Abstract
The acrS regulatory gene is located upstream of the acrEF multidrug efflux system genes. However, the roles of AcrS in regulation of drug efflux pumps have not been clearly understood. Here we show that AcrS represses other multidrug efflux genes, acrAB, which encode a major efflux system in Escherichia coli.
One of the important mechanisms underlying resistance to antibiotics involves extrusion of the compounds by drug efflux pumps. Drug efflux pumps are found in a variety of bacterial species, and their expression is often controlled by cognate regulatory proteins (14, 19). In Escherichia coli, a major drug efflux system, AcrAB, has a broad substrate range and confers intrinsic drug resistance (12-14). The acrR gene is located upstream of acrA, and the AcrR protein represses expression of the acrAB operon. Deletion or inactivation of acrR results in enhanced expression of acrAB and increases fluoroquinolone resistance in clinical E. coli strains (6, 23).
AcrEF also has a broad substrate range, similar to AcrAB. In contrast to acrAB, the expression level of acrEF is very low because of global repression by a histonelike protein, H-NS (15, 16). The acrS (formerly envR) gene is located upstream of acrE and encodes a putative repressor (9, 18).
In order to investigate the effects of AcrS and AcrR on the drug susceptibility of E. coli cells, the acrS or acrR gene was cloned into the pTrc99A expression vector. The resulting plasmids were transformed into the W3104 wild-type strain, and then the MICs of toxic compounds for these transformants were determined as described previously (15). When AcrS was overexpressed, the intrinsic tolerance of W3104 for several toxic compounds was drastically decreased (Table 1). On the other hand, AcrR overexpression did not affect the MICs, except for eightfold decreases in the crystal violet and methylene blue MICs. The ΔacrAB mutant was hypersensitive to various antibiotics, as shown in Table 1. Overexpression of acrS or acrR did not affect the drug susceptibility of ΔacrAB, indicating that the effect of AcrS overexpression on the drug tolerance of the wild-type strain is mediated by AcrAB. We previously reported that deletion of the hns gene increases acrEF expression and results in an AcrEF-dependent multidrug resistance phenotype in the ΔacrAB genetic background (16). The drug susceptibilities of W3104 Δhns ΔacrAB were hardly affected even when AcrS and AcrR were overexpressed (Table 1), suggesting that overexpression of neither AcrS nor AcrR suppresses the expression of acrEF. We also examined the effect of deletion of acrR and/or acrS. The deletion mutants were constructed by a gene replacement method using the pKO3 plasmid (8). Neither deletion of acrR nor deletion of acrS affected the drug susceptibilities, with the exception of susceptibility to novobiocin. Deletion of acrS increased the MIC of novobiocin for W3104 (data not shown).
TABLE 1.
Susceptibility of E. coli repressor-overproducing strains to antibiotics and toxic compounds
| Compound | MIC (μg/ml)a
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Wild type strain W3104
|
ΔacrAB mutant
|
Δhns ΔacrAB mutant
|
|||||||
| No repressor | AcrR repressor | AcrS repressor | No repressor | AcrR repressor | AcrS repressor | No repressor | AcrR repressor | AcrS repressor | |
| Chloramphenicol | 6.25 | 3.13 | 0.78 | 0.78 | 0.78 | 0.78 | 1.56 | 0.78 | 1.56 |
| Tetracycline | 6.25 | 3.13 | 1.56 | 1.56 | 1.56 | 1.56 | 1.56 | 1.56 | 1.56 |
| Erythromycin | 100 | 100 | 6.25 | 6.25 | 6.25 | 6.25 | 25 | 25 | 25 |
| Nalidixic acid | 3.13 | 1.56 | 0.78 | 0.78 | 0.78 | 0.78 | 1.56 | 0.78 | 1.56 |
| Norfloxacin | 0.05 | 0.025 | 0.025 | 0.025 | 0.025 | 0.025 | 0.05 | 0.025 | 0.05 |
| Novobiocin | 1,600 | 800 | 25 | 12.5 | 12.5 | 12.5 | 50 | 50 | 50 |
| Acriflavine | 200 | 100 | 12.5 | 6.25 | 12.5 | 6.25 | 25 | 25 | 25 |
| Crystal violet | 25 | 3.13 | 0.78 | 0.78 | 1.56 | 0.78 | 6.25 | 3.13 | 3.13 |
| Ethidium | 400 | 200 | 25 | 12.5 | 12.5 | 12.5 | 200 | 200 | 200 |
| Methylene blue | >1,600 | 400 | 12.5 | 6.25 | 6.25 | 6.25 | 400 | 400 | 400 |
| Rhodamine 6G | 400 | 200 | 3.13 | 3.13 | 6.25 | 3.13 | 200 | 200 | 200 |
| Tetraphenylphosphonium | 1,600 | 800 | 12.5 | 12.5 | 6.25 | 12.5 | 50 | 25 | 25 |
| Benzalkonium | 50 | 25 | 3.13 | 3.13 | 3.13 | 3.13 | 12.5 | 12.5 | 6.25 |
| Sodium dodecyl sulfate | >25,600 | >25,600 | 200 | 50 | 50 | 50 | 200 | 200 | 100 |
| Deoxycholate | >40,000 | >40,000 | 2,500 | 2,500 | 5,000 | 2,500 | 40,000 | 40,000 | 20,000 |
MIC determination experiments were repeated at least three times.
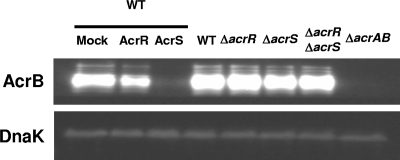
Immunoblotting with anti-AcrB antibody showed that overexpression of acrS decreased the level of production of the AcrB protein (Fig. 1). On the other hand, in cells overexpressing acrR, the level of production of AcrB was moderately decreased. The transcription level of each transporter gene was also examined by quantitative real-time PCR (as described previously) (3) using an AcrEF-overproducing strain, W3104 Δhns. The presence of the acrS expression plasmid decreased the transcriptional level of acrA 310-fold, while the decrease was only moderate with acrR-expressing plasmids (2.4-fold). On the other hand, the acrE transcriptional level was slightly or hardly decreased by acrS and acrR overexpression (2.8- and 1.3-fold decrease, respectively). These results are consistent with greater potency of AcrS for acrA repression than for acrE repression. AcrS also represses the expression of acrA more efficiently than AcrR does. It is known that acrAB expression is also controlled by the global regulators MarA, SoxS, and Rob (4, 5, 10, 21). However, the expression of these regulators was not affected by AcrS and AcrR (data not shown), indicating that the acrAB repression by AcrR and AcrS is unlikely to be mediated by MarA, SoxS, or Rob. Thus, AcrS is an effective repressor of acrAB but not of acrEF. Therefore, the low level of expression of AcrEF is not due to AcrS. Moreover, our observations are consistent with the results of a study that showed that AcrS does not appear to act as a local repressor of acrEF in Salmonella enterica serovar Typhimurium (17).
FIG. 1.
Detection of AcrB expression in the repressor-overexpressing strain. W3104 (which harbors pTrc99A, pTrc99acrR, and pTrc99acrS), W3104 ΔacrR, W3104 ΔacrS, W3104 ΔacrR ΔacrS, and W3104 ΔacrAB were grown to an optical density at 600 nm of 0.8 in LB medium containing 0.1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) and harvested. AcrB and DnaK (control) in 3 μg of cell lysate protein were analyzed by Western blotting with polyclonal anti-AcrB antibodies for AcrB or a monoclonal anti-DnaK antibody (Calbiochem) and alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin G for AcrB (Bio-Rad Laboratories, Hercules, CA) or anti-mouse immunoglobulin G for DnaK, and these proteins were detected by using the CDP-Star substrate (GE Healthcare BioScience). WT, wild type.
To evaluate whether AcrR and AcrS directly regulate acrAB expression, a DNase I footprinting analysis was performed. AcrR-His6 and AcrS-His6 fusion proteins were purified from E. coli crude soluble lysate using nickel affinity resin (GE Healthcare BioScience). The 312-bp DNA fragments including the acrA promoter (229 bp of the upstream region and 83 bp of the coding region) were labeled with 6-carboxyfluorescein (6-FAM) fluorophores. The probes (0.45 pmol) were mixed and incubated for 20 min at room temperature with AcrR-His6 and AcrS-His6, and then DNase I footprinting analysis was performed using a previously described nonradiochemical capillary electrophoresis method and an ABI PRISM 310 sequencer/genetic analyzer equipped with an ABI PRISM 310 GeneScan (2, 24). Both AcrR and AcrS directly bound to the acrA promoter containing the previously predicted 24-bp palindrome sequence (TACATACATT-TATG-AATGTATGTA) (20). This region was protected from DNase I digestion by adding 4.3 pmol of AcrR or AcrS (Fig. 2). To compare the binding affinity of AcrS with the binding affinity of AcrR, we performed an electrophoretic mobility shift assay. A total of 312 bp, including 229 bp upstream and 83 bp of the coding region, and 276 bp upstream of the start codon were used as acrA and acrD DNA fragments, respectively. The acrA and acrD probes (0.15 pmol) were mixed and incubated for 20 min at room temperature with AcrR-His6 and AcrS-His6, respectively. Samples were electrophoresed, and SYBR green I (Lonza)-stained DNAs were visualized under blue incident light at 460 nm using an LAS-3000 luminescent image analyzer (Fujifilm). The electrophoretic mobility shift assay revealed that the acrA probe was almost completely shifted in the presence of 4.5 pmol AcrS, whereas the shift of the acrA probe was not observed at the same concentration of AcrR (Fig. 3). For detection of the shift, 13.5 pmol AcrR was required, indicating that the binding affinity of AcrS for the acrA promoter region is higher than that of AcrR. Hence, the differences in the degree of acrA repression between AcrS and AcrR can be explained in part by the difference in their binding affinities.
FIG. 2.
DNase I footprinting analysis of AcrR or AcrS binding to the acrA promoter region. A DNA fragment (0.45 pmol) including the acrA promoter region was labeled with 6-FAM at the 5′ end, incubated with AcrR-His6 or AcrS-His6 (4.3 to 69 pmol) in a reaction solution containing 20 mM HEPES-Na (pH 7.5) and 1 mM dithiothreitol, and then subjected to DNase I footprinting assays. The fluorescence intensity (ordinate) of 6-FAM-labeled DNA fragments is plotted against the sequence of the fragment (abscissa). Protein-binding sites are enclosed in rectangles.
FIG. 3.
Electrophoretic mobility shift assay for AcrR and AcrS binding to the acrA promoter. DNA fragments (0.15 pmol) including the acrA (312 bp) and acrD (276 bp; control) promoter regions were incubated without or with various concentrations of AcrR-His6 and AcrS-His6 in a reaction solution containing 20 mM HEPES-Na (pH 7.5) and 1 mM dithiothreitol. Lane 1, no repressor; lanes 2, 3, 4, and 8, AcrR protein (lane 2, 1.5 pmol; lane 3, 4.5 pmol; lanes 4 and 8, 13.5 pmol); lanes 5, 6, 7, and 9, AcrS protein (lane 5, 1.5 pmol; lane 6, 4.5 pmol; lanes 7 and 9, 13.5 pmol). Samples were electrophoresed on a 5% nondenaturing polyacrylamide gel.
acrS is adjacent to acrE, and each gene is divergently transcribed. We examined the relationship between the promoter activities of acrS and acrE. For construction of reporter plasmids, DNA fragments containing the acrE promoter (previously constructed by Hirakawa et al. and Kobayashi et al. [2, 7]) and the acrS promoter (400 bp upstream of the start codon) were cloned in front of the β-galactosidase-encoding lacZ reporter gene in a single-copy pNN387 vector (1). The resulting plasmids were introduced into endogenous LacZ-negative strains (MC4100 and MC4100 Δhns), and β-galactosidase activity was assayed in cell lysates using o-nitrophenyl-β-d-galactopyranoside as the substrate (11). In the wild-type strain, the promoter activity of acrS was very low and similar to that of acrE (less than 1.0 Miller unit). The lack of an effect of acrS deletion on AcrAB repression was probably due to the low level of expression of AcrS. When the hns gene was deleted, the promoter activity of acrS greatly increased, indicating that transcription of acrS was stimulated simultaneously with the increase in AcrE expression (11.1 Miller units for acrE and 8.4 Miller units for acrS).
AcrAB and AcrEF may have common physiological roles and features because they have similar broad substrate spectra and high sequence homology (15). Therefore, when the expression of AcrEF is induced, AcrAB may no longer be required and the production is shut down to prevent excess protein production by AcrS. Thus, we believe that AcrS functions as a switch for the alternative expression of AcrAB and AcrEF. However, what compound and/or condition induces the expression of AcrS remains unknown. The cross-regulation of RND efflux pumps involving their local regulators has been reported for Pseudomonas putida (22). In the future we plan to investigate the physiological implications of the switch for alternative expression of AcrAB and AcrEF.
Acknowledgments
We thank George M. Church for providing plasmid pKO3, Ronald W. Davis for providing plasmid pNN387, Takeshi Honda and Toshio Kodama for their technical support during the analysis of the sequence of the AcrS and AcrR proteins, and E. Peter Greenberg for constructive comments.
Hidetada Hirakawa was supported by a research fellowship from the Japan Society for the Promotion of Science for Young Scientists. This work was supported by a grant-in-aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan; by the Japan Society for the Promotion of Science; by CREST and PRESTO, Japan Science and Technology Agency, Japan; by the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation; by the Japan Research Foundation for Clinical Pharmacology; by the Takeda Science Foundation; and by the Inamori Foundation.
Footnotes
Published ahead of print on 20 June 2008.
REFERENCES
- 1.Elledge, S. J., and R. W. Davis. 1989. Position and density effects on repression by stationary and mobile DNA-binding proteins. Genes Dev. 3185-197. [DOI] [PubMed] [Google Scholar]
- 2.Hirakawa, H., Y. Inazumi, T. Masaki, T. Hirata, and A. Yamaguchi. 2005. Indole induces the expression of multidrug exporter genes in Escherichia coli. Mol. Microbiol. 551113-1126. [DOI] [PubMed] [Google Scholar]
- 3.Hirakawa, H., K. Nishino, T. Hirata, and A. Yamaguchi. 2003. Comprehensive studies of drug resistance mediated by overexpression of response regulators of two-component signal transduction systems in Escherichia coli. J. Bacteriol. 1851851-1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jair, K. W., W. P. Fawcett, N. Fujita, A. Ishihama, and R. E. Wolf, Jr. 1996. Ambidextrous transcriptional activation by SoxS: requirement for the C-terminal domain of the RNA polymerase alpha subunit in a subset of Escherichia coli superoxide-inducible genes. Mol. Microbiol. 19307-317. [DOI] [PubMed] [Google Scholar]
- 5.Jair, K. W., X. Yu, K. Skarstad, B. Thony, N. Fujita, A. Ishihama, and R. E. Wolf, Jr. 1996. Transcriptional activation of promoters of the superoxide and multiple antibiotic resistance regulons by Rob, a binding protein of the Escherichia coli origin of chromosomal replication. J. Bacteriol. 1782507-2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jellen-Ritter, A. S., and W. V. Kern. 2001. Enhanced expression of the multidrug efflux pumps AcrAB and AcrEF associated with insertion element transposition in Escherichia coli mutants selected with a fluoroquinolone. Antimicrob. Agents Chemother. 451467-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobayashi, A., H. Hirakawa, T. Hirata, K. Nishino, and A. Yamaguchi. 2006. The growth phase-dependent expression of drug exporters in Escherichia coli and its contribution to the drug tolerance. J. Bacteriol. 1885693-5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Link, A. J., D. Phillips, and G. M. Church. 1997. Methods for generating precise deletions and insertions in the genome of wild-type Escherichia coli: application to open reading frame characterization. J. Bacteriol. 1796228-6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma, D., D. N. Cook, J. E. Hearst, and H. Nikaido. 1994. Efflux pumps and drug resistance in gram-negative bacteria. Trends Microbiol. 2489-493. [DOI] [PubMed] [Google Scholar]
- 10.Martin, R. G., K. W. Jair, R. E. Wolf, Jr., and J. L. Rosner. 1996. Autoactivation of the marRAB multiple antibiotic resistance operon by the MarA transcriptional activator in Escherichia coli. J. Bacteriol. 1782216-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller, J. H. 1992. A short course in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 12.Murakami, S., R. Nakashima, E. Yamashita, T. Matsumoto, and A. Yamaguchi. 2006. Crystal structures of a multidrug transporter reveal a functionally rotating mechanism. Nature 443173-179. [DOI] [PubMed] [Google Scholar]
- 13.Murakami, S., R. Nakashima, E. Yamashita, and A. Yamaguchi. 2002. Crystal structure of bacterial multidrug efflux transporter AcrB. Nature 419587-593. [DOI] [PubMed] [Google Scholar]
- 14.Nikaido, H. 1998. Multiple antibiotic resistance and efflux. Curr. Opin. Microbiol. 1516-523. [DOI] [PubMed] [Google Scholar]
- 15.Nishino, K., and A. Yamaguchi. 2001. Analysis of a complete library of putative drug transporter genes in Escherichia coli. J. Bacteriol. 1835803-5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishino, K., and A. Yamaguchi. 2004. Role of histone-like protein H-NS in multidrug resistance of Escherichia coli. J. Bacteriol. 1861423-1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olliver, A., M. Valle, E. Chaslus-Dancla, and A. Cloeckaert. 2005. Overexpression of the multidrug efflux operon acrEF by insertional activation with IS1 or IS10 elements in Salmonella enterica serovar typhimurium DT204 acrB mutants selected with fluoroquinolones. Antimicrob. Agents Chemother. 49289-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan, W., and B. G. Spratt. 1994. Regulation of the permeability of the gonococcal cell envelope by the mtr system. Mol. Microbiol. 11769-775. [DOI] [PubMed] [Google Scholar]
- 19.Poole, K. 2004. Efflux-mediated multiresistance in Gram-negative bacteria. Clin. Microbiol. Infect. 1012-26. [DOI] [PubMed] [Google Scholar]
- 20.Rodionov, D. A., M. S. Gelfand, A. A. Mironov, and A. B. Rakhmaninova. 2001. Comparative approach to analysis of regulation in complete genomes: multidrug resistance systems in gamma-proteobacteria. J. Mol. Microbiol. Biotechnol. 3319-324. [PubMed] [Google Scholar]
- 21.Rosenberg, E. Y., D. Bertenthal, M. L. Nilles, K. P. Bertrand, and H. Nikaido. 2003. Bile salts and fatty acids induce the expression of Escherichia coli AcrAB multidrug efflux pump through their interaction with Rob regulatory protein. Mol. Microbiol. 481609-1619. [DOI] [PubMed] [Google Scholar]
- 22.Terán, W., A. Felipe, S. Fillet, M. Guazzaroni, T. Krell, R. Ruiz, J. Ramos, and M. Gallegos. 2007. Complexity in efflux pump control: cross-regulation by the paralogues TtgV and TtgT. Mol. Microbiol. 661416-1428. [DOI] [PubMed] [Google Scholar]
- 23.Wang, H., J. L. Dzink-Fox, M. Chen, and S. B. Levy. 2001. Genetic characterization of highly fluoroquinolone-resistant clinical Escherichia coli strains from China: role of acrR mutations. Antimicrob. Agents Chemother. 451515-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson, D. O., P. Johnson, and B. R. McCord. 2001. Nonradiochemical DNase I footprinting by capillary electrophoresis. Electrophoresis 221979-1986. [DOI] [PubMed] [Google Scholar]