Abstract
Vaccination with the recombinant N terminus of the candidal adhesin Als3p (rAls3p-N) protects mice from lethal candidemia. Candidal Als3p also is structurally similar to the microbial surface components recognizing adhesive matrix molecule adhesin, clumping factor, from Staphylococcus aureus. To determine the potential for cross-kingdom vaccination, we immunized mice with rAls3p-N or negative control proteins and challenged them via the tail vein with S. aureus or other gram-positive or gram-negative pathogens. The rAls3p-N vaccine, but neither tetanus toxoid nor a related Als protein (Als5p), improved the survival of vaccinated mice subsequently infected with multiple clinical isolates of S. aureus, including methicillin-resistant strains. The rAls3p-N vaccine was effective against S. aureus when combined with aluminum hydroxide adjuvant. However, the vaccine did not improve the survival of mice infected with other bacterial pathogens. Vaccinated, infected mice mounted moderated type 1 immune responses. T lymphocyte-deficient mice were more susceptible to S. aureus infection, but B lymphocyte-deficient mice were not. Furthermore, T but not B lymphocytes from vaccinated mice mediated protection in adoptive transfer studies. The passive transfer of immune serum was not protective. These data provide the foundation for cross-kingdom vaccine development against S. aureus and Candida, which collectively cause 200,000 bloodstream infections resulting in ≥40,000 to 50,000 deaths annually in the United States alone.
We have previously shown that vaccines derived from the recombinant N termini of the candidal surface adhesins Als1p (rAls1p-N) and Als3p (rAls3p-N) protect mice from otherwise lethal disseminated candidiasis and mitigate the severity of oropharyngeal and vaginal candidiasis (13, 14, 30, 31). Because the efficacy of rAls3p-N was greater than that of rAls1p-N in mucosal models of infection, and because we recently have identified Als3p as a critical candidal invasin in pathogenesis studies (21), subsequent vaccine studies have focused on rAls3p-N (30).
Of considerable interest was our recent discovery that, by molecular modeling, candidal Als1p and Als3p adhesins are predicted to have three-dimensional structural similarity to clumping factor (ClfA), which is a member of a family of surface adhesins expressed by Staphylococcus aureus known as microbial surface components recognizing adhesive matrix molecules (MSCRAMMs) (26). Given the structural similarities between candidal Als3p and S. aureus ClfA, we sought to determine the potential of the rAls3p-N vaccine to mediate protection against S. aureus infection in mice. Furthermore, we sought to define key elements of host defense required for the rAls3p-N vaccine to protect mice from staphylococcemia.
MATERIALS AND METHODS
S. aureus and mouse strains.
S. aureus 67-0, 601, COL, LAC (all methicillin-resistant strains; the latter is a USA300 community-acquired strain generously provided by Frank Deleo), and 29213 (methicillin susceptible; from the ATCC) are clinical isolates that are well characterized and known to cause lethal, disseminated infection when inoculated intravenously (i.v.) in animal models (2, 15, 17, 27, 35). Staphylococcus epidermidis 35984 (4, 8) and Enterococcus faecalis TX2484 (vancomycin resistant) (3) are clinical bloodstream isolates also known to cause lethal infection in animal models at high inocula. Pseudomonas aeruginosa PA-01 is a clinical wound isolate (33) known to cause lethal infection in animals (9). All bacterial isolates were grown overnight in a shaking incubator in brain heart infusion (BHI) broth at 37°C, passaged until log-phase growth, prepared for inoculation by using the optical density (OD) with confirmation of the inoculum by colony counting, and washed in phosphate-buffered saline (PBS) prior to inoculation in 0.5 ml of PBS.
Adult BALB/c mice (National Cancer Institute, Bethesda, MD) were used for most studies. For some experiments, congenic T-cell-deficient (C.Cg/AnBomTac-Foxn1nuN20) or B-cell-deficient (C.129B6-IgH-Jhdtm1Dhu) mice (8 to 12 weeks old; Taconic Farms, Germantown, NY) were used. All procedures involving mice were approved by the institutional animal use and care committee and followed the National Institutes of Health guidelines for animal housing and care.
Immunization.
All immunizations were subcutaneous in a 0.2-ml volume at the base of the neck. rAls3p-N (amino acids 17 to 432 of Als3p) and rAls5p (amino acids 20 to 664) were produced in Saccharomyces cerevisiae and purified via nickel-agarose columns as described previously (22, 30). Initial vaccination experiments were done with complete Freund's adjuvant (CFA), with a booster dose of incomplete Freund's adjuvant (IFA). Subsequent vaccinations were completed with 0.1% Al(OH)3, (Alhydrogel; Brenntag Biosector, Frederikssund, Denmark) in PBS. Tetanus toxoid (Tetguard; Boeringher Ingelheim) was administered at a dose of 0.05 ml. Mice were infected 2 weeks following the boost or 3 weeks following the single dose (vaccinations were staggered so that all mice were infected on the same day). In some experiments, mice were vaccinated as described above without a boost and were infected 8 weeks later.
Splenic lymphocyte priming and response.
S. aureus cell wall extract was prepared by lysostaphin degradation as described previously (7). rClfA-N (ClfA amino acids 40 to 458) was cloned from S. aureus 67-0 genomic DNA using a high-fidelity PCR kit (Roche). The primers used for PCR were CGGGATCCGCAGTGAAAATAGTGTTACGCAATCTG and CCCAAGCTTCACACTATTAGTGACATCCTCAA. The PCR fragment was digested with BamHI and HindIII and cloned into pQE-32 (Qiagen). The resulting construct was transformed into Escherichia coli strain SG130009 for isopropyl β-d-1-thiogalactopyranoside (IPTG)-induced expression according to the manufacturer's instructions and was expressed as a fusion protein with an N-terminal His tag. Purification was performed by Ni column binding of the His tag label on rClfA-N in a fashion identical to that for the purification of rAls3p-N.
The His tag then was removed from rClfA-N with the TAGZyme kit (Qiagen) per the manufacturer's instructions. Endotoxin was removed from rClfA-N by the use of a Detoxi-gel endotoxin removal kit (Pierce, Woburn, MA) per the manufacturer's instructions. The final endotoxin concentration in detagged rClfA-N was 0.08 ng/ml according to the limulus amoebocyte assay (Charles Rivers Laboratories, Savanna).
Splenocytes (2 × 105) from vaccinated or control mice were stimulated in 96-well plates with cell wall extract from 2 × 106 S. aureus organisms per well, 50 μg/ml of ClfA-N, 2 flocculation units/ml of tetanus toxoid (as a negative control; Cylex Inc., Columbia), or with medium alone. Endotoxin-free hamster anti-mouse CD28 antibody (clone 37.51; BD Pharmingen, La Jolla, CA) was added to all wells at 0.5 μg/ml. After 3 days, splenocytes were pulsed with tritiated thymidine (6.7 μCi/mol) and processed on a CombiCell harvester 6 h later.
To quantify the ex vivo priming of splenic lymphocytes by the rAls3p-N vaccine, 2 weeks following the boost splenocytes were harvested as described above. The cells were cultured in complete medium for 72 h in the presence of 100 μg/ml of rAls3p-N. The wells were pulsed for 6 h with 1 μCi of tritiated thymidine (Amersham, Piscataway, NJ) before being harvested to quantify proliferation.
rAls3p-N and rClfA-N ELISA.
Antibody titers were determined by enzyme-linked immunosorbent assay (ELISA) in 96-well plates as previously described (13, 14, 30, 31). The ELISA titer was taken as the reciprocal of the last serum dilution with an OD reading equal to or greater than the mean OD of negative control samples plus twice the standard deviation.
Tissue bacterial burden and cytokine analysis.
On day 5 postinfection, spleens and kidneys were harvested and cut in half for simultaneous bacterial burden and cytokine analyses. For the determination of bacterial burden, organs were homogenized in saline and quantitatively cultured on tryptic soy agar. Whole-organ cytokines were analyzed from splenic and kidney homogenates with the murine type 1/type 2 cytometric bead array kit (BD Pharmingen, La Jolla, CA) per the manufacturer's instructions.
Intracellular cytokines from splenic lymphocytes were analyzed as previously described (32). No exogenous stimulus was added to the cells so that Th1-Th2 frequencies would reflect their frequencies in vivo during staphylococcemia. Three-color flow cytometry was performed on a Becton-Dickinson FACScan instrument calibrated with CaliBRITE beads (Becton Dickinson, San Jose, CA) using FACSComp software per the manufacturer's recommendations. Data for each sample were acquired until 10,000 CD4+ lymphocytes were analyzed. Th1 cells were defined as CD4+, gamma interferon positive (IFN-γ+), and interleukin-4 negative (IL-4−), and Th2 cells were defined as CD4+ IFN-γ− IL-4+. Results are presented as the median ± 25th and 75th quartiles of the percentages of all gated lymphocytes that were Th1 or Th2 cells.
Adoptive transfer and passive immunization.
For adoptive transfer and passive immunization, mice were vaccinated with rAls3p-N (300 μg) plus Al(OH)3 or with Al(OH)3 alone. Two weeks following the boost, sera were pooled from vaccinated or control mice, and splenocytes were harvested as we have previously described (14). CD3+, CD4+, or CD8+ T lymphocytes or B220+ B lymphocytes were purified by the use of the IMag system (BD Pharmingen). The purity (>95%) of the cells was confirmed by surface staining followed by flow cytometry.
Purified lymphocytes (107 per mouse for CD3+ or B220+ cells and 5 × 106 per mouse for CD4+ or CD8+ cells) were administered i.v. to congenic, unvaccinated recipient mice. Serum was administered intraperitoneally to other recipient mice. Mice were infected via the tail vein with S. aureus 67-0 either 24 h after splenocyte adoptive transfer or 3 h after serum administration. Serum doses were repeated weekly in surviving mice.
Statistical analysis.
The Mann-Whitney U test was utilized to compare tissue bacterial burden, Th1/Th2, and cytokine ratios. The nonparametric log-rank test or Cox proportionate hazards test was utilized to determine differences in survival times. P < 0.05 was considered significant.
RESULTS
rAls3p-N priming of murine splenocytes against S. aureus cell wall.
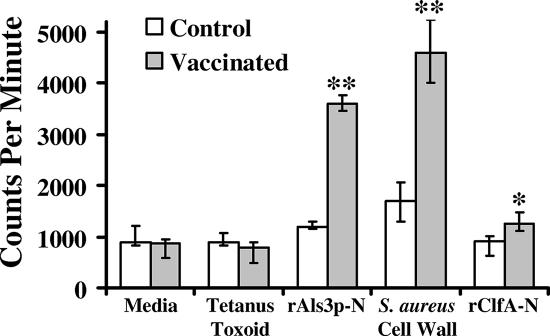
To determine if the rAls3p-N vaccine primed splenocytes against S. aureus, mice were vaccinated with 20 μg of rAls3p-N in CFA, with a booster dose at 3 weeks in IFA. Control mice received adjuvant alone. Splenocytes were harvested 2 weeks following the boost and stimulated ex vivo with rAls3p-N, S. aureus cell wall extract, or tetanus toxoid as a negative control. In addition, splenocytes were stimulated with rClfA-N, as clumping factor A was predicted to have a three-dimensional shape similar to that of rAls3p-N in modeling studies (26). Splenocytes from mice vaccinated with rAls3p-N proliferated significantly more in response to rAls3p-N and S. aureus cell wall extract than did splenocytes from control mice that received adjuvant alone (P < 0.001 for both comparisons) (Fig. 1) . In contrast, tetanus toxoid (the negative control) did not stimulate splenocyte proliferation from mice receiving either vaccine or adjuvant alone. While rClfA-N stimulated proliferation significantly more in splenocytes from vaccinated than from control mice (P = 0.02), the overall magnitude of the response was low (Fig. 1).
FIG. 1.
Candidal rAls3p-N vaccine primes murine splenocytes against the S. aureus cell wall. Splenocytes from mice vaccinated with CFA alone or CFA plus rAls3p-N were stimulated with medium alone, tetanus toxoid, rAls3p-N, S. aureus cell wall extract, or rClfA-N. The cells were pulsed with tritiated thymidine for the last 6 h of culture and harvested for counting of radioactive β-particles. Median and interquartile ranges are shown. **, P < 0.03 compared to results for medium, tetanus toxoid, and rClfA-N; *, P < 0.03 compared to results for CFA alone by the Mann-Whitney U test.
The median anti-rAls3p-N immunoglobulin G titer in vaccinated mice was 1:51,200; it was 1:600 in control mice. In the same serum, anti-rClfA-N serum immunoglobulin G titers from either vaccinated or control mice were <1:50, reflecting an absence of serological cross-reactivity between rAls3p-N and rClfA-N (data not shown).
Efficacy of rAls3p-N against murine staphylococcemia.
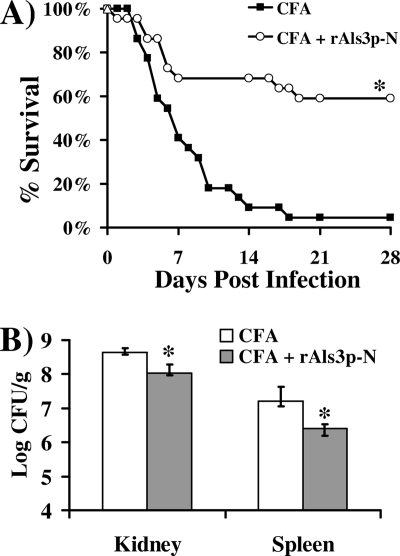
To determine if the cross-priming against S. aureus cell wall extract induced protection in infected mice, mice were vaccinated as described above. Fourteen days after the boost, mice were infected via the tail vein with 2 × 107 cells of S. aureus 67-0, a methicillin-resistant, clinical isolate. Vaccination with rAls3p-N markedly improved survival compared to that with adjuvant alone (60 and 5% survival at 28 days, respectively) (Fig. 2A). Also, at 5 days postinfection, mice that received the rAls3p-N vaccine had a 4- and 10-fold reduction in kidney and spleen bacterial burdens, respectively, compared to those of control mice (Fig. 2B).
FIG. 2.
Candidal rAls3p-N vaccine improved survival and decreased bacterial burden in mice with methicillin-resistant S. aureus bacteremia. (A) Survival of vaccinated or control mice (n = 22 per group from 2 experiments) infected i.v. with S. aureus 67-0 (2 × 107 inoculum), a methicillin-resistant, clinical isolate. *, P = 0.0001 compared to results for CFA alone by the log-rank test. The experiment was terminated on day 28, with all remaining mice appearing well. (B) Day-5 kidney or spleen bacterial CFU per gram of organ. n = 5 for CFA treatment; n = 7 for rAls3p-N vaccination. Median and interquartile ranges are shown. *, P < 0.03 compared to results for CFA by the Mann-Whitney U test.
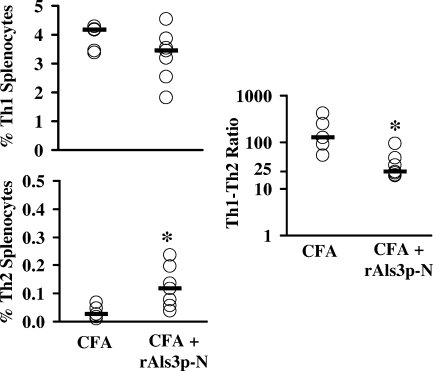
To evaluate in vivo immune responses during infection, we quantified Th1 and Th2 splenocytes at day 5 of bacteremia. Staphylococcemic mice receiving CFA alone had few Th2 splenocytes, resulting in extremely high ratios of Th1/Th2 splenocytes (Fig. 3). Spleens from vaccinated, staphylococcemic mice contained similar frequencies of Th1 cells but higher frequencies of Th2 cells than those of mice receiving CFA alone (P = 0.02 by the Mann-Whitney U test) (Fig. 3). Nevertheless, the ratio of Th1/Th2 cells in vaccinated mice (median, 23:1; 75th quartile, 39:1; 25th quartile, 21:1) was still strongly in favor of Th1 cells (Fig. 3), as a ratio of ∼10:1 is indicative of an unpolarized response in our assay (32).
FIG. 3.
rAls3p-N vaccine induced a moderated type 1 immune response during murine staphylococcemia. Mice were vaccinated with CFA alone (n = 5) or CFA plus rAls3p-N (n = 7) and infected i.v. with S. aureus 67-0 (2 × 107 organisms). Organs were harvested on day 5 postinfection. Frequencies of Th1 (CD4+ IFN-γ+ IL-4−) or Th2 (CD4+ IFN-γ− IL-4+) splenocytes or Th1/Th2 ratios were determined by intracellular cytokine staining and flow cytometry. Bars reflect median values. *, P < 0.05 compared to results for CFA alone by the Mann-Whitney U test.
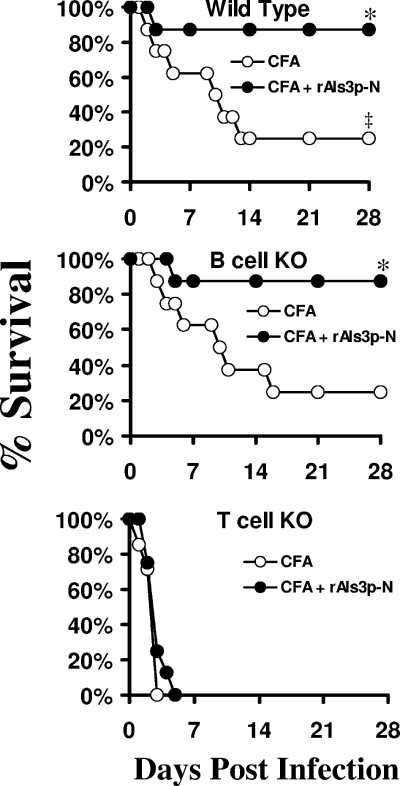
To define the relative requirements for cell-mediated versus humoral immunity, the rAls3p-N vaccine was tested for efficacy in wild-type, congenic T-cell-deficient, and congenic B-cell-deficient mice. Control T-cell-deficient mice (receiving CFA alone) were significantly more susceptible to staphylococcemia than control wild-type mice (P = 0.009 by the log-rank test) (Fig. 4). In contrast, control B-cell-deficient mice were not more susceptible than wild-type mice (P = 0.9) (Fig. 4). The vaccine did not improve the survival of T-cell-deficient mice (P = 0.3 for vaccinated mice compared to those receiving CFA) but did improve the survival of B-cell-deficient mice (P = 0.02 for vaccinated mice compared to those receiving CFA). The survival of vaccinated B-cell-deficient mice was not significantly different than the survival of vaccinated wild-type mice (P = 0.98).
FIG. 4.
T cells, but not B cells, were required for rAls3p-N-mediated protection. Mice were vaccinated with CFA alone or CFA plus rAls3p-N and infected i.v. with S. aureus 67-0 (2 × 107 organisms). n = 8 mice per group. *, P < 0.02 compared to results for CFA alone; ‡, P = 0.009 for wild-type mice receiving CFA compared to results for T-cell knockout (KO) mice receiving CFA; P = 0.9 for wild-type mice receiving CFA compared to results for B-cell knockout mice receiving CFA by the log-rank test.
rAls3p-N immunogenicity and efficacy with aluminum hydroxide [Al(OH)3] adjuvant.
To confirm the potential for clinical utility of an Als vaccine against S. aureus, we tested the rAls3p-N vaccine's efficacy against S. aureus when combined with Al(OH)3, a standard aluminum adjuvant preparation used in approved vaccines. We have previously determined that higher doses of Als vaccines were required to mediate protection against Candida albicans when combined with Al(OH)3 than those when the vaccine was combined with Freund's adjuvant, and that optimal protection against C. albicans occurred at a dose of 300 μg of rAls3p-N (29, 30). Therefore, BALB/c mice were vaccinated with 300 μg of rAls3p-N plus Al(OH)3, or with Al(OH)3 alone, with a booster dose administered at 3 weeks.
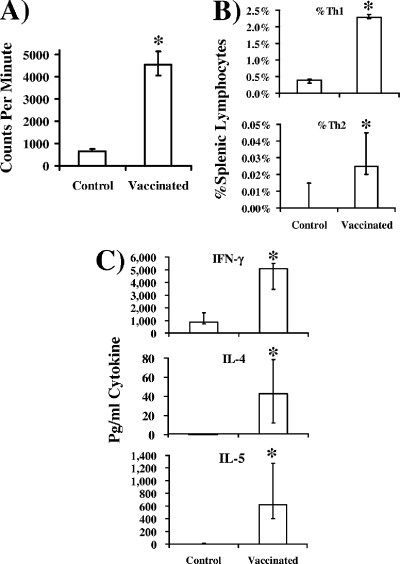
To confirm vaccine priming with Al(OH)3 adjuvant, splenocytes were harvested 2 weeks after the boost and stimulated with rAls3p-N ex vivo. Splenocytes from vaccinated mice proliferated significantly more in response to rAls3p-N than did splenocytes from control mice (Fig. 5A), confirming the immunogenicity of the vaccine in combination with Al(OH)3. Furthermore, there were significantly higher frequencies of Th1 and Th2 splenic lymphocytes from vaccinated mice than controls (Fig. 5B), and splenocytes from vaccinated mice produced significantly more IFN-γ, IL-4, and IL-5 in response to rAls3p-N than did splenocytes from control mice (Fig. 5C).
FIG. 5.
rAls3p-N vaccine administered with Al(OH)3 adjuvant primes murine lymphocytes for both Th1 and Th2 responses. Two weeks following the booster dose, splenocytes were cultured from BALB/c mice (n = 10 per group) vaccinated with rAls3p-N (300 μg) plus Al(OH)3 or Al(OH)3 alone. Cells were stimulated ex vivo with rAls3p-N (100 μg/ml). (A) Proliferation of splenic lymphocytes from vaccinated or control mice after 3 days of coculture with rAls3p-N. (B) Frequency of Th1 (CD4+ IFN-γ+) or Th2 (CD4+ IL-4+) splenocytes after 4 days of coculture with rAls3p-N, as determined by intracellular cytokine analysis. (C) Cytokine content in supernatants after 4 days of splenocyte coculture with rAls3p-N. Median and interquartile ranges are shown. *, P < 0.05 compared to results for alum-administered control mice by the Mann-Whitney U test.
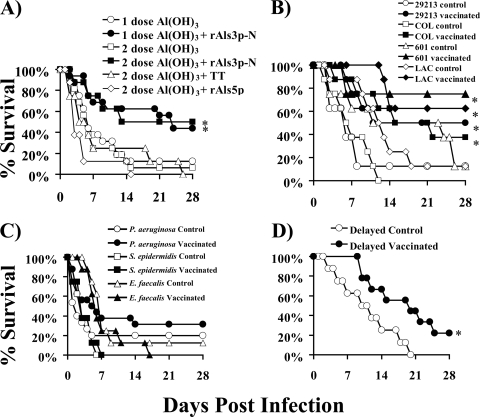
To determine the efficacy of the vaccine with Al(OH)3, mice were vaccinated either with or without a booster dose. As a negative control, some mice were vaccinated and boosted with commercially available tetanus toxoid. In addition, to test the specificity of the vaccine, mice were vaccinated with a different member of the Als family, rAls5p. Both the single-dose and boosted regimens of rAls3p-N significantly improved the survival of mice infected with methicillin-resistant S. aureus compared to that of mice receiving Al(OH)3 alone, tetanus toxoid, or rAls5p (Fig. 6A). Two doses of vaccine did not significantly improve survival compared to that after one dose. However, surviving mice in the one-dose group continued to appear ill at 28 days (i.e., ruffled fur and decreased activity), when the experiment was terminated. In contrast, the mice in the two-dose group appeared clinically well (i.e., no ruffled fur and normal activity) at the end of the experiment. Vaccination with neither tetanus toxoid nor rAls5p improved survival.
FIG. 6.
rAls3p-N vaccine is efficacious in combination with aluminum hydroxide adjuvant. BALB/c mice were vaccinated subcutaneously with one or two doses of rAls3p-N (300 μg) plus aluminum hydroxide [Al(OH)3], with two doses of tetanus toxoid (TT) or rAls5p plus Al(OH)3, or with one or two doses of Al(OH)3 alone. (A) Mice were infected i.v. with S. aureus 67-0 (2 × 107 organisms) 3 weeks following the first dose or 2 weeks following the boost. n = 16 mice per group for two experiments for rAls3p-N; n = 8 mice per group for Als5p and TT. *, P < 0.03 compared to results for all other groups by the log-rank test. (B) Vaccinated (all with two doses) or control mice were infected i.v. with S. aureus 29213 (5 × 106 organisms), COL (1.4 × 108 organisms), 601 (1.3 × 107 organisms), or LAC (8 × 106 organisms). n = 8 mice per group. *, P < 0.05 compared to results for the control by the log-rank test. (C) Vaccinated (all with two doses) or control mice were infected 2 weeks following the boost with 8 × 108 S. epidermidis 35984 cells, 3 × 108 E. faecalis TX2484 cells, or 3 × 107 P. aeruginosa PA01 cells. (D) Mice vaccinated with only one dose of rAls3p-N plus Al(OH)3 or Al(OH)3 alone were infected 8 weeks later with S. aureus 67-0 (1.9 × 107 cells). *, P < 0.05 compared to results for the control by the log-rank test.
To determine the breadth of vaccine-mediated protection, we tested vaccine efficacy against several other S. aureus strains. Vaccinated mice infected with all strains had significantly improved survival compared to that of control mice (Fig. 6B).
In contrast, the vaccine did not improve survival in mice infected with P. aeruginosa, S. epidermidis, or E. faecalis (Fig. 6C). Finally, the efficacy of the vaccine was tested in a delayed infection model. Mice were vaccinated with a single dose of rAls3p-N plus Al(OH)3 or Al(OH)3 alone. Eight weeks following a single dose of vaccine, mice were infected via the tail vein with S. aureus 67-0. Vaccinated mice had significant improvements in survival compared to that of control mice (Fig. 6D).
Lymphocyte adoptive transfer and passive immunization.
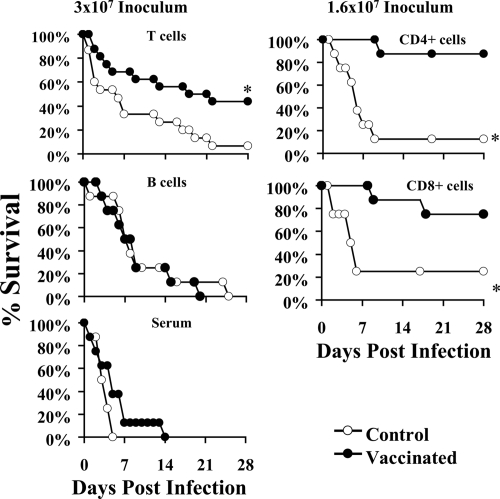
To confirm the relative roles of T and B lymphocytes in vaccine-mediated efficacy, BALB/c mice were vaccinated subcutaneously with rAls3p-N (300 μg) plus Al(OH)3 or Al(OH)3 alone as described above. Two weeks following the boost, vaccinated or control mice were bled to obtain serum, and their splenocytes were harvested. In the first experiment, congenic recipient mice were treated i.v. with B220+ or CD3+ lymphocytes or with serum (found to have a titer of 1:51,200 against rAls3p-N) from vaccinated or alum control donor mice. Recipient mice then were infected with 3 × 107 S. aureus 67-0 cells. The transfer of CD3+ T lymphocytes from vaccinated, but not control, donor mice significantly improved the survival of infected recipient mice (Fig. 7). Subsequently, adoptive transfer was performed with immune or control CD4+ or CD8+ T lymphocytes, and mice were infected with 1.6 × 107 S. aureus 67-0 cells. Both immune but not control CD4+ or CD8+ T lymphocytes transferred protection (Fig. 7).
FIG. 7.
Vaccine-induced protection is mediated by T lymphocytes but not B220+ B lymphocytes or antibody. CD3+ T or B220+ B lymphocytes (107) or CD4+ or CD8+ T lymphocytes (5 × 106) from spleens of mice vaccinated with rAls3p-N plus Al(OH)3 or Al(OH)3 alone were adoptively transferred i.v. via the tail vein to congenic, female BALB/c mice 24 h prior to infection (n = 16 per group from two experiments for CD3+ T-cell transfer; n = 8 mice per group for all others). Pooled sera (250 μl) from vaccinated or control donor mice were administered intraperitoneally 3 h before infection, with doses repeated weekly (n = 8 mice per group). Mice were infected with 3 × 107 or 1.6 × 107 S. aureus 67-0 cells. *, P ≤ 0.02 for the recipients of vaccine or control donor cells or serum by the log-rank test.
In contrast, the adoptive transfer of B lymphocytes or passive immunization with 250 μl of pooled immune serum did not significantly improve survival compared to that of the controls. To evaluate for a subtle effect of immune serum, the passive immunization study was repeated at a lower infectious inoculum (1.3 × 107 S. aureus 67-0 cells) and with several doses of serum (a 750-μl dose, a repeat of the 250-μl dose, and a 25-μl dose to rule out a prozone effect at higher doses). No dose of serum was protective, even against the lower inoculum of infection (data not shown).
DISCUSSION
We have previously shown that the rAls3p-N vaccine protects mice against otherwise lethal disseminated candidiasis (30). We now show that the same vaccine mediates protection during staphylococcemia in mice. Thus, the rAls3p-N vaccine mediated protection in mice against a fungal pathogen and a bacterial pathogen that jointly cause at least 200,000 clinical bloodstream infections, resulting in billions of dollars of health care expenditures and ≥40,000 to 50,000 deaths per year in the United States alone (5, 6, 10, 12, 19, 20, 25, 28, 37, 38).
T lymphocytes were necessary and sufficient for rAls3p-N vaccine-mediated protection, while B lymphocytes were neither necessary nor sufficient. The transfer of immune serum did not improve the survival of mice subsequently infected with S. aureus. Furthermore, mice treated with 250 μl of immune serum had worse survival than those treated with 25 μl of serum. The decreased survival in mice receiving the higher dose of serum may reflect a prozone effect. Our data are consistent with those of Taborda et al., who found that the same antibody administered to mice at different doses or against different infectious inocula can either enhance or ameliorate the severity of the target infection (34). Our data also are concordant with those of Lee et al., who found that T lymphocytes can directly modulate disease outcome during S. aureus infections (16), and those of McLoughlin et al., who found that T-cell-derived IFN-γ is critical to upregulate the neutrophil killing of S. aureus in vivo in mice (18).
The rAls3p-N vaccine induced a moderated type 1 immune response, with high splenic Th1/Th2 ratios during staphylococcemia, but also an increase in Th2 splenocyte frequencies and Th2 cytokine production compared to that of control mice. These results are concordant with those of prior investigations in which the abrogation of IFN-γ improved survival and improved inflammatory-related necrosis in the kidneys of staphylococcemic mice (23, 36, 39). Nevertheless, as mentioned, the critical role of IFN-γ in acquired immunity to S. aureus has been reported (18, 24). Hence, in aggregate, these data suggest that while vaccine-induced type 1 immunity can improve the survival of staphylococcemic mice, the moderation of the severity of inflammation helps maximize survival. Furthermore, the data suggest that the enhancement of type 1 immunity (e.g., by the use of other adjuvants or recombinant IFN-γ or IL-12) is important to explore as a way to advance current anti-staphylococcal vaccine strategies.
We found that the rAls3p-N vaccine primed splenocytes to respond to S. aureus cell wall extract. Furthermore, vaccination with tetanus toxoid or a related Als protein, rAls5p, did not induce protection. Also, the rAls3p-N vaccine did not induce protection against other gram-positive or gram-negative bacteria, and the vaccine remained effective even in an 8-week delayed model of infection, indicating that a memory response to the vaccine had occurred. Finally, protection was provided in adoptive transfer studies by CD3+, CD4+, or CD8+ lymphocytes. Collectively, these data indicate that rAls3p-N vaccine-mediated protection was specific for S. aureus and was not simply a nonspecific response to the antigen.
Prior predictions, based on computer modeling, of a three-dimensional conformational similarity of rAls3-pN, S. aureus clumping factor, and related MSCRAMM proteins (26) influenced us to test the possibility of cross-kingdom protection against C. albicans and S. aureus. However, surprisingly, we found minimal cross-immunization between rAls3p-N and clumping factor. Intensive investigation to elucidate the cross-reacting antigens responsible for protection is ongoing. Furthermore, due to the predicted structural similarity, we initially hypothesized that the protection seen would be antibody mediated. However, as stated above, we found that B cells were neither necessary nor sufficient for protection, and that T lymphocytes were both necessary and sufficient.
We found that a dose of 300 μg of rAls3p-N mediated protection when combined with Al(OH)3 adjuvant, whereas rAls3p-N with 20 μg CFA did not. It is important to emphasize that in translating vaccines from mice to humans, one should not extrapolate on a milligram-per-kilogram basis the dose of protein that will be effective. Rather, doses used in mice tend to reflect those that are effective in humans. Both a widely used FDA guidance (11) and the World Health Organization guidelines on the nonclinical evaluation of vaccines (1) indicate that the highest dose planned for testing in humans is the dose to be used in preclinical vaccine studies. For example, the doses of the hepatitis B surface antigen, tetanus toxoid, and diphtheria and pertussis components of the Tdap vaccine approved for use in humans generally are similar to those tested in preclinical rodent studies. Hence, the efficacy of a 300-μg dose in mice indicates that future clinical trials should use an upper limit of a 300-μg dose of rAls3p-N and does not represent a barrier to testing the vaccine in humans.
In summary, we report that the anti-Candida rAls3p-N vaccine mediates cross-kingdom protection against virulent, drug-resistant, clinical isolates of the ubiquitous bacterial pathogen S. aureus. Of particular importance is the efficacy of the rAls3p-N vaccine when combined with aluminum-based adjuvants, which remain to date the only adjuvants in vaccines approved for use in humans by the FDA. These data underscore the promise of developing rAls3p-N into a clinically useful vaccine targeting these extremely common, increasingly antibiotic-resistant, and highly lethal pathogens.
Acknowledgments
We gratefully acknowledge the technical assistance of Deborah Kupferwasser and Tiffany Ho.
B.S., A.S.I., Y.F., M.R.Y., S.G.F., and J.E.E. own equity in NovaDigm Therapeutics, Inc., which is developing vaccine technologies. NovaDigm Therapeutics, Inc., provided no financial support for these studies.
This work was supported by Public Health Service grants R01 AI19990, R01 AI063382, and R41 AI071554 to J.E.E.; K08 AI060641 and R01 AI072052 to B.S.; R01 AI063503 to A.S.I.; R01 A1054928 and R01 DE017088 to S.G.F.; R01 AI48031 and R01 AI39108 to M.R.Y.; and R01 AI39108 to A.S.B. J.E.E. also is supported by an unrestricted Freedom to Discover Grant for Infectious Disease from Bristol Myers Squibb. A.S.I. also is supported by a Burroughs Wellcome New Investigator Award in Molecular Pathogenic Mycology. B.S. and Y.F. are supported by American Heart Association beginning grants-in-aid 0665154Y and 0665041Y, respectively. B.S. also is supported by the LA Biomedical Research Institute Liu Young Investigator Award.
Editor: A. Camilli
Footnotes
Published ahead of print on 21 July 2008.
REFERENCES
- 1.Anonymous. 21 November 2003, posting date. Annex 1: guidelines on nonclinical evaluation of vaccines. World Health Organization, Geneva, Switzerland. http://www.who.int/biologicals/publications/trs/areas/vaccines/dna/Annex%201_DNA%20vaccines.pdf.
- 2.Andes, D., and W. A. Craig. 2006. Pharmacodynamics of a new streptogramin, XRP 2868, in murine thigh and lung infection models. Antimicrob. Agents Chemother. 50243-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arias, C. A., K. V. Singh, D. Panesso, and B. E. Murray. 2007. Time-kill and synergism studies of ceftobiprole against Enterococcus faecalis, including beta-lactamase-producing and vancomycin-resistant isolates. Antimicrob. Agents Chemother. 512043-2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baddour, L. M., G. D. Christensen, M. G. Hester, and A. L. Bisno. 1984. Production of experimental endocarditis by coagulase-negative staphylococci: variability in species virulence. J. Infect. Dis. 150721-727. [DOI] [PubMed] [Google Scholar]
- 5.Chambers, H. F. 2001. The changing epidemiology of Staphylococcus aureus? Emerg. Infect. Dis. 7178-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chambers, H. F. 2005. Community-associated MRSA—resistance and virulence converge. N. Engl. J. Med. 3521485-1487. [DOI] [PubMed] [Google Scholar]
- 7.Cheung, A. L., A. S. Bayer, J. Peters, and J. I. Ward. 1987. Analysis by gel electrophoresis, Western blot, and peptide mapping of protein A heterogeneity in Staphylococcus aureus strains. Infect. Immun. 55843-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christensen, G. D., A. L. Bisno, J. T. Parisi, B. McLaughlin, M. G. Hester, and R. W. Luther. 1982. Nosocomial septicemia due to multiply antibiotic-resistant Staphylococcus epidermidis. Ann. Intern. Med. 961-10. [DOI] [PubMed] [Google Scholar]
- 9.Deslouches, B., I. A. Gonzalez, D. DeAlmeida, K. Islam, C. Steele, R. C. Montelaro, and T. A. Mietzner. 2007. De novo-derived cationic antimicrobial peptide activity in a murine model of Pseudomonas aeruginosa bacteraemia. J. Antimicrob. Chemother. 60669-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fridkin, S. K., J. C. Hageman, M. Morrison, L. T. Sanza, K. Como-Sabetti, J. A. Jernigan, K. Harriman, L. H. Harrison, R. Lynfield, and M. M. Farley. 2005. Methicillin-resistant Staphylococcus aureus disease in three communities. N. Engl. J. Med. 3521436-1444. [DOI] [PubMed] [Google Scholar]
- 11.Gruber, M. F. 2003. Non-clinical safety assessment of vaccines. Center for Biological Evaluation and Research, U.S. Food and Drug Administration, Washington, DC.
- 12.Hoen, B., F. Alla, C. Selton-Suty, I. Beguinot, A. Bouvet, S. Briancon, J. P. Casalta, N. Danchin, F. Delahaye, J. Etienne, V. Le Moing, C. Leport, J. L. Mainardi, R. Ruimy, and F. Vandenesch. 2002. Changing profile of infective endocarditis: results of a 1-year survey in France. JAMA 28875-81. [DOI] [PubMed] [Google Scholar]
- 13.Ibrahim, A. S., B. J. Spellberg, V. Avanesian, Y. Fu, and J. E. Edwards, Jr. 2006. The anti-Candida vaccine based on the recombinant N-terminal domain of Als1p is broadly active against disseminated candidiasis. Infect. Immun. 743039-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ibrahim, A. S., B. J. Spellberg, V. Avenissian, Y. Fu, S. G. Filler, and J. E. Edwards, Jr. 2005. Vaccination with recombinant N-terminal domain of Als1p improves survival during murine disseminated candidiasis by enhancing cell-mediated, not humoral, immunity. Infect. Immun. 73999-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Josefsson, E., O. Hartford, L. O'Brien, J. M. Patti, and T. Foster. 2001. Protection against experimental Staphylococcus aureus arthritis by vaccination with clumping factor A, a novel virulence determinant. J. Infect. Dis. 1841572-1580. [DOI] [PubMed] [Google Scholar]
- 16.Lee, L. Y., Y. J. Miyamoto, B. W. McIntyre, M. Hook, K. W. McCrea, D. McDevitt, and E. L. Brown. 2002. The Staphylococcus aureus Map protein is an immunomodulator that interferes with T cell-mediated responses. J. Clin. Investig. 1101461-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madrigal, A. G., L. Basuino, and H. F. Chambers. 2005. Efficacy of telavancin in a rabbit model of aortic valve endocarditis due to methicillin-resistant Staphylococcus aureus or vancomycin-intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 493163-3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLoughlin, R. M., J. C. Lee, D. L. Kasper, and A. O. Tzianabos. 2008. IFN-γ regulated chemokine production determines the outcome of Staphylococcus aureus infection. J. Immunol. 1811323-1332. [DOI] [PubMed] [Google Scholar]
- 19.Moran, G. J., A. Krishnadasan, R. J. Gorwitz, G. E. Fosheim, L. K. McDougal, R. B. Carey, and D. A. Talan. 2006. Methicillin-resistant S. aureus infections among patients in the emergency department. N. Engl. J. Med. 355666-674. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen, D. M., E. Bancroft, L. Mascola, R. Guevara, and L. Yasuda. 2007. Risk factors for neonatal methicillin-resistant Staphylococcus aureus infection in a well-infant nursery. Infect. Control Hosp. Epidemiol. 28406-411. [DOI] [PubMed] [Google Scholar]
- 21.Phan, Q. T., C. L. Myers, Y. Fu, D. C. Sheppard, M. R. Yeaman, W. H. Welch, A. S. Ibrahim, J. E. Edwards, and S. G. Filler. 2007. Als3 is a Candida albicans invasin that binds to cadherins and induces endocytosis by host cells. PLoS Biol. 5e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rauceo, J. M., R. De Armond, H. Otoo, P. C. Kahn, S. A. Klotz, N. K. Gaur, and P. N. Lipke. 2006. Threonine-rich repeats increase fibronectin binding in the Candida albicans adhesin Als5p. Eukaryot. Cell 51664-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sasaki, S., S. Nishikawa, T. Miura, M. Mizuki, K. Yamada, H. Madarame, Y. I. Tagawa, Y. Iwakura, and A. Nakane. 2000. Interleukin-4 and interleukin-10 are involved in host resistance to Staphylococcus aureus infection through regulation of gamma interferon. Infect. Immun. 682424-2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sasaki, S., Y. Tagawa, Y. Iwakura, and A. Nakane. 2006. The role of gamma interferon in acquired host resistance against Staphylococcus aureus infection in mice. FEMS Immunol. Med. Microbiol. 46367-374. [DOI] [PubMed] [Google Scholar]
- 25.Sattler, C. A., E. O. Mason, Jr., and S. L. Kaplan. 2002. Prospective comparison of risk factors and demographic and clinical characteristics of community-acquired, methicillin-resistant versus methicillin-susceptible Staphylococcus aureus infection in children. Pediatr. Infect. Dis. J. 21910-917. [DOI] [PubMed] [Google Scholar]
- 26.Sheppard, D. C., M. R. Yeaman, W. H. Welch, Q. T. Phan, Y. Fu, A. S. Ibrahim, S. G. Filler, M. Zhang, A. J. Waring, and J. E. Edwards, Jr. 2004. Functional and structural diversity in the Als protein family of Candida albicans. J. Biol. Chem. 27930480-30489. [DOI] [PubMed] [Google Scholar]
- 27.Smeltzer, M. S., F. L. Pratt, A. F. Gillaspy, and L. A. Young. 1996. Genomic fingerprinting for epidemiological differentiation of Staphylococcus aureus clinical isolates. J. Clin. Microbiol. 341364-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spellberg, B., S. G. Filler, and J. E. Edwards, Jr. 2006. Current treatment strategies for disseminated candidiasis. Clin. Infect. Dis. 42244-251. [DOI] [PubMed] [Google Scholar]
- 29.Spellberg, B., A. S. Ibrahim, L. Lin, V. Avanesian, Y. Fu, P. Lipke, H. Otoo, T. Ho, and J. E. Edwards, Jr. 2008. Antibody titer threshold predicts anti-candidal vaccine efficacy even though the mechanism of protection is induction of cell-mediated immunity. J. Infect. Dis. 197967-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spellberg, B. J., A. S. Ibrahim, V. Avanesian, Y. Fu, C. Myers, Q. T. Phan, S. G. Filler, M. R. Yeaman, and J. E. Edwards, Jr. 2006. Efficacy of the anti-Candida rAls3p-N or rAls1p-N vaccines against disseminated and mucosal candidiasis. J. Infect. Dis. 194256-260. [DOI] [PubMed] [Google Scholar]
- 31.Spellberg, B. J., A. S. Ibrahim, V. Avenissian, S. G. Filler, C. L. Myers, Y. Fu, and J. E. Edwards, Jr. 2005. The anti-Candida albicans vaccine composed of the recombinant N terminus of Als1p reduces fungal burden and improves survival in both immunocompetent and immunocompromised mice. Infect. Immun. 736191-6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spellberg, B. J., D. Johnston, Q. T. Phan, J. E. Edwards, Jr., S. W. French, A. Ibrahim, and S. G. Filler. 2003. Parenchymal organ, and not splenic, immunity correlates with host survival during disseminated candidiasis. Infect. Immun. 715756-5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. Lim, K. Smith, D. Spencer, G. K. Wong, Z. Wu, I. T. Paulsen, J. Reizer, M. H. Saier, R. E. Hancock, S. Lory, and M. V. Olson. 2000. Complete genome sequence of Pseudomonas aeruginosa PA01, an opportunistic pathogen. Nature 406959-964. [DOI] [PubMed] [Google Scholar]
- 34.Taborda, C. P., J. Rivera, O. Zaragoza, and A. Casadevall. 2003. More is not necessarily better: prozone-like effects in passive immunization with IgG. J. Immunol. 1703621-3630. [DOI] [PubMed] [Google Scholar]
- 35.Vernachio, J., A. S. Bayer, T. Le, Y. L. Chai, B. Prater, A. Schneider, B. Ames, P. Syribeys, J. Robbins, and J. M. Patti. 2003. Anti-clumping factor A immunoglobulin reduces the duration of methicillin-resistant Staphylococcus aureus bacteremia in an experimental model of infective endocarditis. Antimicrob. Agents Chemother. 473400-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei, X. Q., B. P. Leung, W. Niedbala, D. Piedrafita, G. J. Feng, M. Sweet, L. Dobbie, A. J. Smith, and F. Y. Liew. 1999. Altered immune responses and susceptibility to Leishmania major and Staphylococcus aureus infection in IL-18-deficient mice. J. Immunol. 1632821-2828. [PubMed] [Google Scholar]
- 37.Wilson, L. S., C. M. Reyes, M. Stolpman, J. Speckman, K. Allen, and J. Beney. 2002. The direct cost and incidence of systemic fungal infections. Value Health 526-34. [DOI] [PubMed] [Google Scholar]
- 38.Wisplinghoff, H., T. Bischoff, S. M. Tallent, H. Seifert, R. P. Wenzel, and M. B. Edmond. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 39309-317. [DOI] [PubMed] [Google Scholar]
- 39.Zhao, Y. X., I. M. Nilsson, and A. Tarkowski. 1998. The dual role of interferon-gamma in experimental Staphylococcus aureus septicaemia versus arthritis. Immunology 9380-85. [DOI] [PMC free article] [PubMed] [Google Scholar]