormston demonstrated that exogenous ALA stimulates porphyrin biosynthesis in several bacteria, including E. coli (10, 27). They also showed that the synthesized porphyrin(ogen)s then are exported into the medium. ALA is metabolized to porphobilinogen by porphobilinogen synthase, which is encoded by the hemB gene (18). Further, porphobilinogen is metabolized to heme via uroporphyrinogen III, coproporphyrinogen III, and protoporphyrinogen IX (26). As shown in Fig. 2, the tolC mutant cells grown in the presence of ALA showed a reddish brown color under visible light and a strong red fluorescence under near-UV irradiation. We presumed that the tolC mutant cells accumulated porphyrin(ogen)s intracellularly, because porphyrin(ogen)s are pigments that yield a red fluorescence. Hence, we measured the amount of accumulated porphyrin(ogen)s in the E. coli cells grown in the presence of ALA. As expected, the tolC mutant had accumulated a large amount of porphyrin(ogen)s intracellularly, while the wild-type cells had not. In contrast, the wild-type E. coli cells grown in the presence of ALA excreted porphyrins in the medium. A large amount of porphyrins was detected in the wild-type culture medium but not in the tolC mutant culture medium. These results suggest that the ALA incorporated into the cells was metabolized to porphyrin(ogen)s, and the excess porphyrin(ogen)s were exported by the TolC-dependent efflux system.
Protoporphyrin(ogen) is known to function as a photosensitizer. It has been reported that the E. coli hemH mutant, which accumulates protoporphyrin(ogen) in its cells, shows photosensitivity (8, 21, 22, 45). In this study, we showed that the tolC mutant cells grown in the presence of ALA exhibited decreased survival following the near-UV irradiation, while the wild-type cells survived under the same conditions. Since the tolC mutant cells mainly accumulated coproporphyrin(ogen), the intracellular coproporphyrin(ogen) functioned as a photosensitizer like protoporphyrin(ogen). Heme biosynthesis is regulated at the ALA synthesis step through feedback inhibition that is dependent on the heme concentration (42, 44). In the presence of exogenous ALA, however, heme biosynthesis is not repressed, and therefore a large amount of heme precursor porphyrin(ogen)s is produced (32). It also has been reported that protoporphyrinogen synthase, which is encoded by hemF, is inhibited by protoporphyrin (6). This is probably the reason why mainly coproporphyrin(ogen) was accumulated in the ALA-treated cells. These findings match well with previous observations (10, 27). The function of the TolC-dependent efflux system may be to prevent the intracellular accumulation of excess porphyrin(ogen)s when cells are exposed to high concentrations of ALA. This is a new physiological role of the TolC-dependent efflux system in E. coli.
The TolC outer membrane channel-tunnel protein functions together with inner membrane efflux pump proteins. Therefore, an inner membrane pump(s) or exporter(s) also should be involved in porphyrin exclusion in combination with TolC. It was shown that a substantial portion of porphyrins that accumulated in the tolC mutant cells was recovered from the periplasmic cell fraction. This indicates that porphyrin(ogen) exclusion is a two-step process, as is the case for enterobactin excretion. In this process, porphyrin(ogen)s are transported to the periplasm by a TolC-independent mechanism and then are transported across the outer membrane by the TolC-dependent efflux system. It has been reported that the cytochrome c maturation system CcmABCDEFGH may be involved in the incorporation of heme into apo cytochromes in the periplasmic space (31). In this system, porphyrin molecules are to be transported from the cytoplasm to the outer side of the cytoplasmic membrane. However, the manner in which porphyrin molecules are transported from the cytoplasm to the periplasmic space across the inner membrane remains unknown (7). We examined the ALA sensitivity and porphyrin(ogen) accumulation in the ccm mutant strains; none of the mutant strains exhibited increased ALA sensitivity or porphyrin(ogen) accumulation (data not shown). We also examined the ALA sensitivity and porphyrin(ogen) accumulation in mutant strains with a known drug efflux pump mutation that is predicted to function along with TolC. Among the strains that we examined, namely, acrAB, acrD, acrEF, emrAB, macAB, mdtEF (yhiUV), mdtABC (yegMNO), and emrKY mutant strains, no mutant strains showed increased ALA sensitivity or porphyrin(ogen) accumulation (data not shown). In particular, it has been reported that Staphylococcus aureus HrtAB, which belongs to the MacAB family of ABC-type efflux carriers, is a possible heme transporter (38). However, macAB mutation showed no effect on porphyrin accumulation in E. coli, as mentioned above. An inner membrane pump that remains to be identified may be involved in porphyrin exclusion, or several pump proteins may simultaneously function as porphyrin exporters.
Acknowledgments
This work was supported in part by a grant-in-aid for scientific research (B) (20380047 to M.W.) from the Japan Society for Promotion of Science and a grant from the Global COE Program of the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Footnotes
Published ahead of print on 18 July 2008.
REFERENCES
- 1.Aono, R., N. Tsukagoshi, and M. Yamamoto. 1998. Involvement of outer membrane protein TolC, a possible member of the mar-sox regulon, in maintenance and improvement of organic solvent tolerance of Escherichia coli K-12. J. Bacteriol. 180938-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avissar, Y. J., and S. I. Beale. 1989. Identification of the enzymatic basis for δ-aminolevulinic acid auxotrophy in a hemA mutant of Escherichia coli. J. Bacteriol. 1712919-2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Awa, Y., N. Iwai, T. Ueda, K. Suzuki, S. Asano, J. Yamagishi, K. Nagai, and M. Wachi. 2005. Isolation of a new antibiotic, alaremycin, structurally related to 5-aminolevulinic acid from Streptomyces sp. A012304. Biosci. Biotechnol. Biochem. 691721-1725. [DOI] [PubMed] [Google Scholar]
- 4.Bechara, E. J. H., F. Dutra, V. E. Cardoso, A. Sartori, K. P. Olympio, C. A. Penatti, A. Adhikari, and N. A. Assunção. 2006. The dual face of endogenous α-aminoketones: pro-oxidizing metabolic weapons. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 14688-110. [DOI] [PubMed] [Google Scholar]
- 5.Bleuel, C., C. Grobe, N. Taudte, J. Scherer, D. Wesenberg, G. J. Kraub, D. H. Nies, and G. Grass. 2005. TolC is involved in enterobactin efflux across the outer membrane of Escherichia coli. J. Bacteriol. 1876701-6707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breckau, D., E. Mahlitz, A. Sauerwald, G. Layer, and D. Jahn. 2003. Oxygen-dependent coproporphyrinogen III oxidase (HemF) from Escherichia coli is stimulated by manganese. J. Biol. Chem. 27846625-46631. [DOI] [PubMed] [Google Scholar]
- 7.Christensen, O., E. M. Harvat, L. Thöny-Meyer, S. J. Ferguson, and J. M. Stevens. 2007. Loss of ATP hydrolysis activity by CcmAB results in loss of c-type cytochrome synthesis and incomplete processing of CcmE. FEBS J. 2742322-2332. [DOI] [PubMed] [Google Scholar]
- 8.Cox, R., and H. P. Charles. 1973. Porphyrin-accumulating mutants of Escherichia coli. J. Bacteriol. 113122-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dailey, H. A. 2002. Terminal steps of haem biosynthesis. Biochem. Soc. Trans. 30590-595. [DOI] [PubMed] [Google Scholar]
- 10.Doss, M., and W. K. Philipp-Dormston. 1971. Porphyrin and heme biosynthesis from endogenous and exogenous δ-aminolevulinic acid in Escherichia coli, Pseudomonas aeruginosa, and Achromobacter metalcaigenes. Hoppe-Seyler's Z. Physiol. Chem. 352725-733. [DOI] [PubMed] [Google Scholar]
- 11.Douki, T., J. Onuki, M. H. Medeiros, E. J. Bechara, J. Cadet, and P. Di Mascio. 1998. DNA alkylation by 4,5-dioxovaleric acid, the final oxidation product of 5-aminolevulinic acid. Chem. Res. Toxicol. 11150-157. [DOI] [PubMed] [Google Scholar]
- 12.Frustaci, J. M., and M. R. O'Brian. 1993. The Escherichia coli visA gene encodes ferrochelatase, the final enzyme of the heme biosynthetic pathway. J. Bacteriol. 1752154-2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ilag, L. L., D. Jahn, G. Eggertsson, and D. Söll. 1991. The Escherichia coli hemL gene encodes glutamate 1-semialdehyde aminotransferase. J. Bacteriol. 1733408-3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ineichen, G., and A. J. Biel. 1993. Location of the hemE gene on the physical map of Escherichia coli. J. Bacteriol. 1757749-7750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanjo, N., K. Nakahigashi, K. Oeda, and H. Inokuchi. 2001. Isolation and characterization of a cDNA from soybean and its homolog from Escherichia coli, which both complement the light sensitivity of Escherichia coli hemH mutant strain VS101. Genes Genet. Syst. 76327-334. [DOI] [PubMed] [Google Scholar]
- 16.Kawamura-Sato, K., K. Shibayama, T. Horii, Y. Iimuma, Y. Arakawa, and M. Ohta. 1999. Role of multiple efflux pumps in Escherichia coli in indole expulsion. FEMS. Microbiol. Lett. 179345-352. [DOI] [PubMed] [Google Scholar]
- 17.Koronakis, V., A. Sharff, E. Koronakis, B. Luisi, and C. Hughes. 2000. Crystal structure of the bacterial membrane protein TolC central to multidrug efflux and protein export. Nature 405914-919. [DOI] [PubMed] [Google Scholar]
- 18.Li, J. M., H. Umanoff, R. Proenca, C. S. Russell, and S. D. Cosloy. 1988. Cloning of the Escherichia coli K-12 hemB gene. J. Bacteriol. 1701021-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loewen, P. 1996. Probing the structure of catalase HPII of Escherichia coli—a review. Gene 17939-44. [DOI] [PubMed] [Google Scholar]
- 20.Ma, D., D. N. Cook, M. Alberti, N. G. Pon, H. Nikaido, and J. E. Hearst. 1993. Molecular cloning and characterization of acrA and acrE genes of Escherichia coli. J. Bacteriol. 1756299-6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyamoto, K., K. Nishimura, T. Masuda, H. Tsuji, and H. Inokuchi. 1992. Accumulation of protoporphyrin IX in light-sensitive mutants of Escherichia coli. FEBS Lett. 310246-248. [DOI] [PubMed] [Google Scholar]
- 22.Nakahigashi, K., K. Nishimura, K. Miyamoto, and H. Inokuchi. 1991. Photosensitivity of a protoporphyrin-accumulating, light-sensitive mutant (visA) of Escherichia coli K-12. Proc. Natl. Acad. Sci. USA 8810520-10524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nikaido, H. 1996. Multidrug efflux pumps of gram-negative bacteria. J. Bacteriol. 1785853-5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishimura, K., T. Nakayashiki, and H. Inokuchi. 1995. Cloning and identification of the hemG gene encoding protoporphyrinogen oxidase (PPO) of Escherichia coli K-12. DNA Res. 21-8. [DOI] [PubMed] [Google Scholar]
- 25.Nitzan, Y., M. Salmon-Divon, E. Sphoren, and Z. Malik. 2004. ALA induced photodynamic effects on gram positive and negative bacteria. Photochem. Photobiol. Sci. 3430-435. [DOI] [PubMed] [Google Scholar]
- 26.Panek, H., and M. R. O'Brian. 2002. A whole genome view of prokaryotic haeme biosynthesis. Microbiology. 1482273-2282. [DOI] [PubMed] [Google Scholar]
- 27.Philipp-Dormston, W. K., and M. Doss. 1973. Composition of porphyrin and heme biosynthesis in various heterotrophic bacteria. Enzyme 1657-64. [DOI] [PubMed] [Google Scholar]
- 28.Rocha, M. E., F. Dutra, B. Bandy, R. L. Baldini, S. L. Gomes, A. Faljoni-Alário, C. W. Liria, M. T. Miranda, and E. J. Bechara. 2003. Oxidative damage to ferritin by 5-aminolevulinic acid. Arch. Biochem. Biophys. 409349-356. [DOI] [PubMed] [Google Scholar]
- 29.Sasarman, A., A. Nepveu, Y. Echelard, J. Dymetryszyn, M. Drolet, and C. Goyer. 1987. Molecular cloning and sequencing of the hemD gene of Escherichia coli K-12 and preliminary data on the Uro operon. J. Bacteriol. 1694257-4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sãsãrman, A., P. Chartrand, R. Proschek, M. Desrochers, D. Tardif, and C. Lapointe. 1975. Uroporphyrin-accumulating mutant of Escherichia coli K-12. J. Bacteriol. 1241205-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stevens, J. M., T. Uchida, O. Daltrop, and S. J. Ferguson. 2005. Covalent cofactor attachment to proteins: cytochrome c biogenesis. Biochem. Soc. Trans. 33792-795. [DOI] [PubMed] [Google Scholar]
- 32.Szocs, K., F. Gabor, G. Csik, and J. Fidy. 1999. δ-Aminolaevulinic acid-induced porphyrin synthesis and photodynamic inactivation of Escherichia coli B. J. Photochem. Photobiol. B 508-17. [DOI] [PubMed] [Google Scholar]
- 33.Tegos, G. P., and M. R. Hamblin. 2006. Phenothiazinium antimicrobial photosensitizers are substrates of bacterial multidrug resistance pumps. Antimicrob. Agents Chemother. 50196-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thanassi, D. G., L. W. Cheng, and H. Nikaido. 1997. Active efflux of bile salts by Escherichia coli. J. Bacteriol. 1792512-2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas, S. D., and P. M. Jordan. 1986. Nucleotide sequence of the hemC locus encoding porphobilinogen deaminase of Escherichia coli K12. Nucleic Acids Res. 146215-6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thöny-Meyer, L. 1997. Biogenesis of respiratory cytochromes in bacteria. Microbiol. Mol. Biol. Rev. 61337-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tikhonova, E. B., and H. I. Zgurskaya. 2004. AcrA, AcrB, and TolC of Escherichia coli form a stable intermembrane multidrug efflux complex. J. Biol. Chem. 27932116-32124. [DOI] [PubMed] [Google Scholar]
- 38.Torres, V. J., D. L. Stauff, G. Pishchany, J. S. Bezbradica, L. E. Gordy, J. Iturregui, K. L. Anderson, P. M. Dunman, S. Joyce, and E. P. Skaar. 2007. A Staphylococcus aureus regulatory system that responds to host heme and modulates virulence. Cell Host Microbe 1109-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Touzé, T., J. Eswaran, E. Bokma, E. Koronakis, C. Hughes, and V. Koronakis. 2004. Interactions underlying assembly of the Escherichia coli AcrAB-TolC multidrug efflux system. Mol. Microbiol. 53697-706. [DOI] [PubMed] [Google Scholar]
- 40.Troup, B., C. Hungerer, and D. Jahn. 1995. Cloning and characterization of the Escherichia coli hemN gene encoding the oxygen-independent coproporphyrinogen III oxidase. J. Bacteriol. 1773326-3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Troup, B., M. Jahn, C. Hungerer, and D. Jahn. 1994. Isolation of the hemF operon containing the gene for the Escherichia coli aerobic coproporphyrinogen III oxidase by in vivo complementation of a yeast HEM13 mutant. J. Bacteriol. 176673-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verderber, E., L. J. Lucast, J. A. Van Dehy, P. Cozart, J. B. Etter, and E. A. Best. 1997. Role of the hemA gene product and δ-aminolevulinic acid in regulation of Escherichia coli heme synthesis. J. Bacteriol. 1794583-4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verkamp, E., V. M. Backman, J. M. Björnsson, D. Söll, and G. Eggertsson. 1993. The periplasmic dipeptide permease system transports 5-aminolevulinic acid in Escherichia coli. J. Bacteriol. 1751452-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Woodard, S. I., and H. A. Dailey. 1995. Regulation of heme biosynthesis in Escherichia coli. Arch. Biochem. Biophys. 316110-115. [DOI] [PubMed] [Google Scholar]
- 45.Yang, H., H. Inokuchi, and J. Adler. 1995. Phototaxis away from blue light by an Escherichia coli mutant accumulating protoporphyrin IX. Proc. Natl. Acad. Sci. USA 927332-7336. [DOI] [PMC free article] [PubMed] [Google Scholar]