Abstract
Enteroaggregative Escherichia coli (EAEC) adherence to human intestinal tissue is mediated by aggregative adherence fimbriae (AAF); however, the receptors involved in EAEC adherence remain uncharacterized. Adhesion to extracellular matrix proteins is commonly observed among enteric pathogens, so we addressed the hypothesis that EAEC may bind to extracellular matrix proteins commonly found in the intestine. We found that EAEC prototype strain 042 adhered more abundantly to surfaces that were precoated with the extracellular matrix proteins fibronectin, laminin, and type IV collagen. Differences in fibronectin binding of almost 2 orders of magnitude were observed between EAEC 042 and a mutant in the AAF/II major pilin gene, aafA. Purified AafA, refolded as a donor strand complementation construct, bound fibronectin in a dose-dependent manner. Addition of fibronectin to the apical surfaces of polarized T84 cell monolayers augmented EAEC 042 adherence, and this effect required expression of aafA. Finally, increased bacterial adherence was observed when apical secretion of fibronectin was induced by adenosine in polarized T84 cells. Binding to fibronectin may contribute to colonization of the gastrointestinal tract by EAEC.
Enteroaggregative Escherichia coli (EAEC) has been associated with acute diarrheal illness in diverse populations in both developing and industrialized regions (13). The pathogen is defined by its distinctive “stacked-brick” aggregative adherence pattern to semiconfluent HEp-2 cells, including adherence to the cells, other bacteria, and the supporting abiotic substratum (19). The pathogenesis of EAEC diarrhea is thought to comprise colonization of the intestinal mucosa, followed by elaboration of enterotoxins and cytotoxins and release of proinflammatory cytokines from infected epithelial cells (10). Clinically, EAEC infection produces watery diarrhea, occasionally with blood and mucus, and patients typically manifest mild intestinal inflammation (30).
Colonization of the gastrointestinal tract by diarrheagenic E. coli is mediated by fimbrial or afimbrial adhesins (31). The principal adhesins for EAEC are the four types of aggregative adherence fimbriae (AAFs), all encoded by 55- to 65-MDa plasmids (designated pAA) (3, 5, 6, 21). The biogenesis of AAFs employs the chaperone-usher pathway, and the organization of AAF-encoding genes is similar to those of the Afa/Dr family of E. coli adhesins; this organization comprises, in order, chaperone, usher, minor pilin subunit, and major pilin subunit (26). The Afa/Dr adhesins bind to several receptors, including decay-accelerating factor (DAF), as well as extracellular matrix (ECM) proteins, particularly type IV collagen (35).
For prototype EAEC strain 042, adherence to cells and abiotic surfaces requires the AAF pilus variant called AAF/II. The AAF/II organelle comprises two structural subunits: the major subunit, AafA, and the minor subunit, AafB, which is hypothesized but not proven to be located at the pilus tip. AafA is required for adhesion to epithelial cell monolayers, whereas AafB has been associated with the release of cytokines (6, 9, 11). Even though AAF subunits are phylogenetically related to those of the Afa/Dr family, they have not been shown to bind to DAF or, as yet, any other mucosal receptor.
The ECM harbors a diverse set of proteins that function as a barrier and support for epithelial cells and that are responsible for the development, growth, and maintenance of mammalian tissues (4). The composition of the ECM differs among various organs, but fibronectin (Fn), collagen types I to XV, laminin, and glycosaminoglycans, such heparan sulfate and chondroitin sulfate, are common constituents (8). Fn is a glycoprotein composed of two nearly identical 220-kDa disulfide-linked protein subunits and comprises several structurally distinct domains that can bind to cellular constituents, including fibrin, collagens, DNA, gelatin, integrins, heparin, and heparan sulfate (23). Fn and other ECM proteins are commonly recognized by bacterial adhesins (15, 33), and Fn was the first ECM protein shown to act as a receptor for bacterial adherence to eukaryotic cells (17). Although ECM proteins are generally localized to the basement membrane, interaction with bacterial enteric pathogens can occur during inflammation or opening of tight junctions (32). Thereupon, binding to ECM proteins may facilitate colonization, invasion, and/or signaling by intestinal pathogens (7, 16, 33).
In this study, we investigated whether prototype EAEC strain 042 binds ECM components. We observed binding of EAEC to Fn and several other constituents of the ECM. We also report that the presence of Fn augments EAEC adherence to polarized cells in culture, providing a plausible mechanism for the adherence of EAEC in an intestinal biofilm.
MATERIALS AND METHODS
Bacterial strains and reagents.
Prototype EAEC strain 042 (O44:H18) was originally isolated from a child with diarrhea in Lima, Peru. Strains 042 aafA and 042 aafB are EAEC 042 isogenic mutants harboring an insertion of TnphoA into the aafA gene or suicide plasmid pJP5603 into the aafB gene, respectively (6, 9). All bacteria were grown under static conditions overnight in Luria-Bertani (LB) broth with the addition of streptomycin (100 μg/ml) and kanamycin (50 μg/ml) to maintain plasmids when appropriate.
Solid-phase binding assay.
Microtiter plates (Thermo Labsystems, Franklin, MA) were coated with a solution of 25 μg/ml of protein in 100 mM Tris-HCl buffer, pH 8.0, overnight at 4°C. Unbound protein was removed by washing the plates eight times with phosphate-buffered saline (PBS) containing 0.05% Tween and was subsequently blocked with 3% bovine serum albumin (BSA) in PBS for 1 h at room temperature. The blocking buffer was removed, and the wells were washed five times prior to the addition of the bacteria in a 100-μl final volume. Incubation with the bacteria was performed for 3 h at 37°C. The plates were washed, rabbit anti-O44 serum (Denka Seiken Co., Ltd, Tokyo, Japan) diluted 1:200 was added to the wells, and the mixtures were incubated for 2 h at room temperature. Anti-rabbit horseradish peroxidase conjugate (KPL, Gaithersburg, MD) was added following another wash step with PBS containing 0.05% Tween. The peroxidase activity associated with each well was detected by the addition of TMB substrate solution (KPL). Optical densities were read at 450 nm with a 96-well plate reader (Thermo Labsystems). To analyze binding data, the background absorbance from wells to which bacteria were not added was subtracted from the absorbance in the test wells. For AafAdsc (donor strand-complemented AafA) binding, 10 μg/ml of Aafdsc protein was added to ECM protein-coated wells and incubated for 3 h at room temperature; anti-AafA antiserum raised in rabbits (diluted 1:2,000) was used to detect the bound protein. Fn (from human plasma), laminin (Engelbreth-Holm-Swarm murine sarcoma), type IV collagen (human placenta), type I collagen (calf skin), and BSA were purchased from Sigma (St. Louis, MO). Deglycosylation of nondenatured Fn was carried out by digestion at 37°C for 4 days with N-glycosidase F, endo-α-N-acetylgalactosaminidase, α2-3,6,8,9-neuraminidase, β1,4-galactosidase, and β-N-acetylglucosaminidase according to the manufacturer's protocols (Calbiochem, San Diego, CA).
AafAdsc purification.
Monomeric AafAdsc protein was constructed via the donor-strand complementation (dsc) method as previously described (2). The folding and structural characterization of AafAdsc will be described elsewhere. Briefly, an autonomously folding monomeric form of AafA was engineered by deleting the N-terminal donor strand and fusing it to the C terminus, preceded by a 4-residue turn. A synthetic gene encoding this AafAdsc construct was subcloned into the pRSET vector (Invitrogen, Carlsbad, CA) for expression in BL21(DE3) cells and induced with isopropyl-β-d-thiogalactopyranoside (IPTG). Cell pellets were lysed in 20 mM sodium phosphate buffer, pH 7.6, containing 8 M urea, 500 mM NaCl, and 5 mM Tris and purified on Co2+-nitrilotriacetic acid resin. The urea was removed by a linear gradient, and the protein was eluted in buffer containing 20 mM sodium acetate, pH 4.0, with 500 mM NaCl. The soluble but unfolded protein was dialyzed in the presence of dithiothreitol and then in its absence to allow correct refolding prior to the formation of disulfide bonds. The purified, folded AafAdsc was concentrated by polyethylene glycol dialysis.
Quantification of bacterial binding.
The wells of microtiter plates coated with ECM proteins were incubated with 1 ml Dulbecco's modified Eagle's medium (DMEM)/0.5% glucose medium containing ∼1 × 108 bacteria at 37°C for 4 h. After the wells were washed with PBS, the bacterial cells that adhered to the wells were collected by scraping them into PBS with 0.1% (vol/vol) Triton X-100; serial dilutions were plated onto LB agar plates supplemented with streptomycin. The number of adherent bacteria was determined by counting the resulting colonies in duplicate.
Polarized T84 adherence assays.
The T84 adherence assay was performed with T84 cells cultivated to polarization on 12-well polycarbonate Transwell filters with 0.4-μm pores (Corning, Acton, MA) as previously described (11). Prior to bacterial inoculation, monolayers were incubated with DMEM-F12 medium plus 1% methyl-α-d-mannopyranoside for 30 min. Medium was aspirated from the apical compartment, and 100 μl containing ∼1 × 108 CFU/ml bacteria was added to the monolayer. The plates were incubated at 37°C in 5% CO2 for 3 h and then washed five times with PBS. The cells were lysed with a solution of 1% (vol/vol) Triton X-100, and serial dilutions of the lysates were plated on LB agar plates supplemented with streptomycin. The number of adherent bacteria was determined by counting the resulting colonies in duplicate. Apical secretion of Fn was stimulated with adenosine (100 μM; Calbiochem) for 24 h prior to bacterial inoculation as described previously (32).
Immunofluorescence.
Glass coverslips in 24-well plates were coated with Fn as described above. After being washed with PBS, 1 ml of DMEM/0.5% glucose containing ∼1 × 108 bacteria (EAEC 042 and its aafA mutant) was added, and the plate was incubated at 37°C for 4 h. Samples were fixed with paraformaldehyde and double stained with rabbit O44 antiserum and mouse anti-Fn antiserum. Samples were incubated with goat anti-rabbit Alexafluor 488 and goat anti-mouse Alexafluor 568 (Invitrogen), respectively, and then analyzed by confocal microscopy.
Pull-down analysis.
Strain 042 and its aafA mutant were grown in DMEM/0.5% glucose, and approximately 1 × 108 bacteria were collected by centrifugation and washed twice in 0.5 ml of PBS. Nonspecific binding sites were blocked in 0.5 ml PBS containing 3% BSA for 1 h at room temperature. The cells were collected again by centrifugation, resuspended in a solution of 100-μg/ml Fn, and incubated for 3 h at room temperature on a tabletop rotator. Unbound Fn was removed by washing the cells five times in PBS. Cell-associated Fn was detected by separation of whole-cell lysates on 10% sodium dodecyl sulfate-polyacrylamide gels, followed by staining them with Coomassie blue (Bio-Rad, Hercules, CA).
Statistical analysis.
Statistical significance between means was analyzed using the unpaired Student's t test with a threshold P value of <0.05. Values are expressed as the means ± the standard errors of the mean of three experiments.
RESULTS
EAEC 042 binds to Fn, laminin, and type IV collagen.
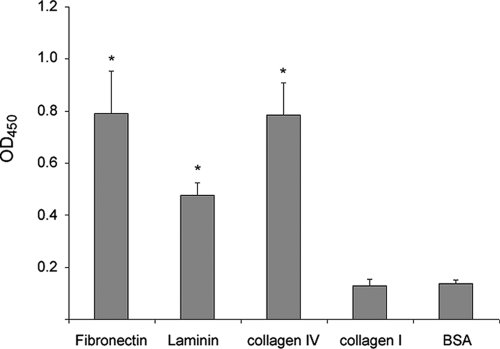
We examined the ability of EAEC 042 to adhere to the major ECM proteins present in the intestinal tract. EAEC 042 was added to 96-well plates previously coated with either Fn, collagen I, collagen IV, laminin, or BSA (employed as a negative control). After 4 h of incubation at 37°C, the wells were washed, and retained bacteria were detected by enzyme-linked immunosorbent assay (ELISA) using a commercial antiserum against the bacterial O antigen (serogroup O44). Compared with BSA, several ECM proteins increased binding of EAEC 042 (Fig. 1); they included Fn, laminin, and type IV collagen. In contrast, binding to type I collagen was similar to that observed in wells coated with BSA.
FIG. 1.
EAEC 042 binds to ECM proteins. EAEC 042 was added to 96-well plates coated separately with 25 μg/ml of each ECM protein, and the binding was detected by ELISA using anti-O44 primary antibodies. The bars represent the means for three experiments, with the error bars indicating 1 standard deviation. *, significantly different from BSA-coated control (P < 0.05). OD450, optical density at 450 nm.
The major subunit of AAF/II fimbriae is required for Fn binding.
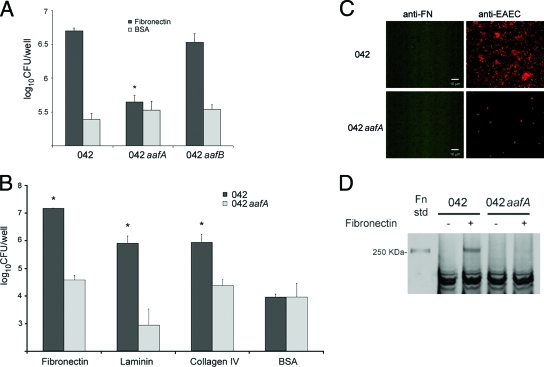
We sought to identify the adherence factor(s) mediating binding of EAEC 042 to ECM proteins. We focused on the major mucosal adhesin of EAEC 042, AAF/II fimbriae. We quantified the adherence of EAEC 042 mutants in the AafA or AafB subunits. 042 aafB exhibited binding to Fn (Fig. 2A), laminin, and type IV collagen similar to that of its wild-type parent (data not shown). In contrast, the aafA mutant bound less to Fn, laminin, and type IV collagen by approximately 2 orders of magnitude (Fig. 2B). The effect was not observed for BSA-coated wells. These observations were confirmed by fluorescence microscopy of fixed samples double stained with anti-O44 antiserum and anti-Fn antibodies (Fig. 2C).
FIG. 2.
Involvement of AAF/II in EAEC binding to ECM proteins. (A) EAEC 042 and its aafA and aafB mutants were added to 24-well plates coated with 25 μg/ml of Fn or BSA, and the bacteria bound were determined by plating. *, significantly different from EAEC 042 and its aafB mutant binding to Fn (P < 0.05). (B) EAEC 042 and its aafA mutant were added to 24-well plates coated with each ECM protein, and the bacteria bound were enumerated by plating. The bars represent the means of three experiments, with the error bars indicating 1 standard deviation. *, significantly different from BSA-coated control and 042 aafA binding (P < 0.05). (C) Immunofluorescence images of EAEC 042 and its aafA mutant binding to Fn after 4 h of incubation at 37°C. Bacterial cells that adhered to wells were visualized by confocal microscopy with rabbit antisomatic O44 antiserum (red signal on right) and mouse anti-Fn antiserum (green signal on left). (D) EAEC 042 or its aafA mutant were incubated either with 3% BSA blocking buffer (−) or with blocking buffer supplemented with 50 μg of Fn (+). After the cells were washed, cell-associated Fn was detected by separation of the whole-cell protein on a 10% polyacrylamide gel. Fn std, Fn standard.
We also tested the ability of anti-AafA antiserum to inhibit Fn binding. EAEC 042 was preincubated with mouse antiserum raised against the purified AafA protein, and bacteria were added to Fn-coated wells. Bacterial binding was significantly reduced when the bacteria were preincubated with anti-AafA antiserum compared with preimmune mouse serum (data not shown).
We utilized a pull-down method to confirm the binding of AAF/II to Fn. Cultures of EAEC 042 or 042 aafA grown under AAF-inducing conditions were incubated with Fn and washed extensively, and then bacteria were pelleted by centrifugation. Lysis of the bacterial cells and subsequent sodium dodecyl sulfate-polyacrylamide gel electrophoresis analyses revealed that only the wild-type bacteria bound Fn (Fig. 2D).
Taken together, these results implicate the AAF/II major subunit in Fn binding of EAEC 042.
Recombinant AafAdsc protein binds to Fn.
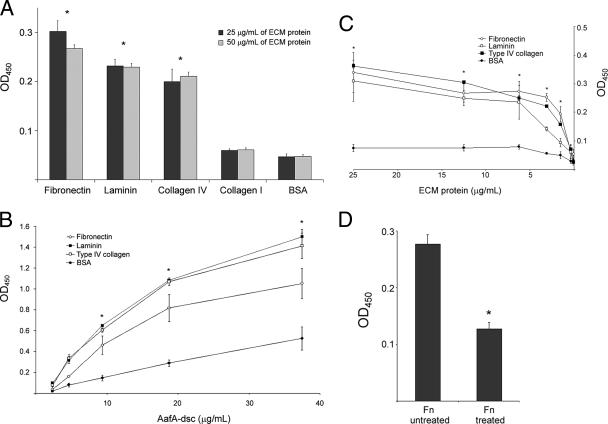
To demonstrate direct binding of the AafA major fimbrial subunit protein to ECM proteins, we purified and refolded the AafA protein using the dsc method (2). Proton (1H) nuclear magnetic resonance spectra displayed characteristics of a well-folded and rapidly tumbling (monomeric) protein, with amide line widths of approximately 30 Hz and chemical shift dispersion from 5.9 to 10.2 ppm (data not shown). The solution structure of AafAdsc will be published elsewhere. The purified and refolded protein was added to 96-well plates precoated with Fn, collagen I, collagen IV, laminin, or BSA (negative control), and the binding to these ECM proteins was assayed by ELISA using a rabbit antiserum raised against AafAdsc. Binding to Fn, laminin, and type IV collagen was significantly greater than that to type I collagen or BSA (Fig. 3A); this binding was blocked by preincubation of the AafAdsc protein with mouse anti-AafA (data not shown). When increasing concentrations of AafAdsc protein were added to ECM protein-coated wells, dose-dependent binding to Fn, laminin, and type IV collagen compared to BSA was observed (Fig. 3B). In the converse experiment, AafAdsc binding to 96-well plates coated with serial dilutions of ECM proteins also showed dose-dependent binding (Fig. 3C).
FIG. 3.
Binding of AafAdsc to ECM proteins. (A) AafAdsc protein was added at 10 μg/ml to wells of 96-well plates coated separately with either 25 μg/ml or 50 μg/ml of each ECM protein, and the binding was determined by ELISA using anti-AafA serum. The bars represent the means of three experiments, with the error bars indicating 1 standard deviation. *, significantly different from BSA-coated control (P < 0.05). (B) Binding of increasing concentrations of AafAdsc to Fn, laminin, type IV collagen, and BSA determined by ELISA using anti-AafA serum. ECM proteins were added to the wells at a standard concentration of 25 μg/ml. The data are shown as means of three experiments, with the error bars indicating 1 standard deviation. *, significantly different from BSA-coated control (P < 0.05). (C) Binding of AafAdsc to different concentrations of Fn, laminin, type IV collagen, and BSA determined by ELISA using anti-AafA serum. AafAdsc was added at a uniform concentration of 10 μg/ml to all wells after they were coated with the concentration of protein indicated. The data are shown as means of three experiments, with the error bars indicating 1 standard deviation. *, significantly different from BSA-coated control (P < 0.05). (D) Ninety-six-well plates were coated with Fn that had been treated with a mixture of N- and O-glycosydases, and the binding of AafAdsc was determined by ELISA using anti-AafA serum. The data are shown as means of three experiments, with the error bars indicating 1 standard deviation. *, significantly different from untreated Fn (P < 0.05). OD450, optical density at 450 nm.
Considering that Fn is a glycoprotein and that carbohydrate residues have been shown to be involved in fimbrial binding to Fn (24), we addressed the possible involvement of Fn glycans in AafAdsc binding. Deglycosylation of O-linked and N-linked Fn oligosaccharides conferred a significant reduction in AafAdsc binding compared to untreated Fn (Fig. 4D).
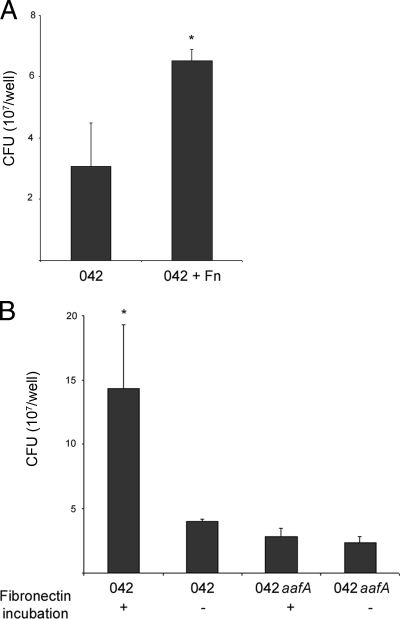
FIG. 4.
Fn promotes EAEC 042 adherence to polarized T84 cells. (A) Numbers of adherent bacteria after infection by EAEC 042 of polarized T84 cells preincubated (042 + FN) or not (042) with 5 μg/ml Fn before the infection. *, significantly different compared with EAEC 042 infection of polarized T84 cells without Fn incubation (P < 0.05). (B) Numbers of adherent bacteria after infection of polarized T84 cells with EAEC 042 or its aafA mutant strains preincubated (+) or not (−) with Fn for 3 h. The bars represent the means of three experiments, with the error bars indicating 1 standard deviation. *, significantly different from either EAEC 042 or its aafA mutant strain (P < 0.05).
Enhanced secretion of Fn increases EAEC binding to polarized T84 cells.
We asked whether Fn could enhance the adherence of EAEC to polarized intestinal-cell monolayers. We have previously described the polarized human intestinal epithelial T84 cell line as a model for EAEC adherence, mucosal toxicity, and inflammation (20). To test whether Fn would increase the number of bacteria adhering to confluent polarized T84 monolayers, we incubated T84 cells with 5 μg/ml Fn before EAEC infection. Addition of Fn significantly increased EAEC binding to the T84 monolayers (Fig. 4A). In the converse experiment, EAEC 042 or its aafA mutant were preincubated with 100 μg/ml Fn in PBS or PBS alone for 3 h at room temperature. The bacteria were extensively washed and then applied to the monolayers. Pretreatment with Fn increased adherence compared with binding of EAEC 042 incubated only with PBS (Fig. 4B). Fn pretreatment did not increase binding by 042 aafA. These results indicate that the presence of Fn in the extracellular milieu increases AAF/II-dependent binding of EAEC to the apical surfaces of T84 cells.
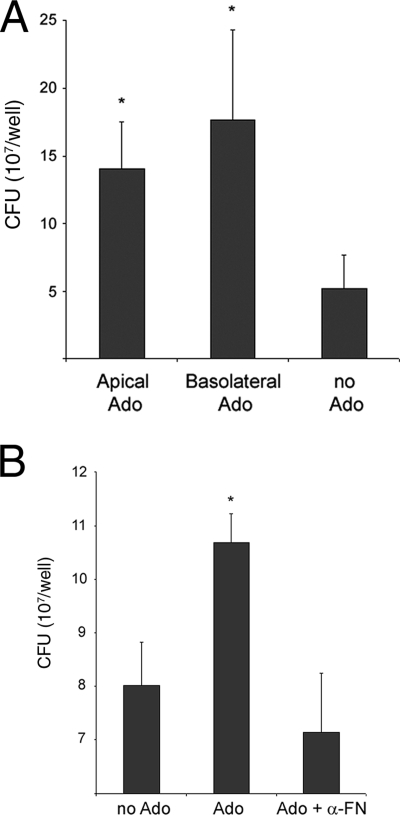
Previous studies demonstrated that apical secretion of Fn could be induced by molecules involved in tissue inflammation, such as adenosine (32). To test whether Fn secretion from the apical surface at physiological concentrations would increase binding of EAEC, polarized T84 monolayers were incubated with medium or medium plus adenosine applied either apically or basolaterally for a period of 24 h. After this time, the monolayer was infected with EAEC 042 for 3 h, and adherent bacteria were enumerated. A significant increase in the number of adherent bacteria was found in cells incubated with adenosine when the latter was applied either apically or basolaterally compared with cells incubated with medium only (Fig. 5A). Adenosine had no effect on adherence by 042 aafA (data not shown). To prove that the increased EAEC adhesion to polarized T84 cells was due specifically to Fn secretion induced by adenosine, the infection was carried out in the presence of anti-Fn antibodies. Under these conditions, the number of adherent bacteria was significantly reduced compared to that found in the absence of anti-Fn antibodies (Fig. 5B).
FIG. 5.
Adenosine promotes EAEC 042 adherence to polarized T84 cells. (A) Adenosine (Ado) was added to the apical or basolateral chambers of polarized monolayers of T84 cells in cell culture medium at a concentration of 100 μM for 24 h prior to infection with EAEC 042 for 3 h. The bars represent the mean counts of adherent bacteria for three experiments, with the error bars indicating 1 standard deviation. *, significantly different from adherence to T84 cells incubated with medium only (P < 0.05). (B) Same experiment described in the legend to panel A, except that rabbit anti-Fn antibodies (1:20 dilution) were added to the apical chamber simultaneously with the addition of bacteria. The bars represent the means of three experiments, with the error bars indicating 1 standard deviation. *, significantly different from the adherence observed for EAEC infection in the presence of anti-Fn antibodies and untreated cells (P < 0.05).
DISCUSSION
Adherence to the intestinal mucosa is a key feature of EAEC infection. This adherence requires the AAF adhesins, which are expressed by the majority of EAEC strains. Though several studies support a role for AAF in EAEC pathogenesis, the cellular receptors for these fimbrial structures are unknown. Our data suggest that ECM proteins may be receptors for EAEC. Prototype EAEC strain 042 binds to several major ECM proteins present in the intestinal epithelium, such as Fn, laminin, and type IV collagen (Fig. 1); this binding is mediated by the major subunit of AAF/II, the AafA protein (Fig. 2).
We focused on binding to Fn, recognizing that several other bacterial adhesins bind to this ECM protein (14, 18, 33). Fn is a glycoprotein that contains 4 to 9% carbohydrate, and glycosylation is either N linked or O linked (23). While it stabilizes Fn against hydrolysis, glycosylation may also make Fn more vulnerable to fimbria-mediated bacterial binding (24). Deglycosylation of Fn resulted in significant reduction in AafAdsc binding (Fig. 3D), implicating Fn oligosaccharides in AAF/II binding.
To assess the role of Fn at an epithelial surface, we employed the polarized T84 monolayer model. This model recapitulates several steps potentially relevant to pathogenesis, including adherence, mucosal toxicity, and inflammation (20). Addition of Fn to the apical sides of polarized T84 cells augmented bacterial adherence, and this effect was shown to require AafA (Fig. 4). Interestingly, transmission electron microscopy of polarized T84 cells infected with EAEC 042 in a prior work suggested that the bacteria do not adhere intimately to the monolayer but may remain anchored at distances of up to 1 μm (20). Thus, Fn, and possibly other secreted ECM proteins, could serve to anchor the bacterial biofilm to the mucosal surface. This was proposed previously for Campylobacter jejuni, which binds Fn via the CadF protein (18). We also note that other E. coli fimbriae have been implicated in ECM binding, including P fimbriae, S fimbriae, and type I fimbriae (24, 29, 34).
Fn binding could play a fundamental role in pathogenesis, perhaps beyond simple adherence. Fn has been demonstrated to be important in both adhesion and invasion by gram-positive pathogenic cocci (22). These biological effects could involve the major Fn receptor α5β1 integrin, which modulates intracellular integrin-mediated signal transduction pathways (27). In Staphylococcus aureus, the engagement of integrin α5β1 by the bacteria induces the formation of fibrillar-adhesion-like protein complexes at the site of bacterial attachment (1). These events lead to activation of signal transduction pathways, which direct internalization of the bacteria by the host cells (12). We have not observed internalization of EAEC by T84 monolayers at the 3-h time point, but the role of Fn binding in cell signaling deserves further investigation.
Fn and other ECM proteins are generally localized to the epithelial basement membrane, where they are not available for interaction with luminal bacteria. However, previous reports suggested that adenosine, a product of neutrophil-derived 5′ AMP, which is actively secreted during intestinal inflammation, is a potent inducer of apical Fn secretion in the polarized T84 cell model (28, 32). We found increased EAEC 042 adhesion to polarized T84 cells incubated with adenosine compared with untreated cells (Fig. 5A). The binding observed after adenosine incubation was due, at least in part, to apical secretion of Fn, because anti-Fn antibodies reduced cell binding to levels observed on untreated cells (Fig. 5B); however, the participation of other apically secreted factors by adenosine cannot be ruled out.
EAEC has been shown to cause inflammatory enteritis in infected patients, and we have reported that strain 042 induces the release of proinflammatory cytokines from polarized T84 monolayers (11). The inflammatory process can trigger signal transduction cascades that induce apical secretion or relocation of Fn. This hypothesis is supported by the observation of induced Fn secretion in intestinal biopsy specimens obtained from patients with Crohn's disease (14). In these biopsy specimens, Enterococcus faecalis attachment was increased in the presence of increased Fn secretion (14). By inducing an inflammatory response, EAEC may be creating an environment promoting further colonization and more severe infection. The release of Fn as a possible consequence of EAEC infection deserves further attention.
Our results also showed that EAEC binds to laminin and type IV collagen in an AAF/II-dependent manner. Previous studies demonstrated that a member of the Afa/Dr adhesin family, Dr adhesin, binds to type IV collagen and that this binding is mediated by the major adhesin subunit, DraE (25). Such binding may be an essential step for establishing chronic renal infection by Dr+ uropathogenic E. coli strains (25). Mutation analyses showed that replacement of a single amino acid at position 113 of the DraE subunit results in loss of type IV collagen binding. Also, inhibition of binding to type IV collagen was observed when the Dr adhesin was incubated with chloramphenicol (25). However, we found that AafA binding to type IV collagen is not affected by chloramphenicol (data not shown), indicating that the binding to this ECM protein may be different from that induced by the Dr adhesins. Elucidation of the solution structure of AafAdsc will facilitate the identification of regions involved in adherence to ECM proteins.
Our studies provide new insights into the adhesion and pathogenesis of EAEC. However, several steps are not yet clarified. Our data suggest that Fn could anchor the growing EAEC biofilm to the intestinal surface through the AAF/II fimbriae, an event that may activate signaling pathways in the host epithelium. Further studies are under way to elucidate the potential roles of ECM proteins in EAEC pathogenesis.
Acknowledgments
This work was supported by grant NIH AI33096 to J.P.N. M.J.F. was supported by the Chilean National Scholarship Program for Graduate Studies.
We thank Miguel O'Ryan and Fernando Ruiz for critical reading of the manuscript.
Editor: A. J. Bäumler
Footnotes
Published ahead of print on 30 June 2008.
REFERENCES
- 1.Agerer, F., S. Lux, A. Michel, M. Rohde, K. Ohlsen, and C. R. Hauck. 2005. Cellular invasion by Staphylococcus aureus reveals a functional link between focal adhesion kinase and cortactin in integrin-mediated internalisation. J. Cell Sci. 1182189-2200. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, K. L., J. Billington, D. Pettigrew, E. Cota, P. Simpson, P. Roversi, H. A. Chen, P. Urvil, L. du Merle, P. N. Barlow, M. E. Medof, R. A. Smith, B. Nowicki, C. Le Bouguenec, S. M. Lea, and S. Matthews. 2004. An atomic resolution model for assembly, architecture, and function of the Dr adhesins. Mol. Cell 15647-657. [DOI] [PubMed] [Google Scholar]
- 3.Bernier, C., P. Gounon, and C. Le Bouguenec. 2002. Identification of an aggregative adhesion fimbria (AAF) type III-encoding operon in enteroaggregative Escherichia coli as a sensitive probe for detecting the AAF-encoding operon family. Infect. Immun. 704302-4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berrier, A. L., and K. M. Yamada. 2007. Cell-matrix adhesion. J. Cell Physiol. 213565-573. [DOI] [PubMed] [Google Scholar]
- 5.Boisen, N., C. Struve, F. Sheutz, K. A. Krogfelt, and J. P. Nataro. 2008. A new adhesin of enteroaggregative Escherichia coli related to the Afa/Dr/AAF family. Infect. Immun. 763281-3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Czeczulin, J. R., S. Balepur, S. Hicks, A. Phillips, R. Hall, M. H. Kothary, F. Navarro-Garcia, and J. P. Nataro. 1997. Aggregative adherence fimbria II, a second fimbrial antigen mediating aggregative adherence in enteroaggregative Escherichia coli. Infect. Immun. 654135-4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorsey, C. W., M. C. Laarakker, A. D. Humphries, E. H. Weening, and A. J. Baumler. 2005. Salmonella enterica serotype Typhimurium MisL is an intestinal colonization factor that binds fibronectin. Mol. Microbiol. 57196-211. [DOI] [PubMed] [Google Scholar]
- 8.Dubreuil, J. D., G. D. Giudice, and R. Rappuoli. 2002. Helicobacter pylori interactions with host serum and extracellular matrix proteins: potential role in the infectious process. Microbiol. Mol. Biol. Rev. 66617-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elias, W. P., S. F. Barros, C. G. Moreira, L. R. Trabulsi, and T. A. Gomes. 2002. Enteroaggregative Escherichia coli strains among classical enteropathogenic Escherichia coli O serogroups. J. Clin. Microbiol. 403540-3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harrington, S. M., E. G. Dudley, and J. P. Nataro. 2006. Pathogenesis of enteroaggregative Escherichia coli infection. FEMS Microbiol. Lett. 25412-18. [DOI] [PubMed] [Google Scholar]
- 11.Harrington, S. M., M. C. Strauman, C. M. Abe, and J. P. Nataro. 2005. Aggregative adherence fimbriae contribute to the inflammatory response of epithelial cells infected with enteroaggregative Escherichia coli. Cell Microbiol. 71565-1578. [DOI] [PubMed] [Google Scholar]
- 12.Hauck, C. R., and K. Ohlsen. 2006. Sticky connections: extracellular matrix protein recognition and integrin-mediated cellular invasion by Staphylococcus aureus. Curr. Opin. Microbiol. 95-11. [DOI] [PubMed] [Google Scholar]
- 13.Huang, D. B., J. P. Nataro, H. L. DuPont, P. P. Kamat, A. D. Mhatre, P. C. Okhuysen, and T. Chiang. 2006. Enteroaggregative Escherichia coli is a cause of acute diarrheal illness: a meta-analysis. Clin. Infect. Dis. 43556-563. [DOI] [PubMed] [Google Scholar]
- 14.Isenmann, R., M. Schwarz, E. Rozdzinski, C. Christ, E. Schmidt, P. Augat, R. Marre, and H. G. Beger. 2002. Interaction of fibronectin and aggregation substance promotes adherence of Enterococcus faecalis to human colon. Dig. Dis. Sci. 47462-468. [DOI] [PubMed] [Google Scholar]
- 15.Konkel, M. E., J. E. Christensen, A. M. Keech, M. R. Monteville, J. D. Klena, and S. G. Garvis. 2005. Identification of a fibronectin-binding domain within the Campylobacter jejuni CadF protein. Mol. Microbiol. 571022-1035. [DOI] [PubMed] [Google Scholar]
- 16.Konkel, M. E., S. G. Garvis, S. L. Tipton, D. E. Anderson, Jr., and W. Cieplak, Jr. 1997. Identification and molecular cloning of a gene encoding a fibronectin-binding protein (CadF) from Campylobacter jejuni. Mol. Microbiol. 24953-963. [DOI] [PubMed] [Google Scholar]
- 17.Kuusela, P. 1978. Fibronectin binds to Staphylococcus aureus. Nature 276718-720. [DOI] [PubMed] [Google Scholar]
- 18.Monteville, M. R., and M. E. Konkel. 2002. Fibronectin-facilitated invasion of T84 eukaryotic cells by Campylobacter jejuni occurs preferentially at the basolateral cell surface. Infect. Immun. 706665-6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nataro, J. P. 2005. Enteroaggregative Escherichia coli pathogenesis. Curr. Opin. Gastroenterol. 214-8. [PubMed] [Google Scholar]
- 20.Nataro, J. P., S. Hicks, A. D. Phillips, P. A. Vial, and C. L. Sears. 1996. T84 cells in culture as a model for enteroaggregative Escherichia coli pathogenesis. Infect. Immun. 644761-4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nataro, J. P., D. Yikang, J. A. Giron, S. J. Savarino, M. H. Kothary, and R. Hall. 1993. Aggregative adherence fimbria I expression in enteroaggregative Escherichia coli requires two unlinked plasmid regions. Infect. Immun. 611126-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nitsche-Schmitz, D. P., M. Rohde, and G. S. Chhatwal. 2007. Invasion mechanisms of Gram-positive pathogenic cocci. Thromb. Haemost. 98488-496. [PubMed] [Google Scholar]
- 23.Pankov, R., and K. M. Yamada. 2002. Fibronectin at a glance. J. Cell Sci. 1153861-3863. [DOI] [PubMed] [Google Scholar]
- 24.Saren, A., R. Virkola, J. Hacker, and T. K. Korhonen. 1999. The cellular form of human fibronectin as an adhesion target for the S fimbriae of meningitis-associated Escherichia coli. Infect. Immun. 672671-2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Selvarangan, R., P. Goluszko, J. Singhal, C. Carnoy, S. Moseley, B. Hudson, S. Nowicki, and B. Nowicki. 2004. Interaction of Dr adhesin with collagen type IV is a critical step in Escherichia coli renal persistence. Infect. Immun. 724827-4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Servin, A. L. 2005. Pathogenesis of Afa/Dr diffusely adhering Escherichia coli. Clin. Microbiol. Rev. 18264-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sinha, B., P. P. Francois, O. Nusse, M. Foti, O. M. Hartford, P. Vaudaux, T. J. Foster, D. P. Lew, M. Herrmann, and K. H. Krause. 1999. Fibronectin-binding protein acts as Staphylococcus aureus invasin via fibronectin bridging to integrin α5β1. Cell Microbiol. 1101-117. [DOI] [PubMed] [Google Scholar]
- 28.Sitaraman, S. V., D. Merlin, L. Wang, M. Wong, A. T. Gewirtz, M. Si-Tahar, and J. L. Madara. 2001. Neutrophil-epithelial crosstalk at the intestinal lumenal surface mediated by reciprocal secretion of adenosine and IL-6. J. Clin. Invesigt. 107861-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sokurenko, E. V., H. S. Courtney, S. N. Abraham, P. Klemm, and D. L. Hasty. 1992. Functional heterogeneity of type 1 fimbriae of Escherichia coli. Infect. Immun. 604709-4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steiner, T. S., A. A. Lima, J. P. Nataro, and R. L. Guerrant. 1998. Enteroaggregative Escherichia coli produce intestinal inflammation and growth impairment and cause interleukin-8 release from intestinal epithelial cells. J. Infect. Dis. 17788-96. [DOI] [PubMed] [Google Scholar]
- 31.Torres, A. G., X. Zhou, and J. B. Kaper. 2005. Adherence of diarrheagenic Escherichia coli strains to epithelial cells. Infect. Immun. 7318-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walia, B., F. E. Castaneda, L. Wang, V. L. Kolachala, R. Bajaj, J. Roman, D. Merlin, A. T. Gewirtz, and S. V. Sitaraman. 2004. Polarized fibronectin secretion induced by adenosine regulates bacterial-epithelial interaction in human intestinal epithelial cells. Biochem. J. 382589-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Westerlund, B., and T. K. Korhonen. 1993. Bacterial proteins binding to the mammalian extracellular matrix. Mol. Microbiol. 9687-694. [DOI] [PubMed] [Google Scholar]
- 34.Westerlund, B., I. Van Die, W. Hoekstra, R. Virkola, and T. K. Korhonen. 1993. P fimbriae of uropathogenic Escherichia coli as multifunctional adherence organelles. Zentralbl. Bakteriol. 278229-237. [DOI] [PubMed] [Google Scholar]
- 35.Zalewska, B., J. Stangret, K. Bury, M. Wojciechowski, J. Kur, and R. Piatek. 2007. DAF- and collagen-binding properties of chimeric Dr fimbriae. Microbiology 1532733-2742. [DOI] [PubMed] [Google Scholar]