Abstract
Mycobacterium avium is an opportunistic infectious agent in immunocompromised patients, living inside macrophage phagosomes. As for other mycobacterial species, iron availability is a critical factor for M. avium survival and multiplication. Indeed, an association between host secondary iron overload and increased susceptibility to these mycobacteria is generally acknowledged. However, studies on the impact of primary iron overload on M. avium infection have not been performed. In this work, we used animal models of primary iron overload that mimic the human disease hereditary hemochromatosis. This pathology is characterized by increased serum transferrin saturation with iron deposition in parenchymal cells, mainly in the liver, and is most often associated with mutations in the gene encoding the molecule HFE. In this paper, we demonstrate that mice of two genetically determined primary iron overload phenotypes, Hfe−/− and β2m−/−, show an increased susceptibility to experimental infection with M. avium and that during infection these animals accumulate iron inside granuloma macrophages. β2m−/− mice were found to be more susceptible than Hfe−/− mice, but depleting Hfe−/− mice of CD8+ cells had no effect on resistance to infection. Overall, our results suggest that serum iron, rather than total liver iron, levels have a considerable impact on susceptibility to M. avium infection.
Iron is an essential element required for the proper metabolism of almost every cell. It is thus not surprising to acknowledge the diverse strategies adopted by bacteria to access this nutrient, namely, when infecting a host organism. This is clearly illustrated by mycobacteria that have developed specific siderophores to acquire iron within infected macrophages. Data from both human and animal studies have shown that the host's iron status is critical for the outcome of mycobacterial infections. Iron excess markedly enhances Mycobacterium tuberculosis growth in vitro (20, 26) and aggravates the disease in humans (9, 12) and rodents (14). Likewise, Mycobacterium avium proliferation is increased in animals or macrophages that receive an exogenous iron surplus (11). Once inside the host, mycobacteria seem to have access to iron both from extracellular transferrin, the main serum iron carrier, and from endogenous macrophage sources (18).
Iron overload of a mammalian organism is rare, given the strict regulation of iron metabolism at the level of intestinal absorption, although it can be found under specific conditions (4). An additional situation where iron overload occurs can be found in certain genetic diseases such as hereditary hemochromatosis (HH), the most common inherited single gene disorder in people of Northern and Western Europe. This disease is most often associated with mutations in a molecule, HFE, homologous to class I major histocompatibility complex (MHC) alpha chains. Although different genetic defects are known to cause HH, about 80% of the individuals of European descent with HH are homozygous for a mutated Hfe gene encoding a cysteine-to-tyrosine substitution (C282Y) (22). Patients develop progressive accumulation of iron in key target organs such as the liver, heart, and pancreas, where iron can be easily detected in increased amounts in parenchymal cells.
There are a number of animal models of primary iron overload, including mice deficient in HFE or in the HFE-binding protein beta-2-microglobulin (β2m). Similarly to human HH patients, β2m−/− and Hfe−/− mice spontaneously develop hepatic iron overload, with iron accumulation in hepatocytes but not in macrophages (2, 6, 31).
These genetically engineered mouse strains are thus useful models to assess the impact of the metabolism of iron in infection. While the association between host secondary iron overload and increased susceptibility to mycobacteria is generally acknowledged (3), primary iron overload, namely, that resulting from mutations in the Hfe gene, is not recognized as causing an increase in susceptibility to this type of infection. On the contrary, because there are in vitro data indicating that monocytes with mutated Hfe have decreased intracellular iron levels (5, 17, 19, 21), some authors speculate that Hfe mutations have been maintained in human populations because they may confer protection against tuberculosis and other infections (15). A few studies have already addressed the susceptibility of β2m-deficient mice to mycobacteria. In one of those studies, the authors concluded that β2m-deficient mice were as resistant to M. avium as wild-type controls (3). In the other study, β2m−/− mice were found to be more susceptible than control mice to M. tuberculosis (25). Interestingly, the latter work also showed that β2m−/− mice were more susceptible to M. tuberculosis than MHC class I-deficient animals, indicating that β2m−/− mouse susceptibility to infection is not entirely due to the lack of antigen presentation to CD8+ T cells. In line with these data, Schaible and colleagues (25) showed that the administration of apolactoferrin to M. tuberculosis-infected mice reduced the bacterial loads in β2m-deficient animals but had no effect in control mice, clearly suggesting a link between susceptibility to infection and the role of β2m in the regulation of iron metabolism, namely, through its physical association with the HFE molecule.
In the present work, we evaluated the impact on M. avium infections of a genetic deficiency in either β2m or HFE, two instances of primary iron overload. The two models allow for discrimination between a predominant iron metabolism defect, in HFE-deficient animals, and a more complex disturbance of iron metabolism and immune function, in β2m deficiency.
MATERIALS AND METHODS
Mouse strains.
β2m- and HFE-deficient mice (both on a C57BL/6 background) and C57BL/6 wild-type mice were bred and housed at the Institute for Molecular and Cell Biology animal facility. Animals were kept under standard hygiene conditions in HEPA filter top cages, fed commercial chow, and given acidified drinking water.
All animal experiments were carried out in compliance with the animal ethics guidelines at the institute.
Infection and other treatments.
Mice were intravenously infected with 1 × 106 CFU of M. avium 2447SmT when they were 7 to 12 weeks old. To induce systemic iron overload, wild-type animals were injected with ferric hydroxide-dextran complex (Sigma, St. Louis, MO), by the intraperitoneal route, 20 days before infection (equivalent to 4 mg iron/animal). HFE-deficient animals were depleted of CD8+ cells with anti-CD8 antibodies (rat immunoglobulin G2b) purified from culture supernatants of the 2.43 hybridoma cell line (ATCC) growing in bioreactors (Integra Bioscience). After antibody purification by protein G-agarose affinity chromatography and saline dialysis, anti-CD8 or rat immunoglobulins (control group) were injected intraperitoneally every 10 days, starting 3 days before infection and continuing for the entire infection period, at a dose of 0.7 mg per animal.
Infected mice were sacrificed at 30, 60, or 120 days postinfection, and spleens and livers were collected for mycobacterial quantification by the CFU method. Serial dilutions of tissue homogenates were plated in Middlebrook 7H10 medium (Difco), and bacterial colonies were counted after incubation at 37°C for 10 days.
Iron quantification in hepatic and splenic tissues.
Organ nonheme iron content was measured by the colorimetric bathophenanthroline method (28).
Serum iron quantification.
Blood was obtained by retro-orbital puncture. The total iron in serum (serum Fe) and unsaturated iron-binding capacity were measured with the colorimetric FerroZine assay (Thermo Electron Corporation). Total iron-biding capacity (TIBC) was calculated as the sum of serum Fe and unsaturated iron-binding capacity. Transferrin saturation was calculated as serum Fe/TIBC × 100.
Histological analysis.
Liver and spleen sections were fixed in buffered formaldehyde and embedded in paraffin. Tissue sections were stained with Perls' blue stain for iron detection. For simultaneous detection of mycobacteria and iron, tissue sections were stained by a modified Ziehl-Neelsen-Perls technique. Briefly, sections were stained with a basic fuchsin solution, and after incubation in 37% Formol, Perls' staining was performed.
Flow cytometry.
In order to confirm the CD8+ cell depletion, splenic and peripheral blood samples were analyzed by flow cytometry. For immunofluorescence staining, 106 cells were incubated with phycoerythrin-conjugated anti-CD8 antibody (dilution, 1:200) in phosphate-buffered saline (PBS) containing 3% fetal calf serum [FCS]). Splenic cells were also incubated with fluorescein isothiocyanate-conjugated anti-CD4 antibody (dilution, 1:100), fluorescein isothiocyanate-conjugated anti-CD19 antibody (dilution, 1:100), and phycoerythrin-conjugated anti-CD3 antibody (dilution, 1:200) in PBS containing 3% FCS. Antibodies were from BD Pharmingen (San Diego, CA). The cells were washed twice with PBS containing 3% FCS. The analysis of cell populations was based on the acquisition of 10,000 events in a Becton Dickinson FACSort instrument equipped with CellQuest software.
Statistical analysis.
All of the results are presented as the mean ± 1 standard deviation for each experimental group. Comparisons between groups were done by one-way analysis of variance. Statistical significance of the differences was assessed by Bonferroni and Tukey tests.
RESULTS
β2m-deficient mice exhibit increased susceptibility to infection by M. avium.
In order to assess their relative susceptibility or resistance, we infected β2m−/− and age-matched C57BL/6 control mice with a strain of M. avium of intermediate virulence, strain 2447SmT. At 30 and 60 days postinfection, the animals were killed and the bacterial loads were evaluated in the liver and spleen. As shown in Table 1, the bacterial loads were similar in wild-type and β2m−/− mice. At 120 days after infection, β2m−/− mice had significantly increased bacterial loads in the two organs analyzed compared to the control group. Similar results were obtained in two independent experiments.
TABLE 1.
Mice deficient in the expression of β2m are more susceptible to M. avium than control C57BL/6 micea
| Expt no, time (days) postinfection | Organ | C57BL/6 | B6.β2m−/− | P value |
|---|---|---|---|---|
| 1, 30 | Liver | 6.17 ± 0.55 | 6.36 ± 0.10 | 0.09 (NS)b |
| Spleen | 6.06 ± 0.33 | 5.97 ± 0.27 | 0.16 (NS) | |
| 2, 60 | Liver | 6.72 ± 0.31 | 6.99 ± 0.13 | 0.11 (NS) |
| Spleen | 6.25 ± 0.16 | 6.46 ± 0.06 | 0.06 (NS) | |
| 3, 120 | Liver | 6.09 ± 0.14 | 6.42 ± 0.19 | <0.01 |
| Spleen | 6.21 ± 0.13 | 6.64 ± 0.19 | <0.01 | |
| 4, 120 | Liver | 5.67 ± 0.11 | 6.33 ± 0.23 | <0.01 |
| Spleen | 5.62 ± 0.18 | 6.81 ± 0.27 | <0.01 |
Groups of five or six mice were intravenously infected with 106 CFU M. avium strain 2447SmT, and the bacterial loads were assessed in the liver and spleen 30, 60, or 120 days later. Data from four independent experiments are presented as the geometric mean log10 number of CFU per organ ± 1 standard deviation. Statistical analysis is based on Bonferroni and Tukey tests.
NS, no statistically significant difference.
Primary iron overload contributes to the increased susceptibility of β2m−/− animals to M. avium infection.
In addition to leading to systemic iron overload by mechanisms that include, although are not limited to, the lack of a functional HFE protein (29), β2m deficiency abrogates antigen presentation in the context of MHC class I.
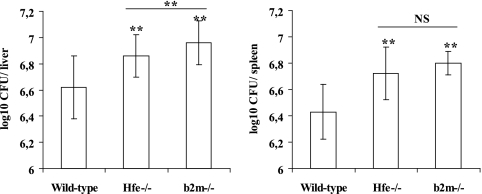
In order to evaluate the contribution of iron overload to the increased susceptibility of β2m−/− animals to M. avium, we compared the bacterial growth in this strain of mice with that in mice deficient in HFE. Table 2 shows that both Hfe−/− and β2m−/− mice have liver iron overload, as well as increased serum iron and transferrin saturation. However, the phenotype in Hfe−/− mice is not as severe as in β2m−/− animals. Iron accumulation in the livers of uninfected Hfe−/− and β2m−/− mice occurred almost exclusively in hepatocytes (data not shown), as has been previously described for these mouse strains (23). After infection with M. avium 2447SmT, Hfe−/− mice had significantly increased bacterial loads in the liver and spleen, relative to wild-type mice. However, compared to β2m−/− mice, HFE-deficient animals had significantly lower bacterial loads in the liver, while in the spleen the difference between these two strains was not significant (Fig. 1).
TABLE 2.
Serum and tissue iron levels of uninfected, 27- to 33-week-old wild-type, Hfe−/−, and β2m−/− micea
| Strain | Serum iron concn (mg/ml) | TIBC (mg/ml) | % Tf satb | [Fe] (mg/g [dry wt])
|
|
|---|---|---|---|---|---|
| Spleen | Liver | ||||
| Wild type | 1.62 ± 0.14 | 3.98 ± 0.24 | 40.8 ± 2.4 | 1,325 ± 65 | 559 ± 67 |
| Hfe−/− | 2.85 ± 0.10 (<0.01)c | 4.00 ± 0.20 (NS)c,d | 71.4 ± 1.9 (<0.01)c | 1,314 ± 190 (NS)c | 2,056 ± 278 (<0.01)c |
| β2m−/− | 3.68 ± 0.22 (<0.01, <0.01)e | 4.38 ± 0.30 (<0.05, NS)e | 84.5 ± 8.8 (<0.01, NS)e | 1,611 ± 35 (<0.05, NS)e | 2,770 ± 255 (<0.01, <0.05)e |
Values are presented as the mean ± 1 standard deviation for four or five mice per group. Statistical analysis is based on Bonferroni and Tukey tests.
Tf sat, transferrin saturation.
P value relative to wild type.
NS, no statistically significant difference (P ≥ 0.05).
P values relative to wild type, relative to Hfe−/−.
FIG. 1.
C57BL/6, B6.Hfe−/−, and B6.β2m−/− age-matched mice were infected with 106 CFU of M. avium 2447SmT by the intravenous route. At 120 days later, mice were killed and the bacterial loads in their organs were quantified. The results are shown as the geometric mean log10 number of CFU per organ ± 1 standard deviation for groups of six mice of each strain. Double asterisks indicate a statistically significant difference (P < 0.01) by the Bonferroni and Tukey tests (analysis of variance). NS indicates that there is no statistically significant difference.
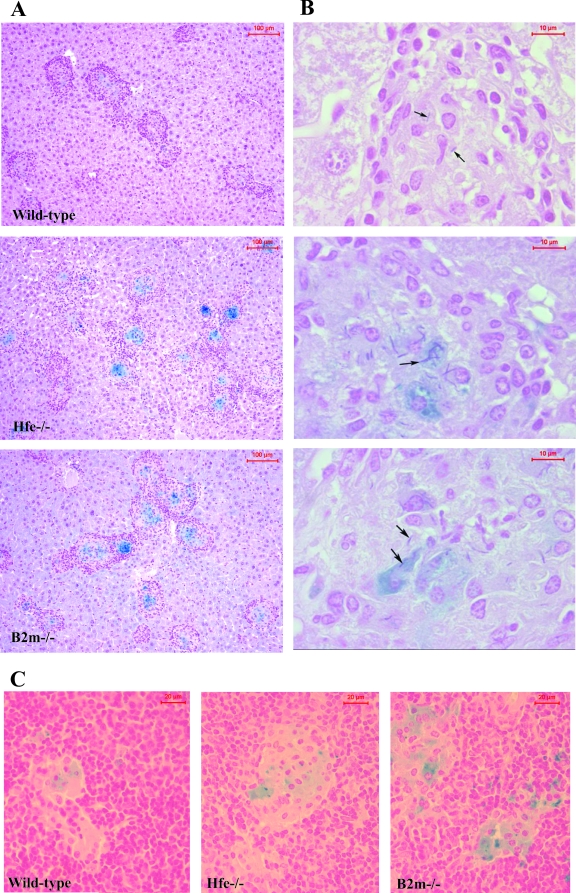
Interestingly, when we analyzed liver and spleen sections from each mouse strain for iron distribution, we could observe the accumulation of iron inside the granulomas formed at infectious foci (Fig. 2A and C). Although iron could be found inside granulomas of wild-type animals, histochemical staining was more intense in both Hfe−/− and β2m−/− mice (Fig. 2). In these mouse strains, mycobacteria could clearly be found associated with iron-overloaded cells (Fig. 2B).
FIG. 2.
C57BL/6, B6.Hfe−/−, and B6.β2m−/− age-matched mice were infected with 106 CFU of M. avium 2447SmT by the intravenous route. At 120 days later, mice were killed and liver (A and B) and spleen (C) sections were collected and processed for histological analysis. Perls' blue stain was used to detect iron distribution (A and C). Liver sections were also stained by a modified Ziehl-Neelsen-Perls technique to show mycobacteria and iron simultaneously (B) (see Materials and Methods). Mycobacteria were found within granulomas (arrows), with some inside iron-overloaded cells. Shown is one representative section for each mouse group.
The increased susceptibility of β2m−/− over Hfe−/− mice is not explained by distinct iron levels or by the lack of CD8+ T cells.
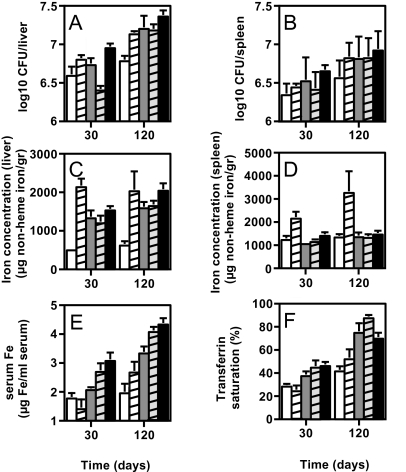
As shown in the previous section, although both β2m−/− and Hfe−/− mice show increased susceptibility to M. avium, the former mouse strain exhibits higher bacterial loads in the liver than the latter. Since β2m−/−, but not Hfe−/−, mice lack functional CD8+ T cells, we decided to test the contribution of this defect to susceptibility to M. avium. For that purpose, we treated Hfe−/− animals with anti-CD8 antibodies, during a 4-months period of infection. Analysis of both spleen and peripheral blood cells by flow cytometry confirmed a 95% reduction in CD8+ cells (not shown). The depletion of CD8+ cells did not result in any significant increase in the susceptibility of Hfe−/− mice to infection by M. avium (Fig. 3A and B).
FIG. 3.
Untreated C57BL/6 (white columns), B6.Hfe−/− (gray columns), and B6.β2m−/− (black columns), as well as experimentally iron-overloaded C57BL/6 (dashed white columns) and CD8-depleted B6.Hfe−/− (dashed gray columns), animals (see Materials and Methods for details of treatments) were infected with M. avium 2447SmT by the intravenous route. At 30 or 120 days later, groups of five mice were killed and the bacterial loads in their organs were determined as CFU counts. The results are shown as the geometric mean log10 number of CFU per organ ± 1 standard deviation (A and B). At the time of sacrifice, liver and spleen samples were collected for iron determination. The results are shown in panels C and D as iron concentrations (micrograms of nonheme iron per gram of tissue). Blood was collected, and serum was separated for the determination of iron parameters. Serum iron (E, micrograms of iron per milliliter of serum) and transferrin saturation (F, percent) were determined as described in Materials and Methods.
Since β2m−/− mice also exhibit increased iron deposition in the liver and spleen compared to Hfe−/− mice (Table 2), we investigated the role of tissue iron levels in the degree of susceptibility to M. avium. C57BL/6 wild-type mice were intraperitoneally injected with iron-dextran (4 mg of iron/animal) 20 days before infection. This treatment led to tissue iron levels that were at least as high as those in β2m−/− mice (Fig. 3C and D). We could not find a direct correlation between tissue iron levels and bacterial loads. In the spleen, the highest iron levels were observed in the animals injected with iron-dextran (the only group with statistically significantly elevated tissue iron; Fig. 3D), while the highest bacterial loads were found in β2m−/− mice. In the liver, all of the experimental groups showed statistically significantly elevated iron levels compared with wild-type controls, with the highest levels found again in animals treated with iron-dextran (Fig. 3C). However, despite a higher iron load, iron-dextran-treated mice do not show higher bacterial loads in the liver than Hfe−/− mice. Bacterial loads in the livers of iron-dextran-treated mice also tended to be lower than those observed in β2m−/− mice (P = 0.085 at 30 days and P < 0.01 at 120 days), despite higher iron levels (Fig. 3A and C).
Infected mice with either experimentally or genetically induced iron overload were also analyzed regarding serum iron parameters. As shown in Fig. 3E, serum iron levels showed a positive correlation with susceptibility to M. avium, in that β2m−/− mice showed the highest values, Hfe−/− mice showed intermediate values, and experimentally iron-overloaded animals show serum iron values close to those of untreated wild-type controls. In contrast, transferrin saturation (Fig. 3F) and TIBC (not shown) did not correlate with the relative susceptibilities of the different groups of mice to infection.
DISCUSSION
Iron availability is a crucial factor in determining the course of an infection. Several studies have shown that experimental iron overload leads to increased susceptibility to infections caused by mycobacteria (11, 14, 25). In contrast, the consequences of genetically determined primary iron overload for the outcome of mycobacterial infections are poorly understood. In the present work, we used gene disruption-containing mice to show that HFE-related host iron overload increases susceptibility to mycobacterial infections. This is, to our knowledge, the first time that HFE-deficient mice were used to study mycobacterial infection, despite the high frequency of the Hfe mutation in the human population worldwide and its clear role in the development of primary iron overload.
Individuals with hemochromatosis caused by mutations in the Hfe gene present a systemic iron overload, with iron deposition occurring mainly in parenchymal cells. However, monocyte-derived macrophages from the same patients have a decreased intracellular labile iron pool (5, 17). Consequently, one would expect that mycobacteria would grow less efficiently inside HFE-deficient macrophages. This has indeed been found in vitro by studying the growth of M. tuberculosis inside monocyte-derived macrophages from hemochromatosis patients (19). In the present study, we did not find any difference between the growth of M. avium inside bone marrow-derived macrophages from HFE-deficient mice and that inside macrophages from wild-type control mice (data not shown). Nevertheless, when susceptibility to infection was assessed in vivo, in terms of bacterial loads in the liver and spleen, our results indicate a clear increase in susceptibility to M. avium in Hfe−/− mice, compared to wild-type mice. In the absence of any evidence of immune system alterations in Hfe−/− mice (2), we suggest that the increased susceptibility to infection is associated with the host's overall iron overload phenotype.
HFE is a nonclassical MHC protein which depends on interaction with β2m for correct expression and activity. The formation of a complex between these molecules and the transferrin receptor negatively regulates iron uptake, as HFE protein decreases the affinity of transferrin for the transferrin receptor approximately 10-fold (7, 22). Accordingly, mice deficient in the β2m protein also lack functional HFE and present tissue iron overload in a phenotype that resembles HFE deficiency in many, but not all, respects (6, 23).
However, β2m deficiency also leads to the impairment of antigen presentation in the context of MHC class I and consequently of CD8+ T-cell development (13, 32), making it difficult to interpret the results of experimental infection in this model. β2m−/− mice have been infected with M. tuberculosis and with M. avium, leading to contrasting results, adding to a large body of evidence indicating that CD8+ T cells are more critical for the development of host protection against M. tuberculosis than against M. avium (3, 25).
Flynn et al., in 1992 (8), were the first to show that β2m−/− mice had increased susceptibility to M. tuberculosis. This increase in susceptibility was attributed to the lack of CD8+ T cells, the importance of which has been corroborated by several other studies (16, 27, 30). However, Schaible et al., in 2002, showed that β2m−/− mice were more susceptible to M. tuberculosis than MHC class I-deficient animals, suggesting that iron overload could be contributing to susceptibility. This hypothesis was further supported by the fact that correction of extracellular iron overload with lactoferrin lowered the bacterial burden to values similar to that observed in MHC class I knockout animals (25).
In the present work, we show that β2m−/− mice are more susceptible to M. avium infection than wild-type mice are (and also more susceptible than Hfe−/− mice, as discussed below). These data are in clear contrast to those of Bermudez and Petrofsky (3), who found no difference in susceptibility to M. avium 101 in β2m−/− mice relative to wild-type controls. However, these authors used a more virulent strain and a higher inoculum dose. The high bacterial loads reached in wild-type mice may make this model less suitable for the detection of a further increase caused by a genetic alteration such as a deficiency in β2m. Maybe more importantly, Bermudez and Petrofsky (3) studied a relatively short period of infection (8 weeks). When we measured the growth of M. avium 2447SmT in β2m−/− mice at 4 or 8 weeks of infection, we found increased values relative to wild-type controls, although the differences did not always reach statistical significance (Table 1 and Fig. 3). At 16 weeks of infection, however, there is a clear increase in the susceptibility of β2m−/− animals relative to normal mice.
In the present study, we directly compared HFE- and β2m-deficient mice and found the latter to have a higher susceptibility to M. avium than the former.
Since one obvious difference between the two mouse strains is the development of CD8+ T cells, we investigated the role of this population by treating Hfe−/− mice with anti-CD8 antibodies for the extent of the infection period. CD8+ T-cell depletion did not further increase the susceptibility of Hfe−/− mice (Fig. 3). These results are in agreement with earlier findings demonstrating that CD8+ T cells are not essential for resistance to M. avium infection in mice (1, 24). Furthermore, they indicate that the increased susceptibility of β2m−/− relative to Hfe−/− mice is not due to the lack of CD8+ T cells and suggest that differences in iron status may underlie the differences in susceptibility to infection.
The iron overload phenotype of β2m−/− mice has been demonstrated to be more severe than that of Hfe−/− mice (23). In the present study, we have confirmed that both before and after infection, β2m−/− mice have higher levels of iron deposition in the liver than Hfe−/− mice. To investigate if this difference could explain the increased susceptibility of β2m−/− mice, we included one experimental group that was subjected to iron-dextran injection, leading to total liver iron levels comparable to those of β2m−/− mice. It should be noted that upon iron-dextran injection, iron accumulates mainly on macrophages and not in hepatocytes, in contrast to what is found in β2m−/− mice. This localization should favor mycobacterial growth. Indeed, mice injected with iron-dextran exhibited higher bacterial loads in the liver than control animals, confirming previous work by our group (11), but did not reach the levels found in β2m−/− mice, showing that the total amount of iron found in the liver is not the critical factor determining bacterial growth.
The spontaneous iron overload found in the livers of Hfe−/− and β2m−/− mice occurs predominantly inside hepatocytes. Since M. avium grows inside macrophages, one could expect that this type of iron accumulation would not affect its growth. However, we show in this work that this is not the case. Furthermore, when we stained liver sections from Hfe−/− and β2m−/− mice infected with M. avium for 4 months, we could clearly see a high accumulation of iron inside the macrophages of mycobacterium-induced granulomas. This observation suggests that during infection there is an iron redistribution in the organism of the mouse. This type of redistribution, toward accumulation inside macrophages, is consistent with the so-called “iron-withholding” system or the process of “anemia of infection.” This group of alterations, which is thought to result from the interleukin-6-induced hepcidin production and the consequent decrease in iron release from macrophages and enterocytes, is presumed to contribute to host protection against infection by decreasing circulating iron levels (10). However, in the case of mycobacterial infection in the context of a previously iron-overloaded animal, these same alterations seem to promote the growth of the pathogen.
Interestingly, the levels of total serum iron remain abnormally high in Hfe−/− and β2m−/− mice, even at 4 months of infection. Furthermore, there is a direct correlation between serum iron levels and the bacterial loads found in the livers of infected mice. This correlation suggests that circulating iron is accessible to intramacrophagic mycobacteria and that serum iron levels are important in determining the susceptibility of mice to M. avium.
In this paper, we demonstrate that mice of two genetically determined iron overload phenotypes, Hfe−/− and β2m−/−, show an increased susceptibility to experimental infection with M. avium and that during infection these animals accumulate iron inside granuloma macrophages. β2m−/− mice were found to be more susceptible than Hfe−/− mice, but depleting Hfe−/− mice of CD8+ cells had no effect on resistance to infection. Overall, our results suggest that serum iron, rather than total liver iron, levels have a considerable impact on susceptibility to M. avium infection.
Acknowledgments
S. Gomes-Pereira received a fellowship (SFRH/BPD-/14979/2004) from the Portuguese Science and Technology Foundation (Portugal).
We are indebted to Alexandra Rêma, Fátima Faria, and Célia Lopes (ICBAS, Universidade do Porto, Porto, Portugal) for their technical help and to M. de Sousa (Iron Genes and Immune System) and Anna Olsson (Laboratory Animal Science), IBMC, Universidade do Porto, Porto, Portugal, for providing the experimental animals.
Editor: A. J. Bäumler
Footnotes
Published ahead of print on 11 August 2008.
REFERENCES
- 1.Appelberg, R., A. G. Castro, J. Pedrosa, R. A. Silva, I. M. Orme, and P. Minóprio. 1994. Role of gamma interferon and tumor necrosis factor alpha during T-cell-independent and -dependent phases of Mycobacterium avium infection. Infect. Immun. 623962-3971. (Erratum, 63:1145, 1995.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bahram, S., S. Gilfillan, L. C. Kühn, R. Moret, J. B. Schulzei, A. Lebeau, and K. Schümanni. 1999. Experimental hemochromatosis due to MHC class I HFE deficiency: immune status and iron metabolism. Proc. Natl. Acad. Sci. USA 9613312-13317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bermudez, L. E., and M. Petrofsky. 1999. Host defense against Mycobacterium avium does not have an absolute requirement for major histocompatibility complex class I-restricted T cells. Infect. Immun. 673108-3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boelaert, J. R., S. J. Vandecasteele, R. Appelberg, and V. R. Gordeuk. 2007. The effect of the host's iron status on tuberculosis. J. Infect. Dis. 1951745-1753. [DOI] [PubMed] [Google Scholar]
- 5.Cairo, G., S. Recalcati, G. Montosi, E. Castrusini, D. Conte, and A. Pietrangelo. 1997. Inappropriately high iron regulatory protein activity in monocytes of patients with genetic hemochromatosis. Blood 892546-2553. [PubMed] [Google Scholar]
- 6.de Sousa, M., R. Reimão, R. Lacerda, P. Hugo, S. H. Kaufmann, and G. Porto. 1994. Iron overload in beta 2-microglobulin-deficient mice. Immunol. Lett. 39105-111. [DOI] [PubMed] [Google Scholar]
- 7.Feder, J. N., Z. Tsuchihashi, A. Irrinki, V. K. Lee, F. A. Mapa, E. Morikang, C. E. Prass, S. M. Starnes, K. W. Roger, S. Parkkila, W. S. Sly, and R. C. Schatzman. 1997. The hemochromatosis founder mutation in HLA-H disrupts β2-microglobulin interaction and cell surface expression. J. Biol. Chem. 27214025-14028. [DOI] [PubMed] [Google Scholar]
- 8.Flynn, J. L., M. M. Goldstein, K. Triebold, B. Koller, and B. Bloom. 1992. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc. Natl. Acad. Sci. USA 8912013-12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gangaidzo, I. T., V. M. Moyo, E. Mvundura, G. Aggrey, N. L. Murphree, H. Khumalo, T. Saungweme, I. Kasvosve, Z. A. Gomo, and T. Rouault. 2001. Association of pulmonary tuberculosis with increased dietary iron. J. Infect. Dis. 184936-939. [DOI] [PubMed] [Google Scholar]
- 10.Ganz, T. 2006. Hepcidin—a peptide hormone at the interface of innate immunity and iron metabolism. Curr. Top. Microbiol. Immunol. 306183-198. [DOI] [PubMed] [Google Scholar]
- 11.Gomes, M. S., J. R. Boelaert, and R. Appelberg. 2001. Role of iron in experimental Mycobacterium avium infection. J. Clin. Virol. 20117-122. [DOI] [PubMed] [Google Scholar]
- 12.Gordeuk, V. R., C. E. McLaren, A. P. MacPhail, G. Deichsel, and T. H. Bothwell. 1996. Associations of iron overload in Africa with hepatocellular carcinoma and tuberculosis: Strachan's 1929 thesis revisited. Blood 873470-3476. [PubMed] [Google Scholar]
- 13.Koller, B. H., P. Marrack, J. W. Kappler, and O. Smithies. 1990. Normal development of mice deficient in beta 2M, MHC class I proteins, and CD8+ T cells. Science 2481227-1230. [DOI] [PubMed] [Google Scholar]
- 14.Lounis, N., C. Truffot-Pernot, J. Grosset, V. R. Gordeuk, and J. R. Boelaert. 2001. Iron and Mycobacterium tuberculosis infection. J. Clin. Virol. 20123-126. [DOI] [PubMed] [Google Scholar]
- 15.Moalem, S., E. D. Weinberg, and M. E. Percy. 2004. Hemochromatosis and the enigma of misplaced iron: implications for infectious disease and survival. Biometals 17135-139. [DOI] [PubMed] [Google Scholar]
- 16.Mogues, T., M. E. Goodrich, L. Ryan, R. LaCourse, and R. J. North. 2001. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J. Exp. Med. 193271-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moura, E., M. A. Noordermeer, N. Verhoeven, A. F. Verheul, and J. J. Marx. 1998. Iron release from human monocytes after erythrophagocytosis in vitro: an investigation in normal subjects and hereditary hemochromatosis patients. Blood 922511-2519. [PubMed] [Google Scholar]
- 18.Olakanmi, O., L. S. Schlesinger, A. Ahmed, and B. E. Britigan. 2002. Intraphagosomal Mycobacterium tuberculosis acquires iron from both extracellular transferrin and intracellular iron pools. Impact of interferon-γ and hemochromatosis. J. Biol. Chem. 27749727-49734. [DOI] [PubMed] [Google Scholar]
- 19.Olakanmi, O., L. S. Schlesinger, and B. E. Britigan. 2007. Hereditary hemochromatosis results in decreased iron acquisition and growth by Mycobacterium tuberculosis within human macrophages. J. Leukoc. Biol. 81195-204. [DOI] [PubMed] [Google Scholar]
- 20.Raghu, B., G. R. Sarma, and P. Venkatesan. 1993. Effect of iron on the growth and siderophore production of mycobacteria. Biochem. Mol. Biol. Int. 31341-348. [PubMed] [Google Scholar]
- 21.Recalcati, S., A. Alberghini, A. Campanella, U. Gianelli, E. De Camilli, D. Conte, and G. Cairo. 2006. Iron regulatory proteins 1 and 2 in human monocytes, macrophages and duodenum: expression and regulation in hereditary hemochromatosis and iron deficiency. Haematologica 91303-310. [PubMed] [Google Scholar]
- 22.Robson, K. J., A. T. Merryweather-Clarke, E. Cadet, V. Viprakasit, M. G. Zaahl, J. J. Pointon, D. J. Weatherall, and J. Rochette. 2004. Recent advances in understanding haemochromatosis: a transition state. J. Med. Genet. 41721-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodrigues, P., C. Lopes, C. Mascarenhas, P. Arosio, G. Porto, and M. De Sousa. 2006. Comparative study between Hfe−/− and β2m−/− mice: progression with age of iron status and liver pathology. Int. J. Exp. Pathol. 87317-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saunders, B. M., Y. Zhan, and C. Cheers. 1995. Endogenous interleukin-12 is involved in resistance of mice to Mycobacterium avium complex infection. Infect. Immun. 634011-4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schaible, U. E., H. L. Collins, F. Priem, and S. H. E. Kaufmann. 2002. Correction of the iron overload defect in beta-2-microglobulin knockout mice by lactoferrin abolishes their increased susceptibility to tuberculosis. J. Exp. Med. 1961507-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serafín-López, J., R. Chacon-Salinas, S. Munoz-Cruz, J. A. Enciso-Moreno, S. A. Estrada-Parra, and I. Estrada-Garcia. 2004. The effect of iron in the expression of cytokines in macrophages infected with Mycobacterium tuberculosis. Scand. J. Immunol. 60329-337. [DOI] [PubMed] [Google Scholar]
- 27.Sousa, A. O., R. J. Mazzaccaro, R. G. Russell, F. K. Lee, O. C. Turner, S. Hong, L. Van Kaer, and B. R. Bloom. 2000. Relative contributions of distinct MHC class I-dependent cell populations in protection to tuberculosis infection in mice. Proc. Natl. Acad. Sci. USA 974204-4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Torrance, J. D., and T. H. Bothwell. 1980. Tissue iron stores, p. 104-109. In J. D. Cook (ed.), Methods in hematology. Churchill Livingstone Press, New York, NY.
- 29.Waheed, A., J. H. Grubb, X. Y. Zhou, S. Tomatsu, R. E. Fleming, M. E. Costaldi, R. S. Britton, B. R. Bacon, and W. S. Sly. 2002. Regulation of transferrin-mediated iron uptake by HFE, the protein defective in hereditary hemochromatosis. Proc. Natl. Acad. Sci. USA 993117-3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woodworth, J. S., and S. M. Behar. 2006. Mycobacterium tuberculosis-specific CD8+ T cells and their role in immunity. Crit. Rev. Immunol. 26317-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou, X. Y., S. Tomatsu, R. E. Fleming, S. Parkkila, A. Waheed, J. Jiang, Y. Fei, E. M. Brunt, D. A. Ruddy, C. E. Prass, R. C. Schatzman, R. O'Neill, R. S. Britton, B. R. Bacon, and W. S. Sly. 1998. HFE gene knockout produces mouse model of hereditary hemochromatosis. Proc. Natl. Acad. Sci. USA 952492-2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zijlstra, M., M. Bix, N. E. Simister, J. M. Loring, D. H. Raulet, and R. Jaenisch. 1990. β2-Microglobulin deficient mice lack CD4−8+ cytolytic T cells. Nature 344742-746. [DOI] [PubMed] [Google Scholar]