Abstract
Campylobacter jejuni and Campylobacter coli colonize and infect the intestinal epithelium and cause acute inflammatory diarrhea. The intestinal epithelium serves as a physical barrier to, and a sensor of, bacterial infection by secreting proinflammatory cytokines. This study examined the mechanisms for Campylobacter-induced secretion of the proinflammatory chemokine interleukin-8 (IL-8) by using polarized T84 human colonic epithelial cells as a model. C. jejuni increased the secretion of both IL-8 and tumor necrosis factor alpha (TNF-α) in polarized epithelial cells. However, the increase in IL-8 secretion was independent of Campylobacter-stimulated TNF-α secretion. Polarized T84 cells secreted IL-8 predominantly to the basolateral medium independently of the inoculation direction. While there was a significant correlation between the levels of IL-8 secretion and Campylobacter invasion, all 11 strains tested increased IL-8 secretion by polarized T84 cells despite their differences in adherence, invasion, and transcytosis efficiencies. Cell-free supernatants of Campylobacter-T84-cell culture increased IL-8 secretion to levels similar to those induced by live bacterial inoculation. The ability of the supernatant to induce IL-8 secretion was reduced by flagellum and cytolethal distending toxin (CDT) gene mutants, treatment of the supernatant with protease K or heat, or treatment of T84 cells with the Toll-like receptor (TLR) inhibitor MyD88 inhibitory peptide or chloroquine. NF-κB inhibitors or cdtB mutation plus MyD88 inhibitor, but not flaA cdtB double mutations, abolished the ability of the supernatant to induce IL-8 secretion. Taken together, our results demonstrate that Campylobacter-induced IL-8 secretion requires functional flagella and CDT and depends on the activation of NF-κB through TLR signaling and CDT in human intestinal epithelial cells.
Campylobacter jejuni and Campylobacter coli are leading causes of food-borne diarrheal diseases worldwide, with an estimated 2.4 million cases each year in the United States (55). Campylobacter infection usually causes acute self-limiting gastroenteritis; however, severe and prolonged cases of enteritis, bacteremia, septic arthritis, and other extraintestinal infections, particularly in immunocompromised patients, have also been reported (52). The pathogenic mechanisms responsible for Campylobacter gastroenteritis are not yet fully understood.
Campylobacter infection is initiated at the intestinal epithelium, the first line of defense against enteric pathogens. The intestinal epithelium not only provides a physical barrier against bacterial infection but also functions as a sensor of bacterial infection for the immune system. The interaction of bacteria with the intestinal epithelium induces the secretion of a panel of proinflammatory cytokines, which provide the earliest warning signs for the immune system (12, 13, 28). One of the major proinflammatory cytokines secreted by the intestinal epithelial cells is interleukin-8 (IL-8) (12, 13, 28), a chemoattractant that recruits neutrophils to the infected site. Previous studies have shown that the interaction of enteric pathogens, such as Salmonella enterica serovar Typhimurium (37), Shigella flexneri (5), and Yersinia enterocolitica (50), with T84 polarized human intestinal epithelial cells induced the secretion of IL-8 and the transmigration of subepithelial neutrophils to the apical surface (5, 37, 38). Like those bacteria, campylobacters colonize, invade, and transmigrate across polarized human intestinal epithelial cells (30, 42) and induce IL-8 secretion in nonpolarized and polarized human intestinal epithelial cell lines (9, 21, 36).
The molecular mechanisms for Campylobacter-induced IL-8 secretion in epithelial cells have not been well studied. Common bacterial components, such as flagellin (53), lipopolysaccharide (LPS) (47), DNAs that contain unmethylated CpG motifs (3), and peptidoglycan (51), can evoke epithelial IL-8 production by activating Toll-like receptors (TLRs), a family of pattern recognition receptors that bind to conserved microbial structures and activate innate immunity (4, 40). For example, the flagellin of Salmonella serovar Typhimurium (17) and the LPS of S. flexneri (5) have been shown to be inducers of IL-8. However, the flagellin of C. jejuni has been reported to be ineffective in the induction of IL-8 secretion in human intestinal epithelial cells (27, 58). Campylobacter-secreted cytolethal distending toxin (CDT) can induce IL-8 secretion (22). Additionally, C. jejuni-induced IL-8 secretion requires the viability of the bacteria (9, 21) and depends on C. jejuni gene products that are expressed upon contact with epithelial cells and on the activation of the mitogen-activated protein kinase extracellular signal-regulated kinase in epithelial cells (58).
Nuclear factor κB (NF-κB) is a major transcriptional regulator of proinflammatory cytokines in the intestinal epithelial cells (14, 44) and a main downstream target of TLR signaling pathways (4). The interaction of mucosal pathogens, such as Salmonella serovar Typhimurium (23), Neisseria gonorrhoeae (45), S. flexneri (11), and Escherichia coli (49), with epithelial cells activates NF-κB. C. jejuni has been shown to activate NF-κB in HeLa cells and HCA-7 human colonic epithelial cells (41). C. jejuni-induced secretion of the proinflammatory chemokines GROα, γIP-10, and monocyte chemoattractant protein-1 depends on NF-κB activation (24). C. jejuni-induced gastroenteritis was much more severe in NF-κB-deficient mice than in wild-type (wt) mice (15), supporting the importance of NF-κB in the host responses to C. jejuni infection.
In this study, we examined bacterial and host factors that are required for the induction of IL-8 secretion in Campylobacter-infected polarized human intestinal epithelial cells. We demonstrated that Campylobacter-induced polarized IL-8 secretion involved Campylobacter flagellum and CDT, but not host cell-secreted tumor necrosis factor alpha (TNF-α), and required the activation of the TLR signaling adaptor MyD88 and NF-κB.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Campylobacter was routinely grown on Mueller-Hinton (MH) agar (Difco Laboratories, Detroit, MI) containing 5% (vol/vol) lysed horse blood at 37°C under a microaerophilic atmosphere (85% N2, 10% CO2, and 5% O2). A human clinical strain, C. jejuni 81-176 (19, 46), its flaA insertion mutant K2-32, which does not express flagellin A, and its pflA insertion mutant K2-55, which expresses flagellin but is not motile (59), were kindly provided by Patricia Guerry-Kopecko of the Naval Medical Research Center (Bethesda, MD). A cdtB mutant of C. jejuni 81-176 (15) was kindly provided by James Fox of MIT (Cambridge, MA). Five C. jejuni and 3 C. coli strains used in the study were selected from 378 Campylobacter isolates recovered from retail raw chicken meat in the Washington, DC, area from June 1999 to July 2000, based on their distinct DNA fingerprinting profiles by pulsed-field gel electrophoresis (60) and their adherence and invasion abilities in nonpolarized epithelial cells (61). All of these strains had intact CDT genes (61).
Construction of the C. jejuni 81-176 flaA cdtB double mutant.
A 2.7-kb DNA fragment that contained the cdtB gene with the chloramphenicol resistance gene inserted was first amplified from a J81-176 cdtB mutant strain by using a pair of primers (forward primer, 5′-ATTTGAAGATAC TGATCCTT-3′; reverse primer, 5′-TTGCACAGCTGAAGTTGT-3′) (15) and then cloned into the pGEM-T Easy vector (Promega). The clones containing the mutated cdtB gene were selected by ampicillin and chloramphenicol resistance. The mutated cdtB gene was introduced into the C. jejuni flaA mutant strain by natural transformation (15). The transformants were selected with kanamycin and chloramphenicol (each at 25 μg/ml), and the mutations of both flaA and cdtB were verified by PCR.
Cell culture.
T84 cells (a human colonic epithelial cell line; ATCC CCL-248) were maintained in a 1:1 mixture of Dulbecco's modified Eagle medium and Ham's F-12 medium containing 2.5 mM l-glutamine, 15 mM HEPES, and 0.5 mM sodium pyruvate (Invitrogen, San Diego, CA) supplemented with 5% (vol/vol) fetal bovine serum, penicillin, and streptomycin (5% DME/F12), as recommended by the ATCC (Manassas, VA). To establish polarized monolayers, T84 cells (2 × 105) were seeded onto transwells (0.33 cm2) with 3.0-μm-pore-size clear polyester membranes (Corning Costar Corp., Cambridge, MA). The polarization of the epithelial monolayers was monitored by transepithelial resistance, measured by Millicell-ERS (Millipore, Billerica, MA). The polarized T84 cells were not used until the transepithelial resistance of the monolayers reached 1,400 Ω/cm2 or higher.
Inhibitors.
The NF-κB inhibitors SN50 [6-amino-4-(4-phenoxyphenylethylamino)quinazoline] (34) and quinazoline (56) were purchased from Calbiochem (San Diego, CA), and TPCK (N-α-p-tosyl-l-phenylalanine chloromethyl ketone) (7) was purchased from Sigma-Aldrich (St. Louis, MO). Chloroquine and the MyD88 homodimerization inhibitory peptide set were purchased from Sigma-Aldrich and Imgenex (San Diego, CA), respectively. Polarized T84 cells were preincubated with SN50 (18 μM), quinazoline (28 μM), TPCK (50 μM), or chloroquine (5 μM) for 30 min or with MyD88 inhibitor or a control peptide (100 μM) for 24 h. The inhibitors were also included in the inoculation medium. The transepithelial resistance was monitored before and after incubation, and no significant effect of these inhibitors on transepithelial resistance was detected. Cycloheximide (39), an inhibitor of eukaryotic but not prokaryotic protein synthesis, and polymyxin B (25, 51), which binds and neutralizes LPS, were purchased from Sigma-Aldrich.
Analysis for IL-8 and TNF-α secretion.
Bacteria were cultured on the plates for 18 h and were harvested using phosphate-buffered saline. Polarized T84 monolayers cultured on transwells were washed with the invasion medium (5% DME/F12 without antibiotics) and treated with one of the following: (i) Campylobacter (2 × 106 CFU; multiplicity of infection [MOI], ∼10) in the invasion medium inoculated either from the apical or from the basolateral chamber of transwells, (ii) conditioned supernatants, generated from the apical or basolateral culture medium, applied to the corresponding culture chambers, or (iii) a DNA extract of C. jejuni 81-176. The epithelial cells were incubated for the times indicated in a 5% CO2 incubator at 37°C. The culture media from the apical and basolateral chambers were collected individually and centrifuged for 20 min at 13,000 × g to remove bacteria. A cocktail of protease inhibitors (Sigma-Aldrich) was added to the supernatants in order to protect the proteins from degradation. The amounts of IL-8 and TNF-α in the supernatants were determined by enzyme-linked immunosorbent assays (ELISA). Mouse anti-human IL-8 and anti-human TNF-α monoclonal antibodies (BD Bioscience, San Jose, CA) were used as capturing antibodies; biotinylated mouse anti-human IL-8 and anti-human TNF-α monoclonal antibodies (BD Bioscience) were used as detecting antibodies; and streptavidin-alkaline phosphatase (SouthernBiotech Inc., Birmingham, AL) and the substrate p-nitrophenylphosphate disodium (Sigma-Aldrich) were used to visualize bound antibodies. Recombinant human IL-8 and TNF-α (BD Bioscience) with known concentrations were used to establish standard curves.
Analysis of Campylobacter adherence, invasion, and transcytosis.
The adherence, invasion, and transcytosis of Campylobacter in polarized epithelial cells were analyzed as previously described (42). Briefly, polarized T84 epithelial-cell monolayers were washed with invasion medium and incubated with Campylobacter (∼2 × 106 CFU; MOI, ∼10) apically at 37°C for 4 h. The transepithelial resistance of the epithelial-cell monolayers was checked before and after incubation with the bacteria, and no significant change was detected. After the 4-h incubation, bacteria in the basolateral medium were enumerated to determine the number of bacteria that had transmigrated from the apical to the basolateral chamber. After a wash to remove unassociated bacteria, cell-associated bacteria were released by incubating cells with 1% saponin for 5 min and vortexing vigorously for 2 min. Serial dilutions of the lysates were plated onto MH-blood plates. To determine the number of bacteria that had invaded T84 cells, T84 epithelial-cell monolayers were washed and incubated with gentamicin (100 μg/ml) in invasion medium at both the apical and basolateral chambers for 2 h to kill extracellular bacteria. Then cells were washed, lysed, and plated onto MH-blood plates. The number of bacteria that had adhered, been internalized, or been transcytosed was plotted as a percentage of the starting viable inoculum.
Preparation of conditioned supernatants from the Campylobacter-epithelial-cell coculture.
Conditioned supernatants were prepared using a previously described procedure with minor modifications (17). Typically, polarized T84 epithelial-cell monolayers were incubated apically with C. jejuni 81-176 or its mutant (around 2 × 106 CFU) at 37°C for 4 h. After the 4-h incubation, the apical (apical conditioned supernatant) and basolateral (basolateral conditioned supernatant) media were collected individually and centrifuged for 20 min at 13,000 × g to remove the bacteria, and the supernatants were stored at −20°C for future use. The same amount of bacteria was suspended in 0.1 ml invasion medium and incubated alone for 4 h at 37°C. The cultures were centrifuged to remove bacteria, and the supernatant was collected as the bacterial culture medium. To inactivate heat-sensitive proteins, the collected conditioned supernatant was boiled for 20 min. To remove most of the protein factors, the conditioned supernatant was treated with protease K (100 μg/ml; Invitrogen) at 56°C overnight, followed by a 20-min incubation at 100°C to inactivate protease K. To remove bacterial DNA, the conditioned supernatant was treated with DNase I (10 U/ml; New England Biolabs, Ipswich, MA) at 37°C for 2 h. The treated supernatants were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (12%) and agarose gel electrophoresis, confirming the removal of proteins by protease K and of bacterial DNA by DNase I (data not shown). The conditioned supernatant was incubated with polymyxin B (20 μg/ml) at 37°C for 30 min to neutralize LPS.
CDT cytotoxicity assay.
The cytotoxicity of CDT in the bacterial culture supernatants was analyzed by using Vero cells as previously described (16). Briefly, Vero cells were seeded onto 96-well plates (∼104 cells/well) 24 h before the analysis. Vero cells were incubated with serially diluted C. jejuni culture supernatants (100 μl/well) that had been filtrated (pore size, 0.22 μm) and treated with polymyxin B (2 mg/ml) (18). The culture medium of filtrated and polymyxin-treated E. coli O157:H7 EDL933 was used as a positive control, and polymyxin B-treated broth was used as a negative control. After a 48-h incubation, the viability of Vero cells was monitored using an MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] assay (10). The CDT titers were expressed as the reciprocal of the highest dilution that caused 50% Vero cell death, compared to untreated cells.
Analysis for NF-κB activation.
The activation of NF-κB was analyzed by following the degradation of the NF-κB inhibitor IκB-α. Epithelial cells were incubated with C. jejuni 81-176 (MOI, ∼10) in the presence of cycloheximide (10 ng/ml; Sigma-Aldrich), which blocks protein synthesis in epithelial cells, for varying lengths of time. The epithelial cells were washed and lysed (20 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1 mM EGTA, 1% NP-40, 1 mM MgCl2, 50 mM NaF, 1 mM Na3VO4, 10 mM Na4P2O7, and protease inhibitor cocktail) at 4°C. Equal amounts (20 μg) of cell lysates were subjected to SDS-PAGE and Western blotting and were probed with a rabbit anti-IκB-α antibody (Santa Cruz Biotechnology, Santa Cruz, CA). The blots were stripped and reprobed with an anti-α-tubulin antibody (Invitrogen) as a loading control.
Statistical analysis.
All statistical analyses were performed using SPSS statistical software (version 10.0; SPSS Inc., Chicago, IL). Pearson's correlation coefficients between the levels of IL-8 secretion and those of Campylobacter adherence, invasion, and transcytosis (percentages of inocula) were calculated. The significance of the differences between IL-8 secretion levels induced by different treatments was analyzed using one-way analysis of variance, followed by a Tukey posthoc test. The P value was determined using a two-tailed test.
RESULTS
Campylobacter increases IL-8 secretion in polarized human intestinal epithelial cells independently of TNF-α induction.
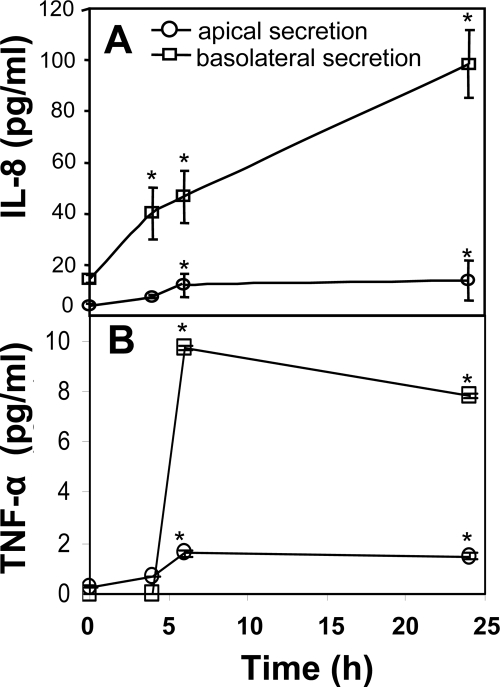
The levels of secretion of IL-8 and TNF-α in the apical and basolateral chambers of polarized human intestinal epithelial cells were determined after T84 cells had been incubated with C. jejuni 81-176 at an MOI of 10:1 at the apical surface for 4, 6, and 24 h. As in previous reports, polarized T84 cells secreted IL-8 predominantly to the basolateral side at low levels in the absence of the bacteria (9, 37) (Fig. 1A). Inoculation of C. jejuni 81-176 increased IL-8 secretion on both the apical and basolateral sides. The secretion of IL-8 increased from 15 pg/ml to 100 pg/ml (6.7-fold) at the basolateral side of the medium and from 4 pg/ml to 14 pg/ml (3.5-fold) at the apical side of the medium by 24 h (Fig. 1A). In the absence of the bacteria, the levels of TNF-α secreted in the apical and basolateral media of polarized T84 cells were nearly undetectable. Campylobacter-inoculation increased TNF-α secretion to 7.8 pg/ml and 1.5 pg/ml in the basolateral and apical media of polarized T84 cells, respectively (Fig. 1B). These results indicate that Campylobacter increases both IL-8 and TNF-α secretion in polarized T84 cells.
FIG. 1.
Campylobacter inoculation increased the secretion of IL-8 and TNF-α in polarized human intestinal epithelial T84 cells. T84 cells were cultured on transwells until the transepithelial resistance reached 1,400 Ω/cm2. C. jejuni 81-176 was inoculated from the apical chamber at an MOI of ∼10 and incubated at 37°C for 0, 4, 6, or 24 h. For the 24-h time point, the cells were washed at 6 h to remove unattached bacteria and cultured in fresh medium for another 18 h. No significant difference was detected between the transepithelial resistance before incubation and that after incubation at any time point. The supernatants from the apical and basolateral chambers were collected separately; the bacteria were removed by centrifugation; and protease inhibitors were added to prevent protein degradation. The concentrations of IL-8 (A) and TNF-α (B) were determined by ELISA. Averages for three independent experiments are shown; error bars, standard deviations. Asterisks represent significant (P < 0.05) differences from control cells that were not inoculated with the bacteria.
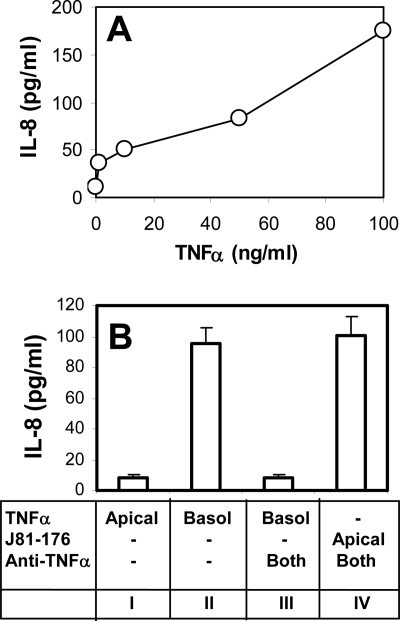
Previous studies showed that TNF-α could induce IL-8 secretion in epithelial cells (12, 26). To test whether Campylobacter-induced IL-8 secretion was due to the secretion of TNF-α, we determined the concentration of TNF-α required for stimulating IL-8 secretion in T84 cells and the effect of a TNF-α neutralizing antibody on Campylobacter-stimulated IL-8 secretion. Treatment of T84 cells with TNF-α increased IL-8 secretion in a dose-dependent manner. Increasing IL-8 secretion to a level similar to that in Campylobacter infection required more than 50 ng/ml of TNF-α (Fig. 2A), which was much greater than the amount of TNF-α secretion induced by Campylobacter (Fig. 1B). It was noticed that only basolateral and not apical treatment with TNF-α effectively induced IL-8 secretion (Fig. 2B), suggesting a polarized expression of TNF-α receptors at the basolateral surface of polarized T84 cells. Treatment of polarized T84 cells with a TNF-α neutralizing antibody in both the apical and basolateral media abolished TNF-α-induced IL-8 secretion, indicating the efficacy of the neutralizing antibody. However, the same TNF-α neutralizing antibody treatment did not affect the level of IL-8 secretion stimulated by apical inoculation of C. jejuni 81-176 (Fig. 2B). Therefore, Campylobacter-induced IL-8 secretion was not the result of TNF-α induction.
FIG. 2.
Campylobacter-stimulated IL-8 secretion is not dependent on the secretion of TNF-α. (A) Polarized T84 cells were incubated with different concentrations of TNF-α (0, 5, 10, 50, and 100 ng/ml) basolaterally at 37°C for 24 h. The basolateral media were collected, and the concentrations of IL-8 were measured using ELISA. (B) IL-8 concentrations in the basolateral medium were measured using ELISA at 24 h after T84 cells were incubated under the following conditions: TNF-α (50 ng/ml) applied apically (Apical) (bar I), TNF-α applied basolaterally (Basol) (bar II), (III) TNF-α applied basolaterally plus mouse anti-human TNF-α antibody (5 μg/ml) applied both apically and basolaterally (Both) (bar III), or live C. jejuni 81-176 applied apically plus mouse anti-human TNF-α antibody applied both apically and basolaterally (bar IV). Averages from three independent experiments are shown; error bars, standard deviations.
Campylobacter adherence, invasion, transcytosis, and inoculation direction are not essential for stimulating IL-8 secretion.
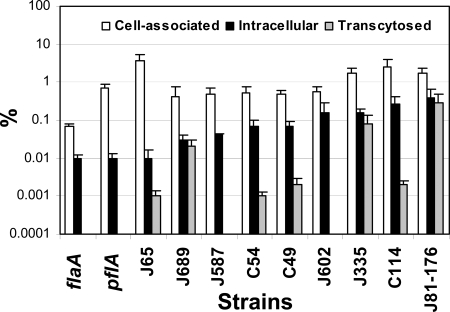
To test whether the adherence, invasion and transcytosis abilities of Campylobacter are important for the increase in IL-8 secretion, we compared the adherence, invasion, and transcytosis abilities of the following strains to their IL-8 induction abilities: the human clinical isolate C. jejuni 81-176, its flaA (K2-32) and pflA (K2-55) mutants, and five C. jejuni and three C. coli isolates recovered from retail chicken meat. We did not detect any significant difference between the growth rates of the different strains used when they were cultured in the invasion medium (data not shown). When inoculated from the apical surface of polarized T84 cells, these bacterial strains displayed different adherence, invasion, and transcytosis abilities (Fig. 3). C. jejuni 81-176 showed the highest invasion and transcytosis efficiencies among the strains tested. The invasion ability of C. jejuni 65 was 40-fold less than that of C. jejuni 81-176, and no transcytosis was detected with C. jejuni 587 or 602 (Fig. 3). In results similar to those with nonpolarized intestinal epithelial cells, the flaA mutant, which is nonmotile and lacks flagellin A but still expresses flagellin B (59), adhered to and invaded polarized T84 cells at a much lower level than wt C. jejuni 81-176, and the pflA mutant, which still assembles flagella but is nonmotile, adhered at a level similar to that of the wt strain but invaded polarized T84 cells at a much lower level than the wt strain (Fig. 3). Both of these noninvasive mutants failed to transcytose across polarized T84 cells. Despite their differences in adherence, invasion, and transcytosis efficiencies, all strains tested were able to increase IL-8 secretion on both the apical and basolateral sides (Fig. 4A), suggesting that Campylobacter adherence, invasion, and transcytosis are not essential for the stimulation of IL-8 secretion. Statistical analysis (Pearson's correlation coefficients) showed a significant positive correlation (r = 0.785; P < 0.007) between the levels of Campylobacter-induced IL-8 secretion and Campylobacter invasion but much weaker correlations between Campylobacter-induced IL-8 secretion and Campylobacter transcytosis (r = 0.439; P < 0.204) and adherence (r = 0.222; P < 0.538) (Fig. 4C). This suggests that while it is not essential, Campylobacter invasion is involved in the stimulation of IL-8 secretion by polarized epithelial cells.
FIG. 3.
Adherence, invasion, and transcytosis abilities of different Campylobacter strains in polarized human intestinal epithelial cells. Polarized T84 cells were inoculated with different chicken isolates (C. coli [C] or C. jejuni [J] strains) or with human clinical isolate C. jejuni 81-176 or its flaA or pflA mutant from the apical chambers of transwells at an MOI of ∼10 and were incubated at 37°C for 4 h. No significant difference was detected between the transepithelial resistance before incubation and that after incubation. The medium from the basolateral chamber was collected to determine the number of transcytosed bacteria. T84 cells were washed and lysed to determine the number of host cell-associated bacteria. Parallel transwells were treated with 100 μg/ml gentamicin, washed, and lysed to determine the number of intracellular bacteria. The data are presented as percentages of the inocula and are averages for three independent experiments with triplicate samples. Error bars, standard deviations.
FIG. 4.
Comparison of IL-8 secretion induced by different Campylobacter strains inoculated apically or basolaterally. (A and B) Polarized T84 cells were inoculated from either the apical (A) or the basolateral (B) side with chicken meat isolates (C. coli [C] or C. jejuni [J] strains) or with C. jejuni 81-176 or its flaA mutant at an MOI of ∼10 and were incubated for 24 h. At 6 h, T84 cells were washed and placed in fresh medium. No significant difference was detected between the transepithelial resistance before incubation and that after incubation. Uninfected T84 cells were used as controls. The apical and basolateral media were collected separately at 24 h, and the concentrations of IL-8 were measured by ELISA. Averages for three independent experiments are shown; error bars, standard deviations. (C) Pearson's correlation coefficients between IL-8 secretion and Campylobacter adherence, invasion, and transcytosis efficiencies (expressed as percentages of the inocula) were calculated and tested for significance (α = 0.05 by a two-tailed test).
To test whether Campylobacter-stimulated IL-8 secretion depends on which surface of the polarized epithelial cells the bacteria colonize and invade from, we compared the IL-8 secretion levels induced by basolateral inoculation of Campylobacter with those induced by apical inoculation. Both apical (Fig. 4A) and basolateral (Fig. 4B) inoculation of the polarized T84 cells with the 10 Campylobacter strains resulted in significant increases in IL-8 secretion over that in uninfected T84 cells (Fig. 4). The levels of IL-8 induced by the basolateral inoculation of Campylobacter (Fig. 4B) were slightly higher than those induced by apical inoculation (Fig. 4A). Like apical inoculation, basolateral inoculation increased IL-8 secretion on both the apical and basolateral sides without altering its basolateral dominance (Fig. 4B). These data indicate that Campylobacter can increase IL-8 secretion and that this IL-8 secretion maintains its basolateral dominance regardless of whether the bacteria are inoculated from the apical or the basolateral surface of polarized epithelial cells.
Protein factors secreted by Campylobacter and/or host cells are involved in IL-8 induction.
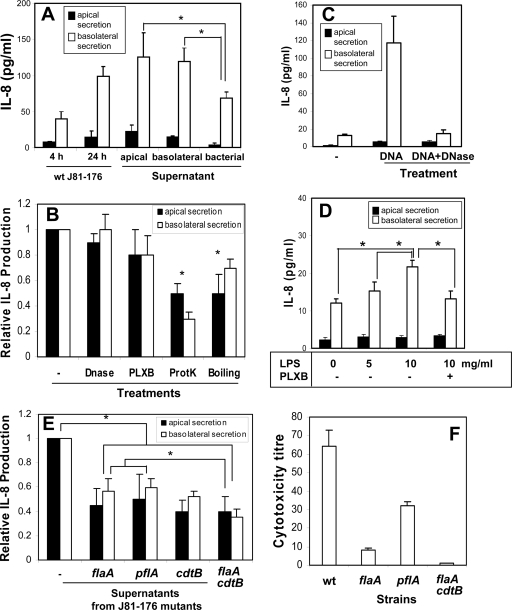
To identify the factors responsible for the induction of IL-8 secretion, we tested the effect of Campylobacter-T84 cell coculture supernatants on IL-8 secretion. The bacterium-free conditioned supernatants were generated from the apical or basolateral medium of T84 cells that were infected with C. jejuni 81-176 apically for 4 h, and the bacterial-culture supernatant was generated from the same number of bacteria cultured in the invasion medium for 4 h. Polarized T84 cells were treated apically with the apical conditioned supernatant, basolaterally with the basolateral conditioned supernatant, or apically with the bacterial-culture supernatant for 24 h. All three types of supernatant significantly increased IL-8 secretion by polarized T84 cells over the control level secreted by T84 cells inoculated with the bacteria for 4 h (Fig. 5A). The levels of IL-8 induced by the apical and basolateral conditioned supernatants were similar to that induced by wt C. jejuni 81-176 after a 24-h inoculation and higher than that induced by the bacterial-culture supernatant (Fig. 5A). These results suggest that factors constitutively secreted by Campylobacter and factors secreted by the bacteria and/or the host cells upon Campylobacter-T84 cell interaction are responsible for the stimulation of IL-8 secretion.
FIG. 5.
IL-8 secretion induced by conditioned supernatants generated from Campylobacter-T84 cell coculture. (A) Polarized T84 cells were incubated for 24 h either with bacterium-free conditioned supernatants generated from the apical or basolateral supernatant of polarized T84 cells that had been inoculated with C. jejuni 81-176 for 4 h or with C. jejuni 81-176 alone cultured in the invasion medium for 4 h (bacterial). T84 cells were incubated with the bacterial culture medium in the apical chamber, with the apical conditioned supernatant in the apical chamber, or with the basolateral conditioned supernatant in the basolateral chamber. Polarized T84 cells inoculated apically with live C. jejuni 81-176 for 4 h and 24 h were used as controls. (B) The basolateral conditioned supernatant generated from C. jejuni 81-176-T84 cell coculture either was not pretreated (−) or was pretreated with either DNase I (10 U/ml) at 37°C for 2 h, polymyxin B (20 μg/ml) at 37°C for 30 min (PLXB), protease K (100 μg/ml) overnight followed by a 20-min incubation at 100°C (ProtK), or a 20-min incubation at 100°C without protease K (Boiling). Polarized T84 cells were incubated with the pretreated or untreated conditioned supernatants from wt C. jejuni 81-176-T84 cell coculture in the basolateral chamber at 37°C for 24 h. (C) Polarized T84 cells were treated basolaterally with a C. jejuni 81-176 DNA extract (25 μg/ml) (DNA) and DNase I-treated C. jejuni 81-176 DNA (DNA + DNase) at 37°C for 24 h. (D) Polarized T84 cells were incubated basolaterally with different concentrations of E. coli LPS in the presence or absence of 20 μg/ml PLXB. (E) Polarized T84 cells were incubated with conditioned supernatants from a coculture of T84 cells with wt C. jejuni 81-176 or its flaA, pflA, cdtB, or flaA cdtB mutant in the basolateral chamber at 37°C for 24 h. After different treatments, the apical (filled bars) and basolateral (open bars) media were collected. IL-8 concentrations were determined by ELISA. The data are means for three independent experiments; error bars, standard deviations. The data in panels B and E are expressed as the ratio of the IL-8 level induced by a treated basolateral conditioned supernatant to that induced by an untreated supernatant and as the ratio of the IL-8 level induced by a mutant basolateral conditioned supernatant to that induced by a wt supernatant. *, P < 0.05. (F) The cytotoxicity of CDT was measured by incubating Vero cells with serially diluted C. jejuni culture supernatants that were filtrated and treated with polymyxin B for 48 h. The viability of the Vero cells was monitored using an MTT assay. The CDT titers were expressed as the reciprocal of the highest dilution that caused 50% Vero cell death compared with the level in untreated cells. Data are averages for three independent cytotoxicity assays; error bars, standard deviations.
To test which secreted factors in the conditioned media contribute to the induction of IL-8 secretion, we treated the basolateral conditioned supernatant with DNase I to remove bacterial DNA (agonists of TLR9), with polymyxin B to neutralize LPS (agonists of TLR4), with protease K to remove proteins, or with heat to denature proteins. Since the basolateral conditioned medium induced a level of IL-8 secretion similar to that induced by the apical conditioned medium, we chose to use the basolateral medium, which avoided the indirect effect caused by the bacterial metabolites produced due to bacterial overgrowth. Either boiling or protease K treatment significantly decreased the IL-8 secretion level (Fig. 5B). Protease K was the most effective of all treatments at reducing IL-8 secretion, with a ∼70% reduction in the basolateral secretion of IL-8 (Fig. 5B). Incubation of polarized T84 cells with DNA extracted from C. jejuni 81-176 significantly increased IL-8 secretion, and this increase was abolished by DNase I treatment (Fig. 5C), demonstrating the efficacy of DNase treatment in removing stimulatory DNA. However, treatment of the basolateral conditioned supernatant with DNase I did not significantly change its ability to induce IL-8 secretion (Fig. 5B). It has been reported that intestinal epithelial cells do not respond to LPS due to their lack of expression of the TLR4 coreceptor MD4 (1, 2, 54). Consistent with these reports, incubation of T84 cells with 10 μg/ml of purified E. coli LPS increased the IL-8 secretion level only slightly. This increase was sensitive to polymyxin B treatment (Fig. 5D), showing the effectiveness of polymyxin B at removing LPS. However, polymyxin B had no significant effect on the ability of the conditioned medium to induce IL-8 secretion (Fig. 5B).
The sensitivity of the conditioned medium to protease K and heat, but not to DNase and polymyxin B, suggests that proteins secreted by the bacteria and/or host cells were responsible for the induction of IL-8 secretion. To determine whether flagellin and CDT, two proteins secreted by Campylobacter, are involved in the induction of IL-8 secretion, we determined the effects of two flagellum mutants (the flaA [K2-32] and pflA [K2-55] mutants), a cdtB mutant that does not secrete CDT, and a flaA cdtB double mutant on IL-8 secretion. The mutations in the flagellum genes (flaA or pflA) and the cdtB gene each caused a ∼45% reduction in the ability of conditioned supernatants to increase IL-8 secretion (Fig. 5E). The flaA cdtB double mutant further reduced IL-8 secretion to 35% of the level induced by the wt (Fig. 5E). By determining the cytotoxicity of CDT secreted to the bacterial culture medium, we found that the flaA and pflA flagellum mutants caused ∼90% and ∼50% reductions, respectively, in the cytotoxicity of secreted CDT (Fig. 5F). This suggests that flagellum mutants can inhibit IL-8 secretion by interfering with CDT secretion. Taken together, our results suggest that while multiple factors could be involved, proteins secreted by host cells and Campylobacter, such as CDT and flagellum, are the major factors responsible for the induction of IL-8 secretion in polarized T84 cells.
Campylobacter-induced IL-8 secretion in human intestinal epithelial cells is MyD88 dependent.
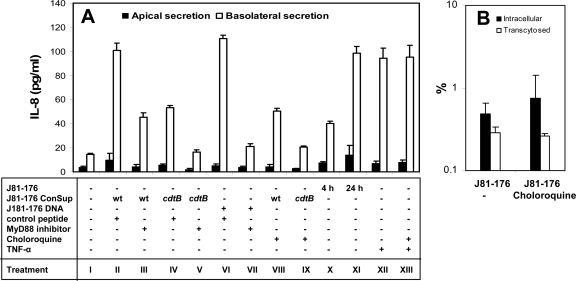
To investigate the role of TLRs in the induction of IL-8 secretion, we examined the effects of two TLR signaling inhibitors on IL-8 secretion stimulated by conditioned supernatants generated from wt C. jejuni 81-176 and its cdtB mutant. MyD88 is an important adaptor molecule in the upstream signaling cascades of almost all TLRs. MyD88 inhibitory peptide inhibits MyD88-dependent signaling activity by interfering with MyD88 homodimer formation (35). Chloroquine inhibits endosome maturation, which is required for the activation of intracellular TLRs, such as TLR7 and TLR9 (32, 33, 48). While the control peptide had no significant effect on the IL-8 induction abilities of the conditioned supernatant (Fig. 6A, bars II) and Campylobacter DNA (Fig. 6A, bars VI), MyD88 inhibitory peptide almost completely abolished Campylobacter DNA-induced IL-8 production (Fig. 6A, bars VII), indicating its efficacy at inhibiting TLR signaling pathways. The inhibitory peptide resulted in a ∼60% reduction in the secretion of IL-8 induced by the conditioned supernatant generated from C. jejuni 81-176 (Fig. 6A, bars III) and abolished the remaining IL-8 induction ability of the conditioned supernatant generated from the cdtB mutant (Fig. 6A, bars V). Like treatment with the MyD88 inhibitory peptide, chloroquine treatment resulted in a ∼50% reduction in IL-8 secretion induced by the conditioned medium of the wt (Fig. 6A, bars VIII) and abolished the remaining IL-8 induction ability of the conditioned supernatant of the cdtB mutant (Fig. 6A, bars IX). Treatment of T84 cells with chloroquine had no significant effect on TNF-α-induced IL-8 secretion (Fig. 6A, bars XII and XIII) or on invasion and transcytosis by C. jejuni 81-176 (Fig. 6B), indicating that the inhibitory effect of chloroquine on Campylobacter-induced IL-8 secretion is not due to any interference by chloroquine with Campylobacter invasion and transcytosis or with the cellular machinery for IL-8 secretion. These results demonstrate that Campylobacter-induced IL-8 secretion depends on both CDT and TLRs.
FIG. 6.
Campylobacter-induced IL-8 secretion depends on Campylobacter-secreted CDT and the TLR signaling adaptor MyD88 in polarized intestinal epithelial cells. (A) Polarized T84 cells were pretreated with MyD88 inhibitory peptide (100 μM) (bars III, V, and VII) or its control peptide (bars II, IV, and VI) for 24 h or with chloroquine (5 μM) (bars VIII, IX, and XIII) for 30 min before being incubated with the conditioned supernatant (ConSup) from the coculture of T84 cells with wt C. jejuni 81-176 (bars II, III, and VIII), its cdtB mutant (bars IV, V, and IX), or a C. jejuni 81-176 DNA extract (25 μg/ml) (bars VI and VII) at 37°C for 24 h. MyD88 inhibitory peptide, its control peptide, or chloroquine was also included during the 24-h incubation. Polarized T84 cells incubated with TNF-α only (bar XII) or TNF-α plus chloroquine (bar XIII) were used as controls for the effect of chloroquine on IL-8 secretion. Polarized T84 cells incubated with the medium alone for 24 h (bars I) or with live C. jejuni 81-176 for 4 h (bars X), after which the conditioned supernatants were collected, or 24 h (bars XI) were used as controls. In the 24-h incubation with live bacteria, unbound bacteria were removed at 6 h. No significant difference was detected between the transepithelial resistance before incubation and that after incubation. The apical and basolateral media were collected individually, and the IL-8 concentrations were determined by ELISA. The data are means for three independent experiments; error bars, standard deviations. (B) Polarized T84 cells were pretreated with chloroquine (5 μM) for 30 min before incubation with C. jejuni 81-176 (MOI, ∼10) at 37°C for 4 h. The abilities of C. jejuni 81-176 to adhere to, invade, and transcytose across chloroquine-treated T84 cells were analyzed as described in the legend to Fig. 3. Averages for three independent experiments with triplicate samples are shown; error bars, standard deviations.
Campylobacter-induced IL-8 secretion in human intestinal epithelial cells is NF-κB dependent.
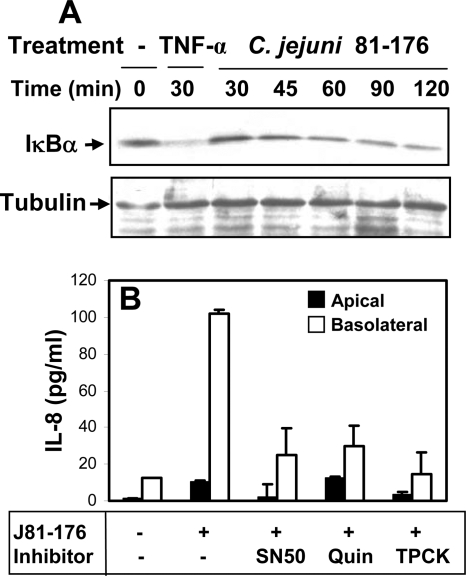
NF-κB is an important transcription regulator of IL-8 (14, 44). To determine whether Campylobacter-induced IL-8 secretion is dependent on NF-κB activation, we first tested whether Campylobacter could induce the activation of NF-κB. The activation of NF-κB was followed by the degradation of the NF-κB inhibitor IκB-α as determined by Western blotting (43). TNF-α treatment significantly decreased the IκB-α level at 30 min (Fig. 7A), indicating the activation of NF-κB. Inoculation with C. jejuni 81-176 induced the degradation of IκB-α in T84 cells in a time-dependent manner. After incubation with the bacteria for 120 min, the amount of IκB-α in T84 cells decreased to a level similar to that in T84 cells treated with TNF-α for 30 min (Fig. 7A). This indicated that Campylobacter inoculation induced NF-κB activation in T84 cells. Next, we tested the effects of three different NF-κB inhibitors, which block different steps of NF-κB activation, on Campylobacter-induced IL-8 secretion. SN50 is a cell-permeant peptide that inhibits the translocation of the active NF-κB complex into the nucleus (34). Quinazoline inhibits NF-κB transcriptional activation (56). TPCK, a proteasome inhibitor, inhibits IκB-α degradation (20). T84 cells were treated with one of the three inhibitors in both the apical and basolateral media for 30 min before and during Campylobacter inoculation. Treatment with these inhibitors had no significant effect on the transepithelial resistance of the polarized epithelial-cell monolayer (data not shown), indicating that these inhibitors did not disturb the integrity of epithelial-cell monolayers. All three inhibitors reduced Campylobacter-induced IL-8 secretion to levels similar to that in uninfected T84 cells (Fig. 7B). This result indicates that NF-κB activation is required for Campylobacter-induced secretion of IL-8.
FIG. 7.
Campylobacter-induced IL-8 secretion is dependent on NF-κB activation. (A) T84 cells were incubated with C. jejuni 81-176 (MOI, ∼10) in the presence of cycloheximide for varying lengths of time. T84 cells treated with TNF-α were used as a positive control. −, no treatment. The cells were washed and lysed, and the cell lysates were analyzed by SDS-PAGE and Western blotting with probing for IκB-α. The blots were stripped and reprobed for tubulin as a loading control. Representative results from three independent experiments are shown. (B) Polarized T84 cells were pretreated with the NF-κB inhibitor SN50 (18 μM), quinazoline (28 μM), or TPCK (50 μM) for 30 min and then incubated with C. jejuni 81-176 in the presence of the inhibitor at 37°C for 24 h. No significant difference was detected between the transepithelial resistance before incubation and that after incubation. The apical and basolateral media were collected individually, and the IL-8 concentrations were determined by ELISA. The data are means for three independent experiments; error bars, standard deviations.
DISCUSSION
IL-8 is one of the earliest cytokines that are induced by enteric bacteria (12, 13, 28). IL-8 chemoattracts neutrophils to infection sites, initiating local inflammation (29, 31). It has been reported previously that the interaction of enteric pathogens, including Campylobacter, with T84 polarized human intestinal epithelial cells increases IL-8 secretion (5, 9, 37, 50). Here, we found that Campylobacter-stimulated IL-8 secretion maintained its basolateral dominance, independent of the Campylobacter inoculation direction. Furthermore, Campylobacter strains that failed to efficiently transcytose across polarized T84 cells were able to increase the basolaterally dominated secretion of IL-8 upon apical inoculation. These data suggest that the basolateral secretion of IL-8 does not require the transcytosis of C. jejuni from the apical to the basolateral side during infection and that the polarity of IL-8 secretion is dependent on an intrinsic mechanism of polarized epithelial cells that specifically targets IL-8 to the basolateral surface, but not on the polarity of stimulation. The interaction of Campylobacter with polarized epithelial cells at the apical surface may trigger signals that stimulate IL-8 secretion at the basolateral surface. The independence of the basolateral secretion of IL-8 from Campylobacter transcytosis could allow for the effective chemoattraction of neutrophils from the underlying tissues and blood vessels and the induction of inflammatory responses, despite the differing abilities of infecting Campylobacter strains to transcytose across the intestinal epithelium.
In this study, we found that all the Campylobacter strains used here stimulated IL-8 production in polarized T84 cells, in spite of their different adherence, invasion, and transcytosis abilities. This indicates that Campylobacter adherence, invasion, and transcytosis abilities are not essential for stimulating IL-8 secretion in polarized T84 cells. The finding of a significant positive correlation between IL-8 secretion levels and Campylobacter invasion but not adherence and transcytosis efficacies suggests that invasion is involved in IL-8 induction in polarized intestinal epithelial cells. This finding is in line with those of a previous study using INT407 nonpolarized human embryo intestinal epithelial cells as a model (21). The ability of Campylobacter to invade intestinal epithelial cells distinguishes this enteric pathogen from commensal bacteria in the gut and could render this pathogen able to effectively trigger intracellularly expressed TLRs, leading to NF-κB activation and IL-8 secretion.
Our data revealed that bacterium-free conditioned supernatants generated from the basolateral and apical media of T84 cells that were inoculated with C. jejuni 81-176 for 4 h induced IL-8 secretion to levels similar to that induced by live bacterial inoculation. This indicates that the induction of IL-8 secretion in T84 cells involves molecules secreted by C. jejuni 81-176 and/or T84 cells. The supernatant of bacterial culture alone increased IL-8 secretion in T84 cells, indicating that Campylobacter constitutively secretes factors that are involved in stimulating IL-8 secretion. The conditioned supernatants from Campylobacter-T84 cell coculture were more effective than the bacterial-culture supernatant at the induction of IL-8 secretion, suggesting that the interaction between the bacteria and T84 cells induces the secretion of bacterial and/or host factors that are important for IL-8 induction. The basolateral and apical conditioned supernatants induced IL-8 secretion to similar levels, in spite of the fact that there were many fewer bacteria in the basolateral medium than in the apical medium, since only <1% of inoculated Campylobacter bacteria transmigrated from the apical into the basolateral chamber. This may result from a rapid transcytosis of Campylobacter-secreted factors from the apical to the basolateral surface independent of bacterial transcytosis or from a direct secretion of host factors by epithelial cells from the basolateral surface upon interaction with the bacteria at the apical surface.
We found that protease K- and heat-sensitive factors in the conditioned supernatants were involved in IL-8 induction. TNF-α secreted by host epithelial cells can stimulate IL-8 secretion by intestinal epithelial cells (12). However, we showed that a TNF-α neutralization antibody that effectively blocked TNF-α-induced IL-8 secretion failed to reduce Campylobacter-induced IL-8 secretion by T84 cells, indicating that TNF-α is not the major host-secreted factor responsible for IL-8 induction. One of the bacterially secreted, heat-labile protein factors that have been shown to induce IL-8 secretion in the absence of live, intact bacteria is CDT (22). Our finding that the conditioned supernatant of the C. jejuni 81-176 cdtB mutant exhibited a ∼50% reduction in IL-8 induction further confirms this notion and also suggests that additional factors are required for IL-8 induction. Another candidate is flagellin, a potential ligand for TLR5. However, previous studies showed that purified Campylobacter flagellin failed to induce IL-8 secretion in polarized T84 cells (9, 58). Our finding that the flaA mutant, which is nonmotile and lacks flagellin A but not flagellin B expression, and the pflA mutant, which expresses flagellin but is not motile, caused similar (∼50%) reductions in IL-8 induction further argues against a role for secreted flagellin A in stimulating IL-8 secretion through TLR5. Campylobacter flagella are required for invasion of and transcytosis across human epithelial cells and also provide a type III secretory organelle in the absence of a specialized secretion system. A previous study showed that the flaA mutant maintained its activity in secreting Cia proteins (27). Here we found that flagellum mutations significantly decreased the cytotoxicity of CDT secreted into the C. jejuni culture medium, suggesting that C. jejuni CDT secretion depended on flagella. However, the flaA (∼90%) and pflA (∼50%) mutants, which caused different levels of reduction in CDT secretion, reduced IL-8 induction to similar levels, suggesting a role of flagella in addition to mediating CDT secretion. These findings together support the hypothesis that the flagellum can participate in the induction of IL-8 secretion by mediating CDT secretion and proper Campylobacter-host cell interaction.
Treatment with protease K did not completely abolish IL-8 secretion, suggesting the involvement of factors that are not proteins. DNA and LPS are two major nonprotein factors secreted by bacteria that are potential stimulators of IL-8 secretion. Indeed, treatment of polarized T84 cells with purified Campylobacter DNA, a potential ligand of TLR9, significantly increased IL-8 secretion. However, DNase I treatment, which blocked Campylobacter DNA-induced IL-8 secretion, had no significant effect on the ability of the conditioned supernatant to induce IL-8 secretion. This indicates that Campylobacter DNA, which can be removed by DNase I, is not important for IL-8 induction. This does not exclude the possibility of the presence of DNase-resistant DNA-protein complexes that are able to stimulate IL-8 secretion. It has been reported that intestinal epithelial cells, including the T84 cell line, do not express the key coreceptor of TLR4, MD-2, and do not respond to LPS (1, 2, 54), providing the intestinal epithelium with tolerance to gram-negative commensal bacteria. Our results showed that treatment of T84 cells with a relatively high concentration of E. coli LPS (10 μg/ml) only slightly increased IL-8 secretion, consistent with these previous reports. Treatment of the conditioned supernatants with the LPS neutralization reagent polymyxin B, which has been used previously to neutralize LPS (6) and release Campylobacter enterotoxins (57), had no effect on Campylobacter-induced IL-8 secretion. These results further argue against a role for Campylobacter LPS in stimulating IL-8 secretion by human intestinal epithelial cells. Our finding that the flaA, pflA, cdtB, and flaA cdtB mutations of C. jejuni 81-176, as well as protease K treatment, all caused partial reductions in IL-8 induction suggests that multiple bacterial or host factors are involved in the induction of IL-8 secretion and that the optimal induction of IL-8 secretion requires other factors in addition to CDT and a complete flagellar apparatus.
TLRs are important innate immune receptors that play an essential role in initiating inflammatory responses. Here, we revealed for the first time that MyD88, the key signaling adaptor for most TLRs, was required for Campylobacter-induced IL-8 secretion, demonstrating an essential role of TLRs in triggering IL-8 secretion by human intestinal epithelial cells. The sensitivity of Campylobacter DNA-induced IL-8 secretion to MyD88 inhibitory peptide confirmed the effectiveness of the inhibitor. The inhibitory effect of chloroquine, which blocks the activation of intracellular TLRs by interfering with endosome maturation, on IL-8 secretion further supported a role for TLRs in IL-8 induction. While the MyD88 inhibitor or the cdtB mutation alone only partially inhibited Campylobacter-induced IL-8 secretion, the combination of the MyD88 inhibitor and the cdtB mutation brought the IL-8 secretion down to the baseline level. This demonstrates that both Campylobacter CDT and host cell TLRs are required for optimal IL-8 induction and suggests that CDT and TLRs induce IL-8 secretion using different pathways. The findings that Campylobacter-secreted flagellin, LPS, and DNA (which can be removed by DNase I) are not important for IL-8 induction suggest that these are not the main activators of TLRs. How and which TLRs are activated during Campylobacter infection remain to be elucidated.
Previous studies showed that Campylobacter-induced NF-κB activation was required for the induction of IL-8 secretion (24, 41). Here we further supported this notion by showing that all three different NF-κB inhibitors completely blocked Campylobacter-induced IL-8 secretion. The blockage of Campylobacter-induced IL-8 secretion by NF-κB inhibitors suggests that both CDT- and TLR-triggered IL-8 secretion depends on NF-κB activation. While TLR-mediated NF-κB activation has been well studied (8), whether and how CDT activates NF-κB is not known.
The studies presented here show that Campylobacter increases IL-8 secretion by polarized human intestinal epithelial cells. Campylobacter adherence, invasion, and transcytosis abilities are not essential for stimulating IL-8 secretion. Protein factors secreted by Campylobacter and/or host cells are required for stimulating IL-8 secretion. CDT, which is secreted by Campylobacter, probably through flagella, but not epithelial-cell-secreted TNF-α, is important for Campylobacter-induced IL-8 secretion. Campylobacter-induced IL-8 secretion depends on the activation of NF-κB by TLR and CDT in polarized epithelial cells. This reveals the underlying mechanism for the induction of IL-8 secretion in human intestinal epithelial cells. Further studies are required to identify the factors that are secreted by Campylobacter or intestinal epithelial cells upon bacterium-host cell interaction and trigger TLR and NF-κB activation and to understand the mechanisms for TLR- and CDT-induced IL-8 secretion in human intestinal epithelial cells.
Acknowledgments
This work was supported by a research grant from the Joint Institute of Food Safety and Applied Nutrition, University of Maryland and Food and Drug Administration.
We thank Patricia Guerry-Kopecko and James Fox for providing mutant strains and Karen Swanson and Margaret Seeley for critical reading of the manuscript.
Editor: V. J. DiRita
Footnotes
Published ahead of print on 21 July 2008.
REFERENCES
- 1.Abreu, M. T., M. Fukata, and M. Arditi. 2005. TLR signaling in the gut in health and disease. J. Immunol. 1744453-4460. [DOI] [PubMed] [Google Scholar]
- 2.Abreu, M. T., P. Vora, E. Faure, L. S. Thomas, E. T. Arnold, and M. Arditi. 2001. Decreased expression of Toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccharide. J. Immunol. 1671609-1616. [DOI] [PubMed] [Google Scholar]
- 3.Akhtar, M., J. L. Watson, A. Nazli, and D. M. McKay. 2003. Bacterial DNA evokes epithelial IL-8 production by a MAPK-dependent, NF-κB-independent pathway. FASEB J. 171319-1321. [DOI] [PubMed] [Google Scholar]
- 4.Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2675-680. [DOI] [PubMed] [Google Scholar]
- 5.Beatty, W. L., and P. J. Sansonetti. 1997. Role of lipopolysaccharide in signaling to subepithelial polymorphonuclear leukocytes. Infect. Immun. 654395-4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhor, V. M., C. J. Thomas, N. Surolia, and A. Surolia. 2005. Polymyxin B: an ode to an old antidote for endotoxic shock. Mol. Biosyst. 1213-222. [DOI] [PubMed] [Google Scholar]
- 7.Brown, K., S. Gerstberger, L. Carlson, G. Franzoso, and U. Siebenlist. 1995. Control of IκB-α proteolysis by site-specific, signal-induced phosphorylation. Science 2671485-1488. [DOI] [PubMed] [Google Scholar]
- 8.Carmody, R. J., and Y. H. Chen. 2007. Nuclear factor-κB: activation and regulation during toll-like receptor signaling. Cell. Mol. Immunol. 431-41. [PubMed] [Google Scholar]
- 9.Chen, M. L., Z. Ge, J. G. Fox, and D. B. Schauer. 2006. Disruption of tight junctions and induction of proinflammatory cytokine responses in colonic epithelial cells by Campylobacter jejuni. Infect. Immun. 746581-6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coote, J. G., D. E. Stewart-Tull, R. J. Owen, F. J. Bolton, B. L. Siemer, D. Candlish, D. H. Thompson, A. C. Wardlaw, S. L. On, A. Candlish, B. Billcliffe, P. J. Jordan, K. Kristiansen, and P. Borman. 2007. Comparison of virulence-associated in vitro properties of typed strains of Campylobacter jejuni from different sources. J. Med. Microbiol. 56722-732. [DOI] [PubMed] [Google Scholar]
- 11.Dyer, R. B., C. R. Collaco, D. W. Niesel, and N. K. Herzog. 1993. Shigella flexneri invasion of HeLa cells induces NF-κB DNA-binding activity. Infect. Immun. 614427-4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eckmann, L., H. C. Jung, C. Schurer-Maly, A. Panja, E. Morzycka-Wroblewska, and M. F. Kagnoff. 1993. Differential cytokine expression by human intestinal epithelial cell lines: regulated expression of interleukin 8. Gastroenterology 1051689-1697. [DOI] [PubMed] [Google Scholar]
- 13.Eckmann, L., M. F. Kagnoff, and J. Fierer. 1993. Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infect. Immun. 614569-4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elewaut, D., J. A. DiDonato, J. M. Kim, F. Truong, L. Eckmann, and M. F. Kagnoff. 1999. NF-κB is a central regulator of the intestinal epithelial cell innate immune response induced by infection with enteroinvasive bacteria. J. Immunol. 1631457-1466. [PubMed] [Google Scholar]
- 15.Fox, J. G., A. B. Rogers, M. T. Whary, Z. Ge, N. S. Taylor, S. Xu, B. H. Horwitz, and S. E. Erdman. 2004. Gastroenteritis in NF-κB-deficient mice is produced with wild-type Camplyobacter jejuni but not with C. jejuni lacking cytolethal distending toxin despite persistent colonization with both strains. Infect. Immun. 721116-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gentry, M. K., and J. M. Dalrymple. 1980. Quantitative microtiter cytotoxicity assay for Shigella toxin. J. Clin. Microbiol. 12361-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gewirtz, A. T., P. O. Simon, Jr., C. K. Schmitt, L. J. Taylor, C. H. Hagedorn, A. D. O'Brien, A. S. Neish, and J. L. Madara. 2001. Salmonella typhimurium translocates flagellin across intestinal epithelia, inducing a proinflammatory response. J. Clin. Investig. 10799-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guerrant, R. L., C. A. Wanke, R. A. Pennie, L. J. Barrett, A. A. Lima, and A. D. O'Brien. 1987. Production of a unique cytotoxin by Campylobacter jejuni. Infect. Immun. 552526-2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guerry, P., P. M. Pope, D. H. Burr, J. Leifer, S. W. Joseph, and A. L. Bourgeois. 1994. Development and characterization of recA mutants of Campylobacter jejuni for inclusion in attenuated vaccines. Infect. Immun. 62426-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henkel, T., T. Machleidt, I. Alkalay, M. Kronke, Y. Ben-Neriah, and P. A. Baeuerle. 1993. Rapid proteolysis of IκB-α is necessary for activation of transcription factor NF-κB. Nature 365182-185. [DOI] [PubMed] [Google Scholar]
- 21.Hickey, T. E., S. Baqar, A. L. Bourgeois, C. P. Ewing, and P. Guerry. 1999. Campylobacter jejuni-stimulated secretion of interleukin-8 by INT407 cells. Infect. Immun. 6788-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hickey, T. E., A. L. McVeigh, D. A. Scott, R. E. Michielutti, A. Bixby, S. A. Carroll, A. L. Bourgeois, and P. Guerry. 2000. Campylobacter jejuni cytolethal distending toxin mediates release of interleukin-8 from intestinal epithelial cells. Infect. Immun. 686535-6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hobbie, S., L. M. Chen, R. J. Davis, and J. E. Galan. 1997. Involvement of mitogen-activated protein kinase pathways in the nuclear responses and cytokine production induced by Salmonella typhimurium in cultured intestinal epithelial cells. J. Immunol. 1595550-5559. [PubMed] [Google Scholar]
- 24.Hu, L., and T. E. Hickey. 2005. Campylobacter jejuni induces secretion of proinflammatory chemokines from human intestinal epithelial cells. Infect. Immun. 734437-4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jerala, R., and M. Porro. 2004. Endotoxin neutralizing peptides. Curr. Top. Med. Chem. 41173-1184. [DOI] [PubMed] [Google Scholar]
- 26.Jijon, H. B., W. J. Panenka, K. L. Madsen, and H. G. Parsons. 2002. MAP kinases contribute to IL-8 secretion by intestinal epithelial cells via a posttranscriptional mechanism. Am. J. Physiol. Cell Physiol. 283C31-C41. [DOI] [PubMed] [Google Scholar]
- 27.Johanesen, P. A., and M. B. Dwinell. 2006. Flagellin-independent regulation of chemokine host defense in Campylobacter jejuni-infected intestinal epithelium. Infect. Immun. 743437-3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jung, H. C., L. Eckmann, S. K. Yang, A. Panja, J. Fierer, E. Morzycka-Wroblewska, and M. F. Kagnoff. 1995. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J. Clin. Investig. 9555-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelly, C. P., S. Keates, D. Siegenberg, J. K. Linevsky, C. Pothoulakis, and H. R. Brady. 1994. IL-8 secretion and neutrophil activation by HT-29 colonic epithelial cells. Am. J. Physiol. 267G991-G997. [DOI] [PubMed] [Google Scholar]
- 30.Konkel, M. E., D. J. Mead, S. F. Hayes, and W. Cieplak, Jr. 1992. Translocation of Campylobacter jejuni across human polarized epithelial cell monolayer cultures. J. Infect. Dis. 166308-315. [DOI] [PubMed] [Google Scholar]
- 31.Kucharzik, T., and I. R. Williams. 2002. Neutrophil migration across the intestinal epithelial barrier—summary of in vitro data and description of a new transgenic mouse model with doxycycline-inducible interleukin-8 expression in intestinal epithelial cells. Pathobiology 70143-149. [DOI] [PubMed] [Google Scholar]
- 32.Latz, E., A. Schoenemeyer, A. Visintin, K. A. Fitzgerald, B. G. Monks, C. F. Knetter, E. Lien, N. J. Nilsen, T. Espevik, and D. T. Golenbock. 2004. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat. Immunol. 5190-198. [DOI] [PubMed] [Google Scholar]
- 33.Lee, J., T. H. Chuang, V. Redecke, L. She, P. M. Pitha, D. A. Carson, E. Raz, and H. B. Cottam. 2003. Molecular basis for the immunostimulatory activity of guanine nucleoside analogs: activation of Toll-like receptor 7. Proc. Natl. Acad. Sci. USA 1006646-6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin, Y. Z., S. Y. Yao, R. A. Veach, T. R. Torgerson, and J. Hawiger. 1995. Inhibition of nuclear translocation of transcription factor NF-κB by a synthetic peptide containing a cell membrane-permeable motif and nuclear localization sequence. J. Biol. Chem. 27014255-14258. [DOI] [PubMed] [Google Scholar]
- 35.Loiarro, M., C. Sette, G. Gallo, A. Ciacci, N. Fanto, D. Mastroianni, P. Carminati, and V. Ruggiero. 2005. Peptide-mediated interference of TIR domain dimerization in MyD88 inhibits interleukin-1-dependent activation of NF-κB. J. Biol. Chem. 28015809-15814. [DOI] [PubMed] [Google Scholar]
- 36.MacCallum, A. J., D. Harris, G. Haddock, and P. H. Everest. 2006. Campylobacter jejuni-infected human epithelial cell lines vary in their ability to secrete interleukin-8 compared to in vitro-infected primary human intestinal tissue. Microbiology 1523661-3665. [DOI] [PubMed] [Google Scholar]
- 37.McCormick, B. A., S. P. Colgan, C. Delp-Archer, S. I. Miller, and J. L. Madara. 1993. Salmonella typhimurium attachment to human intestinal epithelial monolayers: transcellular signalling to subepithelial neutrophils. J. Cell Biol. 123895-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCormick, B. A., S. I. Miller, D. Carnes, and J. L. Madara. 1995. Transepithelial signaling to neutrophils by salmonellae: a novel virulence mechanism for gastroenteritis. Infect. Immun. 632302-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McRoberts, J. A., R. Aranda, N. Riley, and H. Kang. 1990. Insulin regulates the paracellular permeability of cultured intestinal epithelial cell monolayers. J. Clin. Investig. 851127-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Medzhitov, R. 2001. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1135-145. [DOI] [PubMed] [Google Scholar]
- 41.Mellits, K. H., J. Mullen, M. Wand, G. Armbruster, A. Patel, P. L. Connerton, M. Skelly, and I. F. Connerton. 2002. Activation of the transcription factor NF-κB by Campylobacter jejuni. Microbiology 1482753-2763. [DOI] [PubMed] [Google Scholar]
- 42.Monteville, M. R., and M. E. Konkel. 2002. Fibronectin-facilitated invasion of T84 eukaryotic cells by Campylobacter jejuni occurs preferentially at the basolateral cell surface. Infect. Immun. 706665-6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moynagh, P. N. 2005. The NF-κB pathway. J. Cell Sci. 1184589-4592. [DOI] [PubMed] [Google Scholar]
- 44.Mukaida, N., Y. Mahe, and K. Matsushima. 1990. Cooperative interaction of nuclear factor-κB- and cis-regulatory enhancer binding protein-like factor binding elements in activating the interleukin-8 gene by pro-inflammatory cytokines. J. Biol. Chem. 26521128-21133. [PubMed] [Google Scholar]
- 45.Naumann, M., S. Wessler, C. Bartsch, B. Wieland, and T. F. Meyer. 1997. Neisseria gonorrhoeae epithelial cell interaction leads to the activation of the transcription factors nuclear factor κB and activator protein 1 and the induction of inflammatory cytokines. J. Exp. Med. 186247-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Newell, D. G., H. McBride, and J. M. Dolby. 1985. Investigations on the role of flagella in the colonization of infant mice with Campylobacter jejuni and attachment of Campylobacter jejuni to human epithelial cell lines. J. Hyg. (London) 95217-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Philpott, D. J., S. Yamaoka, A. Israel, and P. J. Sansonetti. 2000. Invasive Shigella flexneri activates NF-κB through a lipopolysaccharide-dependent innate intracellular response and leads to IL-8 expression in epithelial cells. J. Immunol. 165903-914. [DOI] [PubMed] [Google Scholar]
- 48.Rutz, M., J. Metzger, T. Gellert, P. Luppa, G. B. Lipford, H. Wagner, and S. Bauer. 2004. Toll-like receptor 9 binds single-stranded CpG-DNA in a sequence- and pH-dependent manner. Eur. J. Immunol. 342541-2550. [DOI] [PubMed] [Google Scholar]
- 49.Savkovic, S. D., A. Koutsouris, and G. Hecht. 1997. Activation of NF-κB in intestinal epithelial cells by enteropathogenic Escherichia coli. Am. J. Physiol. 273C1160-C1167. [DOI] [PubMed] [Google Scholar]
- 50.Schulte, R., and I. B. Autenrieth. 1998. Yersinia enterocolitica-induced interleukin-8 secretion by human intestinal epithelial cells depends on cell differentiation. Infect. Immun. 661216-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwandner, R., R. Dziarski, H. Wesche, M. Rothe, and C. J. Kirschning. 1999. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J. Biol. Chem. 27417406-17409. [DOI] [PubMed] [Google Scholar]
- 52.Skirrow, M. B., and M. J. Blaser. 2000. Clinical aspects of Campylobacter infection, p. 69-88. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd ed. ASM Press, Washington, DC.
- 53.Smith, K. D., and A. Ozinsky. 2002. Toll-like receptor-5 and the innate immune response to bacterial flagellin. Curr. Top. Microbiol. Immunol. 27093-108. [DOI] [PubMed] [Google Scholar]
- 54.Smith, M. F., Jr., A. Mitchell, G. Li, S. Ding, A. M. Fitzmaurice, K. Ryan, S. Crowe, and J. B. Goldberg. 2003. Toll-like receptor (TLR) 2 and TLR5, but not TLR4, are required for Helicobacter pylori-induced NF-κB activation and chemokine expression by epithelial cells. J. Biol. Chem. 27832552-32560. [DOI] [PubMed] [Google Scholar]
- 55.Tauxe, R. V. 1992. Epidemiology of Campylobacter jejuni infections in the United States and other industrialized nations. ASM Press, Washington, DC.
- 56.Tobe, M., Y. Isobe, H. Tomizawa, T. Nagasaki, H. Takahashi, T. Fukazawa, and H. Hayashi. 2003. Discovery of quinazolines as a novel structural class of potent inhibitors of NF-κB activation. Bioorg. Med. Chem. 11383-391. [DOI] [PubMed] [Google Scholar]
- 57.Wassenaar, T. M. 1997. Toxin production by Campylobacter spp. Clin. Microbiol. Rev. 10466-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Watson, R. O., and J. E. Galan. 2005. Signal transduction in Campylobacter jejuni-induced cytokine production. Cell. Microbiol. 7655-665. [DOI] [PubMed] [Google Scholar]
- 59.Yao, R., D. H. Burr, P. Doig, T. J. Trust, H. Niu, and P. Guerry. 1994. Isolation of motile and non-motile insertional mutants of Campylobacter jejuni: the role of motility in adherence and invasion of eukaryotic cells. Mol. Microbiol. 14883-893. [DOI] [PubMed] [Google Scholar]
- 60.Zhao, C., B. Ge, J. De Villena, R. Sudler, E. Yeh, S. Zhao, D. G. White, D. Wagner, and J. Meng. 2001. Prevalence of Campylobacter spp., Escherichia coli, and Salmonella serovars in retail chicken, turkey, pork, and beef from the Greater Washington, D.C., area. Appl. Environ. Microbiol. 675431-5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zheng, J., J. Meng, S. Zhao, R. Singh, and W. Song. 2006. Adherence to and invasion of human intestinal epithelial cells by Campylobacter jejuni and Campylobacter coli isolates from retail meat products. J. Food Prot. 69768-774. [DOI] [PubMed] [Google Scholar]