Abstract
Campylobacter jejuni is a frequent cause of bacterial gastroenteritis worldwide. Lipooligosaccharide (LOS) has been identified as an important virulence factor that may play a role in microbial adhesion and invasion. Here we specifically address the question of whether LOS sialylation affects the interaction of C. jejuni with human epithelial cells. For this purpose, 14 strains associated with Guillain-Barré syndrome (GBS), 34 enteritis-associated strains, the 81-176 reference strain, and 6 Penner serotype strains were tested for invasion of two epithelial cell lines. C. jejuni strains expressing sialylated LOS (classes A, B, and C) invaded cells significantly more frequently than strains expressing nonsialylated LOS (classes D and E) (P < 0.0001). To further explore this observation, we inactivated the LOS sialyltransferase (Cst-II) via knockout mutagenesis in three GBS-associated C. jejuni strains expressing sialylated LOS (GB2, GB11, and GB19). All knockout strains displayed significantly lower levels of invasion than the respective wild types. Complementation of a Δcst-II mutant strain restored LOS sialylation and reset the invasiveness to wild-type levels. Finally, formalin-fixed wild-type strains GB2, GB11 and GB19, but not the isogenic Δcst-II mutants that lack sialic acid, were able to inhibit epithelial invasion by viable GB2, GB11, and GB19 strains. We conclude that sialylation of the LOS outer core contributes significantly to epithelial invasion by C. jejuni and may thus play a role in subsequent postinfectious pathologies.
Campylobacter jejuni is recognized as a leading cause of bacterial gastroenteritis worldwide. Poorly handled or improperly cooked poultry meat, raw milk, pets, and untreated water are thought to be sources of infection (28). The disease spectrum caused by C. jejuni ranges from asymptomatic infection to severe inflammatory bloody diarrhea (19). Furthermore, C. jejuni infection has been associated with the development of postinfectious complications such as the Guillain-Barré syndrome (GBS) (26). The apparent variation in gastrointestinal disease outcome is likely to be affected by the expression of virulence factors that are associated with specific pathogenic mechanisms, e.g., C. jejuni motility (24), attachment (18), and invasion (6, 16, 38). Motility and chemotaxis appear to be necessary for the epithelial adherence of C. jejuni, whereas the expression of functional flagella may determine the capacities of C. jejuni to invade the epithelium and to effectively colonize the mouse intestine (38, 41, 44, 45).
Next to the role of flagella in the regulation of C. jejuni invasiveness, lipooligosaccharide (LOS) structures have generally been implicated in microbial invasion (15, 17, 21, 25, 33, 35, 37). To date, five major and distinctive LOS biosynthesis gene clusters, referred to here as LOS classes, have been described for C. jejuni (31), and this number continues to increase (30). Sequencing and microarray analysis of the LOS biosynthesis gene locus of the C. jejuni genome have also revealed this locus to be highly variable (11, 15), which may contribute to the variation in C. jejuni-associated pathologies. Furthermore, it has been shown that C. jejuni strains may also acquire these LOS synthesis genes from other C. jejuni strains by means of horizontal exchange (10, 34).
A subgroup of C. jejuni strains that express the LOS class A, B, or C gene locus harbor genes involved in sialic acid biosynthesis and are therefore able to synthesize sialylated LOS (9, 11, 12, 14). The cst-II gene encodes a sialyltransferase (7) that is necessary for the transfer of sialic acid onto the LOS core in C. jejuni class A and B strains. C. jejuni class C strains depend on the cst-III gene for LOS sialylation. Hence, only C. jejuni strains expressing LOS class A, B, or C are capable of LOS sialylation. Previously, we have shown that the presence and expression of the cst-II gene is specifically associated with GBS and is required for the induction of antiganglioside antibody responses, which are the hallmark of this postinfectious complication (12, 39). Based on this prior work, we hypothesized that LOS sialylation (and consequently C. jejuni LOS subclasses) may be involved in C. jejuni invasiveness.
Therefore, a panel of 48 human isolates and 7 human control strains was assessed for invasiveness for two human epithelial carcinoma cell lines (Caco-2 and T84). To specifically explore the role of sialylation, we generated three GBS-associated sialyltransferase (Cst-II) knockout C. jejuni strains (GB2 Δcst-II, GB11 Δcst-II, and GB19 Δcst-II). These GB2 Δcst-II, GB11 Δcst-II, and GB19 Δcst-II mutants were tested for their abilities to adhere to and invade Caco-2 cells. Finally, we investigated whether complementation of the Δcst-II mutant would restore the invasion-associated function of this gene product.
MATERIALS AND METHODS
Bacterial strains.
Fourteen GBS- and 34 enteritis-associated C. jejuni strains isolated from Dutch patients, 6 Penner serotype strains, and the 81-176 enteritis reference strain were used in this study (see Table 1). To minimize in vitro passages, C. jejuni strains were recovered from the original patient-isolated glycerol stock by culturing on Butzler agar plates (Becton Dickinson, Breda, The Netherlands). A second passage was allowed for optimal vitality before these strains were used in experiments. After recovery, cells were harvested in Hanks balanced salt solution (Life Technology, Breda, The Netherlands), and densities were adjusted according to the optical density at 600 nm (OD600).
TABLE 1.
C. jejuni strains and their invasiveness for Caco-2 cells
| Straina | LOS locus | % Invasionb | No. of invading C. jejuni organisms per 100 cells | Presence of NeuAcc | Ganglioside mimicd | Illness |
|---|---|---|---|---|---|---|
| GB2 | A | 3.4 ± 0.55 | 285-395 | Yes | GM1a, GD1a | GBS |
| GB11 | A | 2.2 ± 0.7 | 150-290 | Yes | GM1a, GD1a | GBS |
| GB19 | A | 0.8 ± 0.29 | 51-109 | Yes | GD1c | GBS |
| GB3 | A | 0.12 ± 0.046 | 7-16 | Yes | GM1a, GD1a | GBS |
| GB22 | A | 0.05 ± 0.026 | 3-7 | Yes | GM1a, GD1a | GBS |
| GB23 | A | 1.17 ± 0.14 | 103-131 | Yes | GM2 | GBS |
| GB29 | A | 0.73 ± 0.06 | 67-79 | GBS | ||
| E990521 | A | 3.0 ± 1.15 | 185-415 | Enteritis | ||
| E991095 | A | 1.9 ± 0.81 | 110-271 | Enteritis | ||
| E9126 | A | 1.2 ± 0.58 | 70-178 | Enteritis | ||
| P19 | A | 4.7 ± 1.4 | 330-610 | Yes | GM1a, GD1a | Enteritis |
| P10 | A | 4.23 ± 1.86 | 237-609 | Yes | GD3 | Enteritis |
| P4 | A | 0.0054 ± 0.00092 | 0.44-0.63 | Yes | GM1a, GD1a | Enteritis |
| GB17 | B | 3.05 ± 1.75 | 130-480 | Yes | GM1b, GD1c | GBS |
| GB25 | B | 0.27 ± 0.13 | 14-40 | Yes | GM1b, GD1c | GBS |
| GB31 | B | 0.97 ± 0.15 | 82-112 | Yes | GM1a, GD1a | GBS |
| GB37 | B | 0.16 ± 0.03 | 13-19 | GBS | ||
| Rivm 16 | B | 1.98 ± 0.7 | 192-205 | Enteritis | ||
| Rivm 38 | B | 0.037 ± 0.023 | 1.0-6.0 | Enteritis | ||
| Rivm 129 | B | 0.084 ± 0.026 | 5.0-11 | Enteritis | ||
| E989123 | B | 0.29 ± 0.011 | 18-40 | Enteritis | ||
| E981033 | B | 0.26 ± 0.075 | 18-33 | Yes | GM1a | Enteritis |
| E98652 | B | 0.028 ± 0.006 | 2-4 | Yes | GM1a, GQ1b | Enteritis |
| 81-176 | B | 0.26 ± 0.06 | 20-32 | Yes | GM2, GM3 | Enteritis |
| GB13 | C | 0.2 ± 0.017 | 18-22 | Yes | GM1a | GBS |
| GB38 | C | 1.8 ± 0.77 | 103-257 | GBS | ||
| Rivm 15 | C | 0.00075 ± 0.00014 | 0.061-0.089 | Enteritis | ||
| Rivm 83 | C | 2.75 ± 1.28 | 147-403 | Enteritis | ||
| Rivm 93 | C | 3.5 ± 1.15 | 235-465 | Enteritis | ||
| Rivm 109 | C | 1.22 ± 0.44 | 78-166 | Enteritis | ||
| Rivm 116 | C | 0.25 ± 0.13 | 12-38 | Enteritis | ||
| E98682 | C | 0.010 ± 0.0036 | 0.6-1.4 | Yes | GM1a, GQ1b | Enteritis |
| E981087 | C | 0.13 ± 0.031 | 10-16 | Yes | GM1a | Enteritis |
| P1 | C | 0.01 ± 0.001 | 0.9-1.1 | Yes | GM2 | Enteritis |
| P2 | C | 0.005 ± 0.0017 | 0.33-0.67 | Yes | GM1b | Enteritis |
| Rivm 3 | D | 0.005 ± 0.0012 | 0.38-0.62 | Enteritis | ||
| Rivm 33 | D | 0.017 ± 0.0045 | 1-2 | Enteritis | ||
| Rivm 65 | D | 0.018 ± 0.0026 | 1-2 | Enteritis | ||
| Rivm 67 | D | 0.0097 ± 0.0013 | 0.5-1 | Enteritis | ||
| Rivm 95 | D | 0.019 ± 0.003 | 1-2 | Enteritis | ||
| Rivm 104 | D | 0.0082 ± 0.0014 | 0.68-0.96 | Enteritis | ||
| E98706 | D | 0.014 ± 0.0025 | 1.15-1.65 | No | None | Enteritis |
| E970873 | D | 0.14 ± 0.02 | 12-16 | Enteritis | ||
| GB4 | E | 0.009 ± 0.003 | 0.5-1 | No | None | GBS |
| Rivm 37 | E | 0.081 ± 0.029 | 5-11 | Enteritis | ||
| Rivm 46 | E | 0.0065 ± 0.0027 | 0.38-0.92 | |||
| Rivm 47 | E | 0.097 ± 0.028 | 6-12 | Enteritis | ||
| Rivm 50 | E | 0.0065 ± 0.00096 | 0.56-0.74 | Enteritis | ||
| Rivm 61 | E | 0.011 ± 0.0066 | 1-2 | Enteritis | ||
| E9141 | E | 0.074 ± 0.013 | 5-9 | Enteritis | ||
| E9144 | E | 0.14 ± 0.03 | 11-17 | Enteritis | ||
| E9146 | E | 0.08 ± 0.015 | 6-10 | Enteritis | ||
| E98623 | E | 0.004 ± 0.0015 | 0.2-0.5 | No | None | Enteritis |
| E98624 | E | 0.003 ± 0.00075 | 0.23-0.4 | No | None | Enteritis |
| P3 | E | 0.0045 ± 0.0013 | 0.32-0.58 | No | None | Enteritis |
GB, GBS-associated strain; E, enteritis-related strain; P, Penner serotype strain. Strain 81-176 was used as a positive control.
Data are means ± standard deviations for at least three independent experiments and are calculated as the percentage of bacteria that survived the gentamicin treatment.
Determined by mass spectrometry or immunological methods for the 25 strains for which results are shown. Data were not available for the other strains.
The LOS structures showing the ganglioside mimics of 18 strains were elucidated by mass spectrometry and immunological methods; for 7 strains, LOS structures were elucidated by immunological methods only. Data were not available for the other strains.
Typing of the LOS biosynthesis gene cluster.
To determine the class of LOS locus present in each C. jejuni strain, genomic DNA was isolated using the DNeasy tissue kit (Qiagen, Venlo, The Netherlands). PCR analysis was done with primer sets specific for classes A, B, C, D, and E as previously described (12). PCR assays were performed in a Perkin-Elmer GeneAmp PCR system, model 9700 (Applied Biosystems, Nieuwerkerk aan de IJssel, The Netherlands), with 35 cycles of 1 min at 94°C, 1 min at 52°C, and 2 min at 72°C.
Knockout mutagenesis.
Strains GB2 and GB11 and their Δcst-II mutants, GB2 Δcst-II and GB11 Δcst-II, respectively, have been described previously (12). A Δcst-II mutant of a third GBS-related strain that is described here, GB19, was generated by the same procedure that was used for the knockout mutagenesis of strains GB2 and GB11 (12). Briefly, the target gene (cst-II) and approximately 700 bp of upstream and downstream flanking sequences were amplified and cloned into the pGem-T Easy vector (Promega Corp., Leiden, The Netherlands). Inverse PCR was used to introduce a BamHI restriction site and a deletion of approximately 800 bp in the target gene. Inverse PCR products were digested with BamHI (Fermentas, St. Leon-Rot, Germany) and ligated to the BamHI-digested chloramphenicol resistance (Cmr) cassette. Constructs were electroporated into electrocompetent GB19 C. jejuni cells, and recombinants were selected on Mueller-Hinton plates (Becton Dickinson, Breda, The Netherlands) containing 20 μg/ml chloramphenicol (Difco, Alphen aan den Rijn, The Netherlands).
Mass spectrometry.
Samples were prepared for LOS mass spectrometric analysis by overnight growth of C. jejuni strains at 37°C on Butzler agar plates under a microaerobic atmosphere. Material from one confluent agar plate under a microaerobic atmosphere was harvested and treated with proteinase K at 60 μg/ml, RNase A at 200 μg/ml, and DNase I at 100 μg/ml (Promega, Leiden, The Netherlands). O-deacylated LOS samples were prepared and analyzed by capillary electrophoresis coupled to electrospray ionization mass spectrometry (23).
Complementation of the cst-II gene.
We used site-specific homologous recombination to restore the wild-type phenotype of the GB11 Δcst-II mutant strain (unpublished data). Briefly, a construct containing the cst-II gene together with its promoter region and a gene encoding erythromycin resistance were cloned in the same orientation and were transformed by electroporation into electrocompetent GB11 Δcst-II mutant cells. The electroporated cells were plated onto selective blood agar plates containing 10 μg/ml erythromycin (Sigma-Aldrich, Zwijndrecht, The Netherlands) and were incubated at 42°C under a microaerobic environment. Colonies formed were subcultured to purity and stored at −80°C until further use.
SDS-PAGE and Western blot assay.
To analyze C. jejuni LOS sialylation, a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel was run. Strains were harvested from an overnight Butzler agar plate, and concentrations were equalized by OD600 measurement. Bacterial cell suspensions were lysed using glass beads (MP Biomedicals, Solon, OH). Lysates were digested with proteinase K at 60 μg/ml for 4 h at 56°C, and equal amounts were run on a 10% SDS-PAGE Tris-HCl gel for 2 h. As a standard, the prestained SDS-PAGE broad-range molecular weight marker was used (Bio-Rad, Nazareth Eke, Belgium). After electrophoresis, the LOS was transferred to a nitrocellulose membrane (Amersham Biosciences, Piscataway, NJ) for a Western blot assay. The nitrocellulose membrane was blocked overnight with 0.05% (vol/vol) Tween 20 (Sigma-Aldrich, Zwijndrecht, The Netherlands) and 5% (wt/vol) nonfat milk (Bio-Rad, Nazareth Eke, Belgium). The next day, the membranes were washed three times for 10 min each with phosphate-buffered saline (PBS) and incubated with horseradish peroxidase (HRP)-labeled cholera toxin (Sigma-Aldrich, Zwijndrecht, The Netherlands) in 1% blocking buffer as a detection agent. The presence or absence of sialylated LOS was visualized with an ECL detection kit (Biocompare, San Francisco, CA) and Kodak photo film (Roche-Diagnostics, Almere, The Netherlands) according to the manufacturers' protocol.
Bacterial growth assay.
The bacterial growth characteristics of the clinical isolates and their corresponding mutants were determined in Mueller-Hinton broth (Becton Dickinson, Breda, The Netherlands) and in a specific antibiotic-free cell culture medium, which was used in the gentamicin exclusion assay. Bacterial strains were inoculated at equal OD600s, equivalent to 5.0 × 104 CFU/ml, and incubated at 37°C with gentle shaking under a microaerobic environment. Bacterial cell counts and OD600 values were determined at 4, 8, 18, 24, 36, and 42 h postinoculation.
Intestinal epithelial cell line.
Caco-2 and T84 human intestinal epithelial cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and 1% nonessential amino acids (all medium components were from Invitrogen, Breda, The Netherlands). The cells were routinely grown in a 75-cm2 flask (Greiner Bio-One, Alphen a/d Rijn, The Netherlands) at 37°C in a humidified 5% CO2-95% air incubator. Confluent stock cultures were washed with PBS (Invitrogen, Breda, The Netherlands) and trypsinized with trypsin-EDTA (Lonza, Verviers, Belgium), and 5.0 × 105 cells were seeded in a new 75-cm2 flask.
Adhesion and invasion.
The adherence and invasion of C. jejuni were determined by growing the intestinal epithelial cells (Caco-2 or T84) to confluence for 48 h at a final approximate density of 5.0 × 106 cells per well (Greiner Bio-One, Alphen a/d Rijn, The Netherlands) without allowing them to differentiate in the case of Caco-2 cells. The adherence and invasion assays were performed by incubating the epithelial cells with C. jejuni at a ratio of 1:100. Bacteria and epithelial cells were coincubated for 2 h at 37°C under a 5% CO2-95% air atmosphere to assess adherence. For invasion, a subsequent 2-h incubation of the epithelial cells was allowed. After incubation, monolayers were washed three times with prewarmed PBS. To kill extracellular bacteria, monolayers were treated for 3 h with a bactericidal concentration of gentamicin (480 μg/ml) (Sigma-Aldrich, Zwijndrecht, The Netherlands) in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum and 1% nonessential amino acids as described previously (38). For all strains, sensitivity to this concentration of gentamicin was confirmed. After a wash, epithelial cells were lysed with 0.1% Triton X-100 (Cornell, Philadelphia, PA) in PBS for 15 min at room temperature. The number of C. jejuni bacteria that had invaded the cells was determined by plating serial dilutions of the lysis mix onto freshly prepared blood agar plates. After incubation for 24 to 36 h at 37°C in a microaerobic environment, colonies were counted. The percentage of bacteria that had invaded was calculated by first dividing the number of C. jejuni bacteria that had invaded the cells by the number of C. jejuni bacteria that had been inoculated onto the cells and then multiplying by 100%. For determination of adherence, cells were washed extensively three times with PBS, and the cell monolayer was lysed with 0.1% Triton X-100, after which serial dilutions were plated onto blood agar plates (Becton Dickinson, Breda, The Netherlands).
Inhibition of invasion.
Formalin fixed, wild-type C. jejuni strains and their Δcst-II mutants were used to inhibit invasion by viable C. jejuni GB2, GB11, and GB19. Briefly, GB2, GB11, GB19, and their Δcst-II mutants at a starting concentration of 5.0 × 109 CFU/ml, determined by the OD600, were fixed in 3.6% formalin (Sigma-Aldrich, Zwijndrecht, The Netherlands) in PBS for 10 min. The excess of formalin was removed by washing the fixed cells three times in PBS. The sterility of the control cultures confirmed that fixation was complete. Caco-2 cells at a density of 5.0 × 104 per well were preincubated for 30 min with formalin-killed wild-type or Δcst-II mutant C. jejuni strains at a multiplicity of infection (MOI) ranging from 100 to 5,000. Subsequently, the Caco-2 cells were washed to remove excess dead C. jejuni bacteria, and then fresh medium was added. Viable wild-type cells were added at an MOI of 100, and invasion was assessed by the gentamicin exclusion protocol as described above.
Statistical analysis.
Statistical analysis was performed using Instat software (version 2.05a; GraphPad Software, San Diego, CA). Because the invasiveness of strains differed widely, log transformation was used to equalize variances. Invasiveness was expressed as the geometric mean number of CFU per milliliter retrieved from the infected cell line in all three to six invasion experiments performed per C. jejuni strain. Differences in invasiveness between LOS class A, B, and C strains and LOS class D and E strains, and between GBS-associated and enteritis-associated strains, were tested for significance with a Mann-Whitney U test, since column statistics showed that the Gaussian distribution was unequal for the strains. A two-tailed value with P < 0.05 indicated statistical significance. Statistical analysis for differences in adherence and invasion between wild-type and knockout mutant strains was performed, and differences were tested for significance with a paired t test.
RESULTS
LOS sialylation is associated with increased epithelial cell invasion.
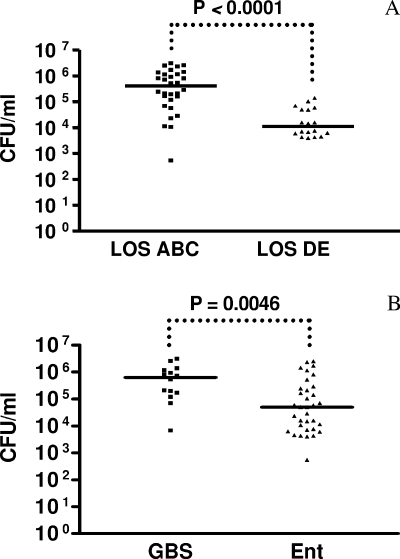
We observed a wide range of invasion capacities among the C. jejuni strains (Table 1). Categorization of C. jejuni strains into those carrying sialylated (n = 30) and nonsialylated (n = 18) LOS established that the sialylated-LOS producers, classes A, B, and C, were more invasive than the nonsialylated-LOS producers, classes D and E (median CFU per milliliter, 408,300 for classes A, B, and C and 11,190 for classes D and E; P < 0.0001) (Fig. 1A). Notably, on average, the GBS-associated strains (n = 14) invaded significantly better than the enteritis-associated strains (n = 34) (median CFU per milliliter, 632,700 versus 49,630, respectively; P = 0.0046) (Fig. 1B). The invasiveness of the C. jejuni Penner serotype strains corresponded with LOS class expression of sialylated or nonsialylated LOS, with the exception of Penner serotype strain O:4. Thus, Penner serotype strain O:4 and also an enteritis-associated strain, Rivm 15, invaded poorly, despite the presumed expression of sialylated LOS due to the presence of a class A or C LOS biosynthesis gene cluster, respectively. Strain 81-176 invaded the Caco-2 cell line as well as it did in previous studies, although most of those invasion studies were performed using a different cell line and a shorter incubation period (see Table 1). All Dutch clinical strains that contain LOS genes of class A, B, or C are thought to express sialylated LOS (12). Characterization of the LOS ganglioside mimic structures and determination of the presence or absence of sialylation for the GBS strains (GB2, GB3, GB4, GB11, GB13, GB17, GB19, GB22, GB23, GB25, and GB31) and enteritis strains (E98-623, 624, 652, 682, 706, 1033, and 1087) were carried out previously by immunological methods (1, 13). These results are shown in Table 1.
FIG. 1.
The invasiveness of C. jejuni is dependent on sialylation of the LOS. Scattergrams show the invasion of Caco-2 cells by Dutch C. jejuni strains, categorized with respect to the type of LOS that is expressed (sialylated LOS of classes A, B, and C [n = 30] versus nonsialylated LOS of classes D and E [n = 18]) (A) or the clinical outcome of infection, i.e., GBS (n = 14) versus uncomplicated gastroenteritis (n = 34) (B). Experiments were performed in triplicate and repeated at least three times. For each strain, a geometric mean outcome (number of CFU per milliliter) was calculated. The differences between the geometric means of groups of strains were tested with the Mann-Whitney U statistic. The median for each group of strains is shown.
LOS phenotype characteristics of different C. jejuni strains and Δcst-II mutants.
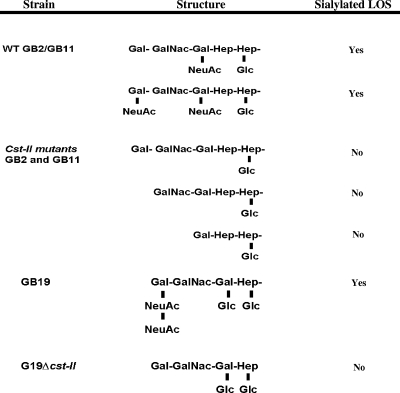
As determined by mass spectrometry analysis, GB19 expressed sialylated LOS in the form of ganglioside mimic GD1c (also referred to as GD3, due to the structural similarity to human GD3). GD1c contains disialic acid bound to the terminal galactose residue. All three Δcst-II mutants were chemically defined and found not to express sialylated LOS. The LOS structures of C. jejuni strains GB2, GB11, and GB19 and their associated Δcst-II mutants are shown in Fig. 2. For a subset of strains, comprising GB3, GB4, GB13, GB17, GB22, GB23, GB25, and GB31, ganglioside mimic structures were determined previously by mass spectrometry (Table 1) (13). The LOS structures of the Penner serotype strains O:1, O:2, O:3, O:4, O:10, O:19, and 81-176 (Table 1) have been characterized previously by other researchers (2-5, 15, 29). As can be seen by the absence of data for some strains in Table 1, mass spectrometry data on LOS structures were not available for all bacteria.
FIG. 2.
Proposed LOS outer core structures as determined by mass spectrometry analysis. Note that GB2 and GB11 express a mixture of the sialylated LOS ganglioside mimics GM1 and GD1a, whereas GB19 expresses sialylated LOS only in the form of GD1c. In all three strains, knockout mutagenesis of cst-II resulted in loss of expression of sialylated LOS.
Knockout mutagenesis of cst-II does not affect the bacterial growth rate significantly.
To exclude the possibility that differences in viability and growth rates would influence the results of our invasion assays, we assessed the growth rates of wild-type strains GB2, GB11, and GB19 and their Δcst-II mutants in Mueller-Hinton medium and in the cell culture medium used in the Caco-2 cell invasion assays. No significant differences in growth rates were observed between the wild-type GB2, GB11, and GB19 strains and their Δcst-II mutants during the time span of our invasion experiments (data not shown).
Disruption of cst-II significantly affects the invasiveness of C. jejuni for intestinal epithelial cells.
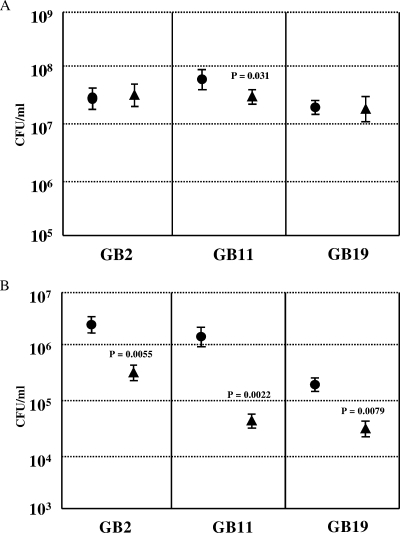
We compared the capacities of the C. jejuni wild-type strains GB2, GB11, and GB19 to adhere to and invade Caco-2 cells with those of their respective Δcst-II mutants. At an MOI of 100, wild-type and mutant strains adhered equally well to the human Caco-2 cell line (Fig. 3A). The only exception was the GB11 Δcst-II strain, which displayed a lower level of adherence than wild-type GB11 (P = 0.031). GB2 Δcst-II, GB11 Δcst-II, and GB19 Δcst-II all showed significant reductions in invasiveness relative to that of their wild-type parent strain (P = 0.005, P = 0.002, and P = 0.008, respectively) (Fig. 3B).
FIG. 3.
LOS sialylation plays an important role in invasion of, but not in adherence to, Caco-2 cells by C. jejuni. C. jejuni wild-type strains GB2, GB11, and GB19 and their respective Δcst-II mutants were studied for adherence to (A) and invasion of (B) human enterocyte-like Caco-2 cells. Circles represent the wild type; triangles represent the mutant. Differences in adhesion and invasion were tested for significance by using the standard t test. Data are expressed as geometric means for at least three experiments, each performed in triplicate. Error bars, standard deviations.
In order to study whether the role of sialic acid in C. jejuni invasion is restricted to interactions with Caco-2 cells, a small selection of C. jejuni strains (P3, GB2, GB11, and GB13) and Δcst-II mutants (GB2 Δcst-II and GB11 Δcst-II) were tested for invasiveness for the T84 human intestinal epithelial cell line (data not shown). The levels of invasiveness of all wild-type strains were similar in both cell types. Again, Δcst-II mutants displayed reduced (by 1 to 1.5 log units) invasion of T84 cells. Together, these data establish that LOS sialylation contributes significantly to the invasion of intestinal epithelial cells by C. jejuni. We excluded variation in microbial motility as the mechanism underlying the reduced invasion of the Δcst-II mutant strains by performing quantitative swarming assays (data not shown).
Complementation of the GB11 Δcst-II mutant restores expression of sialylated LOS.
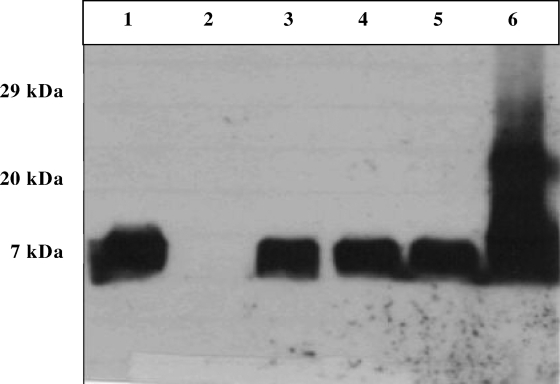
Site-specific homologous recombination was used to reinstall the cst-II gene, together with its promoter region, in the GB11 Δcst-II strain. Using HRP-labeled cholera toxin as a detection agent, we confirmed the expression of sialylated LOS of the wild-type GB11 strain and of three selected clones of the complemented GB11 Δcst-II mutant by a Western blot assay (Fig. 4, lanes 1, 3, 4, and 5, respectively). The GB11 Δcst-II mutant did not express sialylated LOS (Fig. 4, lane 2). LOS isolated from the 11168 genome strain was used as a positive control for the binding of the HRP-labeled cholera toxin (Fig. 4, lane 6).
FIG. 4.
Western blot assay for analysis of cholera toxin binding at the LOS of wild-type GB11, its Δcst-II mutant, and the complemented GB11 Δcst-II mutant strain. Lane 1, LOS of the GB11 wild-type strain; lane 2,LOS of the GB11 Δcst-II mutant strain; lanes 3, 4, and 5, LOS from three selected clones of the complemented GB11 Δcst-II mutant; lane 6, LOS of the 11168 genome strain, used as a positive control. The LOS band is present at around 7 kDa.
Complementation of the GB11 Δcst-II mutant restores invasiveness.
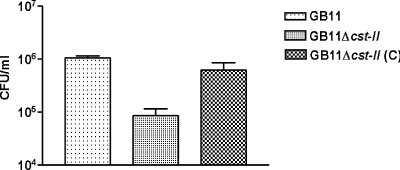
The Western blot assay provided evidence that the complemented mutant was now capable of LOS sialylation. With the gentamicin exclusion assay, we were able to show that this complementation also restored invasiveness to wild-type levels (Fig. 5). These results reiterate the importance of LOS sialylation in invasion.
FIG. 5.
Complementation of the GB11 Δcst-II mutant restores the wild-type phenotype for invasion observed with GB11. The C. jejuni wild-type strain GB11, the GB11 Δcst-II mutant, and the complemented GB11 Δcst-II (C) mutant were studied for invasion of human enterocyte-like Caco-2 cells. Data are geometric means from at least three independent experiments, each performed in duplo. Error bars, standard deviations.
Fixed, sialylated LOS-containing strains inhibit invasion by their viable counterparts.
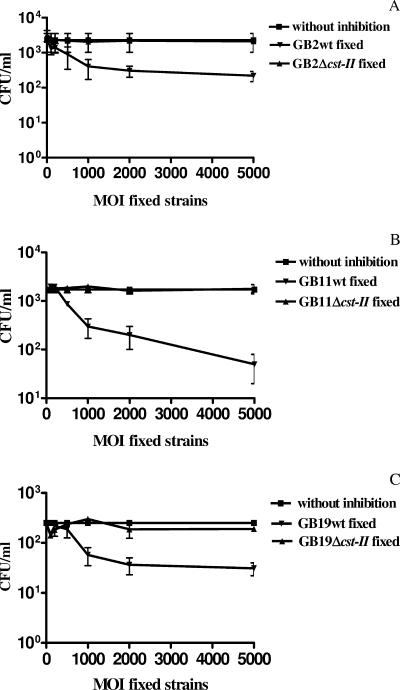
The decreased invasiveness of GB2 Δcst-II, GB11 Δcst-II, and GB19 Δcst-II and the restored wild-type invasion phenotype of the complemented GB11 Δcst-II mutant clearly indicate a role for C. jejuni LOS sialylation in invasion. In order to further address the involvement of LOS sialylation in invasion, we designed an inhibition assay. We preincubated the Caco-2 cells with formalin-fixed, nonviable sialylated wild-type strains (GB2, GB11, and GB19) before incubating the cells with viable sialylated wild-type strains (GB2, GB11, and GB19). We found reductions of as much as 1 to 2 log units in invasion by viable wild-type strains. When Caco-2 cells were preincubated with an excess of formalin-fixed nonsialylated LOS Δcst-II mutants, no differences in invasion were found relative to the invasion control (Fig. 6). The control groups consisted of Caco-2 cells that were incubated only with the viable wild-type strain GB2, GB11, or GB19. These results corroborate that LOS sialylation is an important determinant of epithelial cell invasiveness.
FIG. 6.
C. jejuni strains GB2, GB11, and GB19 invade Caco-2 cells via a sialylated-LOS-dependent mechanism(s). The levels of invasion by viable wild-type strains GB2 (A), GB11 (B), and GB19 (C) were assessed in the presence of either formalin-fixed GB2, GB11, or GB19 wild-type (wt) bacteria (sialylated LOS) or the respective fixed Δcst-II mutants (truncated LOS, nonsialylated). Data are means from at least three independent experiments; error bars, standard deviations.
DISCUSSION
The mucosal epithelial cells are the first to interact with enteric pathogens such as C. jejuni. This microorganism may temporarily colonize the intestines in the absence of any clinical symptom. On the other hand, C. jejuni has been implicated in the pathogenesis of immune-mediated pathologies, e.g., GBS. Because C. jejuni infection can present with a such wide range of symptoms, it is crucial to further identify factors and mechanisms that control C. jejuni epithelial invasion and persistence (42). We hypothesized that the factors that regulate C. jejuni epithelial invasion may contribute directly to postinfectious sequelae, e.g., GBS.
Several C. jejuni outer membrane proteins, e.g., CadF, JlpA, and PEB1, play roles in epithelial adhesion and invasion (8, 20, 32). Recently, PEB1 has also been identified as an amino acid transport system, which is essential for microbial growth (22). Previous studies that identified microbial LOS as a generally important factor for invasion have been confirmed for C. jejuni (15, 17, 25, 33). Here we specifically addressed if and to what extent sialylation of C. jejuni LOS contributes to microbial invasion. Therefore, we performed a large-scale survey by testing a heterogeneous panel of 48 human-isolated C. jejuni strains, 7 human control strains, and 3 sialyltransferase (cst-II) knockout strains. The knockout strains were previously shown to lack the capacity of LOS sialylation (12).
Our studies indicate that LOS sialylation facilitates epithelial invasion (Table 1), since C. jejuni strains expressing sialylated LOS invaded significantly more frequently than nonsialylated LOS strains (P < 0.0001). Two strains with presumed LOS sialylation displayed low invasiveness. These results show that LOS sialylation must be regarded as an important contributor to C. jejuni invasiveness but not the single determinant. Earlier reports support the hypothesis that several factors determine invasiveness (15, 17, 25, 33). Similar contributions of sialic acid to invasiveness have been established for other pathogens (36, 43). In contrast, one study reports on inhibition of invasion by sialic acid (40).
Our experiments with the GB2, GB11, and GB19 sialyltransferase (cst-II) knockout strains further established the importance of LOS sialylation, since these mutated strains expressing nonsialylated LOS displayed significantly lower invasiveness than their respective wild-type controls. The methods for generation of such knockout strains may be accompanied by various technical side effects, e.g., mutation of genes other than the target gene. Furthermore, insertion of an antibiotic resistance cassette may induce expression or silencing of adjacent genes and gene products. Therefore, we set up experiments using a complemented Δcst-II mutant strain. We show that this procedure indeed restored sialylation of the LOS (Fig. 4) and subsequent invasiveness to wild-type levels (Fig. 5).
In our studies, only the GB11 Δcst-II mutant strain showed diminished adherence relative to that of its wild-type parent strain, indicating a less important role for LOS sialylation in epithelial adhesion than in invasion. These findings indicate that adhesion and invasion are regulated by different sets of factors. Adhesion is likely established by proteins such as CadF, JlpA, and PEB1 (8, 20, 32), whereas invasion is more influenced by LOS sialylation in the strains we tested.
To support the hypothesis that invasion is facilitated by LOS sialylation, we established that formalin-fixed wild-type strains GB2, GB11, and GB19, but not the isogenic Δcst-II mutants, were able to inhibit epithelial invasion by viable GB2, GB11, and GB19 strains. These findings may have two implications. First, these data may help to identify novel epithelial invasion receptors. Second, these experiments may lead to the discovery of specific agents that can be used to block microbial invasion.
Previously, sialylation of C. jejuni LOS was associated with GBS (12, 27, 46). Isolates from GBS patients mainly synthesize sialylated LOS of classes A and B (±80%) (13). Strains isolated from enteritis patients show a more mixed LOS composition, with a tendency toward nonsialylated LOS expressed by classes D and E. Notably, the presence of strains expressing LOS classes A and B in enteritis patients is around 20 to 25%. Therefore, the enhanced invasiveness of GBS-associated strains seems to result from the frequent presence of LOS class A and B strains in this patient group (1). We hypothesize that among other risk factors, enhanced invasiveness (e.g., through LOS class A expression) contributes to the development of postinfectious complications such as GBS.
In conclusion, we demonstrate that C. jejuni strains expressing sialylated LOS have an overall increased capacity to invade intestinal epithelial cells. Knockout mutagenesis of the cst-II gene and complementation and blocking experiments provide additional evidence on the role of LOS sialylation in the invasion of the intestinal epithelium. Understanding the function of LOS sialylation in epithelial cell invasion may provide us with potential target structures for future therapeutic interventions in C. jejuni-mediated diarrheal disease and its postinfectious complications.
Acknowledgments
This work was supported by a grant from the Human Frontier Science Program (RGP 38/2003).
We thank Denis Brochu and Jianjun Li (NRC, Ottawa, Canada) for the mass spectrometry analysis of LOS. We thank Eduardo Taboada and John H. E. Nash (NRC, Ottawa, Canada) for their contribution to the microarray analysis. We thank Arnoud van Vliet (Institute of Food Research, Nottingham, England) for kindly providing the pDH20 vector containing the erythromycin gene. We thank the Rijks instituut voor volksgezondheid en mileu (RIVM) for providing the RIVM enteritis strains. Last but not least, we acknowledge the technical assistance of Ytje Oosterhuis, Hans Verhoog, and Jeroen Hol (Erasmus MC, Pediatrics, Rotterdam, The Netherlands).
Editor: J. B. Bliska
Footnotes
Published ahead of print on 21 July 2008.
REFERENCES
- 1.Ang, C. W., J. D. Laman, H. J. Willison, E. R. Wagner, H. P. Endtz, M. A. de Klerk, A. P. Tio-Gillen, N. van den Braak, B. C. Jacobs, and P. A. Van Doorn. 2002. Structure of Campylobacter jejuni lipopolysaccharides determines antiganglioside specificity and clinical features of Guillain-Barré and Miller Fisher patients. Infect. Immun. 701202-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aspinall, G. O., C. M. Lynch, H. Pang, R. T. Shaver, and A. P. Moran. 1995. Chemical structures of the core region of Campylobacter jejuni O:3 lipopolysaccharide and an associated polysaccharide. Eur. J. Biochem. 231570-578. [PubMed] [Google Scholar]
- 3.Aspinall, G. O., A. G. McDonald, and H. Pang. 1994. Lipopolysaccharides of Campylobacter jejuni serotype O:19: structures of O antigen chains from the serostrain and two bacterial isolates from patients with the Guillain-Barré syndrome. Biochemistry 33250-255. [DOI] [PubMed] [Google Scholar]
- 4.Aspinall, G. O., A. G. McDonald, T. S. Raju, H. Pang, L. A. Kurjanczyk, J. L. Penner, and A. P. Moran. 1993. Chemical structure of the core region of Campylobacter jejuni serotype O:2 lipopolysaccharide. Eur. J. Biochem. 2131029-1037. [DOI] [PubMed] [Google Scholar]
- 5.Aspinall, G. O., A. G. McDonald, T. S. Raju, H. Pang, A. P. Moran, and J. L. Penner. 1993. Chemical structures of the core regions of Campylobacter jejuni serotypes O:1, O:4, O:23, and O:36 lipopolysaccharides. Eur. J. Biochem. 2131017-1027. [DOI] [PubMed] [Google Scholar]
- 6.Byrne, C. M., M. Clyne, and B. Bourke. 2007. Campylobacter jejuni adhere to and invade chicken intestinal epithelial cells in vitro. Microbiology 153561-569. [DOI] [PubMed] [Google Scholar]
- 7.Chiu, C. P., A. G. Watts, L. L. Lairson, M. Gilbert, D. Lim, W. W. Wakarchuk, S. G. Withers, and N. C. Strynadka. 2004. Structural analysis of the sialyltransferase Cst-II from Campylobacter jejuni in complex with a substrate analog. Nat. Struct. Mol. Biol. 11163-170. [DOI] [PubMed] [Google Scholar]
- 8.de Melo, M. A., and J. C. Pechere. 1990. Identification of Campylobacter jejuni surface proteins that bind to eucaryotic cells in vitro. Infect. Immun. 581749-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilbert, M., J.-R. Brisson, M.-F. Karwaski, J. Michniewicz, A.-M. Cunningham, Y. Wu, N. M. Young, and W. W. Wakarchuk. 2000. Biosynthesis of ganglioside mimics in Campylobacter jejuni OH4384. Identification of the glycosyltransferase genes, enzymatic synthesis of model compounds, and characterization of nanomole amounts by 600-MHz 1H and 13C NMR analysis. J. Biol. Chem. 2753896-3906. [DOI] [PubMed] [Google Scholar]
- 10.Gilbert, M., P. C. R. Godschalk, M.-F. Karwaski, C. W. Ang, A. van Belkum, J. Li, W. W. Wakarchuk, and H. P. Endtz. 2004. Evidence for acquisition of the lipooligosaccharide biosynthesis locus in Campylobacter jejuni GB11, a strain isolated from a patient with Guillain-Barré syndrome, by horizontal exchange. Infect. Immun. 721162-1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilbert, M., M.-F. Karwaski, S. Bernatchez, N. M. Young, E. Taboada, J. Michniewicz, A.-M. Cunningham, and W. W. Wakarchuk. 2002. The genetic bases for the variation in the lipo-oligosaccharide of the mucosal pathogen, Campylobacter jejuni. Biosynthesis of sialylated ganglioside mimics in the core oligosaccharide. J. Biol. Chem. 277327-337. [DOI] [PubMed] [Google Scholar]
- 12.Godschalk, P. C., A. P. Heikema, M. Gilbert, T. Komagamine, C. W. Ang, J. Glerum, D. Brochu, J. Li, N. Yuki, B. C. Jacobs, A. van Belkum, and H. P. Endtz. 2004. The crucial role of Campylobacter jejuni genes in antiganglioside antibody induction in Guillain-Barré syndrome. J. Clin. Investig. 1141659-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Godschalk, P. C., M. L. Kuijf, J. Li, F. St Michael, C. W. Ang, B. C. Jacobs, M. F. Karwaski, D. Brochu, A. Moterassed, H. P. Endtz, A. van Belkum, and M. Gilbert. 2007. Structural characterization of Campylobacter jejuni lipooligosaccharide outer cores associated with Guillain-Barré and Miller Fisher syndromes. Infect. Immun. 751245-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guerry, P., C. P. Ewing, T. E. Hickey, M. M. Prendergast, and A. P. Moran. 2000. Sialylation of lipooligosaccharide cores affects immunogenicity and serum resistance of Campylobacter jejuni. Infect. Immun. 686656-6662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guerry, P., C. M. Szymanski, M. M. Prendergast, T. E. Hickey, C. P. Ewing, D. L. Pattarini, and A. P. Moran. 2002. Phase variation of Campylobacter jejuni 81-176 lipooligosaccharide affects ganglioside mimicry and invasiveness in vitro. Infect. Immun. 70787-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanel, I., J. Muller, W. Muller, and F. Schulze. 2004. Correlation between invasion of Caco-2 eukaryotic cells and colonization ability in the chick gut in Campylobacter jejuni. Vet. Microbiol. 10175-82. [DOI] [PubMed] [Google Scholar]
- 17.Kanipes, M. I., L. C. Holder, A. T. Corcoran, A. P. Moran, and P. Guerry. 2004. A deep-rough mutant of Campylobacter jejuni 81-176 is noninvasive for intestinal epithelial cells. Infect. Immun. 722452-2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karlyshev, A. V., P. Everest, D. Linton, S. Cawthraw, D. G. Newell, and B. W. Wren. 2004. The Campylobacter jejuni general glycosylation system is important for attachment to human epithelial cells and in the colonization of chicks. Microbiology 1501957-1964. [DOI] [PubMed] [Google Scholar]
- 19.Ketley, J. M. 1997. Pathogenesis of enteric infection by Campylobacter. Microbiology 1435-21. [DOI] [PubMed] [Google Scholar]
- 20.Konkel, M. E., S. G. Garvis, S. L. Tipton, D. E. Anderson, Jr., and W. Cieplak, Jr. 1997. Identification and molecular cloning of a gene encoding a fibronectin-binding protein (CadF) from Campylobacter jejuni. Mol. Microbiol. 24953-963. [DOI] [PubMed] [Google Scholar]
- 21.Lambotin, M., I. Hoffmann, M. P. Laran-Chich, X. Nassif, P. O. Couraud, and S. Bourdoulous. 2005. Invasion of endothelial cells by Neisseria meningitidis requires cortactin recruitment by a phosphoinositide-3-kinase/Rac1 signalling pathway triggered by the lipo-oligosaccharide. J. Cell Sci. 1183805-3816. [DOI] [PubMed] [Google Scholar]
- 22.Leon-Kempis Mdel, R., E. Guccione, F. Mulholland, M. P. Williamson, and D. J. Kelly. 2006. The Campylobacter jejuni PEB1a adhesin is an aspartate/glutamate-binding protein of an ABC transporter essential for microaerobic growth on dicarboxylic amino acids. Mol. Microbiol. 601262-1275. [DOI] [PubMed] [Google Scholar]
- 23.Li, J., M. Koga, D. Brochu, N. Yuki, K. Chan, and M. Gilbert. 2005. Electrophoresis-assisted open-tubular liquid chromatography/mass spectrometry for the analysis of lipo-oligosaccharide expressed by Campylobacter jejuni. Electrophoresis 263360-3368. [DOI] [PubMed] [Google Scholar]
- 24.Morooka, T., A. Umeda, and K. Amako. 1985. Motility as an intestinal colonization factor for Campylobacter jejuni. J. Gen. Microbiol. 1311973-1980. [DOI] [PubMed] [Google Scholar]
- 25.Muller, J., B. Meyer, I. Hanel, and H. Hotzel. 2007. Comparison of lipo-oligosaccharide biosynthesis genes of Campylobacter jejuni strains with varying abilities to colonize the chicken gut and to invade Caco-2 cells. J. Med. Microbiol. 561589-1594. [DOI] [PubMed] [Google Scholar]
- 26.Nachamkin, I., B. Mishu Allos, and T. Ho. 1998. Campylobacter species and Guillain-Barré syndrome. Clin. Microbiol. Rev. 11555-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nachamkin, I., H. Ung, A. P. Moran, D. Yoo, M. M. Prendergast, M. A. Nicholson, K. Sheikh, T. Ho, A. K. Asbury, G. M. McKhann, and J. W. Griffin. 1999. Ganglioside GM1 mimicry in Campylobacter strains from sporadic infections in the United States. J. Infect. Dis. 1791183-1189. [DOI] [PubMed] [Google Scholar]
- 28.Nachamkin, I., and M. J. Blaser. 2000. Campylobacter, 2nd ed. ASM Press, Washington, DC.
- 29.Nam Shin, J. E., S. Ackloo, A. S. Mainkar, M. A. Monteiro, H. Pang, J. L. Penner, and G. O. Aspinall. 1997. Lipo-oligosaccharides of Campylobacter jejuni serotype O:10. Structures of core oligosaccharide regions from a bacterial isolate from a patient with the Miller-Fisher syndrome and from the serotype reference strain. Carbohydr. Res. 305223-232. [DOI] [PubMed] [Google Scholar]
- 30.Parker, C. T., M. Gilbert, N. Yuki, H. P. Endtz, and R. E. Mandrell. 13 June 2008. Characterization of lipooligosaccharide biosynthetic loci of Campylobacter jejuni reveals new lipooligosaccharide classes: evidence of mosaic organizations. J. Bacteriol. doi: 10.1128/JB.00254-08. [DOI] [PMC free article] [PubMed]
- 31.Parker, C. T., S. T. Horn, M. Gilbert, W. G. Miller, D. L. Woodward, and R. E. Mandrell. 2005. Comparison of Campylobacter jejuni lipooligosaccharide biosynthesis loci from a variety of sources. J. Clin. Microbiol. 432771-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pei, Z., C. Burucoa, B. Grignon, S. Baqar, X. Z. Huang, D. J. Kopecko, A. L. Bourgeois, J. L. Fauchere, and M. J. Blaser. 1998. Mutation in the peb1A locus of Campylobacter jejuni reduces interactions with epithelial cells and intestinal colonization of mice. Infect. Immun. 66938-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perera, V. N., I. Nachamkin, H. Ung, J. H. Patterson, M. J. McConville, P. J. Coloe, and B. N. Fry. 2007. Molecular mimicry in Campylobacter jejuni: role of the lipo-oligosaccharide core oligosaccharide in inducing anti-ganglioside antibodies. FEMS Immunol. Med. Microbiol. 5027-36. [DOI] [PubMed] [Google Scholar]
- 34.Phongsisay, V., V. N. Perera, and B. N. Fry. 2006. Exchange of lipooligosaccharide synthesis genes creates potential Guillain-Barré syndrome-inducible strains of Campylobacter jejuni. Infect. Immun. 741368-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Preston, A., R. E. Mandrell, B. W. Gibson, and M. A. Apicella. 1996. The lipo-oligosaccharides of pathogenic gram-negative bacteria. Crit. Rev. Microbiol. 22139-180. [DOI] [PubMed] [Google Scholar]
- 36.Schenkman, R. P., F. Vandekerckhove, and S. Schenkman. 1993. Mammalian cell sialic acid enhances invasion by Trypanosoma cruzi. Infect. Immun. 61898-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swords, W. E., B. A. Buscher, K. Ver Steeg II, A. Preston, W. A. Nichols, J. N. Weiser, B. W. Gibson, and M. A. Apicella. 2000. Non-typeable Haemophilus influenzae adhere to and invade human bronchial epithelial cells via an interaction of lipo-oligosaccharide with the PAF receptor. Mol. Microbiol. 3713-27. [DOI] [PubMed] [Google Scholar]
- 38.Szymanski, C. M., M. King, M. Haardt, and G. D. Armstrong. 1995. Campylobacter jejuni motility and invasion of Caco-2 cells. Infect. Immun. 634295-4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Belkum, A., N. van den Braak, P. Godschalk, W. Ang, B. Jacobs, M. Gilbert, W. Wakarchuk, H. Verbrugh, and H. Endtz. 2001. A Campylobacter jejuni gene associated with immune-mediated neuropathy. Nat. Med. 7752-753. [DOI] [PubMed] [Google Scholar]
- 40.van Putten, J. P., H. U. Grassme, B. D. Robertson, and E. T. Schwan. 1995. Function of lipopolysaccharide in the invasion of Neisseria gonorrhoeae into human mucosal cells. Prog. Clin. Biol. Res. 39249-58. [PubMed] [Google Scholar]
- 41.Wassenaar, T. M., N. M. Bleumink-Pluym, and B. A. van der Zeijst. 1991. Inactivation of Campylobacter jejuni flagellin genes by homologous recombination demonstrates that flaA but not flaB is required for invasion. EMBO J. 102055-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watson, R. O., and J. E. Galan. 2008. Campylobacter jejuni survives within epithelial cells by avoiding delivery to lysosomes. PLoS Pathog. 4e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weis, W., J. H. Brown, S. Cusack, J. C. Paulson, J. J. Skehel, and D. C. Wiley. 1988. Structure of the influenza virus haemagglutinin complexed with its receptor, sialic acid. Nature 333426-431. [DOI] [PubMed] [Google Scholar]
- 44.Yao, R., D. H. Burr, P. Doig, T. J. Trust, H. Niu, and P. Guerry. 1994. Isolation of motile and non-motile insertional mutants of Campylobacter jejuni: the role of motility in adherence and invasion of eukaryotic cells. Mol. Microbiol. 14883-893. [DOI] [PubMed] [Google Scholar]
- 45.Yao, R., D. H. Burr, and P. Guerry. 1997. CheY-mediated modulation of Campylobacter jejuni virulence. Mol. Microbiol. 231021-1031. [DOI] [PubMed] [Google Scholar]
- 46.Yuki, N., Y. Ichihashi, and T. Taki. 1995. Subclass of IgG antibody to GM1 epitope-bearing lipopolysaccharide of Campylobacter jejuni in patients with Guillain-Barré syndrome. J. Neuroimmunol. 60161-164. [DOI] [PubMed] [Google Scholar]