Abstract
Clostridium perfringens type B and type C isolates, which produce beta-toxin (CPB), cause fatal diseases originating in the intestines of humans or livestock. Our previous studies demonstrated that CPB is necessary for type C isolate CN3685 to cause bloody necrotic enteritis in a rabbit ileal loop model and also showed that purified CPB, in the presence of trypsin inhibitor (TI), can reproduce type C pathology in rabbit ileal loops. We report here a more complete characterization of the effects of purified CPB in the rabbit small and large intestines. One microgram of purified CPB, in the presence of TI, was found to be sufficient to cause significant accumulation of hemorrhagic luminal fluid in duodenal, jejunal, or ileal loops treated for 6 h with purified CPB, while no damage was observed in corresponding loops receiving CPB (no TI) or TI alone. In contrast to the CPB sensitivity of the small intestine, the colon was not affected by 6 h of treatment with even 90 μg of purified CPB whether or not TI was present. Time course studies showed that purified CPB begins to induce small intestinal damage within 1 h, at which time the duodenum is less damaged than the jejunum or ileum. These observations help to explain why type B and C infections primarily involve the small intestine, establish CPB as a very potent and fast-acting toxin in the small intestines, and confirm a key role for intestinal trypsin as an innate intestinal defense mechanism against CPB-producing C. perfringens isolates.
Clostridium perfringens is an anaerobic, spore-forming, gram-positive pathogen of humans and domestic animals (10). The virulence of C. perfringens is largely attributable to its prolific secretion of toxins. However, no single isolate produces all of the more than 15 toxins reported in the literature, providing the basis for a commonly used classification scheme that assigns C. perfringens isolates to one of five types (A to E), depending upon their production of four (alpha, beta, epsilon, and iota) typing toxins (10, 15). C. perfringens type B or C isolates both produce alpha- and beta-toxins (CPA and CPB, respectively). In addition, type B isolates also produce epsilon-toxin, a potent neurotoxin listed as a class B select agent by the Centers for Disease Control and Prevention/U. S. Department of Agriculture.
Type B isolates cause an often-fatal hemorrhagic dysentery in sheep, and possibly in other species, that is accompanied by sudden death or acute neurological signs (23). Intestinal lesions of those infected animals are characterized by diffuse necrohemorrhagic enteritis, predominantly in the ileum, with serosanguineous fluid in the abdominal cavity (27). There is currently limited information regarding the pathogenesis of type B-associated diseases, although some evidence indicates that both CPB and epsilon-toxin may contribute to lethality (3).
C. perfringens type C isolates also cause fatal diseases ranging from enteritis to enterotoxemia, predominantly in newborn animals of most livestock species. Infected animals typically show necrohemorrhagic enteritis, which can result in death due to direct intestinal damage or, probably more commonly, from toxemia after the absorption of toxins from the intestines into the circulation (23, 24). Type C-associated diseases annually result in serious economic losses for the agricultural industry.
In humans, C. perfringens type C isolates cause enteritis necroticans (also known as Darmbrand or Pigbel), a disease that is endemic in much of Southeast Asia but particularly Papua New Guinea (6). Although less common, this disease also occurs in diabetic patients from developed countries. Persons suffering from enteritis necroticans often survive less than 48 h after the first appearance of symptoms (9, 16, 21, 26). Histologically, the disease is characterized by necrotic enteritis and the presence of numerous bacteria in the intestinal lumen (29). Immunohistochemistry studies using anti-CPB antibodies showed the presence of CPB on the necrotic intestinal epithelium of humans suffering from type C infection (9).
CPB is a 35-kDa protein that forms pores in the membrane of susceptible cell lines, which leads to swelling and cell lysis (12, 17, 25). CPB is also lethal for mice, with a calculated 50% lethal dose of 1.87 μg/per kg of body weight when administered via the intraperitoneal route. A relatively crude beta-toxoid was shown to protect animals and humans against type C infection (5, 28), suggesting that CPB is important for the virulence of type C isolates. However, the lack of a good small animal model and difficulties in producing C. perfringens mutants had prevented fulfilling molecular Koch's postulates to establish a definitive relationship between CPB and type C virulence. In response, we recently developed a rabbit ileal loop model for type C disease and improved mutagenesis techniques for C. perfringens. These advances were used to construct a series of C. perfringens type C toxin-null mutants, which demonstrated that beta-toxin, but not perfringolysin O (PFO) or CPA, is necessary and sufficient for type C isolates to cause damage in rabbit ileal loops (19). Moreover, CPB is sufficient to damage ileal loops since purified CPB reproduced the natural pathology of type C disease in rabbit ileal loops, provided that trypsin inhibitor (TI) was present to prevent CPB degradation by endogenous trypsin (19).
This TI requirement to obtain CPB activity in rabbit ileal loops reflects natural type C disease in animals and humans. Risk factors for developing necrotizing enteritis from type C isolates include low trypsin production due to a protein-poor diet or pancreatic disease and consumption of foods, such as sweet potato, containing a high concentration of a TI. Also, the colostrum ingested by newborn animals has powerful inhibitory properties against trypsin. These risk factors contribute to the persistence of trypsin-sensitive CPB in the gastrointestinal tract during type C infection (10).
Despite these recent advances, the intestinal effects of CPB remain poorly characterized. Therefore, this study used the rabbit intestinal loop model to investigate the pathological effects of purified CPB in the colons, duodena, jejuna, and ilea of rabbits.
MATERIALS AND METHODS
Strain and bacterial culture media.
C. perfringens type C strain CN3685 (plc+, pfoA+, cpb+, and tpeL+), which was isolated from the peritoneal fluid of a sheep with struck (a type C infection of adult sheep), was used to purify beta-toxin, as described below. The bacterial culture media used throughout the present study included fluid thioglycolate medium (Difco Laboratories), TGY (3% tryptic soy broth [Becton Dickinson], 2% glucose [Sigma Aldrich], 1% yeast extract [Becton Dickinson], 0.1% sodium thioglycolate [Sigma Aldrich]), and TSC agar medium (SFP agar [Difco Laboratories] supplemented with 0.04% d-cycloserine [Sigma Aldrich]).
Purification of CPB protein.
An isolated colony of CN3685 from a TSC agar plate was inoculated into fluid thioglycolate medium and grown overnight at 37°C. An aliquot (0.1 ml) of this overnight culture was then transferred to 30 ml of TGY and grown at 37°C for ∼8 h. The 30-ml culture was transferred to 3 liters of fresh TGY and grown at 37°C for another ∼8 h. The culture was then chilled immediately on ice for 10 min and centrifuged at 10,000 × g for 20 min. Proteins in the culture supernatant were precipitated using 40% ammonium sulfate (Fisher Scientific), with constant stirring, at 4°C for ∼1 h. The precipitate was then collected by centrifugation at 10,000 × g for 30 min. The pellet resulting from the 40% saturation ammonium sulfate cut was resuspended in 40 ml of 30 mM Tris-HCl buffer (pH 7.5) and dialyzed overnight against the same buffer (4 l), with several changes, at 4°C. After the dialyzed solution was again centrifuged at 10,000 × g for 30 min, the supernatant was filtered through a 0.45-μm-pore-size filter (Millipore) and loaded onto a DEAE-CL6B Sepharose column (Sigma). This column was pre-equilibrated with 30 mM Tris-HCl buffer (pH 7.5) in an ÄKTA prime system (Amersham Bioscience). After loading of the sample, the DEAE-CL6B column was washed with 45 ml of 30 mM Tris-HCl buffer (pH 7.5), and bound CPB was then eluted from the column using a gradient of NaCl (0 to100 mM) in 30 mM Tris-HCl buffer (pH 7.5). Fractions were assessed for the presence of CPB by Western blotting using a mouse monoclonal anti-CPB antibody obtained from P. Hauer (Center for Veterinary Biologics, Ames, Iowa). Fractions containing the purified CPB were pooled and dialyzed with ice-cold phosphate-buffered saline (PBS [pH 7.4]) at 4°C overnight. Pooled fractions were then concentrated by ultrafiltration using an Ultrafree 10-kDa cutoff centrifugal filter device (Millipore) and stored at −80°C. The final concentration of purified CPB was estimated by Lowry assay, using bovine serum albumin as the standard (8).
Analysis of the CPB toxin preparation purity.
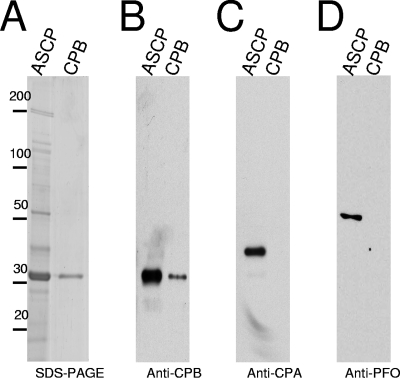
CPB purity was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and densitometric analysis, which showed the preparation to be ∼95% homogeneous. An aliquot (25 μl) of the ammonium sulfate supernatant-concentrated proteins or 1 μg of purified CPB was electrophoresed in a SDS-12% PAGE gel and then either stained with Coomassie blue or electrophoresed and transferred to nitrocellulose membrane. The membrane was then probed using a monoclonal anti-CPB antibody, a monoclonal anti-CPA antibody, or a rabbit polyclonal anti-PFO antibody. These analyses show no contamination in our CPB toxin preparation with CPA or PFO (Fig. 1).
FIG. 1.
Purity analysis of CPB preparations. To purify CPB, proteins in the supernatant of C. perfringens type C isolate CN3586 were concentrated by using 40% of ammonium sulfate saturation. CPB was purified by anion-exchange chromatography as described in Materials and Methods. (A) An aliquot of ammonium sulfate supernatant-concentrated proteins (ASCP) and 1 μg of purified CPB (CPB) were subjected to SDS-12% PAGE and stained with Coomassie blue or electrophoresed and transferred to nitrocellulose membrane. The membrane was immunoblotted with a mouse monoclonal anti-CPB antibody (B), a mouse monoclonal anti-CPA antibody (C), or a rabbit polyclonal anti-PFO antibody (D). Bound antibody was detected with a horseradish peroxidase-conjugated secondary anti-mouse or -rabbit IgG antibody and incubation of blots with a chemiluminescent substrate. The numbers at the left are in kilodaltons.
Effects of purified beta-toxin on rabbit intestinal loops. (i) Inoculum.
In all experiments an ∼1-ml aliquot of a Ringer's solution containing specified amounts of purified CPB, without or with 150 μg of TI/ml, was injected into each intestinal loop. For the dose-response experiments a mixture containing Ringer's solution with 1, 5, 10, or 20 μg of purified CPB and TI/ml was injected into each loop. In time course experiments a mixture of Ringer's solution with 10 μg of purified CPB and TI/ml was injected in each loop. Alternatively, some loops received (i) an injection of Ringer's solution containing only 20 μg of purified CPB (no TI)/ml or (ii) a mixture of Ringer's solution containing TI and purified CPB (10 μg/ml) that had been preincubated for 45 min at room temperature on a rocking platform with a neutralizing anti-CPB monoclonal antibody (MAb; 200 μg) or anti-CPA MAb (200 μg) (provided by P. Hauer). The amount of anti-CPB and anti-CPA used was the minimum amount that was protective in a mouse intravenous bioassay (unpublished observation). In all experiments, control loops received a similar volume (∼1 ml) of sterile Ringer's solution containing 150 μg of TI/ml.
(ii) Rabbit loop model.
Fasted young adult, male or female, New Zealand White rabbits (Charles River, California) were premedicated with acepromazine, xylazine, and burprenorphine. Anesthesia was then induced with ketamine and maintained with inhalatory isofluorane. A laparotomy was performed via the mid line, and the small intestine or colon was exposed. Lengths (∼2 cm) of each individual intestinal section (duodenum, jejunum, ileum, or colon) were isolated by ligation, leaving an empty segment of gut between the loops. Care was taken to avoid overdistension of bowel loops and interference with the blood supply, eliminating a possible ischemic component to the toxin-induced damage. The colon content was washed with saline solution injected into the lumen, followed by a gentle massage before colonic loops were prepared. During surgery, the serosal surface of the loops was kept wet by frequent soaking with normal saline solution. After injecting the inoculum, the abdominal incision was closed by separate muscle and skin sutures, and the animals were kept deeply anesthetized throughout the experiment.
(iii) Measurement of fluid accumulation and histological analyses.
After a 6-h (dose-response experiment) or a 15-, 30-, or 60-min or 6-h (time course study) CPB treatment, the rabbits were euthanized by an overdose of sodium barbiturate (Beuthanasia, Schering-Plough Animal Health, Kenilworth, NJ). The abdominal cavity was then reopened, and the small intestinal loops were excised in the same order that they had been inoculated. Loops were cut out and weighed, before and after the fluid was removed, and the length was measured. Fluid secretion was expressed as the loop weight-to-length ratio (mg/cm).
For histological analysis, all tissues were fixed by immersion in 10% buffered (pH 7.4) formalin for 24 to 48 h, followed by dehydration through graded alcohols to xylene before being embedded in paraffin wax. Sections (4 μm thick) were cut and stained with hematoxylin and eosin according to standard procedures. Tissue sections were examined by a pathologist in a blinded fashion, using a quantitative scoring system as described previously (19). Briefly, the degree of damage was scored by using a scale of 1 to 5. On this scale, a “1” indicates no histologic damage, while “2,” “3,” “4.” and “5” values indicate increasingly severe damage. Histologic parameters considered in this evaluation included mucosal necrosis, desquamation of the epithelium, inflammation, villous blunting, edema, and hemorrhage. Sections of representative treated or control tissues were then photomicrographed using an Olympus microscope (Tokyo, Japan) at a 100× or a 200× final magnification. All procedures were reviewed and approved by the University of California, Davis Committee for Animal Care and Use (permit 04-11593).
Statistical analyses.
To statistically validate our results, each experiment was performed with at least six repetitions in six different animals. All statistical analyses were done by using the Minitab 15 software. The fluid accumulation data were analyzed by using two-way analysis of variance with the post hoc test. The histology data were analyzed by using the Friedman test.
RESULTS
Effects of purified CPB on fluid accumulation in rabbit intestinal loops.
We recently demonstrated that purified CPB can cause intestinal lesions in rabbit ileal loops (19). To characterize and compare the activity of CPB in various parts of the intestine, we first examined the effect of increasing doses of CPB purified (Fig. 1) to near homogeneity (∼95%), along with TI, in loops constructed in the rabbit jejunum, duodenum, ileum, or colon.
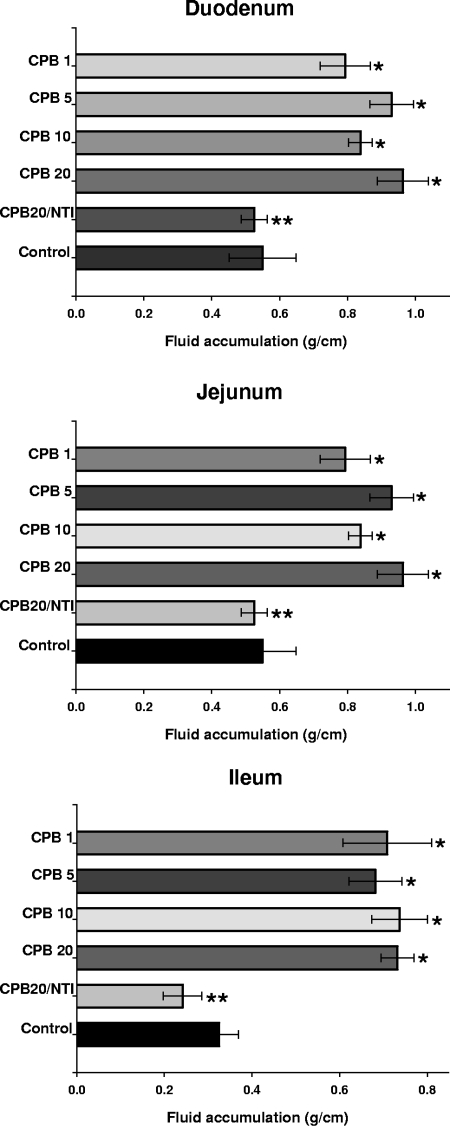
Compared to their corresponding control loops, 6-h treatment with the lowest dose of purified CPB tested (1 μg) induced a very conspicuous fluid accumulation in rabbit loops made in the duodenum, jejunum, or ileum (Fig. 2 and 3). The fluid accumulation with all of the doses of CPB tested was statistically significant in CPB-treated loops versus their corresponding control loops (P < 0.05). However, no statistically significant difference was observed in fluid accumulation between any of the small intestinal segments treated with any of the doses of CPB tested (Fig. 3). This indicated that a 6-h treatment with 1 μg of CPB was sufficient to induce near-maximal luminal fluid accumulation in the rabbit small intestine. The intestinal loop fluid, however, became progressively bloodier as the CPB dose increased.
FIG. 2.
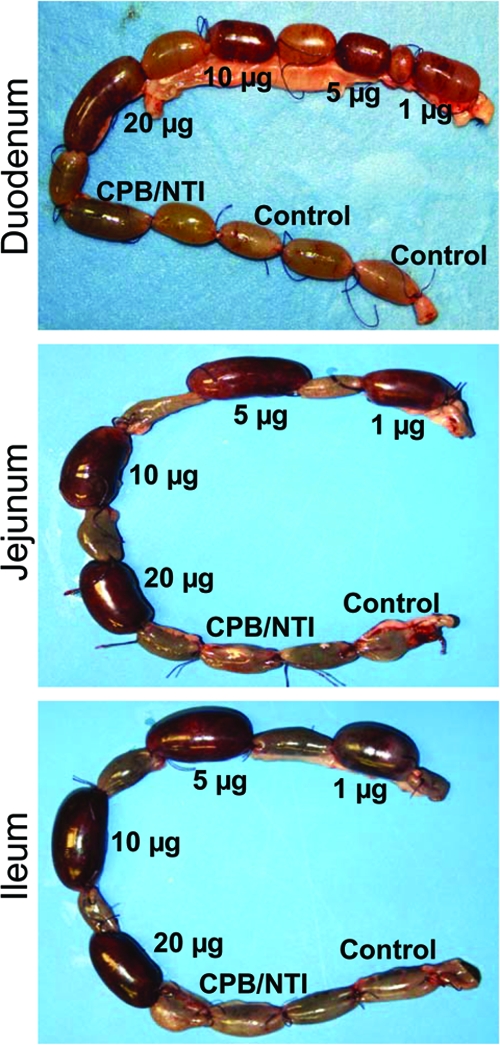
Gross pathology of rabbit duodenal (top), jejunal (middle), or ileal (bottom) loops treated with Ringer's solution containing specified doses (1, 5, 10, or 20 μg) of CPB and TI (150 μg/ml); 20 μg of purified CPB with no TI (CPB/NTI); or 150 μg of TI/ml (control). After treatment, loops were incubated for 6 h. Note that as the dose of CPB increased, the bloody fluid content in all loops receiving both purified CPB and TI also increased. No hemorrhagic fluid was observed in loops receiving an injection of CPB alone (no TI) or TI alone (no CPB). The data shown are representative of six repetitions.
FIG. 3.
Fluid accumulation of rabbit intestinal loops treated with different doses of purified CPB. Rabbit duodenal (top), jejunal (middle), or ileal (bottom) loops were treated with Ringer's solution containing 1 (CPB 1), 5 (CPB 5), 10 (CPB 10), or 20 (CPB 20) μg of purified CPB and 150 μg of TI/ml; 20 μg of purified CPB with no TI (CPB20/NTI); or Ringer's solution and 150 μg of TI/ml (control). After a 6-h treatment, fluid accumulation was recorded as described in Materials and Methods. Fluid accumulation in all loops injected with any dose of CPB plus TI was statistically different from control loops (*, P > 0.05) or loops treated with CPB and no TI (**, P > 0.05). Every experiment was independently performed six times; the data shown represent the mean of these studies, and small bars represent standard errors.
To confirm that the observed fluid accumulation and the presence of luminal blood were induced by CPB rather than by a contaminant, the purified CPB (20 μg) was preincubated with a neutralizing anti-CPB MAb prior to its injection into ileal loops. This CPB MAb preincubation completely eliminated the ability of the toxin preparation to cause bloody fluid accumulation in rabbit ileal loops (data not shown). A similar preincubation of purified CPB with a neutralizing MAb against alpha-toxin had no inhibitory effect on CPB ileal loop activity (data not shown).
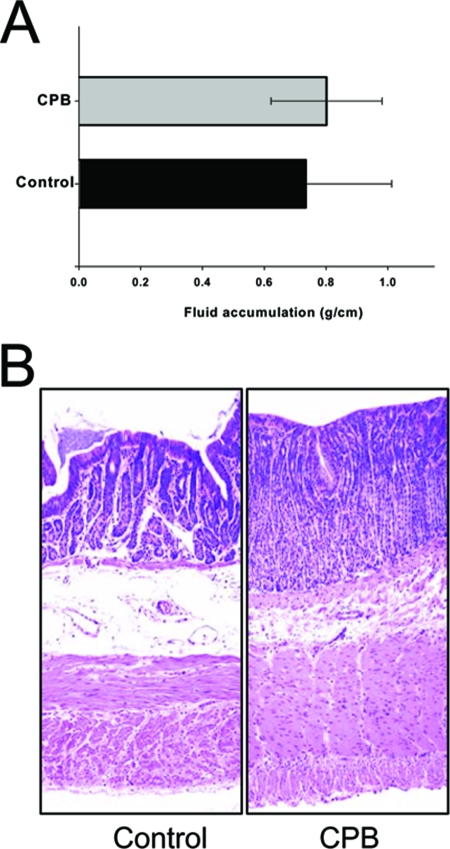
In contrast to the observed effect of very small amounts of purified CPB plus TI on all three small intestinal regions, a 6-h treatment with even 90 μg of purified CPB/ml, whether injected in the presence or absence of TI, did not induce fluid accumulation or hemorrhage in the rabbit colon (Fig. 4A). These results indicated that purified CPB specifically elicits abundant fluid accumulation and hemorrhage in the rabbit small intestine.
FIG. 4.
The rabbit colon is not affected by purified CPB. Rabbit colonic loops were treated for 6 h with Ringer's solution containing 90 μg of purified CPB and TI (CPB) or Ringer's solution and TI (control). (A) Fluid accumulation in colonic loops was recorded as described in Materials and Methods. No statistically significant difference was observed between CPB-treated and control colonic loops. (B) Histology. Colonic loops treated with Ringer's solution containing 90 μg of purified CPB and TI (CPB) or Ringer's solution and TI (control) showed intact intestinal villi with a well-preserved epithelium and lamina propria. Tissues were then processed by histology using hematoxylin and eosin stain. Preparations were photographed at 200× magnification. The data shown are representative of six experimental repetitions.
Evidence suggests that endogenous trypsin plays an important role as an innate intestinal defense mechanism against CPB secreted by C. perfringens type C isolates during natural disease (5, 7). To further corroborate the protective role of trypsin against CPB, small-intestine loops were treated with CPB in the absence of TI. Although rabbit small intestinal loops treated with 20 μg of CPB and TI showed abundant bloody fluid accumulation (Fig. 2), duodenal, jejunal, or ileal loops injected with 20 μg of CPB without TI exhibited no accumulation or bloody fluid (Fig. 2 and 3). Moreover, fluid levels present in loops injected with CPB without TI were similar to those found in negative control loops receiving only an injection of Ringer's solution and TI (Fig. 2 and 3). These results further support endogenous trypsin as playing a decisive protective role against CPB-induced small intestinal damage.
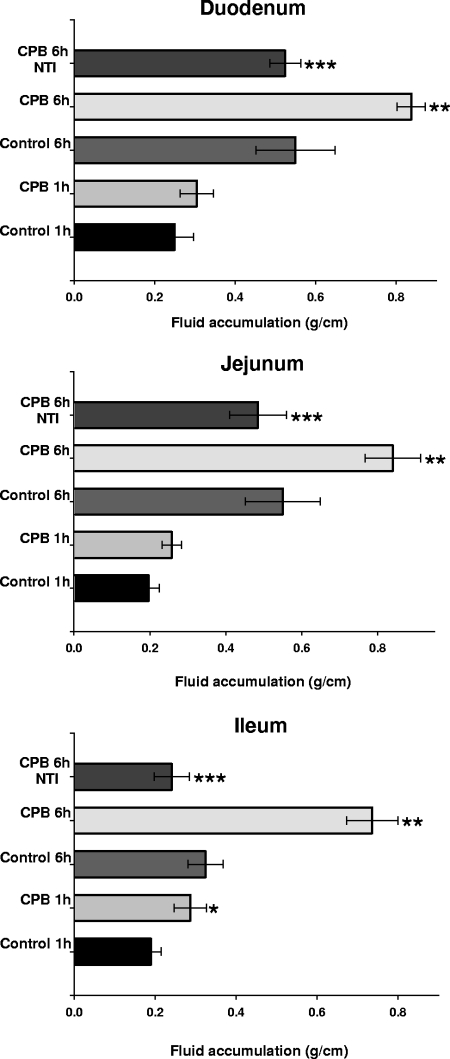
Since fluid accumulation had become prominent in small-intestine loops after 6 h of treatment with CPB and TI, a time course study was performed to evaluate when CPB begins to affect the small intestine. Confirming Fig. 2 and 3, bloody fluid accumulation was again observed after a 6-h treatment of the duodenum, jejunum, or ileum with 10 μg of CPB and TI, but no fluid accumulation beyond control levels (TI without CPB) was observed in any loops treated for 6 h with the same dose of CPB in the absence of TI (Fig. 5). Treatment of different small intestinal segments with purified CPB and TI for 60 min consistently caused some fluid accumulation over matching controls, but this effect reached statistical significance (P < 0.05) only in ileal loops (Fig. 5), which also contained traces of blood in their luminal fluid by this treatment time (not shown). Significant fluid accumulation over matching controls was not detected after 15 or 30 min of combined CPB and TI treatment of any small intestinal region (data not shown). These results indicate that luminal fluid influx develops most quickly in the ileum.
FIG. 5.
Time course fluid accumulation in rabbit intestinal loops. Duodenal (top), jejunal (middle), or ileal (bottom) loops were treated for 1 or 6 h (as indicated) with Ringer's solution containing 10 μg of purified CPB, along with TI (CPB) or Ringer's solution with TI (control). Other loops were treated for 6 h with Ringer's solution containing 10 μg of purified CPB but no TI (CPB 6 h/NTI). Fluid accumulation was recorded as described in Materials and Methods. Statistically significant differences (*, P > 0.05) in fluid accumulation were observed in ileal loops treated for 1 h with CPB and TI versus control loops (control 1 h). Loops (duodenal, jejunal, or ileal) treated for 6 h with purified CPB and TI (CPB 6 h) also showed statistically significant differences (**, P > 0.05) from control loops (control 6 h) or loops treated only with CPB (CPB 6 h NTI) (***, P > 0.05). Every experiment was performed independently six times; the data shown are the mean values. Small bars represent standard errors.
Histopathological damage induced by purified CPB in rabbit intestinal loops.
The results presented above demonstrated that, in the presence of TI, CPB can induce bloody fluid accumulation in all small intestinal regions between ca. 1 and 6 h of treatment. This observation suggested that purified CPB plus TI might be causing intestinal tissue damage. Therefore, histological damage in these loops was assessed. This analysis showed that duodenal, jejunal, or ileal rabbit loops treated with any tested dose of purified CPB plus TI exhibited severe tissue damage by 6 h (Table 1). In all small intestinal regions, the extent of these histological alterations in loops treated with CPB and TI was statistically different from control loops (Ringer's solution plus TI but lacking CPB, Table 1).
TABLE 1.
Rabbit loop pathology in 6-h incubation dose-response experiment
| Site, treatment, and CPB dose (μg)a | Rabbit loop pathology (mean degree of damage ± SD)b
|
||||||
|---|---|---|---|---|---|---|---|
| Desquamation of epithelium | Necrosis of epithelium | Necrosis of lamina propria | Inflammation | Edema | Villous blunting | Overall severity | |
| Duodenum | |||||||
| CPB (1) | 2.2 ± 0.5a | 2.1 ± 0.6a | 1.2 ± 0.5 | 1.0 ± 0.0 | 1.5 ± 0.5a | 1.9 ± 0.4a | 2.0 ± 0.5a |
| CPB (5) | 2.4 ± 0.4a | 2.3 ± 0.4a | 1.5 ± 0.4a | 1.3 ± 0.5a | 1.2 ± 0.4 | 1.5 ± 0.0a | 2.3 ± 0.2a |
| CPB (10) | 3.0 ± 0.3a | 3.0 ± 0.3a | 2.0 ± 0.0a | 1.9 ± 0.2a | 1.8 ± 0.4a | 2.3 ± 0.4a | 3.0 ± 0.3a |
| CPB (20) | 3.6 ± 0.7a,b | 3.6 ± 0.7a,b | 2.8 ± 0.8a,b | 2.1 ± 0.5a,b | 2.2 ± 0.4a,b | 2.4 ± 0.6a,b | 3.6 ± 0.6a,b |
| CPB (20)/NTI | 1.2 ± 0.4 | 1.2 ± 0.4 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.2 ± 0.4 | 1.2 ± 0.4 | 1.4 ± 0.4 |
| Control | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 |
| Jejunum | |||||||
| CPB (1) | 3.3 ± 1.2a | 3.3 ± 1.0a | 3.3 ± 1.0a | 2.5 ± 0.8a | 2.6 ± 0.7a | 3.6 ± 1.4a | 3.3 ± 1a |
| CPB (5) | 3.6 ± 0.7a | 3.7 ± 0.5a | 3.3 ± 1.2a | 2.8 ± 0.4a | 2.8 ± 0.4a | 4.0 ± 1.2a | 3.6 ± 0.6a |
| CPB (10) | 3.9 ± 0.2a | 3.8 ± 0.4a | 3.7 ± 0.8a | 2.8 ± 0.4a | 2.8 ± 0.4a | 4.2 ± 0.8a | 3.9 ± 0.2a |
| CPB (20) | 4.3 ± 0.5a,b | 4.3 ± 0.5a,b | 3.8 ± 0.4a,b | 2.8 ± 0.4a,b | 2.8 ± 0.4a,b | 4.4 ± 0.2a,b | 4.3 ± 0.5a,b |
| CPB (20)/NTI | 2.2 ± 1.3 | 2.2 ± 1.3 | 1.6 ± 0.5 | 1.6 ± 0.5 | 1.8 ± 0.8 | 1.5 ± 0.5 | 2.0 ± 0.8a |
| Control | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.3 ± 0.4 | 1.0 ± 0.0 | 1.0 ± 0.0 |
| Ileum | |||||||
| CPB (1) | 3.1 ± 1.1a | 2.5 ± 1.3a | 2.8 ± 1.4a | 2.0 ± 1.1a | 2.5 ± 0.5a | 3.3 ± 1.4a | 3.1 ± 1.1a |
| CPB (5) | 3.8 ± 0.4a | 3.5 ± 0.6a | 3.8 ± 0.4a | 3.0 ± 0.0a | 3.0 ± 0.0a | 4.3 ± 0.6a | 3.8 ± 0.4a |
| CPB (10) | 3.8 ± 0.4a | 4.0 ± 0.0a | 3.8 ± 0.4a | 3.0 ± 0.0a | 3.0 ± 0.0a | 4.5 ± 0.0a | 3.9 ± 0.2a |
| CPB (20) | 4.2 ± 0.5a,b | 4.3 ± 0.5a,b | 3.8 ± 0.4a,b | 3.0 ± 0.0a,b | 3.0 ± 0.0a,b | 4.5 ± 0.0a,b | 4.2 ± 0.5a,b |
| CPB (20)/NTI | 1.3 ± 0.4 | 1.2 ± 0.3 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.5 ± 0.4 | 1.5 ± 0.6 | 1.3 ± 0.2 |
| Control | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.1 ± 0.2 | 1.0 ± 0.0 | 1.0 ± 0.0 |
NTI, no TI; control, Ringer's solution plus TI (no CPB).
A superscript “A” indicates a statistically significant difference (P < 0.05) relative to the control loop using the Friedman test. A superscript “B” indicates a statistically significant difference (P < 0.05) relative to the loop treated with (CPB [20 μg]) without TI using the Friedman test.
The severity and extent of this CPB-induced 6-h intestinal damage showed a statistically significant dose dependency in duodenal loops (Fig. 6 and Table 1). Dose-dependent histological damage was also observed after a 6-h treatment of the jejunum or ileum with CPB plus TI, although the dose-dependent differences in tissue damage did not reach statistical significance (Table 1). In general, the same dose of purified CPB (plus TI) induced more severe histological damage in the jejunum and ileum versus the duodenum. For example, necrosis of the epithelium or lamina propria, villous blunting, and inflammation were more severe with any given dose of purified CPB plus TI injected into the jejunum or ileum versus the duodenum (Table 1), indicating that these two segments of the small intestine are more susceptible to CPB-induced damage than is the duodenum.
FIG. 6.
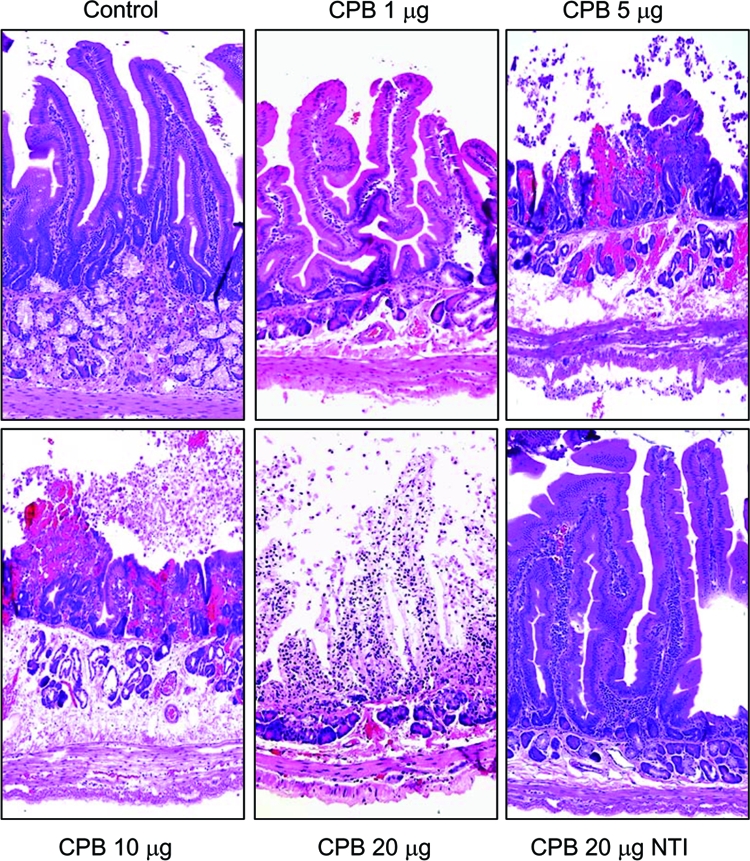
Purified CPB induces dose-dependent histologic damage in duodenal loops. Duodenal loops were treated for 6 h with Ringer's solution containing: 1, 5, 10, or 20 μg of purified CPB along with TI or else TI alone (control). Other loops were treated for 6 h with Ringer's solution containing 20 μg of purified CPB without TI (CPB 20 μg NTI). Loops treated with increasing doses of purified CPB and TI (CPB) showed progressive tissue damage, which included necrosis and loss of epithelium, necrosis of lamina propria, blunting of the villi, hemorrhage of the mucosa, and diffuse neutrophilic infiltration of mucosa and submucosa (see Table 1 for details). In contrast, duodenal loops injected with Ringer's solution and TI (control) or purified CPB without TI (CPB 20 μg NTI) showed intact intestinal villi with a well-preserved epithelium and lamina propria. Tissues were processed by histology using hematoxylin and eosin stain. Sections of treated or control tissues were then photomicrographed at 200× final magnification. Shown are representative photomicrographs of six repetitions for each condition.
Confirming that CPB was the active agent inducing the intestinal damage in Fig. 6, preincubation of purified CPB with a neutralizing monoclonal anti-CPB antibody, but not with a monoclonal anti-CPA antibody, totally abolished the ability of a CPB and TI mixture to cause histological damage (data not shown). In addition, rabbit duodenal, jejunal, or ileal loops treated with purified CPB in the absence of TI exhibited no intestinal lesions, appearing histologically indistinguishable from negative control loops receiving only Ringer's solution and TI (Fig. 6 and 7 and Table 1).
FIG. 7.
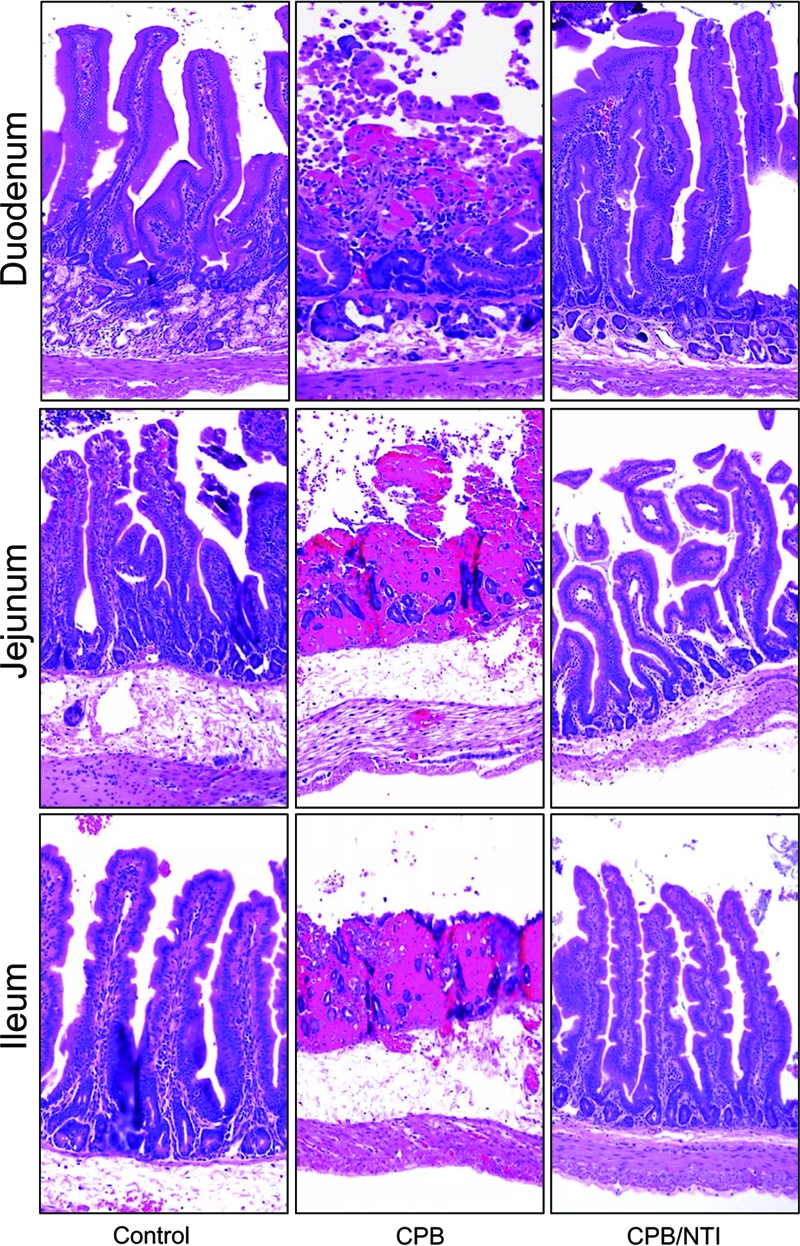
CPB-induced histologic damage in the rabbit small intestine. Intestinal loops constructed in the duodenum (top row), jejunum (middle row), or ileum (bottom row) were treated with Ringer's solution containing 20 μg of purified CPB and TI (CPB), Ringer's solution with TI (control) or Ringer's solution with 20 μg of purified CPB but no TI (CPB/NTI). After 6 h, duodenal, jejunal, or ileal loops treated with purified CPB and TI (CPB) showed severe damage (see Table 1 for details), but loops treated with TI but no CPB (control) or with purified CPB but no TI (CPB/NTI) showed normal, full-length intestinal villi with a well-preserved epithelium and lamina propria. Intestinal tissues were processed by histology using a hematoxylin and eosin stain. Sections of treated or control tissues were then photomicrographed at 200× final magnification. Representative photomicrographs of six repetitions for each condition are shown.
No histologic damage was observed when the colon was treated with CPB (90 μg) and the TI. For example, after 6 h of treatment with CPB and TI, the colon appeared similar to the control colon treated only with Ringer's solution and TI (Fig. 4B). Together, these results indicated that purified CPB, in a reduced trypsin environment, causes histologic damage in the rabbit small intestine but not in the colon, at least under the experimental conditions used in the present study.
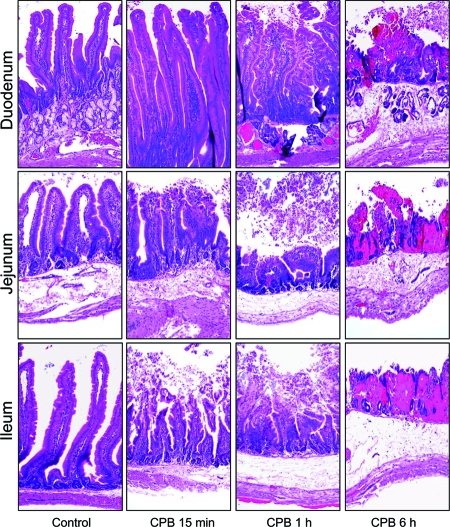
To help clarify whether the CPB-induced bloody fluid accumulation shown in Fig. 2 and 3 might be linked to the toxin's ability to induce severe tissue damage, histological lesions were also assessed at early CPB treatment time points. As shown in Fig. 8, normal histology was observed when the duodenum was treated with 10 μg of purified CPB for 15 min in the presence of TI. However, at the same time point, the jejunum and ileum were already showing slight damage, including some destruction of the villi tips. By 30 min or 1 h of CPB treatment in the presence of TI, the extent of histological alterations in duodenal, jejunal, or ileal loops treated with CPB and TI was statistically different from their corresponding control loops (Fig. 8 and Table 2).
FIG. 8.
Time course development of histologic damage induced by CPB in the rabbit small intestine. Intestinal loops constructed in the duodenum (top row), jejunum (middle row), or ileum (bottom row) were treated with Ringer's solution containing 10 μg of purified CPB and TI (CPB) or Ringer's solution and TI (control). Loops were then incubated for 15 min (CPB 15 min), 1 h (CPB 1 h), or 6 h (CPB 6 h). Note that after 15 min, jejunal or ileal loops treated with purified CPB and TI (CPB 15 min) showed destruction of the villus tips. Duodenal loops remained normal after 15 min of incubation with purified CPB and TI. Histologic damage then increased from moderate (1-h incubation period) to severe (6-h incubation period) in all loops treated with purified CPB and TI. Note the presence of necrosis and loss of epithelium, necrosis of lamina propria, blunting of the villi, hemorrhage of the mucosa, and diffuse neutrophilic infiltration of mucosa and submucosa. Intestinal loops injected with Ringer's solution and TI (control) retained normal, full-length intestinal villi with a well-preserved epithelium and lamina propria. Intestinal tissues were processed by histology using hematoxylin and eosin stain. Sections of representative treated or control tissues were photomicrographed at 200× final magnification. Shown are representative photomicrographs of six repetitions for each condition.
TABLE 2.
Rabbit loop pathology in time-course experiments
| Site and incubation time in min (CPB 10 μg) | Rabbit loop pathology (mean degree of damage ± SD)a
|
||||||
|---|---|---|---|---|---|---|---|
| Desquamation of epithelium | Necrosis of epithelium | Necrosis of lamina propria | Inflammation | Edema | Villous blunting | Overall severity | |
| Duodenum | |||||||
| 15 | 1.4 ± 0.1a | 1.4 ± 0.1a | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.3 ± 0.1 |
| 30 | 1.5 ± 0.3a | 1.5 ± 0.3a | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.3 ± 0.3 | 1.0 ± 0.0 | 1.4 ± 0.1a |
| 60 | 1.8 ± 0.3a | 1.8 ± 0.3a | 1.2 ± 0.4 | 1.0 ± 0.0 | 1.3 ± 0.3 | 1.0 ± 0.0 | 1.6 ± 0.2a |
| Control | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 |
| Jejunum | |||||||
| 15 | 2.0 ± 0.5a | 2.0 ± 0.5a | 1.5 ± 0.5a | 1.5 ± 0.5 | 1.5 ± 0.5 | 1.5 ± 0.5 | 1.9 ± 0.7a |
| 30 | 2.5 ± 0.3a | 2.5 ± 0.3a | 1.8 ± 0.3a | 1.5 ± 0.5 | 1.8 ± 0.3a | 2.3 ± 0.3a | 2.5 ± 0.3a |
| 60 | 2.8 ± 0.6a | 2.8 ± 0.6a | 2.2 ± 0.7a | 1.8 ± 1.0 | 1.8 ± 0.3a | 2.3 ± 0.3a | 2.8 ± 0.6a |
| Control | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 |
| Ileum | |||||||
| 15 | 1.6 ± 0.2a | 1.6 ± 0.2a | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.5 ± 0.3a |
| 30 | 2.3 ± 0.3a | 2.3 ± 0.3a | 1.8 ± 0.3a | 1.5 ± 0.5 | 1.8 ± 0.3a | 2.3 ± 0.3a | 2.3 ± 0.3a |
| 60 | 2.8 ± 0.5a | 2.8 ± 0.5a | 2.0 ± 0.5a | 1.8 ± 0.8 | 2.1 ± 0.7a | 2.8 ± 0.3a | 2.8 ± 0.5a |
| Control | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 |
A superscript “A” indicates a statistically significant difference (P < 0.05) relative to the control loop using the Friedman test.
DISCUSSION
Type B and C diseases originate in the intestines but often later involve sudden death or acute neurological signs resulting from enterotoxemia (absorption of toxins from the intestines into the circulation) (10, 23). In nearly all livestock and humans the intestinal effects of C. perfringens type C isolates involve necrotizing enteritis, which is clinically characterized by abundant bloody diarrhea, abdominal pain, and distension (13, 23, 27). CPB has long been implicated in type B and type C disease, but its contribution to intestinal disease has only recently been demonstrated (19). Although type B and C isolates usually produce between three and five different lethal toxins (3, 5), studies with isogenic toxin-null mutants have demonstrated that CPB is necessary to reproduce the intestinal pathology of type C isolate CN3685 in rabbit ileal loops (19). That study also showed, for the first time, that purified CPB alone is sufficient to cause bloody fluid accumulation and histologic damage in ileal loops (19). The key to demonstrating the enteric activity of purified CPB in that study was the inclusion of TI in the CPB treatment, which mimicked natural type C disease conditions, where high levels of TI and/or low levels of trypsin are present due to diet or disease.
The present study now significantly extends those initial findings by characterizing more completely the intestinal effects of purified CPB. A trypsin inhibition approach was again used to demonstrate that, in addition to the ileum, purified CPB produces bloody fluid accumulation and tissue damage in the rabbit jejunum and duodenum. However, the duodenum was found to be less CPB-sensitive than the jejunum or ileum. These regional CPB sensitivity differences provide one explanation for gastrointestinal aspects of natural type B or type C diseases, which primarily involve the jejuna and ilea of infected humans and animals (2, 9, 13, 27). The reduced CPB sensitivity of the duodenum in animal models and natural disease may be attributable, at least in part, to pancreatic secretion producing higher intestinal trypsin levels in the duodenum, which then reduces CPB activity (30).
Although purified CPB exhibited activity in all sections of the rabbit small intestines, the present study found that the rabbit colon is not affected by even high CPB doses applied in the presence of TI. These differences in CPB sensitivity between the rabbit small intestine versus the colon provide one explanation for the predominant involvement of the small intestine in type B and C infections of both humans and animals. However, there are also now some emerging reports of colonic damage in human type C disease (2, 21), which may involve (i) another type C toxin (not CPB) possessing colonic activity, (ii) species-specific differences in colonic CPB susceptibility, or (iii) CPB colonic damage requiring higher CPB doses or longer treatment times than used in the present study. Sorting out these possibilities will require additional experiments, but it is notable that another C. perfringens toxin, i.e., the enterotoxin, resembles CPB by also failing to damage rabbit colonic loops and yet possessing some limited activity on the human colon in vivo and ex vivo (1, 4, 11).
The present study also shows, for the first time, that CPB acts very quickly in the small intestine, with the duodenum, jejunum, or ileum all exhibiting visible tissue damage within the first hour of CPB treatment (Fig. 8). However, after 1 h of CPB treatment, no fluid accumulation differences were observed between control versus the toxin-treated duodenum and jejunum, and only modest CPB-induced fluid accumulation was detected in the ileum. Since luminal fluid accumulation developed after the onset of CPB-induced damage and usually included the presence of blood, these results are consistent with the accumulation of bloody luminal fluid in CPB-treated small intestine resulting, at least in part, from severe mucosal necrosis. CPB-induced inflammation may also contribute to this intestinal bleeding since there is a well-established association between hemorrhage and inflammation for other enteric toxins and pathogens (20). Similar hemorrhaging and intestinal necrosis as seen with purified CPB has also been reported for rabbit ileal loops injected with whole cultures of type C strain CN3685, but not with its isogenic CPB-null mutants (19), supporting the importance of CPB production in the ability of type C (and possibly type B) isolates to cause enteric effects during natural disease.
The present study also demonstrates that CPB possesses considerable enteric potency. Histologic damage was observed in duodenal, jejunal, and ileal rabbits loops treated for 6 h, in the presence of TI, with only 1 μg of purified CPB. A dose-dependent CPB effect on tissue damage was noted in the relatively CPB-insensitive duodenum. However, the dose dependency of CPB-induced tissue damage in the jejunum or ileum was statistically insignificant, probably because near-maximal effects had already been produced with the lowest CPB dose tested in the present study. Future studies might determine the minimal dose of CPB causing enteric effects in rabbit small-intestinal loops, but the current findings establish that, in the presence of TI, CPB is considerably more potent in rabbit intestinal loops than is C. perfringens enterotoxin (18, 22).
The enteric potency of CPB demonstrated in the present study could help explain, at least in part, the relatively rapid and often fatal progression of type C disease. For example, it is now clear that, in reduced trypsin conditions, even low doses of CPB are sufficient to rapidly induce significant necrosis of the epithelium. Beyond its enteric potency now demonstrated in the present study, CPB possesses the second lowest 50% lethal dose of all C. perfringens toxins when administered intravenously to mice (5). This suggests that the absorption of even small amounts of CPB from a damaged small intestine may be sufficient to cause death or damage to internal organs. It is notable in this respect that no rabbits died during the present experiments, which may suggest that longer treatment times or higher CPB doses are required to cause systemic lethality, at least in the rabbit ileal loop model. The present study's observation of extensive CPB-induced enteric damage in the absence of lethality supports the general view that death during type B or type C diseases mainly results from toxemia rather than from intestinal pathology (23).
CPB is very sensitive to the action of trypsin, although the molecular basis for this sensitivity is unknown. Endogenous intestinal trypsin is known to play an important role as an innate defense mechanism against type C infection (10). Lawrence and Cooke showed that C. perfringens type C could cause a pigbel-like disease in guinea pigs fed with a persistent low protein diet combined with dietary protease inhibitors (7). Enteric lesions similar to those observed in human pigbel cases have also been successfully reproduced by injecting a type C culture into lambs (14) or rabbit ileal loops (19), but only when a TI was given as well. Attempts to reproduce type C disease in animal models injecting CPB alone (no TI) have consistently failed (23). In this report and a previous study (19), we have now demonstrated that the presence of TI allows purified CPB to reproduce type C-like pathology in small intestinal loops. Since CPB is very sensitive to trypsin (5, 17), these results suggest that the presence of even small amounts of endogenous trypsin remaining in washed small intestinal loops can be sufficient to inactivate CPB if a TI is not administered simultaneously with the toxin.
The small-intestine histological alterations observed by using purified CPB in the present study and our previous work (19) are very similar to those described in type C natural diseases. For example, type C isolates produce in sheep intestinal lesions consisting of diffuse or multifocal hemorrhagic and necrotizing enteritis, mainly in the ileum, with excess of sanguineous serous fluid in the abdominal cavity (27). In piglets, type C infection also produces necrotizing enteritis with deep mucosal necrosis and emphysema in small intestine, sometimes extending to the colon (24). The similarity noted in this and our previous studies (3, 5) between intestinal lesions caused by purified CPB and the lesions of natural type B and C disease, coupled with the inability of isogenic CPB-null mutants to cause intestinal pathology (19), further support CPB as a critical virulence factor for type C (and likely type B) disease.
In summary, this research shows that, in the presence of TI, CPB induce enteric effects in the duodenum, jejunum, or ileum that resemble those in type C intestinal disease. However, colonic loops were found to be unaffected by similar treatment. Small-intestinal lesions developed quickly and required only a small amount of the purified toxin, indicating that CPB is highly active in the rabbit small intestine. These findings provide new insights into the overall pathogenic mechanism of fatal diseases induced by C. perfringens type B and C isolates and support the importance of CPB immunity for vaccine-induced protection against type B or C disease.
Acknowledgments
This study was supported by grant R01 AI056177-04 (B.A.M.) from the National Institute of Allergy and Infectious Diseases. J.E.V. received generous support from the Mexican National Council of Science and Technology (CONACyT).
We thank P. Hauer (Center for Veterinary Biologics, Ames, Iowa) for supplying monoclonal antibodies against CPB and CPA, Rod Tweten for supplying PFO antibody, and Jim Cravotta (University of California at Davis) for his substantial assistance in rabbit surgery. We also thank Richard D. Day and The-Minh Luong of the Biostatistics Consulting Service of the University of Pittsburgh for their assistance in statistical analyses.
Editor: S. R. Blanke
Footnotes
Published ahead of print on 14 July 2008.
REFERENCES
- 1.Bos, J., L. Smithee, B. McClane, R. F. Distefano, F. Uzal, J. G. Songer, S. Mallonee, and J. M. Crutcher. 2005. Fatal necrotizing colitis following a foodborne outbreak of enterotoxigenic Clostridium perfringens type A infection. Clin. Infect. Dis. 40e78-e83. [DOI] [PubMed] [Google Scholar]
- 2.Farrant, J. M., Z. Traill, C. Conlon, B. Warren, N. Mortensen, F. V. Gleeson, and D. P. Jewell. 1996. Pigbel-like syndrome in a vegetarian in Oxford. Gut 39336-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernandez-Miyakawa, M. E., D. J. Fisher, R. Poon, S. Sayeed, V. Adams, J. I. Rood, B. A. McClane, and F. A. Uzal. 2007. Both epsilon-toxin and beta-toxin are important for the lethal properties of Clostridium perfringens type B isolates in the mouse intravenous injection model. Infect. Immun. 751443-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernandez-Miyakawa, M. E., V. Pistone-Creydt, F. A. Uzal, B. A. McClane, and C. Ibarra. 2005. Clostridium perfringens enterotoxin damages the human intestine in vitro. Infect. Immun. 738407-8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisher, D. J., M. E. Fernandez-Miyakawa, S. Sayeed, R. Poon, V. Adams, J. I. Rood, F. A. Uzal, and B. A. McClane. 2006. Dissecting the contributions of Clostridium perfringens type C toxins to lethality in the mouse intravenous injection model. Infect. Immun. 745200-5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson, S., and D. N. Gerding. 1997. Enterotoxemic infections, p. 117-140. In J. I. Rood, B. A. McClane, J. G. Songer, and R. W. Titball (ed.), The clostridia: molecular biology and pathogenesis. Academic Press, London, United Kingdom.
- 7.Lawrence, G., and R. Cooke. 1980. Experimental pigbel: the production and pathology of necrotizing enteritis due to Clostridium welchii type C in the guinea pig. Br. J. Exp. Pathol. 61261-271. [PMC free article] [PubMed] [Google Scholar]
- 8.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193265-275. [PubMed] [Google Scholar]
- 9.Matsuda, T., Y. Okada, E. Inagi, Y. Tanabe, Y. Shimizu, K. Nagashima, J. Sakurai, M. Nagahama, and S. Tanaka. 2007. Enteritis necroticans ‘pigbel’ in a Japanese diabetic adult. Pathol. Int. 57622-626. [DOI] [PubMed] [Google Scholar]
- 10.McClane, B. A., F. A. Uzal, M. Fernandez-Miyakawa, D. Lyerly, and T. D. Wilkins. 2004. The enterotoxigenic clostridia, p. 698-752. In S. F. M. Dworkin, E. Rosenburg, K. F. Schleifer, and E. Stackebrandt (ed.), The prokaryotes, vol. 4. Springer-Verlag, New York, NY. [Google Scholar]
- 11.McDonel, J. L., and G. W. Demers. 1982. In vivo effects of enterotoxin from Clostridium perfringens type A in the rabbit colon: binding versus biologic activity. J. Infect. Dis. 145490-494. [DOI] [PubMed] [Google Scholar]
- 12.Nagahama, M., S. Hayashi, S. Morimitsu, and J. Sakurai. 2003. Biological activities and pore formation of Clostridium perfringens beta toxin in HL 60 cells. J. Biol. Chem. 27836934-36941. [DOI] [PubMed] [Google Scholar]
- 13.Niilo, L. 1988. Clostridium perfringens type C Enterotoxemia. Can. Vet. J. 29658-664. [PMC free article] [PubMed] [Google Scholar]
- 14.Niilo, L. 1986. Experimental production of hemorrhagic enterotoxemia by Clostridium perfringens type C in maturing lambs. Can. J. Vet. Res. 5032-35. [PMC free article] [PubMed] [Google Scholar]
- 15.Petit, L., M. Gibert, and M. R. Popoff. 1999. Clostridium perfringens: toxinotype and genotype. Trends. Microbiol. 7104-110. [DOI] [PubMed] [Google Scholar]
- 16.Petrillo, T. M., C. M. Beck-Sague, J. G. Songer, C. Abramowsky, J. D. Fortenberry, L. Meacham, A. G. Dean, H. Lee, D. M. Bueschel, and S. R. Nesheim. 2000. Enteritis necroticans (pigbel) in a diabetic child. N. Engl. J. Med. 3421250-1253. [DOI] [PubMed] [Google Scholar]
- 17.Sakurai, J., and C. L. Duncan. 1978. Some properties of beta-toxin produced by Clostridium perfringens type C. Infect. Immun. 21678-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarker, M. R., R. J. Carman, and B. A. McClane. 1999. Inactivation of the gene (cpe) encoding Clostridium perfringens enterotoxin eliminates the ability of two cpe-positive C. perfringens type A human gastrointestinal disease isolates to affect rabbit ileal loops. Mol. Microbiol. 33946-958. [DOI] [PubMed] [Google Scholar]
- 19.Sayeed, S., F. A. Uzal, D. J. Fisher, J. Saputo, J. E. Vidal, Y. Chen, P. Gupta, J. I. Rood, and B. A. McClane. 2008. Beta toxin is essential for the intestinal virulence of Clostridium perfringens type C disease isolate CN3685 in a rabbit ileal loop model. Mol. Microbiol. 6715-30. [DOI] [PubMed] [Google Scholar]
- 20.Sears, C. L., and J. B. Kaper. 1996. Enteric bacterial toxins: mechanisms of action and linkage to intestinal secretion. Microbiol. Rev. 60167-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Severin, W. P., A. A. de la Fuente, and M. F. Stringer. 1984. Clostridium perfringens type C causing necrotizing enteritis. J. Clin. Pathol. 37942-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sherman, S., E. Klein, and B. A. McClane. 1994. Clostridium perfringens type A enterotoxin induces tissue damage and fluid accumulation in rabbit ileum. J. Diarrhoeal. Dis. Res. 12200-207. [PubMed] [Google Scholar]
- 23.Songer, J. G. 1996. Clostridial enteric diseases of domestic animals. Clin. Microbiol. Rev. 9216-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Songer, J. G., and F. A. Uzal. 2005. Clostridial enteric infections in pigs. J. Vet. Diagn. Investig. 17528-536. [DOI] [PubMed] [Google Scholar]
- 25.Steinthorsdottir, V., H. Halldorsson, and O. S. Andresson. 2000. Clostridium perfringens beta-toxin forms multimeric transmembrane pores in human endothelial cells. Microb. Pathog. 2845-50. [DOI] [PubMed] [Google Scholar]
- 26.Tonnellier, M., E. Maury, J. Guglielminotti, and G. Offenstadt. 2001. A fatal sandwich. Lancet. Infect. Dis. 1202. [DOI] [PubMed] [Google Scholar]
- 27.Uzal, F. A. 2004. Diagnosis of Clostridium perfringens intestinal infections in sheep and goats. Anaerobe 10135-143. [DOI] [PubMed] [Google Scholar]
- 28.Walker, P. D., W. H. Foster, P. A. Knight, D. S. Freestone, and G. Lawrence. 1979. Development, preparation and safety testing of a Clostridium welchii type C toxoid. I: preliminary observations in man in Papua New Guinea. J. Biol. Stand. 7315-323. [DOI] [PubMed] [Google Scholar]
- 29.Walker, P. D., T. G. Murrell, and L. K. Nagy. 1980. Scanning electron microscopy of the jejunum in enteritis necroticans. J. Med. Microbiol. 13445-450. [DOI] [PubMed] [Google Scholar]
- 30.Zeitlin, I. J., and W. Sircus. 1974. Factors influencing duodenal trypsin levels following a standard test meal as a test of pancreatic function. Gut 15173-179. [DOI] [PMC free article] [PubMed] [Google Scholar]