Abstract
Cellular immune responses against protective antigen (PA) of Bacillus anthracis in subjects that received the anthrax vaccine adsorbed (AVA) vaccine were examined. Multiple CD4+ T-cell epitopes within PA were identified by using tetramer-guided epitope mapping. PA-reactive CD4+ T cells with a CD45RA− phenotype were also detected by direct ex vivo staining of peripheral blood mononuclear cells (PBMC) with PA-specific tetramers. Surprisingly, PA-specific T cells were also detected in PBMC of nonvaccinees after a single cycle of in vitro PA stimulation. However, PA-reactive CD4+ T cells in nonvaccinees occurred at lower frequencies than those in vaccinees. The majority of PA-reactive T cells from nonvaccinees were CD45RA+ and exhibited a Th0/Th1 cytokine profile. In contrast, phenotyping and cytokine profile analyses of PA-reactive CD4+ T cells from vaccinees indicated that vaccination leads to commitment of PA-reactive T cells to a Th2 lineage, including generation of PA-specific, pre-Th2 central memory T cells. These results demonstrate that the current AVA vaccine is effective in skewing the development of PA CD4+ T cells to the Th2 lineage. The data also demonstrated the feasibility of using class II tetramers to analyze CD4+ cell responses and lineage development after vaccination.
Anthrax is a potentially fatal disease caused by the gram-positive, spore-forming bacterium Bacillus anthracis. There are three primary forms of anthrax: cutaneous, gastrointestinal, and inhalational (4). In humans, cutaneous anthrax is the most common naturally occurring form of the disease. Though anthrax has been recognized as an occupational hazard for more than a century, it is fairly uncommon in modern society. The recent use of anthrax spores in a bioterrorist attack has brought the disease to the attention of the general public (11, 16).
Anthrax vaccines have been used for prevention of anthrax in both humans and domestic animals since 1930 (7, 23). The currently licensed vaccine in the United States is anthrax vaccine adsorbed (AVA) (BioThrax). The vaccine is produced by harvesting the supernatant of an avirulent strain of B. anthracis, which is filtered and adsorbed onto aluminum hydroxide gel, which acts as a depot and adjuvant for the vaccine. One of the major components of the AVA vaccine is the protective antigen (PA), a subunit of two different exotoxins (lethal toxin and edema toxin) of the bacterium. Military personnel receive this vaccine as a precaution against the use of anthrax spores in biological warfare. The current recommended immunization protocol consists of a total of six doses of vaccine over a 2-year period, followed by an annual booster. Experiments with animal models indicated that induction of neutralization antibody directed against PA in the host is correlated with protective immunity (3, 5, 15, 20, 25, 26). Although there are many studies of humoral responses against PA after vaccination in humans (2, 8, 9, 13, 19), studies of cellular responses against PA after AVA vaccination are quite limited (1, 12, 24). In general, cellular responses elicited by vaccines are not well characterized, due to difficulties in monitoring of cellular immune responses. The initial objective of this study was to investigate whether AVA vaccination effectively elicits PA-specific memory CD4+ T cells. During the course of the study, we observed that PA-reactive T cells were also detected in non-AVA vaccinees. This observation provided an opportunity to explore the effect of AVA vaccine on naïve, PA-reactive T cells. Here, we report that AVA vaccination leads to the induction of PA-specific Th2 CD4+ T cells. The study also demonstrated that tetramers are a useful tool for monitoring CD4+ T-cell responses after vaccination.
MATERIALS AND METHODS
Human subjects.
The study was approved by the Institutional Review Boards of both Benaroya Research Institute and Madigan Army Medical Center. Informed consent was obtained from a total of 44 subjects (36 vaccinated with AVA and 8 unvaccinated controls). All subjects were HLA typed, and those subjects that had the HLA-DRA1*0101/DRB1*0401 (DR0401) or HLA-DRA1*0101/DRB1*0701 (DR0701) haplotype were selected for further study as described in this paper. The HLA haplotypes and the AVA vaccination histories of these subjects are shown in Table 1. For subjects that had received the AVA vaccine, blood samples were obtained from 1 to 5 years after the last vaccination.
TABLE 1.
HLA haplotypes of AVA vaccinees and nonvaccinees
| Group and subject no. | HLA haplotype | No. of AVA doses received |
|---|---|---|
| AVA vaccinees | ||
| 5 | DR0401 | 6 |
| 6 | DR0401 | 7 |
| 10 | DR0701 | 7 |
| 15 | DR0701 | 6 |
| 18 | DR0701 | 5 |
| 25 | DR0701 | 3 |
| 30 | DR0701 | 8 |
| Nonvaccinees | ||
| 11 | DR0701 | 0 |
| 229 | DR0701 | 0 |
| 602 | DR0701 | 0 |
| 899 | DR0701 | 0 |
| 4798 | DR0701 | 0 |
| 40 | DR0401 | 0 |
| 333 | DR0401 | 0 |
| 500 | DR0401 | 0 |
TGEM.
PA protein consists of 764 amino acids (aa). A total of 94 overlapping peptides that covered the entire PA of B. anthracis were purchased from Mimotopes (Clayton, Australia). Each peptide was 20 aa in length, with a 12-aa overlap with the adjacent peptides. These peptides were divided into 19 pools, with five peptides in each pool, with the exception of the last pool, which contained four peptides. Recombinant DR0401 and DR0701 molecules were purified from the supernatants of S2 transfectants (Drosophila melanogaster cells containing a Cu-inducible expression vector), as described previously (17). Each peptide pool was loaded onto the soluble class II molecules to produce pooled peptide tetramers. Tetramers loaded with single peptides were also produced as needed. For tetramer-guided epitope mapping (TGEM), CD4+ T cells were stimulated with the different pooled peptides, and PA responses were analyzed by staining with pooled PA tetramers on day 14 and peptide-specific tetramers on day 17 as previously described (18).
Recombinant-PA-protein stimulation assay.
In some experiments, peripheral blood mononuclear cells (PBMC) were stimulated with recombinant PA (provided by the Biodefense and Emerging Infections Research Repository [BEI Resources]). For these experiments, PBMC were allowed to adhere to 48-well plates for 1 h, nonadherent cells were removed, and the remaining adherent cells were utilized as antigen-presenting cells (APC). These cells were pulsed with 500 μg/ml of PA for 2 h and were washed twice with T-cell medium (10% pooled human serum in RPMI medium containing l-glutamine, HEPES, 0.01 U/ml penicillin, and 0.01 μg/ml streptomycin). CD4+ T cells were isolated from the nonadherent cell fraction with a Miltenyi CD4+ T-cell isolation kit and were added back to the culture as responding T cells. These cells were cultured for 14 days before tetramer staining analysis.
Frequencies and surface phenotypes of PA-specific T cells.
The method for estimating the frequency of PA-specific T cells was as previously described (14). In brief, 6 million PBMC in a volume of 100 μl were stained with 20 μg/ml tetramers at room temperature for 2 h. During the last 20 min, the cells were stained with fluorescein isothiocyanate (FITC)-conjugated anti-CD3 (eBioscience), APC-conjugated anti-CD4 (eBioscience), peridinin chlorophyll protein (PerCP)-conjugated anti-CD14 (BD Pharmingen), and PerCP-conjugated anti-CD19 (BD Pharmingen). Subsequently, the cells were washed and then incubated with anti-phycoerythrin (anti-PE) magnetic beads (Miltenyi Biotec) at 4°C for another 20 min. The cells were washed again, and 1/10 of the fraction was saved for later analysis. The other fraction was passed through a Miltenyi magnetic column. The bound fractions were flushed out and collected. Cells in both the bound fraction and the precolumn fraction were stained with ViaProbe (BD Bioscience) for 10 min before flow cytometry with a FACSCalibur. All data were analyzed using FlowJo (Tree Star). For analysis, cells were gated on forward scatter, side scatter, expression of CD3, and lack of CD14 and CD19 expression. Dead cells were also removed from analysis by using ViaProbe. The frequency was calculated as the total number of tetramer-positive cells in the bound fraction divided by 10 times the total number of CD4+ T cells in the precolumn fraction.
For phenotyping experiments using a FACSCalibur, the following antibodies were used: CD4-APC, CD4-FITC (eBioscience), CD14-PerCP, CD19-PerCP, CD28-FITC (eBioscience), CD45RA-FITC (BD Pharmingen), CD62L-FITC (BD Pharmingen), CCR4-Alexa Fluor 647 (BD Pharmingen), and CCR7-FITC (R&D). For experiments using an LSRII, the following antibodies were used: CD4-Pacific Blue (BD Pharmingen), CD14-PerCP, CD19-PerCP, CD62L-FITC, CCR4-Alexa Fluor 647, and CCR7-PE-Cy7 (BD Pharmingen).
CD45RA fractionation assay.
For CD45RA+ phenotyping of PA-reactive T cells in non-AVA vaccinees, CD4+ T cells were sorted into CD4+ CD45RA+ and CD4+ CD45RA− populations by using a FACS Vantage. Cells were then plated in the presence of APC, stimulated with the appropriate PA peptides at 10 μg/ml, cultured for 14 days, and analyzed by tetramer staining.
Cytokine analysis of PA-specific T cells.
CD4+ T cells were stimulated with 10 μg/ml of PA peptide and cultured for 2 weeks in the presence of APC. Cells were washed twice, and 200,000 cells in 0.1 ml were then transferred to a 96-well plate in which wells were precoated with the corresponding tetramers. Soluble anti-CD28 (1 μg/ml; BD Bioscience) was also added to the culture. Twenty-five microliters of supernatant was harvested 24 h later and transferred to a Meso Scale Th1/Th2 multiplex plate (Meso Scale Discovery). The plate was processed according to the manufacturer's instructions and read using a Meso Scale Sector 2400 imager (Meso Scale Discovery).
Homology search.
To identify homologous peptides shared by the proteins of other pathogens, the antigenic peptides identified within PA were analyzed individually using the NCBI protein BLAST tool. The PSI-BLAST algorithm was used to identify, align, and rank similar peptide sequences. Default parameters were used for this analysis.
Statistical methods.
For comparing the cytokine profiles of T cells from vaccinees and nonvaccinees, a two-tailed, unpaired t test was used to compare the mean gamma interferon (IFN-γ)/interleukin-13 (IL-13) and IFN-γ/IL-5 ratios for the two groups.
RESULTS
Identification of PA-specific T-cell epitopes.
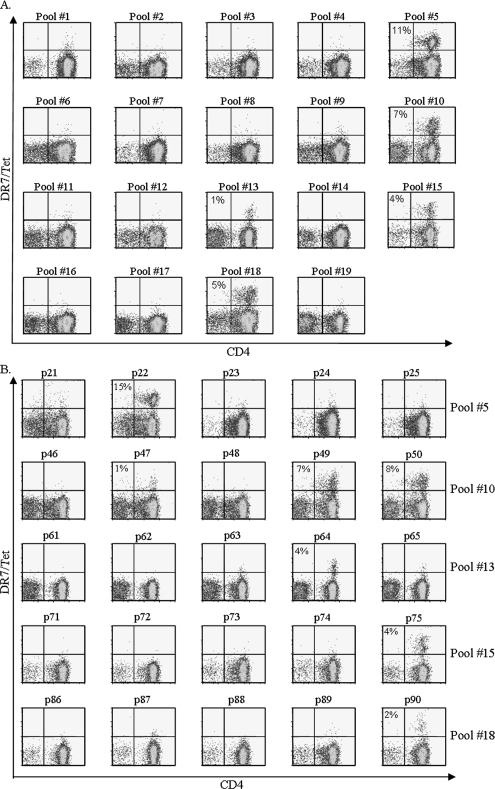
Healthy military and former military personnel that received at least three doses of AVA vaccines and healthy nonvaccinated volunteers were recruited for this study. The HLA haplotypes and the AVA vaccination histories of these subjects are shown in Table 1. The TGEM approach was applied to identify HLA-DR0701- and HLA-DR0401-restricted T-cell epitopes within the PA of B. anthracis. This experimental approach is briefly described in Materials and Methods and has been described in detail elsewhere (18). Experimental results from a representative DR0701 vaccinee are shown in Fig. 1. Staining with pooled DR0701/PA peptide tetramers indicated that peptide pools 5, 10, 13, 15, and 18 gave positive staining results. Subsequent staining with DR0701/PA individual peptide tetramers indicated that PA peptides p22, p47, p49, p50, p64, p75, and p90 contain DR0701-restricted PA epitopes. These peptides correspond to PA169-188, PA369-388, PA385-404, PA393-412, PA505-524, PA593-612, and PA713-732, respectively. All these DR0701 epitopes are naturally processed epitopes, as CD4+ T cells that were stimulated with recombinant PA protein generated identical epitope-specific immune responses, as assayed by staining with DR0701/PA tetramers (data not shown). Similar studies were completed for two additional DR0701 vaccinees and two additional DR0401 vaccinees. These results are summarized in Table 2. All but three of these DR0401 epitopes were also confirmed to be naturally processed epitopes. These results demonstrated the efficacy of the TGEM approach for epitope identification and indicate that PA-specific CD4+ T cells could be detected in the peripheral blood samples of AVA vaccinees.
FIG. 1.
Identification of DR0701-restricted PA epitopes. CD4+ T cells from a DR0701 AVA vaccine were stimulated with 19 peptide pools derived from PA of B. anthracis. (A) Cells were stained with the corresponding pooled DR0701/PA peptide tetramers (Tet) at day 14. Pools 5, 10, 13, 15, and 18 gave positive staining results. (B) Cells stimulated with peptide pools that gave positive staining results were stained with individual DR0701 peptide tetramers at day 17. The percentages of tetramer-positive cells are indicated. The background staining level in this experiment was 0.3% or lower. Tetramers with PA peptides p22, p47, p49, p50, p64, p75, and p90 gave staining results above the background level, indicating that these peptides contain DR0701-restricted epitopes. These peptides correspond to PA169-188, PA369-388, PA385-404, PA393-412, PA505-524, PA593-612, and PA713-732, respectively.
TABLE 2.
DR0401- and DR0701-restricted PA epitopes
| HLA and epitope | Amino acid sequence | Naturally processed | Tetramer stain result for indicated group and subject no.
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vaccinees
|
Nonvaccinees
|
|||||||||||
| 5 | 6 | 10 | 25 | 30 | 333 | 40 | 11 | 229 | 602 | |||
| DR0401 | ||||||||||||
| PA41-60 | SESSSQGLLGYYFSDLNFQA | Yes | + | + | − | − | ||||||
| PA49-68 | LGYYFSDLNFQAPMVVTSST | No | + | + | − | − | ||||||
| PA137-156 | LEKGRLYQIKIQYQRENPTE | NDa | + | − | + | − | ||||||
| PA145-164 | IKIQYQRENPTEKGLDFKLY | No | + | + | + | − | ||||||
| PA353-372 | FSNSNSSTVAIDHSLSLAGE | Yes | + | + | − | + | ||||||
| PA385-404 | DTARLNANIRYVNTGTAPIY | Yes | + | + | − | + | ||||||
| PA393-412 | IRYVNTGTAPIYNVLPTTSL | Yes | + | + | − | + | ||||||
| PA617-636 | NILIRDKRFHYDRNNIAVGA | Yes | + | + | + | + | ||||||
| PA625-644 | FHYDRNNIAVGADESVVKEA | Yes | + | + | + | + | ||||||
| PA713-732 | KLPLYISNPNYKVNVYAVTK | Yes | + | + | + | + | ||||||
| DR0701 | ||||||||||||
| PA169-188 | QNKKEVISSDNLQLPELKQK | Yes | + | + | + | − | + | + | ||||
| PA369-388 | LAGERTWAETMGLNTADTAR | Yes | + | + | + | + | − | − | ||||
| PA385-404 | DTARLNANIRYVNTGTAPIY | Yes | + | + | + | + | + | + | ||||
| PA393-412 | IRYVNTGTAPIYNVLPTTSL | Yes | + | + | + | + | + | + | ||||
| PA505-524 | NWSEVLPQIQETTARIIFNG | Yes | + | + | + | + | − | − | ||||
| PA593-612 | NQLAELNATNIYTVLDKIKL | Yes | − | + | + | + | + | + | ||||
| PA713-732 | KLPLYISNPNYKVNVYAVTK | Yes | + | + | + | − | + | − | ||||
| PA721-740 | PNYKVNVYAVTKENTIINPS | Yes | − | + | + | − | − | − | ||||
ND, not determined.
Surprisingly, PA-specific T cells were also detected within the PBMC of nonvaccinated DR0701 and DR0401 subjects by using the TGEM approach. Representative results for nonvaccinees are shown in Fig. 2 and summarized in Table 2.
FIG. 2.
Detection of PA-specific CD4+ T cells in non-AVA vaccinees. The TGEM approach was applied to PBMC from a DR0701 nonvaccinee (A) and a DR0401 nonvaccinee (B). The figure shows only positive staining samples (PA385-404, PA505-524, PA713-732, and PA617-636) and one representative negative staining sample (PA169-188). Tet, tetramer.
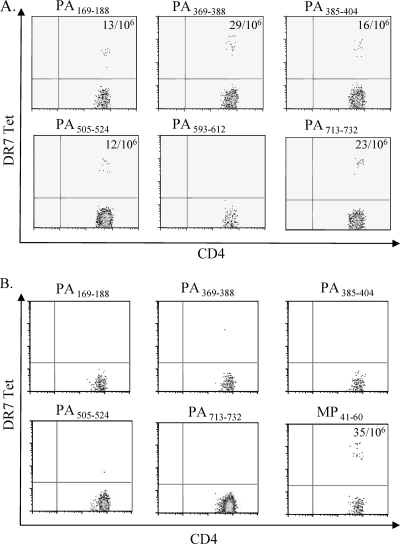
To characterize these PA responses further, the frequencies of PA-specific T cells in both vaccinated and nonvaccinated subjects within unstimulated PBMC were examined. For these experiments, PBMC were stained with PE-labeled tetramers ex vivo by using the anti-PE bead enrichment approach as described in Materials and Methods. Staining results for a DR0701 vaccinee are shown in Fig. 3A. The frequencies of the DR7-restricted, PA-reactive T cells in this subject ranged from 12 per million to 29 per million CD4+ T cells, depending on the epitope tested. The results for this and four other AVA vaccinees are summarized in Table 3. Notably, the frequencies of PA-specific T cells from vaccinees were similar to those reported for influenza virus-specific T cells (6). In contrast, PA-specific T cells could not be detected by the ex vivo staining approach in subjects (a total of four) that had not received the anthrax vaccine. Representative ex vivo staining results for a DR0701 nonvaccinee are shown in Fig. 3B. Staining results for a control influenza matrix protein epitope in this subject were positive. The detection limit of the PE enrichment protocol is approximately 1 in 300,000 CD4+ T cells. Thus, PA-specific T cells in nonvaccinees are present at frequencies lower than 1 in 300,000 CD4+ T cells.
FIG. 3.
Frequencies of PA-specific CD4+ T cells in peripheral blood samples. PBMC were stained with PE-labeled PA tetramers (Tet) as indicated and subsequently with anti-CD4, anti-CD14, and anti-CD19 antibodies. Cells were then captured with anti-PE magnetic beads and enriched using a magnetic column before flow cytometry analyses. Results of staining from an AVA vaccinee with the DR0701 haplotype are shown in panel A, and results from an AVA-naïve DR0701 subject are shown in panel B. Detection of influenza virus A matrix protein-reactive T cells with the DR7/MP41-60 tetramer was used as a positive control. The frequencies of PA-specific T cells per million CD4+ cells are indicated in each of the positive quadrants. The frequencies of PA593-612 in the AVA vaccinee and all PA-reactive T cells in the nonvaccinee were below the sensitivity limit of the assay.
TABLE 3.
Frequencies of PA-specific CD4+ T cells
| Subject no. | HLA | No. of PA-specific cells/million CD4+ T cells for:
|
|||||
|---|---|---|---|---|---|---|---|
| PA169-188 | PA269-388 | PA385-404 | PA505-524 | PA713-732 | PA617-636 | ||
| 15 | DR0701 | 11 | NDa | 29 | 45 | ND | |
| 30 | DR0701 | 13 | 29 | 16 | 12 | 23 | |
| 18 | DR0701 | ND | ND | 20 | 10 | ND | |
| 5 | DR0401 | 20 | |||||
| 6 | DR0401 | 36 | |||||
ND, not determined.
Phenotypes of PA-specific CD4+ T cells.
Ex vivo staining analysis also allowed a direct examination of the surface phenotype of PA-specific T cells by simultaneous staining of PBMC with tetramers and antibodies. The CD45RA phenotype of T cells specific for multiple PA epitopes in seven AVA vaccinees was examined. Examples of results from these experiments are shown in Fig. 4A. All PA-specific T cells from vaccinated subjects were CD45RA−, suggesting that these were memory T cells (more than 20 different staining experiments). The surface phenotype of PA-specific T cells from healthy nonvaccinees could not be examined by ex vivo staining, due to low frequencies. To determine whether these T cells exhibited the CD45RA+ phenotype, CD4+ T cells from three DR0701 nonvaccinees were fractionated into both CD45RA+ and CD45RA− populations and then subjected to in vitro stimulation with PA385-404 andPA505-524 peptides. Subsequent analysis by tetramer staining indicated that PA385-404-reactive T cells from DR0701 nonvaccinees resided in both the CD45RA+ and the CD45RA− populations (Fig. 4B). In contrast, PA505-524-specific T cells primarily resided in the CD45RA+ population. This specific pattern of CD45RA+ and CD45RA− distribution of PA-reactive T cells for the different DR0701 epitopes was observed in all three DR0701 subjects examined. Additional experiments were carried out with two nonvaccinated DR0401 subjects for multiple epitopes; all PA-reactive T cells were detected only in the CD45RA+ fraction (Fig. 4C).
FIG. 4.
CD45RA+ phenotype of PA-specific CD4+ T cells in AVA vaccinees and non-AVA vaccinees. (A) PBMC of an AVA vaccinee with the DR0701 haplotype were stained with PE-labeled tetramers (Tet) as indicated and subsequently stained with anti-CD4, anti-CD45RA, anti-CD14, and anti-CD19 antibodies. Cells were then captured with anti-PE magnetic beads and enriched using a magnetic column before flow cytometry analyses (representative data from four experiments are shown). (B) PBMC of three nonvaccinated subjects with the DR0701 haplotype were sorted into CD4+ CD45RA+ and CD4+ CD45RA− populations. These cells were stimulated with the PA peptides as indicated for 14 days and then analyzed with the corresponding PA tetramers. (C) PBMC of a nonvaccinated subject with the DR0401 haplotype were sorted into CD4+ CD45RA+ and CD4+ CD45RA− populations. These cells were stimulated with the PA peptides as indicated for 14 days and then analyzed with the corresponding PA tetramers (representative data from two experiments are shown).
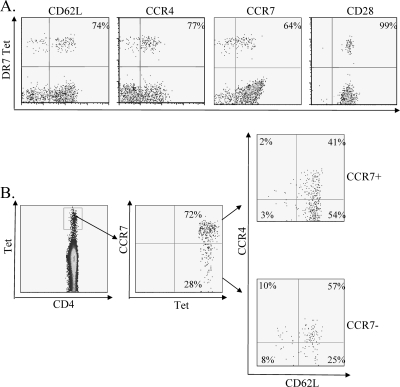
Additional characterizations of the surface phenotype of PA-specific T cells from subjects that received the AVA vaccine are shown in Fig. 5 and Table 4. To effectively visualize these T cells ex vivo, PBMC were simultaneously stained with multiple PE-conjugated tetramers. For DR0701 subjects, the DR0701 tetramers used were DR0701/PA369-388, DR0701/PA385-404, and DR0701/PA505-524. For DR0401 subjects, the tetramers used were DR0401/PA385-404 and DR0401/PA617-636. For the individuals tested, PA-specific T cells were almost exclusively CD28+. PA-specific T cells were more heterogeneous in their CCR4, CD62L, and CCR7 surface expressions. Further experiments demonstrated that the majority of the CCR7+ cells were also CD62L+ (Fig. 5B). In addition, approximately 41% of the CCR7+ CD62L+ PA-specific T cells were also CCR4+ positive (Fig. 5B), a marker that correlates with polarized Th2 response (10).
FIG. 5.
Phenotypes of PA-specific CD4+ T cells in AVA vaccinees. (A) PBMC of a DR0701-vaccinated subject were stained with a pool of DR0701/PA369-388, DR0701/PA385-404, and DR0701/PA505-524 tetramers by using magnetic bead enrichment. Cells were also stained with surface antibodies as indicated. For analysis, cells were gated on side scatter, expression of CD4, and lack of CD14 and CD19 expression. (B) PBMC of a DR0701-vaccinated subject were stained with the same set of pooled tetramers and with anti-CD4, anti-CD14, anti-CD19, anti-CCR4, anti-CCR7, and anti-CD62L. CD4+, CD14−, and CD19− cells were gated and analyzed for tetramer staining and CCR7 staining. Both the CCR7+ and the CCR7− tetramer-positive cells were further gated and analyzed for CCR4 and CD62L expression.
TABLE 4.
Phenotypes of PA-reactive T cells
| Subject no. | % of cells positive for:
|
|||
|---|---|---|---|---|
| CD28 | CD62L | CCR4 | CCR7 | |
| 6 | 100 | 46 | 71 | 30 |
| 10 | 100 | 76 | 55 | 57 |
| 15 | 98 | 74 | 77 | 64 |
Cytokine profiles of PA-specific CD4+ T cells.
To determine the cytokine profiles of PA-specific T cells, PBMC were stimulated with PA peptides and cultured for 14 days. The cells were then reactivated with corresponding plate-bound, PA-specific tetramers for 24 h, and the levels of cytokines IFN-γ, IL-5, and IL-13 in the supernatants were assayed. Examples of results from these experiments are shown in Table 5. Multiple experiments with different AVA vaccinees indicated that the PA-specific T cells secrete IFN-γ, IL-5, and IL-13, with IL-5 and IL-13 as their major cytokines (Table 5 and Fig. 6). The cytokine profiles of PA-specific T cells from nonimmunized subjects were also analyzed. Although these T cells also secreted IFN-γ, IL-5, and IL-13, IFN-γ was the major cytokine observed (Table 5 and Fig. 6). t tests comparing the IFN-γ/IL-13 and IFN-γ/IL-5 ratios (indicating the polarization of the cytokine response) for AVA vaccinees and nonvaccinees demonstrated significant differences (P values of <0.0001 and <0.01, respectively). Thus, PA-specific T cells from vaccinees were Th2-like (predominantly secreting IL-5 and IL-13), while PA-specific T cells from nonvaccinees were more Th0/Th1-like. The Th2-like profile of PA-reactive T cells in AVA vaccinees was antigen specific, as tetanus toxoid-reactive T cells from AVA vaccinees exhibited a Th0 phenotype when identical analysis was used (data not shown).
TABLE 5.
Cytokine profiles of PA-reactive T cells in vaccinees and nonvaccinees
| Group and subject no. | HLA and epitope | Concn (pg/ml) of:
|
Ratio
|
|||
|---|---|---|---|---|---|---|
| IFN-γ | IL-13 | IL-5 | IFN-γ/IL-13 | IFN-γ/IL-5 | ||
| Vaccinees | ||||||
| 15 | DR7/PA385-404 | 1,037 | 3,584 | 5,028 | 0.3 | 0.2 |
| DR7/PA505-524 | 988 | 4,010 | 1,764 | 0.2 | 0.6 | |
| 18 | DR7/PA385-404 | 569 | 4,477 | 2,528 | 0.1 | 0.2 |
| DR7/PA505-524 | 2,011 | 2,127 | 2,515 | 1 | 1 | |
| 30 | DR7/PA385-404 | 459 | 5,984 | 3,130 | <0.1 | 0.1 |
| DR7/PA505-524 | 88 | 4,419 | 2,766 | <0.1 | <0.1 | |
| 6 | DR4/PA393-412 | 964 | 5,534 | 4,651 | 0.2 | 0.2 |
| DR4/PA617-636 | 1,644 | 9,739 | 6,127 | 0.2 | 0.3 | |
| Nonvaccinees | ||||||
| 11 | DR7/PA385-404 | 15,868 | 5,399 | 793 | 3 | 20 |
| DR7/PA505-524 | 24,682 | 9,718 | 2,588 | 3 | 10 | |
| 899 | DR7/PA385-404 | 3,911 | 786 | 21 | 5 | 186 |
| DR7/PA505-524 | 13,950 | 2,155 | 27 | 6 | 516 | |
| 4798 | DR7/PA385-404 | 4,565 | 1,566 | 46 | 3 | 100 |
| DR7/PA505-524 | NDa | ND | ND | |||
| 500 | DR4/PA393-412 | 17,805 | 7,182 | 1,839 | 2 | 10 |
| DR4/PA617-636 | 14,267 | 1,921 | 316 | 7 | 15 | |
ND, not determined.
FIG. 6.
Cytokine profiles of PA-specific CD4+ T cells in AVA vaccinees and nonvaccinees. CD4+ T cells from vaccinated or nonvaccinated subjects were stimulated with the antigenic PA peptides for 14 days. Cells were washed twice, and 200,000 cells in 0.1 ml were transferred to a 96-well plate which had been precoated with the corresponding PA-specific tetramers. IFN-γ, IL-13, and IL-5 levels were assayed 24 h after activation of the plate-bounded tetramer by using a Meso Scale Th1/Th2 plate as described in the text. IFN-γ/IL-13 and IFN-γ/IL-5 ratios were calculated as a measure of the polarization of the cytokine response and compared for vaccinees and nonvaccinees. Statistical significance was determined by the unpaired, two-tailed t test; P values of <0.05 were considered significant.
DISCUSSION
Comparing a group of individuals immunized with anthrax vaccine with a group of healthy, unvaccinated volunteers afforded us the opportunity to examine the CD4+ T-cell responses against PA elicited by AVA vaccination. Experiments were carried out using class II tetramers to examine PA-specific T cells in both AVA vaccinees and nonvaccinees. The major outcomes and observations in this study were as follows: (i) CD4+ T cells that recognized multiple epitopes within PA were detected in both vaccinated and nonvaccinated subjects, (ii) frequencies of PA-specific T cells were higher in vaccinees than in nonvaccinees, (iii) PA-reactive CD4+ T cells exhibit a Th2 cytokine profile in vaccinees and a Th0/Th1 cytokine profile in nonvaccinees, and (iv) PA-specific T cells with a pre-Th2 central memory T-cell (TCM) phenotype were detected in vaccinated subjects.
The TGEM approach was effective in identifying T-cell epitopes within PA and detecting PA-specific CD4+ T cells. Though TGEM may fail to detect some low-avidity epitopes, high-avidity T cells as detected by TGEM are likely to be the most biologically relevant. Surprisingly, PA-specific T cells were detected not only in the PBMC of vaccinated subjects but also in those of nonvaccinated subjects examined after a single cycle of antigen-specific stimulation in vitro. Further study indicated that PA-specific T cells from nonvaccinated subjects were present at frequencies that were lower than 1 in 300,000 CD4+ T cells. Although the majority of PA-reactive T cells from nonvaccinees resided in the CD45RA+ population, it is surprising that PA-reactive CD4+ T cells from all the DR0701 nonvaccinees examined (a total of three) exhibited a memory phenotype for the PA385-404 epitopes. It is possible that the general populations are exposed to minute numbers of Bacillus anthracis spores or bacteria, which can be found naturally in the soil, through inhalation and ingestion. We consider this possibility very unlikely, as PA-reactive T cells that recognized other PA epitopes in the same subjects are naïve T cells. It is more likely that PA385-404-reactive T cells are cross-reactive to other antigenic epitopes which have previously been recognized by the immune system. A homology study revealed that the PA389-404 (LNANIRYVNTGTAPIY) sequence shares a high degree of similarity with the sequences of several clostridial binding toxins, including the C2 toxin of Clostridium botulinum (aa 362 to 377, INPNIRYYNTGTAPVY) and the binary toxin CdtB of Clostridium difficile (aa 389 to 404, INANVRYYNTGTAPMY). Therefore, a previous challenge with one or more of these antigens may generate a memory population capable of recognizing this PA epitope.
PA-specific T cells from vaccinees were detected by direct ex vivo tetramer staining, with frequencies ranging from 1 in 22,000 to 1 in 100,000 CD4+ T cells in the five subjects examined. As PA-reactive T cells could not be detected by ex vivo staining in nonvaccinated subjects, these data imply that there are increases in the frequencies of PA-specific T cells in vaccinated subjects. All of the PA-specific T cells in vaccinated subjects were CD45RA−, indicating that these are memory T cells. Since each of the subjects tested had measurable responses to more than one PA epitope, the data imply that the total frequency of T cells directed against the PA protein restricted by a particular DR allele can be as high as 1 in 10,000 even years after the last vaccination. The observed frequencies of PA-reactive T cells were also similar to those reported for influenza virus A-specific CD4+ T cells (6). Taken together, these results demonstrated that AVA vaccination can elicit strong heterogeneous CD4+ T-cell responses against PA.
Although the epitope recognition patterns are similar in AVA vaccinees and nonvaccinees, PA-specific CD4+ T cells expanded from the PBMC of vaccinated and nonvaccinated subjects exhibited different cytokine profiles upon antigen rechallenge. PA-reactive T cells from AVA vaccinees produced large amounts of IL-5 and IL-13 compared to IFN-γ, while PA-specific T cells from nonvaccinees produced more IFN-γ than IL-5 and IL-13. Because it was technically difficult to detect cytokine secretion from antigen-specific T cells directly ex vivo, these cytokine results were obtained from expanded cells. Thus, the observed differences may be (at least in part) an artifact of in vitro expansion. However, the observation that the phenotype of PA-specific T cells from AVA vaccinees is Th2-like is also supported by the observation that a large percentage of these T cells expressed the CCR4 surface marker in direct ex vivo staining experiments. Though on average only 25% of total CD4+ cells in PBMC express CCR4 on the cell surface (data not shown), 55 to 77% of PA-reactive T cells expressed the CCR4+ marker in the vaccinees studied. These results suggest that AVA vaccination leads to the generation of PA-reactive T cells with a Th2-like phenotype. Previous publications have indicated that T-cell responses to PA contained a mixture of both Th1-like and Th2-like cells (1, 12). However, our current findings clearly indicate that PA-specific memory CD4+ cells are prone to Th2 cytokine production, while PA-specific CD4+ T cells from nonvaccinees are more prone to Th0/Th1 cytokine production. This apparent contrast may be caused by differences in experimental methodology. Allen et al. (1) assayed for IL-4 and IL-10 but not IL-5 or IL-13. Laughlin et al. (12) assayed two expanded T-cell clones, demonstrating that one primarily produced IFN-γ while the other produced IL-5. A Th2-like phenotype would be desirable for the stimulation of B cells, potentially triggering a protective antibody response.
The results of direct ex vivo surface phenotyping indicated generation of PA-specific TCMs (22) in vaccinated subjects, as there were clear populations of CD45RA− CCR7+ CD62L+ PA-reactive T cells in AVA vaccinees. Also of interest, the PA-specific TCMs were elevated in their expression of CCR4, at least in the small number of subjects examined. These observations are an indication that AVA vaccination leads to the generation of pre-Th2 TCMs (21). The presence of these populations of TCMs supports the observation that T cells directed against PA could be detected even in subjects that had received the vaccines 10 to 15 years earlier (1). In conclusion, our findings demonstrated that the AVA vaccine can elicit strong CD4+ T-cell responses in AVA vaccines and that vaccination leads to the generation of PA-specific, pre-Th2 memory T cells.
More generally, this study illustrates the fact that class II tetramers can be used as a tool to monitor CD4+ cell responses after vaccination. Due to the technical challenges associated with detecting antigen-specific CD4+ T cells, studies of the effect of vaccination on antigen specific CD4+ T-cell responses have been limited. In this paper, we showcase the use of class II tetramers to identify T-cell epitopes for an antigen of interest and subsequently to perform direct ex vivo analysis of the frequencies and phenotypes of antigen-specific T cells within vaccinated subjects. This approach should be applicable to any antigen, facilitating the monitoring of CD4+ responses after vaccination. Future efforts in this area will help to decipher the influences of route of immunization, adjuvants, and vaccine composition on the phenotypes and frequencies of resulting memory T-cell responses. The detection of specific T cells recognizing B. anthracis PA within naïve subjects also raises the possibility that it may be feasible to identify the dominant CD4+ T-cell epitopes for a given antigen of a pathogen by measuring the responses of PBMC from healthy subjects that are completely naïve to the antigen of interest.
Acknowledgments
This work was supported in part by NIH contract HHSN266200400028C to W. W. Kwok.
Editor: S. R. Blanke
Footnotes
Published ahead of print on 4 August 2008.
REFERENCES
- 1.Allen, J. S., A. Skowera, G. J. Rubin, S. Wessely, and M. Peakman. 2006. Long-lasting T cell responses to biological warfare vaccines in human vaccinees. Clin. Infect. Dis. 431-7. [DOI] [PubMed] [Google Scholar]
- 2.Baillie, L., T. Townend, N. Walker, U. Eriksson, and D. Williamson. 2004. Characterization of the human immune response to the UK anthrax vaccine. FEMS Immunol. Med. Microbiol. 42267-270. [DOI] [PubMed] [Google Scholar]
- 3.Barnard, J. P., and A. M. Friedlander. 1999. Vaccination against anthrax with attenuated recombinant strains of Bacillus anthracis that produce protective antigen. Infect. Immun. 67562-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cieslak, T. J., and E. M. Eitzen, Jr. 1999. Clinical and epidemiologic principles of anthrax. Emerg. Infect. Dis. 5552-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen, S., I. Mendelson, Z. Altboum, D. Kobiler, E. Elhanany, T. Bino, M. Leitner, I. Inbar, H. Rosenberg, Y. Gozes, R. Barak, M. Fisher, C. Kronman, B. Velan, and A. Shafferman. 2000. Attenuated nontoxinogenic and nonencapsulated recombinant Bacillus anthracis spore vaccines protect against anthrax. Infect. Immun. 684549-4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danke, N. A., and W. W. Kwok. 2003. HLA class II-restricted CD4+ T cell responses directed against influenza viral antigens postinfluenza vaccination. J. Immunol. 1713163-3169. [DOI] [PubMed] [Google Scholar]
- 7.Grabenstein, J. D. 2003. Anthrax vaccine: a review. Immunol. Allergy Clin. North Am. 23713-730. [DOI] [PubMed] [Google Scholar]
- 8.Grunow, R., M. Porsch-Ozcurumez, W. Splettstoesser, A. Buckendahl, U. Hahn, W. Beyer, R. Bohm, M. Huber, U. vd Esche, W. Bessler, D. Frangoulidis, and E. J. Finke. 2007. Monitoring of ELISA-reactive antibodies against anthrax protective antigen (PA), lethal factor (LF), and toxin-neutralising antibodies in serum of individuals vaccinated against anthrax with the PA-based UK anthrax vaccine. Vaccine 253679-3683. [DOI] [PubMed] [Google Scholar]
- 9.Hanson, J. F., S. C. Taft, and A. A. Weiss. 2006. Neutralizing antibodies and persistence of immunity following anthrax vaccination. Clin. Vaccine Immunol. 13208-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim, C. H., L. Rott, E. J. Kunkel, M. C. Genovese, D. P. Andrew, L. Wu, and E. C. Butcher. 2001. Rules of chemokine receptor association with T cell polarization in vivo. J. Clin. Investig. 1081331-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kyriacou, D. N., A. Adamski, and N. Khardori. 2006. Anthrax: from antiquity and obscurity to a front-runner in bioterrorism. Infect. Dis. Clin. North Am. 20227-251. [DOI] [PubMed] [Google Scholar]
- 12.Laughlin, E. M., J. D. Miller, E. James, D. Fillos, C. C. Ibegbu, R. S. Mittler, R. Akondy, W. Kwok, R. Ahmed, and G. Nepom. 2007. Antigen-specific CD4+ T cells recognize epitopes of protective antigen following vaccination with an anthrax vaccine. Infect. Immun. 751852-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lininger, L. A., M. E. Cullum, M. B. Lyles, and D. R. Bienek. 2007. The impact of incomplete vaccination schedules on the magnitude and duration of protective antigen-specific IgG responses in recipients of the US licensed anthrax vaccine. Vaccine 251619-1625. [DOI] [PubMed] [Google Scholar]
- 14.Lucas, M., C. L. Day, J. R. Wyer, S. L. Cunliffe, A. Loughry, A. J. McMichael, and P. Klenerman. 2004. Ex vivo phenotype and frequency of influenza virus-specific CD4 memory T cells. J. Virol. 787284-7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marcus, H., R. Danieli, E. Epstein, B. Velan, A. Shafferman, and S. Reuveny. 2004. Contribution of immunological memory to protective immunity conferred by a Bacillus anthracis protective antigen-based vaccine. Infect. Immun. 723471-3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mock, M., and A. Fouet. 2001. Anthrax. Annu. Rev. Microbiol. 55647-671. [DOI] [PubMed] [Google Scholar]
- 17.Novak, E. J., A. W. Liu, G. T. Nepom, and W. W. Kwok. 1999. MHC class II tetramers identify peptide-specific human CD4(+) T cells proliferating in response to influenza A antigen. J. Clin. Investig. 104R63-R67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Novak, E. J., A. W. Liu, J. A. Gebe, B. A. Falk, G. T. Nepom, D. M. Koelle, and W. W. Kwok. 2001. Tetramer-guided epitope mapping: rapid identification and characterization of immunodominant CD4+ T cell epitopes from complex antigens. J. Immunol. 1666665-6670. [DOI] [PubMed] [Google Scholar]
- 19.Pittman, P. R., S. L. Norris, J. G. Barrera Oro, D. Bedwell, T. L. Cannon, and K. T. McKee, Jr. 2006. Patterns of antibody response in humans to the anthrax vaccine adsorbed (AVA) primary (six-dose) series. Vaccine 243654-3660. [DOI] [PubMed] [Google Scholar]
- 20.Reuveny, S., M. D. White, Y. Y. Adar, Y. Kafri, Z. Altboum, Y. Gozes, D. Kobiler, A. Shafferman, and B. Velan. 2001. Search for correlates of protective immunity conferred by anthrax vaccine. Infect. Immun. 692888-2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rivino, L., M. Messi, D. Jarrossay, A. Lanzavecchia, F. Sallusto, and J. Geginat. 2004. Chemokine receptor expression identifies Pre-T helper (Th)1, Pre-Th2, and nonpolarized cells among human CD4+ central memory T cells. J. Exp. Med. 200725-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sallusto, F., J. Geginat, and A. Lanzavecchia. 2004. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 22745-763. [DOI] [PubMed] [Google Scholar]
- 23.Scorpio, A., T. E. Blank, W. A. Day, and D. J. Chabot. 2006. Anthrax vaccines: Pasteur to the present. Cell. Mol. Life Sci. 632237-2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shinn, A. H., N. C. Bravo, H. T. Maecker, and J. W. Smith. 2003. TNF-alpha detection using a flow cytometric assay to determine cellular responses to anthrax vaccine. J. Immunol. Methods 282169-174. [DOI] [PubMed] [Google Scholar]
- 25.Turnbull, P. C., M. G. Broster, J. A. Carman, R. J. Manchee, and J. Melling. 1986. Development of antibodies to protective antigen and lethal factor components of anthrax toxin in humans and guinea pigs and their relevance to protective immunity. Infect. Immun. 52356-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Welkos, S., S. Little, A. Friedlander, D. Fritz, and P. Fellows. 2001. The role of antibodies to Bacillus anthracis and anthrax toxin components in inhibiting the early stages of infection by anthrax spores. Microbiology 1471677-1685. [DOI] [PubMed] [Google Scholar]