Abstract
Lyme neuroborreliosis is likely caused by inflammatory effects of the tick-borne spirochete Borrelia burgdorferi on the nervous system. Microglia, the resident macrophage cells within the central nervous system (CNS), are important in initiating an immune response to microbial products. In addition, astrocytes, the major CNS glial cell type, also can contribute to brain inflammation. TLRs (Toll-like receptors) are used by glial cells to recognize pathogen-associated molecular patterns (PAMPs), mediate innate responses, and initiate an acquired immune response. Here we hypothesize that because of their PAMP specificities, TLR1, -2, -5, and -9 may be involved in the pathogenesis of Lyme neuroborreliosis. Previous reports have shown that the rhesus monkey is the only animal model to exhibit signs of Lyme neuroborreliosis. Therefore, we used primary cultures of rhesus astrocytes and microglia to determine the role of TLRs in mediating proinflammatory responses to B. burgdorferi. The results indicate that microglia and astrocytes respond to B. burgdorferi through TLR1/2 and TLR5. In addition, we observed that phagocytosis of B. burgdorferi by microglia enhances not only the expression of TLR1, -2, and -5, but also that of TLR4. Taken together, our data provide proof of the concept that astrocyte and microglial TLR1, -2, and -5 are involved in the in vivo response of primate glial cells to B. burgdorferi. The proinflammatory molecules elicited by these TLR-mediated responses could be a significant factor in the pathogenesis of Lyme neuroborreliosis.
Lyme disease, caused by the spirochete Borrelia burgdorferi, is the most frequently reported vector-borne disease in the United States, and it is prevalent worldwide (28). An infection with B. burgdorferi may result in a broad array of clinical manifestations, including erythema migrans, acute or chronic arthritis, carditis, and neuroborreliosis. The latter form of the disease may affect both the central nervous system (CNS) and peripheral nervous system. Clinical manifestations of Lyme neuroborreliosis include lymphocytic meningitis, cranial neuropathy, polyradiculopathy, encephalomyelitis, and loss of memory and other cognitive functions (44).
Inflammation is a significant contributing factor to neurodegenerative disease. In response to injury, infection, or disease, resident CNS cells may generate proinflammatory mediators (e.g., cytokines and chemokines), express adhesion molecules, and recruit immune cells from the periphery (42). Microglia and astrocytes are key players in the immune responses that occur within the CNS (11, 12, 18). Studies have shown that microglia and astrocytes express Toll-like receptors (TLRs) (3, 4, 8, 27, 33, 39). These receptors play a major role in innate immune responses against microbial pathogens, are widely distributed throughout cells of the immune system, and are able to recognize a variety of highly conserved structural motifs or pathogen-associated molecular patterns (PAMPs) (1). So far, 10 TLR types, displaying distinct ligand specificities, have been identified in humans. TLR4 binds to lipopolysaccharide (26). TLR2 recognizes various components, including bacterial peptidoglycan, lipopeptide, and lipoprotein and mycoplasma lipoprotein (25, 47); it requires TLR6 and TLR1 as coreceptors for the recognition of diacylated and triacylated lipoprotein, respectively (48, 49). TLR3 recognizes double-stranded RNA (2), TLR5 binds to bacterial flagellin (22), TLR7 and human TLR8 recognize imidazoquinoline compounds and single-stranded RNA from viruses (23), and TLR9 binds to bacterial and viral CpG DNA motifs (24). In addition, intracellular signaling pathways downstream from TLRs can activate several transcription factors, leading to the expression of a variety of immune response genes.
After infection with B. burgdorferi, the rhesus monkey develops not only typical manifestations of Lyme disease, but peripheral nervous system and CNS involvement as well (14, 35, 37). Based on these studies, we used primary cultures of microglia and astrocytes that had been isolated from the brains of normal rhesus macaques to determine the involvement of primate TLRs in mediating the expression of cytokines and chemokines in response to B. burgdorferi in vitro. Because of their PAMP specificities, we hypothesized that TLR1, -2, -5, and -9 might be involved in the innate response to B. burgdorferi in the CNS and thus in the pathogenesis of Lyme neuroborreliosis.
MATERIALS AND METHODS
Primary cultures of glial cells.
Brain tissues used in this study were collected from adult rhesus macaques (Macaca mulatta) of either Chinese or Indian origin. These animals were not infected with B. burgdorferi and were culled from the breeding colony because of chronic diarrhea or injury. The procedure used for euthanasia was consistent with the recommendations of the American Veterinary Medical Association's Panel on Euthanasia. Tissue was removed from the cortical region of the brain and immediately processed as follows. The meninges and blood vessels were carefully removed, and the tissue was suspended in 20 ml of Dulbecco's modified Eagle's medium (DMEM)-F-12 with l-glutamine and 15 mM HEPES buffer (Invitrogen), 100 U/ml penicillin, and 100 μg/ml streptomycin (Gibco). The tissue was mechanically dissociated, treated with 0.25% trypsin-0.38 g/ml EDTA (Invitrogen) and 0.1% DNase (Sigma-Aldrich), and incubated at 37°C for 40 min. After incubation, the tissue was centrifuged for 10 min at 425 × g. Cells were filtered through a Nitex filter (20 μm) and resuspended in glial culture medium, which was composed of DMEM-F-12 with l-glutamine and HEPES buffer, 10% fetal bovine serum (HyClone), 0.5 ng/ml of granulocyte-macrophage colony-stimulating factor (Sigma-Aldrich), 100 U/ml penicillin, and 100 μg/ml streptomycin. Cells were incubated at 37°C in a humid atmosphere with 5% CO2. After 14 to 21 days in culture, microglia were isolated by vigorously tapping the flasks. Dislodged microglial cells were resuspended in the same medium as for mixed glial cultures. To obtain purified astrocytes, glial cells were incubated for 90 min in 10 mM l-leucine methyl ester (LME) (Sigma-Aldrich). After addition of LME, cultures were visually inspected to ensure maximal microglial lysis with minimal toxicity to astrocytes. Thereafter, astrocytes were washed thoroughly and resuspended in glial culture medium. Purity of astrocytes and microglial cultures was assessed by staining with a specific microglial marker (anti-IBA antibody) and was routinely of 99%.
Bacterial culture.
B. burgdorferi strain B31 (clone 5A19, possessing all plasmids) was cultured in Barbour-Stoenner-Kelly-H (BSK-H) medium (Sigma-Aldrich) at 34°C. For subsequent experiments, B. burgdorferi cells were washed with phosphate-buffered saline (PBS) and resuspended in DMEM-F-12 with l-glutamine and HEPES buffer plus 10% fetal bovine serum. Spirochetal viability was confirmed after 24 h of culture in this medium using the LIVE/DEAD BacLight bacterial viability kit (Molecular Probes) according to the manufacturer's instructions.
RNA isolation.
For quantification of TLR and cytokine mRNA expression, microglia (1 × 105/ml) and astrocytes (5 × 105/ml) were incubated in glial culture medium alone or with added, live B. burgdorferi (10:1 spirochete/cell ratio = a multiplicity of infection [MOI] of 10), sonicated B. burgdorferi cells (quantity equivalent to an MOI of 10), 0.25 μg/ml recombinant lipidated outer surface protein A (L-OspA) (GlaxoSmithKline), 1.0 μM unmethylated CpG oligodeoxynucleotide (ODN) M362 (Invivogen), or 100 ng/ml flagellin (FliC) (isolated from Salmonella enterica serovar Typhimurium) (Alexis Biochemicals). RNA was collected after 2 and 8 h of incubation, except for the quantification of TLR4 transcript, where incubations were for 8, 12, and 24 h and only with live spirochetes (MOI of 10). Total RNA was isolated using the RNeasy kit (Qiagen) following the protocol supplied by the manufacturer.
qRT-PCR.
TLR and interleukin-6 (IL-6) transcripts were quantified through the use of the Sybr green real-time PCR and TaqMan PCR assay. The Sybr green primers as well as the TaqMan primers and probe selected for this study (Tables 1 and 2) were designed for rhesus macaque sequences using Primer 3 (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi). The quantitative reverse transcriptase PCR (qRT-PCR) was performed using either an ABI PRISM 7700 or 7900 thermal cycler system (Applied Biosystems), following the manufacturer's instructions. Cycle threshold (CT) values for specific genes were normalized to the CT value for the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) housekeeping gene and expressed as ΔCT. The specificity of the RT-PCR was controlled using no-template controls.
TABLE 1.
Sequences of the primers used for Sybr green qRT-PCRa
| Gene target | Primer sequence (3′→5′) | Amplicon size (bp) |
|---|---|---|
| TLR1 | CACGATTCTTTCTGGGTGAAGCACCACTCACTCTGGACAA | 186 |
| TLR2 | GATGCCTACTGGGTGGAGAACCACTTGCCAGGAATGAAGTC | 102 |
| TLR5 | AGGACGCCATCTGGAACACTGGTACTGGGACAAGGAC | 164 |
| TLR9 | CTGGGTGTACAATGAGCTTCATCACCAGCACCACGACAT | 254 |
| IL-6 | AATGAGGACACTTGCCTGGTGCAGGGGTGGTTATTGCATCTAG | 186 |
| GAPDH | CAGCCTCAAGATCATCAGCAGTCTTCTGGGTGGCAGTGATG | 136 |
In each case, the annealing temperature was 55°C.
TABLE 2.
Sequences of the primers and probes used for TaqMan qRT-PCR
| Gene target | Sequence (5′→3′)
|
Amplicon size (bp) | |
|---|---|---|---|
| Primer | Probe | ||
| TLR1 | CATTCCGCAGTACTCCATTCAGCCCATGTTTGCTCTTTTC | CAAGCTCAAAAATCTCATGGCCAGGAa | 102 |
| TLR2 | TGATGCTGCCATTCTTGTTCGCCACTCCAGGTAGGTCTTG | CGCTTCTGCAAGCTGCGGAAGATAATa | 107 |
| TLR4 | GAGAACTTCCCCATTGGACACTGGAAAGGTCCAAGTGCTC | TGTGGCTCACAATCTTATCCAGTCTa | 128 |
| TLR5 | GCCAGTCCTGTGTTTGGATTCTCAGCAGGAGCCTCTCAGT | TGCTCCTTTGATGGCCGAATAGCCTa | 119 |
| GAPDH | CTGCACCACCAACTGCTTAGGATGGCATGGACTGTGGTC | CTGGCCAAGGTCATCCATGACAACTb | 91 |
Labeled at the 5′ end with 6-carboxyfluorescein and terminally quenched at the 3′ end with Black Hole Quencher-1.
Labeled at the 5′ end with HEX (6-carboxy-2′,4,4′,5′,7,7′-hexachlorofluorescein) and terminally quenched at the 3′ end with Black Hole Quencher-1.
Western blot analysis.
Microglia (5 × 105/ml) and astrocytes (5 × 105/ml) were incubated with glial culture medium alone or with added, live B. burgdorferi cells (MOI of 10), 0.25 μg/ml L-OspA, or 100 ng/ml FliC for 24 h. Cells were lysed in radioimmunoprecipitation assay buffer with Protease inhibitor cocktail (Pierce Biotechnology). Protein concentration was determined with the bicinchoninic acid protein assay (Pierce Biotechnology). Equal amounts of sample (25 μg/lane) were separated in 12% acrylamide Tris-HCl precast gels (Bio-Rad), transferred to nitrocellulose Protran membrane (Schleicher and Schuell BioScience), and blocked in phosphate-buffered saline (PBS) with 0.05% Tween 20 with 3% bovine serum albumin (BSA) fraction V (Sigma-Aldrich). Membranes were probed with 2 μg/ml of rabbit polyclonal primary antibody against human TLR1, TLR2, or TLR5 (Santa Cruz Biotechnology) and 1 μg/ml of rabbit polyclonal primary antibody to β-actin (ABCAM), followed by incubation with the appropriate secondary antibody (Santa Cruz Biotechnology) conjugated with horseradish peroxidase. Immunoreactive proteins were visualized by using 3,3′-diaminobenzidine as a chromogen.
Flow cytometry.
Mixed glial cells (1 × 107 cells) were incubated with live B. burgdorferi cells (MOI of 10) or 0.25 μg/ml L-OspA for 4 and 24 h. After stimulation, glial cells were washed with ice-cold staining buffer (PBS with 2% fetal bovine serum, 2 mM EDTA, and 0.05% sodium azide). The cell suspensions were filtered with a cell strainer (40-μm pore [BD Falcon]) and then preincubated for 20 min with 0.24 mg/ml of normal mouse immunoglobulin G (IgG) (Invitrogen) at 4°C, to block nonspecific binding. Cells were then incubated with fluorescein isothiocyanate (FITC)-conjugated mouse monoclonal (IgG1) anti-monkey CD45 (Miltenyi Biotec), phycoerythrin-conjugated mouse monoclonal (IgG2a) anti-human TLR2 (Santa Cruz Biotechnology), and allophycocyanin-Cy7-conjugated mouse monoclonal (IgG1) anti-human CD11b (BD Bioscience) for 20 min at 4°C. After washing with staining buffer, the cells were fixed with 1× BD stabilizing fixative solution (BD Biosciences). A phycoerythrin-conjugated isotype-matched control (Santa Cruz Biotechnology) was used as a negative control for TLR2 expression. Samples were analyzed on an LSRII flow cytometer (Becton Dickinson), and data analyses were performed using FlowJo version 6 (Tree Star).
Immunofluorescence assays.
After 4, 12, or 24 h of incubation with TLR ligands, live or sonicated B. burgdorferi cells, or FluoSpheres yellow-green carboxylate-modified microspheres (0.5 μm, 1:10 cell/bead ratio) (Molecular Probes), microglia (1 × 104/ml) and astrocytes (5 × 104/ml) were fixed with 2% p-formaldehyde for 10 min and rinsed with PBS. Permeabilization and blocking were performed with 0.1% Triton X-100-PBS-0.2% fish skin gelatin for 30 min, followed by additional blocking incubation with 10% goat serum-PBS-0.2% fish skin gelatin for 1 h. The cells were then incubated with 5 μg/ml of FITC-conjugated anti-Borrelia species (BacTrace), 1 μg/ml of mouse monoclonal (IgG2a) anti-human IL-6 (ProSpec), 2 μg/ml of rabbit polyclonal anti-human TLR1, TLR2, and TLR5 (Santa Cruz Biotechnology), or rabbit polyclonal anti-TLR4 (ABCAM) as primary antibodies. To identify astrocytes and microglia, a 1/300 dilution of mouse monoclonal (IgG1) anti-human glial fibrillary acidic protein (anti-GFAP) antibody (Sigma-Aldrich) and 2 μg/ml of chicken polyclonal anti-human IBA1 antibody (Aves Labs Inc.), respectively, were used. This procedure was followed by staining with secondary antibody conjugated to Alexa 488-FITC (green), Alexa 633 (far red), or Alexa 568 (red) (Molecular Probes). In each case, a negative control experiment was performed to exclude nonspecific staining. To differentiate among individual cells, we used ToPro3 and BoPro1 nuclear markers (Molecular Probes). Samples were analyzed on a Leica TCS SP2 confocal microscope equipped with three lasers (Leica Microsystem, Exton, PA). NIH Image (version 1.62) and Adobe Photoshop were used to assign colors to the four channels collected.
Measurement of cytokine and chemokine concentrations.
Microglia (1 × 104/ml) and astrocytes (5 × 104/ml) were incubated in glial culture medium alone or with added live B. burgdorferi cells (MOI of 10), sonicated B. burgdorferi cells (MOI of 10), 0.25 μg/ml L-OspA, 0.25 μg/ml recombinant unlipidated outer surface protein A (U-OspA) (GlaxoSmithKline), 12.5 ng/ml tripalmitoyl-S-glyceryl-Cys-Ser-Lys4-OH lipohexapeptide (Pam3Cys) (Boehringer Mannheim), 1.0 μM CpG ODN M362, 1.0 μM unmethylated GpC ODN M362 as a negative control (Invivogen), and 10 ng/ml or 100 ng/ml FliC, for a period of 2, 8, 12, or 24 h, after which cell-free culture supernatants were collected and assayed for the presence of tumor necrosis factor alpha (TNF-α), IL-6, and IL-8 by sandwich enzyme-linked immunosorbent assay (ELISA) using capture and detection monoclonal antibody pairs (BD Bioscience), and CXCL13, CCL3, and CCL4 using sandwich ELISA DuoSet kit (R&D Systems) according to the manufacturers' instructions.
Intracellular cytokine staining.
Microglia (1 × 104/ml) were incubated in glial culture medium alone or with added live B. burgdorferi (MOI of 10) for a period of 12 h. Brefeldin A (Invitrogen) was added directly to the culture medium at a concentration of 5.0 μg/ml, and cells were incubated at 37°C for 2.5 h. Following treatment, cells were used for immunofluorescence analysis.
Determination of endotoxin contamination.
All of the TLR ligands were verified to have endotoxin levels of <0.03 endotoxin units/ml as determined by the Limulus amebocyte lysate assay (Associates of Cape Cod).
Statistical analysis.
Results are presented as means ± standard deviations of the number of determinations specified in each case. Cytokine concentrations were examined by one-way analysis of variance, using GraphPad PRIM 3.0 (GraphPad Software). For qRT-PCR, the relative expression ratio of each gene was calculated by Relative Expression Software Tool (REST) (http://www.wzw.tum.de/gene-quantification/). Differences were considered significant at P < 0.05.
RESULTS
Transcriptional regulation of TLRs in microglia and astrocytes.
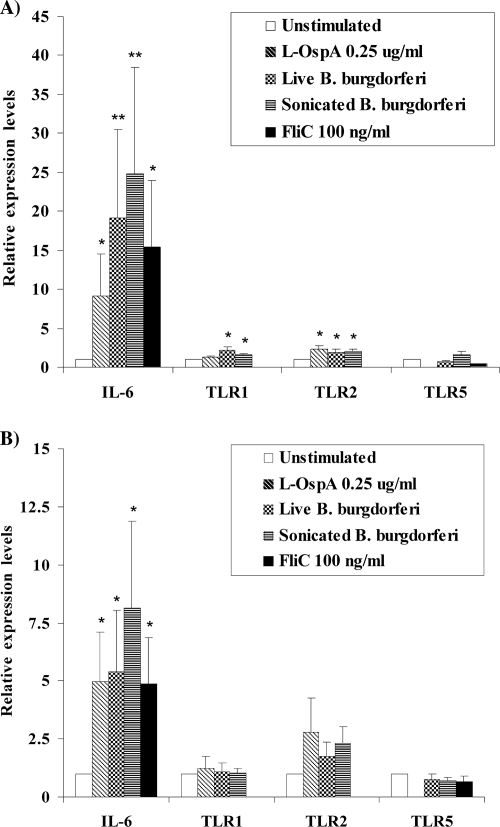
Transcripts of TLR1, -2, -5, and -9 were constitutively expressed in both microglia and astrocytes; however, the expression of TLR9 transcripts was found to be low (mean CT, 31.12 ± 0.46) in comparison to the housekeeping gene (mean CT, 16.4 ± 0.89). Previous studies had indicated that in vitro stimulation with a ligand for TLR2 (27) or with B. burgdorferi whole-cell extracts (39) leads to upregulation of TLR2 transcript (39) or both transcript and protein (27) in human (27) and murine (39) microglia. Murine astrocytes also have been shown to respond to specific ligands for TLR1, -2, -4, -5, and -9 by substantially elevating the level of TLR expression (3, 8). Here we investigated the expression of individual TLRs after 2 and 8 h of stimulation with live and sonicated B. burgdorferi cells, L-OspA, CpG ODN M362, and FliC. We observed a significant upregulation of TLR2 expression in microglia stimulated for 8 h with L-OspA and live or sonicated B. burgdorferi cells (Fig. 1A). In contrast, no significant difference was detected in astrocytes (Fig. 1B). The expression of TLR5 and -9 transcripts in both cell types remained unchanged after stimulation, and the upregulation in TLR1 transcript was restricted to microglia after stimulation with live and sonicated B. burgdorferi cells (Fig. 1A).
FIG. 1.
Relative expression of IL-6 and TLR1, -2 and -5 transcripts in microglia and astrocytes in response to specific TLR ligands and B. burgdorferi. Microglia (A) and astrocytes (B) were treated with L-OspA, live and sonicated B. burgdorferi cells (MOI of 10), and FliC for 8 h. The expression of each TLR transcript was determined using qRT-PCR, and each transcript was normalized with respect to the expression of GAPDH. Presented are the mean values obtained from triplicate specimens ± standard deviations. Asterisks indicate a significant difference between unstimulated and stimulated cells: * and **, P < 0.05 and P < 0.005, respectively. Similar results were obtained with microglial RNA obtained from three additional rhesus monkeys and astrocyte RNA from four additional animals.
In addition, we quantified the IL-6 transcript in astrocytes and microglia incubated for 2 and 8 h with L-OspA, FliC, and live and sonicated B. burgdorferi cells. The ability of B. burgdorferi to induce IL-6 mRNA expression has been previously described in primary murine microglia and astrocytes (39, 40). As shown in Fig. 1A and B, both cell types showed a significant increase of IL-6 transcript expression in response to all stimuli.
TLR protein expression in microglia and astrocytes.
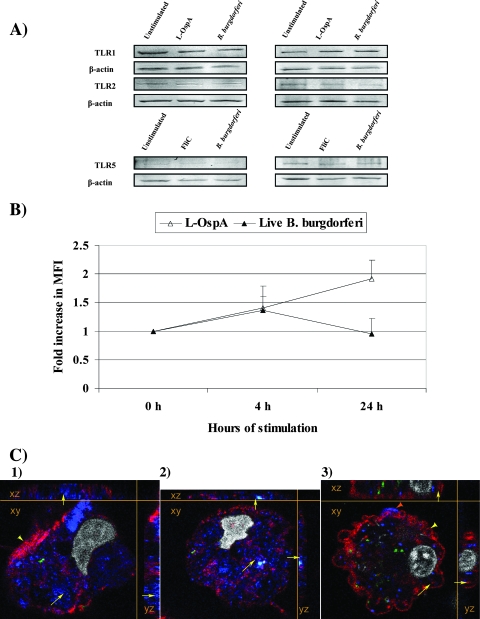
Both microglia and astrocytes showed constitutive expression of TLR1, -2, and -5 as assessed by Western blotting. No detectable upregulation of any of these proteins was observed, regardless of whether the cells were stimulated with L-OspA, flagellin, or live B. burgdorferi cells (Fig. 2A).
FIG. 2.
(A) Western blot analysis of TLR1, TLR2, and TLR5 protein expression in microglia and astrocytes. Microglia (left panels) and astrocytes (right panels) either were left unstimulated or were stimulated with L-OspA (0.25 μg/ml), FliC (100 ng/ml), or live B. burgdorferi cells (MOI of 10) for 24 h. (B) Extracellular expression of TLR2 on activated microglia in response to L-OspA and live B. burgdorferi cells. Cells were stimulated with L-OspA (0.25 μg/ml) and B. burgdorferi (MOI of 10) for 4 and 24 h and evaluated by flow cytometry. The MFI values showed upregulation of TLR2 expression on cells stimulated with L-OspA at 4 and 24 h. An increase of TLR2 expression was observed at 4 h of stimulation with B. burgdorferi, followed by a decrease of expression at 24 h. Data are expressed as means ± standard deviations of three independent experiments (different animals). (C) Surface and intracellular expression of TLR1, TLR2, and TLR5 in microglia. Multilabel images show surface expression of TLR1 (Alexa 568, red [yellow arrowhead]) and intracellular expression of TLR2 (Alexa 633, blue [yellow arrows]) (panel 1); intracellular expression of TLR1 (blue [yellow arrows]), with TLR5 also shown, in red (panel 2); and intracellular expression of TLR5 (red [yellow arrows]) and surface expression of both TLR5 (yellow arrowhead) and TLR2 (blue, orange arrowhead) during phagocytosis of live B. burgdorferi cells (labeled with FITC, green) (panel 3). Cell nuclei were labeled with BoPro1 (gray). The images show a pseudo-three-dimensional representation, with the XY plane in the center, and the XZ and YZ planes on the sides. Similar results were obtained with cells from two additional rhesus monkeys.
In an attempt to better correlate upregulated levels of mRNA encoding TLR2 with increased receptor protein expression in microglia, we performed flow cytometric analysis using preparations of mixed glial cells. Resting microglia are known to express low levels of CD45 and CD11b, while activated microglia gradually increase the expression of both markers (16, 45). Therefore, we used CD45 and CD11b to specifically gate microglia and exclude other cells lacking these markers, such as astrocytes, oligodendrocytes, and neurons (16). CD45+/CD11b+ unstimulated and stimulated mixed glial cells were then colabeled with anti-TLR2 antibody, as described in Materials and Methods. We observed that TLR2 expression (mean fluorescence intensity [MFI]) was upregulated after 4 and 24 h of stimulation with L-OspA and at 4 h with live B. burgdorferi cells (Fig. 2B). TLR2 expression levels were decreased at 24 h of stimulation with B. burgdorferi, perhaps due to the progressive internalization of TLR2.
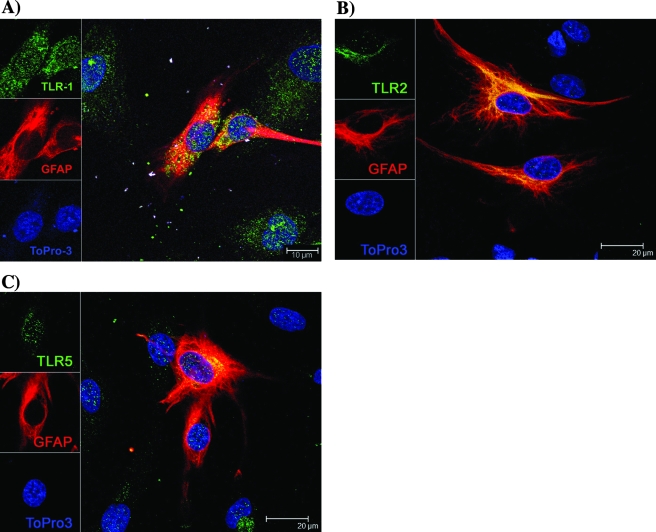
Confocal microscopy was used to further verify the expression of TLR1, -2, and -5 proteins on microglia and astrocytes and to assess their localization in microglia. At 24 h poststimulation with live B. burgdorferi cells, expression of TLR1, TLR2, and TLR5 was detected, respectively, in 6.5%, 9.3%, and 7.1% of total microglia. Activated/phagocytic microglia did simultaneously express TLR1, -2, and -5, and these receptors were shown to be localized on the cell surface and/or internalized (Fig. 2C). Interestingly, these TLRs were upregulated only in microglia that also contained spirochetes or spirochetal fragments. Microglia that had not internalized spirochetal components expressed much lower levels of TLR1 and -5 and no detectable levels of TLR2 (please see Fig. 5A, C, and B, respectively). Astrocytes expressed TLR1, -2, and -5 proteins constitutively (Fig. 3); no differences in the expression levels of these receptors were observed between unstimulated and stimulated cells (data not shown).
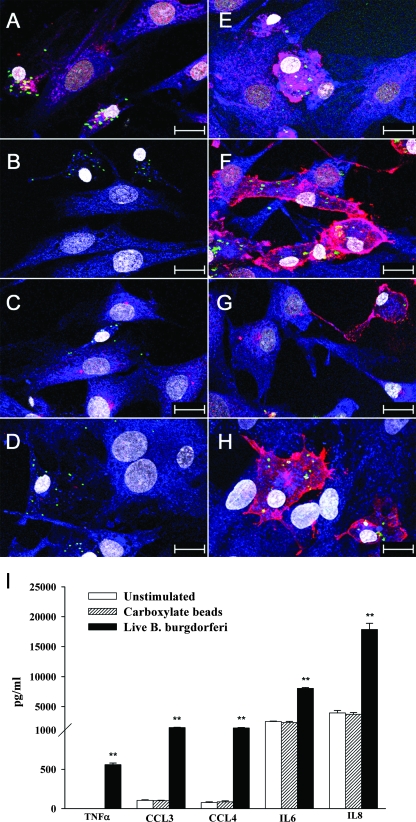
FIG. 5.
Internalization of B. burgdorferi correlates with the enhancement of TLR expression. Microglia were incubated for 12 h with B. burgdorferi cells or carboxylate-modified microspheres. Confocal microscopy images show the expression of TLR1 (A and E), TLR2 (B and F), TLR5 (C and G), and TLR4 (D and H) after stimulation with B. burgdorferi (E, F, G, and H) or carboxylate beads (A, B, C, and D). Antibody to B. burgdorferi was labeled with FITC (green) and 488-nm FluoSpheres (yellow green), TLR1, -2, -5, and -4 were labeled with Alexa 568 (red), IBA1 was labeled with Alexa 633 (blue), and cell nuclei were labeled with BoPro1 (gray). Bars represent 20 μm. (I) TNF-α, IL-6, IL-8, CCL3, and CCL4 production by microglia in response to B. burgdorferi or carboxylate beads. Values are means (n = 3) ± standard deviations. Asterisks (**, P < 0.005) indicate a significant difference between unstimulated and stimulated cells. ELISA results were similar with specimens from three additional animals.
FIG. 3.
Expression of TLR1, -2, and -5 by astrocytes. Confocal microscopy shows that astrocytes constitutively express TLR1 (A), TLR2 (B), and TLR5 (C). Antibody to GFAP was labeled with Alexa 568 (red); TLR1, -2, and -5 were labeled with Alexa 488 (green); and cell nuclei were labeled with ToPro3 (blue). Similar results were obtained with astrocytes isolated from two additional rhesus monkeys.
Inflammatory cytokines and chemokine production by primary microglia and astrocytes upon stimulation with TLR ligands and B. burgdorferi.
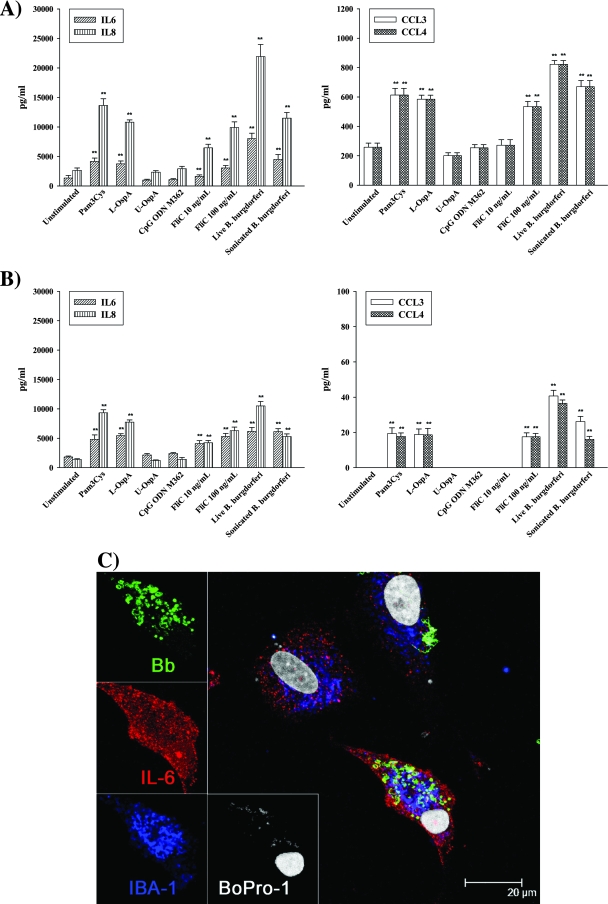
In order to characterize the role of different TLR ligands and B. burgdorferi in the release of the proinflammatory cytokines IL-6 and TNF-α and the chemokines IL-8, CXCL13, CCL3, and CCL4 by primary microglia and astrocytes, specific ELISAs were performed as described in Materials and Methods. Production of all mediators was highest at 24 h, except for TNF-α (see below). Microglia constitutively secreted IL-6, IL-8, CCL3, and CCL4 and significantly enhanced the production of these mediators upon incubation with live and sonicated B. burgdorferi cells and TLR1/2 and TLR5 ligands (Fig. 4A). Similar results were observed for astrocytes, related to the production of IL-6 and IL-8 (Fig. 4B). However, the amounts of CCL3 and CCL4 secreted by astrocytes were smaller than with microglia. There was no significant change in levels of IL-6, IL-8, CCL3, and CCL4 production in both cell types when these were stimulated with CpG ODN, the TLR9 ligand. The secretion of TNF-α by microglia was increased earlier, at 2 to 12 h poststimulation (Fig. 5I). No detectable TNF-α concentrations were measured in unstimulated or stimulated astrocytes. CXCL13 production was undetectable in both cell types.
FIG. 4.
Secretion of proinflammatory mediators by microglia and astrocytes. Concentrations of IL-6, IL-8, CCL3, and CCL4 were determined after 24-h incubations of microglia (A) and astrocytes (B) with no stimulant, 0.25 μg/ml L-OspA, 0.25 μg/ml U-OspA, 12.5 ng/ml Pam3Cys, 1.0 μM CpG ODN M362, 10 or 100 ng/ml FliC, and live and sonicated B. burgdorferi cells (MOI of 10). Shown are the mean values ± standard deviations obtained from triplicate specimens. Asterisks (**, P < 0.005) indicate significant differences between unstimulated and stimulated cells. (C) Multilabel confocal microscopy showing increased cytoplasmic expression of IL-6 in phagocytic microglia after a 12-h stimulation with live B. burgdorferi cells, followed by 2.5 h of incubation with brefeldin A. Antibody to B. burgdorferi (Bb) was labeled with FITC (green), antibody to IL-6 was labeled with Alexa 568 (red), antibody to IBA1 was labeled with Alexa 633 (blue), and cell nuclei were labeled with BoPro1 (gray). Similar results were obtained with supernatants obtained from cells of three additional rhesus macaques.
Confocal microscopy was used to visualize cytoplasmic expression of IL-6 in microglia. Cells were stimulated with live B. burgdorferi cells followed by treatment with brefeldin A. Brefeldin A is a fungal metabolite that inhibits the export of proteins from the Golgi network (41). A more intense accumulation of IL-6 was detected only within activated/phagocytic microglia (Fig. 4C).
Phagocytosis of B. burgdorferi by primary microglia induces expression of TLRs and may amplify the release of inflammatory mediators.
The observation that phagocytosis of B. burgdorferi by microglia appeared to correlate with expression of TLR1, -2, and -5 (Fig. 2C) prompted us to evaluate more thoroughly this phenomenon. Microglia were incubated for 12 h with either live B. burgdorferi cells or with carboxylate-modified microspheres. Images obtained by confocal microscopy revealed that while phagocytosis of the beads did not induce upregulation of TLR1, -2, or -5 expression (Fig. 5A, B, and C), uptake of B. burgdorferi by microglia ostensibly increased the expression of these TLRs (Fig. 5E, F, and G). Surprisingly, uptake of B. burgdorferi, but not of beads, also correlated with elevated expression of TLR4 (Fig. 5D and H). In addition, the production of proinflammatory mediators, such as TNF-α, IL-6, IL-8, CCL3, and, CCL4, was significantly elevated in cells that had taken up or had been exposed to B. burgdorferi compared to unstimulated cells or cells that were exposed to or had incorporated carboxylate beads (Fig. 5I). To further our insight into TLR4 expression by phagocytic microglia, we quantified the TLR4 transcript in these cells after 8, 12, and 24 h of coincubation with live B. burgdorferi cells or in medium alone. Microglia constitutively expressed TLR4 mRNA, but the expression of the latter was not upregulated by coincubation with spirochetes at any of the time points.
DISCUSSION
We had hypothesized that inflammatory responses elicited by B. burgdorferi itself or by antigens from dead spirochetes left in the tissues are a key factor in the pathogenesis of Lyme neuroborreliosis (38). For this study, we focused on the role of TLRs from glial cells in mediating an innate inflammatory response to B. burgdorferi in vitro. We argued that TLR1/2, -5, and -9, because of their PAMP specificities, may be involved in the response to B. burgdorferi. TLR2 was included because of the abundance of lipoprotein open reading frames in the B. burgdorferi genome (17) and the already demonstrated role of these molecules in causing inflammation in experimental Lyme borreliosis (13, 32). TLR1 was chosen as it is bound together with TLR2 by triacylated lipoproteins (49), which is the type of lipoprotein expressed by B. burgdorferi (30). Since B. burgdorferi is flagellated, TLR5 could be bound by spirochetal flagellin that was exposed from a disrupted periplasmic space of fragmented spirochetes or from spirochetes taken up by microglia; TLR9 could be elicited in response to bacterial CpG DNA.
We first tested our hypothesis by assessing TLR transcript upregulation in glial cells stimulated with live or fragmented (sonicated) B. burgdorferi cells. While all of the TLR transcripts analyzed were expressed constitutively, only TLR1 and -2 from microglia were significantly upregulated by spirochetes or spirochetal antigens. This finding indicated that both TLR1 and -2 are involved in the response of glial cells to live B. burgdorferi cells. The relatively subdued or absent upregulation of primate TLR transcripts in response to TLR ligands or B. burgdorferi contrasts with that observed in murine glial cells (39) and is perhaps the consequence of differences between these animal species or in the dose of stimulants used. Induction of TLR1 and TLR2 transcripts had been shown previously in human microglia that were stimulated with L-OspA and whole-cell extracts of B. burgdorferi, but stimulation with live spirochetes was not performed (9). Both microglia and astrocytes significantly increased the expression of IL-6 transcript in response not only to B. burgdorferi but also to TLR1/2 and TLR5 ligands. This indicates that these receptors are functionally expressed not only by microglia but also by astrocytes in the primary cultures we utilized in our experiments.
The constitutive expression of TLR1, -2, and -5 proteins by microglia and astrocytes was evaluated and confirmed by Western blot analysis. Both types of glial cells expressed these receptor molecules. However, the Western blot technique was apparently not sensitive enough to detect the translation into protein of the slight TLR1 and -2 transcript upregulation in microglia. TLR2 protein upregulation was confirmed by flow cytometry upon cell stimulation with both B. burgdorferi and L-OspA. Interestingly, TLR2 expression levels began to decrease by 24 h of stimulation with B. burgdorferi. Previous studies had demonstrated that TLR2 is distributed on the cell surface of macrophages; however, after exposure to pathogens or their different PAMPs, TLR2 can be internalized (51) and/or recruited to the membranes of nascent phagosomes (52). Therefore, the decrease in TLR2 surface expression we observed could be due to the progressive internalization of this receptor in response to B. burgdorferi.
Intracellular (and surface) expression of TLR1, -2, and -5 by microglia was confirmed by confocal microscopy. In microglia, expression of TLR was observed in the fraction of cells whose morphology evinced cellular activation; these cells also exhibited internalized B. burgdorferi antigenic material. The internalization of B. burgdorferi by human glial and monocytic cells has been previously reported (10, 29, 31). Here we show that uptake of B. burgdorferi is concomitant with microglial activation and enhanced expression of TLR1, -2, and -5. However, only a small proportion of microglia was involved in phagocytosis (up to 10%) and thus had enhanced TLR expression, a fraction perhaps not detectable by Western blotting. The increased TLR expression must have involved recognition of B. burgdorferi, by TLR and/or other receptors, in a specific manner, as uptake of carboxylate beads had no effect on TLR expression or release of inflammatory mediators.
While the result of this experiment attests to the involvement of TLR1, -2, and -5 in glial responses to B. burgdorferi, as per our hypothesis, it also entails that this hypothesis needs to be revised, to include the expression of TLR4 in phagocytic microglia. This observation takes on additional importance due to the fact that B. burgdorferi does not contain lipopolysaccharide (46), the ligand of TLR4 and otherwise a major component of the outer membrane of gram-negative bacteria. Several investigators have shown that stimulation of a particular TLR can influence the expression of other TLRs (15, 50, 53). Therefore, we propose that during B. burgdorferi infection, stimulation via TLR1/2 and/or TLR5 may modify the expression of TLR4. Another possibility is that phagocytic receptors, such as C-type lectin, and scavenger receptors may also play a role in modulation and activation of TLR signaling. As with other TLRs, the TLR4 transcript was expressed constitutively by unstimulated microglia. However, no upregulation of this transcript was detected upon coincubation of the cells with live spirochetes, perhaps due to the small proportion of phagocytic microglia in our preparations. Alternatively, it is possible that expression of some TLRs in phagocytic microglia is regulated posttranscriptionally.
As for the functional involvement of glial TLR1, -2, -5, and -9 in the release of inflammatory mediators and thus, possibly, in the pathogenesis of Lyme neuroborreliosis, our data document that primary cultures of rhesus astrocytes and microglia release proinflammatory cytokines and chemokines in response to ligands of TLR1, -2, and -5, but not TLR9. Different types of CpG ODNs (type A, B, and C) are known to induce distinct immune responses. Our results showed that microglia and astrocytes express low levels of TLR9 mRNA, and no transcript upregulation after stimulation with CpG, live, or sonicated B. burgdorferi. In addition, CpG ODN 362 (type C) did not induce the production of cytokines and chemokines in either cell type. To confirm our results, we performed similar stimulations with CpG ODN 2006 (type B) and CpG ODN 2216 (type A). Although CpG ODN 2006 has been shown to elicit B-cell proliferation in nonhuman primates (21), no response was detected in either microglia or astrocytes after stimulation with this specific CpG ODN. Thus, it is likely that CpG ODNs do not stimulate TLR9-dependent NF-κB signaling in rhesus primary glial cells. This data suggests that TLR9 is not involved in the response of glial cells to B. burgdorferi and thus plays no role in the pathogenesis of Lyme neuroborreliosis.
Recognition of flagellin, the major constituent of bacterial flagella, via TLR5 can lead to the activation of the transcription factor NF-κB, resulting in the production of inflammatory mediators. Human astrocytes and microglia have been reported to express TLR5 (27). Our results indicate that rhesus microglia and astrocytes not only express TLR5 but also respond upon stimulation with TLR5 ligand, releasing cytokines and chemokines. Based on the data that we obtained from confocal microscopy, we speculate that these cells may also respond to B. burgdorferi through TLR5.
Most inflammatory mediators can be rapidly induced in response to CNS injury or infection. TNF-α is one of the central mediators of CNS inflammation, and IL-6 appears to be upregulated in most CNS diseases, including Lyme neuroborreliosis (34). The chemokines IL-8, CCL3, and CCL4 play important roles in sustaining inflammation and promoting recruitment of inflammatory cells in the CNS (19). In addition, high levels of CXCL13, a B-cell-attracting chemokine, have been found in the cerebrospinal fluid of Lyme neuroborreliosis patients (43) and in the tissues of nonhuman primates infected with B. burgdorferi (36). Ligands to TLR1, -2, and -5, as well as B. burgdorferi, were able to elicit sizeable amounts of IL-6 and IL-8 in both microglia and astrocytes. Microglia were able to produce TNF-α, mostly early in the stimulation process. Production of this cytokine from astrocyte cultures was not detected.
We had previously reported production of TNF-α by astrocytes stimulated with L-OspA (38). In the procedure to purify astrocytes that we utilized herein, we included an additional step, incubation with LME, to more fully remove microglia (20). While we also took care to assess microglial contamination in our previous work, it is possible, as we argued then (38), that microglia present in the astrocyte cultures (untreated with LME) may have contributed to eliciting the phenomena we observed, including the production of TNF-α.
Production of CCL3 and CCL4 was more pronounced in microglia, with astrocytes releasing about 3 to 5% of the concentration of these chemokines produced by microglia in 24 h. It is possible that astrocytes did not produce CCL3 and CCL4, and the concentrations observed with the stimulants we used may have been due to a residual microglial contamination. No CXCL13 production was detected, which is perhaps an indication that glial cells are either not the source of this chemokine or that additional mediators, available in vivo, are required to elicit CXCL13 production by microglia and/or astrocytes when these cells are exposed to B. burgdorferi.
Taken together, our data provide proof of the concept that astrocyte and microglial TLR1, -2, and -5 are involved in the response of primate glial cells to B. burgdorferi. While B. burgdorferi spirochetes localize primarily to the meninges in the CNS of rhesus macaques, and probably in humans as well, they are also able to migrate to the CNS parenchyma. In a study designed to investigate spirochetal localization in the rhesus CNS and in other organs, spirochetes were detected immunohistochemically chiefly in the leptomeninges, nerve roots, and dorsal root ganglia. By PCR ELISA targeting the OspA gene, a wider distribution of CNS tissue tropisms was determined. Spirochetal DNA was detected in the cerebrum, brainstem and cerebellum, spinal cord, and dura mater, not only in immunosuppressed animals but also in immunocompetent rhesus monkeys, albeit in much smaller amounts (5). Inflammation related to the presence of B. burgdorferi in the tissues occurs in the rhesus monkey CNS not only in the meninges, with the associated pleocytosis, but also in the brain, albeit at a lower frequency than in organs such as skeletal (6) and cardiac (7, 35) muscles. Thus, localization of B. burgdorferi to the CNS parenchyma, while likely a rare event, does occur. The proinflammatory molecules elicited by TLR-mediated responses in glial cells could thus be a factor in the pathogenesis of Lyme neuroborreliosis.
Acknowledgments
We thank Vida Dennis, Monica Embers, Ramesh Ramamoorthy, Aarti Gautam, Mary Jacobs, and Peter Mottram for advice.
This work was supported by grants NS048952 and RR00164 from the National Institutes of Health and UO1-CI000153 from the Centers for Disease Control and Prevention.
We do not have a commercial or other association that might pose a conflict of interest.
Editor: A. J. Bäumler
Footnotes
Published ahead of print on 11 August 2008.
REFERENCES
- 1.Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2675-680. [DOI] [PubMed] [Google Scholar]
- 2.Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413732-738. [DOI] [PubMed] [Google Scholar]
- 3.Bowman, C. C., A. Rasley, S. L. Tranguch, and I. Marriott. 2003. Cultured astrocytes express toll-like receptors for bacterial products. Glia 43281-291. [DOI] [PubMed] [Google Scholar]
- 4.Bsibsi, M., R. Ravid, D. Gveric, and J. M. van Noort. 2002. Broad expression of Toll-like receptors in the human central nervous system. J. Neuropathol. Exp. Neurol. 611013-1021. [DOI] [PubMed] [Google Scholar]
- 5.Cadavid, D., T. O'Neill, H. Schaefer, and A. R. Pachner. 2000. Localization of Borrelia burgdorferi in the nervous system and other organs in a nonhuman primate model of Lyme disease. Lab. Investig. 801043-1054. [DOI] [PubMed] [Google Scholar]
- 6.Cadavid, D., Y. Bai, D. Dail, M. Hurd, K. Narayan, E. Hodzic, S. W. Barthold, and A. R. Pachner. 2003. Infection and inflammation in skeletal muscle from nonhuman primates infected with different genospecies of the Lyme disease spirochete Borrelia burgdorferi. Infect. Immun. 717087-7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cadavid, D., Y. Bai, E. Hodzic, K. Narayan, S. W. Barthold, and A. R. Pachner. 2004. Cardiac involvement in non-human primates infected with the Lyme disease spirochete Borrelia burgdorferi. Lab. Investig. 841439-1450. [DOI] [PubMed] [Google Scholar]
- 8.Carpentier, P. A., W. S. Begolka, J. K. Olson, A. Elhofy, W. J. Karpus, and S. D. Miller. 2005. Differential activation of astrocytes by innate and adaptive immune stimuli. Glia 49360-374. [DOI] [PubMed] [Google Scholar]
- 9.Cassiani-Ingoni, R., E. S. Cabral, J. D. Lünemann, Z. Garza, T. Magnus, H. Gelderblom, P. J. Munson, A. Marques, and R. Martin. 2006. Borrelia burgdorferi induces TLR1 and TLR2 in human microglia and peripheral blood monocytes but differentially regulates HLA-class II expression. J. Neuropathol. Exp. Neurol. 65540-548. [DOI] [PubMed] [Google Scholar]
- 10.Cruz, A. R., M. W. Moore, C. J. La Vake, C. H. Eggers, J. C. Salazar, and J. D. Radolf. 2008. Phagocytosis of Borrelia burgdorferi, the Lyme disease spirochete, potentiates innate immune activation and induces apoptosis in human monocytes. Infect. Immun. 7656-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doetsch, F. 2003. The glial identity of neural stem cells. Nat. Neurosci. 61127-1134. [DOI] [PubMed] [Google Scholar]
- 12.Dong, Y., and E. N. Benveniste. 2001. Immune function of astrocytes. Glia 36180-190. [DOI] [PubMed] [Google Scholar]
- 13.Ebnet, K., K. D. Brown, U. K. Siebenlist, M. M. Simon, and S. Shaw. 1997. Borrelia burgdorferi activates nuclear factor-kappa B and is a potent inducer of chemokine and adhesion molecule gene expression in endothelial cells and fibroblasts. J. Immunol. 1583285-3292. [PubMed] [Google Scholar]
- 14.England, J. D., R. P. Bohm, Jr., E. D. Roberts, and M. T. Philipp. 1997. Lyme neuroborreliosis in the rhesus monkey. Semin. Neurol. 1753-56. [DOI] [PubMed] [Google Scholar]
- 15.Fan, J., R. S. Frey, and A. B. Malik. 2003. TLR4 signaling induces TLR2 expression in endothelial cells via neutrophil NADPH oxidase. J. Clin. Investig. 1121136-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ford, A. L., A. L. Goodsall, W. F. Hickey, and J. D. Sedgwick. 1995. Normal adult ramified microglia separated from other central nervous system macrophages by flow cytometry sorting. Phenotypic differences defined and direct ex vivo antigen presentation to myelin basic protein-reactive CD4+ T cells compared. J. Immunol. 1544309-4321. [PubMed] [Google Scholar]
- 17.Fraser, C. M., S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J. F. Tomb, R. D. Fleischmann, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. van Vugt, N. Palmer, M. D. Adams, J. Gocayne, J. Weidman, T. Utterback, L. Watthey, L. McDonald, P. Artiach, C. Bowman, S. Garland, C. Fuji, M. D. Cotton, K. Horst, K. Roberts, B. Hatch, H. O. Smith, and J. C. Venter. 1997. Genomic sequence of a Lyme disease spirochete, Borrelia burgdorferi. Nature 390580-586. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez-Scarano, F., and G. Baltuch. 1999. Microglia as mediators of inflammatory and degenerative disease. Annu. Rev. Neurosci. 22219-240. [DOI] [PubMed] [Google Scholar]
- 19.Grygorczuk, S., S. Pancewicz, J. Zajkowska, M. Kondrusik, R. Rwierzbinska, and T. Hermanowska-Szpakowicz. 2004. Concentrations of macrophage inflammatory proteins MIP-1alpha and MIP-1beta and interleukin 8 (IL-8) in Lyme borreliosis. Infection 32350-355. [DOI] [PubMed] [Google Scholar]
- 20.Hamby, M. E., T. F. Uliasz, S. J. Hewett, and J. A. Hewett. 2006. Characterization of an improved procedure for the removal of microglia from confluent monolayers of primary astrocytes. J. Neurosci. Methods 150128-137. [DOI] [PubMed] [Google Scholar]
- 21.Hartmann, G., R. D. Weeratna, Z. K. Ballas, P. Payette, S. Blackwell, I. Suparto, W. L. Rasmussen, M. Waldschmidt, D. Sajuthi, R. H. Purcell, H. L. Davis, and A. M. Krieg. 2000. Delineation of a CpG phosphorothioate oligodeoxynucleotide for activating primate immune responses in vitro and in vivo. J. Immunol. 1641617-1624. [DOI] [PubMed] [Google Scholar]
- 22.Hayashi, F., K. D. Smith, A. Ozinsky, T. R. Hawn, E. C. Yi, D. R. Goodlett, J. K. Eng, S. Akira, D. M. Underhill, and A. Aderem. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 4101099-1103. [DOI] [PubMed] [Google Scholar]
- 23.Heil, F., H. Hemmi, H. Hochrein, F. Ampenberger, C. Kirschning, S. Akira, G. Lipford, H. Wagner, and S. Bauer. 2004. Species-specific recognition of single-stranded RNA via Toll-like receptor 7 and 8. Science 3031526-1529. [DOI] [PubMed] [Google Scholar]
- 24.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408740-745. [DOI] [PubMed] [Google Scholar]
- 25.Hirschfeld, M., C. J. Kirschning, R. Schwandner, H. Wesche, J. H. Weis, R. M. Wooten, and J. J. Weis. 1999. Cutting edge: inflammatory signaling by Borrelia burgdorferi lipoprotein is mediated by Toll-like receptor 2. J. Immunol. 1632382-2386. [PubMed] [Google Scholar]
- 26.Hoshino, K., O. Takeuchi, T. Kawai, H. Sanjo, T. Ogawa, Y. Takeda, K. Takeda, and S. Akira. 1999. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 1623749-3752. [PubMed] [Google Scholar]
- 27.Jack, C. S., N. Arbour, J. Manusow, V. Montgrain, M. Blain, E. McCrea, A. Shapiro, and J. P. Antel. TLR signaling tailors innate immune responses in human microglia and astrocytes. J. Immunol. 1754320-4330. [DOI] [PubMed]
- 28.Jaenson, T. G. 1991. The epidemiology of Lyme borreliosis. Parasitol. Today 739-45. [DOI] [PubMed] [Google Scholar]
- 29.Livengood, J. A., and R. D. Gilmore, Jr. 2006. Invasion of human neuronal and glial cells by an infectious strain of Borrelia burgdorferi. Microbes Infect. 82832-2840. [DOI] [PubMed] [Google Scholar]
- 30.Meng, G., A. Grabiec, M. Vallon, B. Ebe, S. Hampel, W. Bessler, H. Wagner, and C. J. Kirschning. 2003. Cellular recognition of tri-/di-palmitoylate peptides is independent from a domain encompassing the N-terminal seven leucine-rich repeat (LRR)/LRR-like motifs of TLR2. J. Biol. Chem. 27839822-39829. [DOI] [PubMed] [Google Scholar]
- 31.Moore, M. W., A. R. Cruz, C. J. LaVake, A. L. Marzo, C. H. Eggers, J. C. Salazar, and J. D. Radolf. 2007. Phagocytosis of Borrelia burgdorferi and Treponema pallidum potentiates innate immune activation and induces gamma interferon production. Infect. Immun. 752046-2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morrison, T. B., J. H. Weis, and J. J. Weis. 1997. Borrelia burgdorferi outer surface protein A (OspA) activates and primes human neutrophils. J. Immunol. 1584838-4845. [PubMed] [Google Scholar]
- 33.Olson, J. K., and S. D. Miller. 2004. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J. Immunol. 1733916-3924. [DOI] [PubMed] [Google Scholar]
- 34.Pachner, A. R., K. Amemiya, E. Delaney, T. O'Neill, C. A. Hughes, and W. F. Zhang. 1997. Interleukin-6 is expressed at high levels in the CNS in Lyme neuroborreliosis. Neurology 49147-152. [DOI] [PubMed] [Google Scholar]
- 35.Pachner, A. R., D. Cadavid, G. Shu, D. Dail, S. Pachner, E. Hodzic, and S. W. Barthold. 2001. Central and peripheral nervous system infection, immunity, and inflammation in the NHP model of Lyme borreliosis. Ann. Neurol. 50300-308. [PubMed] [Google Scholar]
- 36.Pachner, A. R., D. Dail, K. Narayan, K. Dutta, and D. Cadavid. 2002. Increased expression of B-lymphocyte chemoattractant, but not pro-inflammatory cytokines, in muscle tissue in rhesus chronic Lyme borreliosis. Cytokine 19297-307. [DOI] [PubMed] [Google Scholar]
- 37.Pachner, A. R., E. Delaney, and E. T. O'Neill. 1995. Neuroborreliosis in the nonhuman primates: Borrelia burgdorferi persists in the central nervous system. Ann. Neurol. 38667-669. [DOI] [PubMed] [Google Scholar]
- 38.Ramesh, G., A. L. Alvarez, E. D. Roberts, V. A. Dennis, B. L. Lasater, X. Alvarez, and M. T. Philipp. 2003. Pathogenesis of Lyme neuroborreliosis: Borrelia burgdorferi lipoproteins induce both proliferation and apoptosis in rhesus monkey astrocytes. Eur. J. Immunol. 332539-2550. [DOI] [PubMed] [Google Scholar]
- 39.Rasley, A., J. Anguita, and I. Marriott. 2002. Borrelia burgdorferi induces inflammatory mediator production by murine microglia. J. Neuroimmunol. 13022-23. [DOI] [PubMed] [Google Scholar]
- 40.Rasley, A., S. L. Tranguch, D. M. Rati, and I. Marriott. 2006. Murine glia express the immunosuppressive cytokine, interleukin-10, following exposure to Borrelia burgdorferi or Neisseria meningitidis. Glia 53583-592. [DOI] [PubMed] [Google Scholar]
- 41.Reaves, B., and G. Banting. 1992. Perturbation of the morphology of the trans-Golgi network following brefeldin A treatment: redistribution of a TGN-specific integral membrane protein, TGN38. J. Cell Biol. 11685-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rothwell, N. J., and G. N. Luheshi. 2003. Interleukin 1 in the brain: biology, pathology and therapeutic target. Trends Neurosci. 23618-625. [DOI] [PubMed] [Google Scholar]
- 43.Rupprecht, T. A., H. W. Pfister, B. Angele, S. Kastenbauer, B. Wilske, and U. Koedel. 2005. The chemokine CXCL13 (BLC): a putative diagnostic marker for neuroborreliosis. Neurology 65448-450. [DOI] [PubMed] [Google Scholar]
- 44.Steere, A. C. 2001. Lyme disease. N. Engl. J. Med. 345115-125. [DOI] [PubMed] [Google Scholar]
- 45.Stevens, S. L., J. Bao, J. Hollis, N. S. Lessov, W. M. Clark, and M. P. Stenzel-Poore. 2002. The use of flow cytometry to evaluate temporal changes in inflammatory cells following focal cerebral ischemia in mice. Brain Res. 932110-119. [DOI] [PubMed] [Google Scholar]
- 46.Takayama, K., R. J. Rothenberg, and A. G. Barbour. 1987. Absence of lipopolysaccharide in the Lyme disease spirochete, Borrelia burgdorferi. Infect. Immun. 552311-2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takeuchi, O., A. Kaufmann, K. Grote, T. Kawai, K. Hoshino, M. Morr, P. F. Mühlradt, and S. Akira. 2000. Cutting edge: preferentially the R-stereoisomer of the mycoplasmal lipopeptide macrophage-activating lipopeptide-2 activates immune cells through a toll-like receptor 2- and MyD88-dependent signaling pathway. J. Immunol. 164554-557. [DOI] [PubMed] [Google Scholar]
- 48.Takeuchi, O., T. Kawai, P. F. Mühlradt, M. Morr, J. D. Radolf, A. Zychlinsky, K. Takeda, and S. Akira. 2001. Discrimination of bacterial lipoprotein by Toll-like receptor 6. Int. Immunol. 13933-940. [DOI] [PubMed] [Google Scholar]
- 49.Takeuchi, O., S. Sato, T. Horiuchi, K. Horiuchi, K. Takeda, Z. Dong, R. L. Modlin, and S. Akira. 2002. Cutting edge: role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J. Immunol. 16910-14. [DOI] [PubMed] [Google Scholar]
- 50.Tötemeyer, S., N. Foster, P. Kaiser, D. J. Maskell, and C. E. Bryant. 2003. Toll-like receptor expression in C3H/HeN and C3H/HeJ mice during Salmonella enterica serovar Typhimurium infection. Infect. Immun. 716653-6657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Triantafilou, M., F. G. Gamper, R. M. Haston, M. A. Mouratis, S. Morath, T. Hartung, and K. Triantafilou. 2006. Membrane sorting of Toll-like receptor (TLR)-2/6 and TLR2/1 heterodimers at the cell surface determines heterotypic associations with CD36 and intracellular targeting. J. Biol. Chem. 28131002-31011. [DOI] [PubMed] [Google Scholar]
- 52.Underhill, D. M., A. Ozinsky, A. M. Hajjar, A. Stevens, C. B. Wilson, M. Bassetti, and A. Aderem. 1999. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 401811-815. [DOI] [PubMed] [Google Scholar]
- 53.van Aubel, R. A., A. M. Keestra, D. J. Krooshoop, W. van Eden, and J. P. van Putten. 2007. Ligand-induced differential cross-regulation of Toll-like receptor 2, 4 and 5 in intestinal epithelial cells. Mol. Immunol. 443702-3714. [DOI] [PubMed] [Google Scholar]