Abstract
Treatment of ulcerative keratitis due to Pseudomonas aeruginosa is difficult, time-consuming, and uncomfortable owing to the need for the frequent application of antibiotic drops to the infected corneal surface. We examined here whether a fully human immunoglobulin G1 monoclonal antibody (MAb) specific to the conserved alginate surface polysaccharide of P. aeruginosa could mediate protective immunity against typically nonmucoid strains isolated from human cases of keratitis. MAb F429 effectively opsonized alginate-positive, but not alginate-negative, nonmucoid strains in conjunction with phagocytes and complement. Prophylactic administration of MAb F429 18 h prior to infection with two clinical isolates significantly reduced bacterial levels in the eye and the associated corneal pathology. Along similar lines, systemic intraperitoneal injection, as well as topical application of the MAb onto the infected eye, starting 8 h postinfection in both experimental protocols resulted in significant reductions in bacteria in the eye, as well as minimizing pathological damage to the cornea. These findings indicate that MAb F429 could be useful as an additional therapeutic component for the treatment of P. aeruginosa keratitis.
Pseudomonas aeruginosa is usually the most common bacterial pathogen isolated from cases of corneal keratitis (12, 13, 23, 24, 26). Infection poses a serious threat to normal vision and is associated with extended wear of contact lenses, eye trauma (15), eye surgery, and orthokeratology (9, 28), as well as severe burns, ocular irradiation, tracheostomy, exposure to the intensive care environment, or coma (14). Infection can occur following relative minor injury or compromise to the corneal surface and progresses rapidly, with the potential to involve the entire cornea within 2 days. The epithelial injury becomes rapidly necrotic, the underlying stroma becomes edematous, and a mucopurulent discharge develops. More severe eye involvement can then ensue, leading to perforation of the cornea, infection and inflammation in the anterior chamber of the eye, and potentially endophthalmitis (14). Due to the rapid progression of this infection, proper diagnosis and immediate institution of appropriate antimicrobial therapy is essential for resolving the infection and preserving eyesight.
Typically P. aeruginosa keratitis is treated with topical antibiotics including an aminoglycoside such as tobramycin which can be supplemented with another appropriate antibiotic such as piperacillin or ticarcillin. Oral fluoroquinalones may also be used (14). This topical treatment involves applying antibiotic eye drops every 5 min for 1 h and then every 15 to 30 min for 24 to 48 h, an obviously demanding regimen of treatment. Clearly, improvements in this therapeutic approach that would result in less frequent topical applications of antibacterial therapies and function as a supplemental therapy along with antibiotics to improve outcomes could potentially lessen the likelihood that infection progresses to more serious eye pathology.
One potential approach is the use of human monoclonal antibodies (MAbs) to P. aeruginosa antigens that could promote bacterial clearance leading to a more rapid resolution of infection and inflammation, the latter being the primary cause of corneal pathology (4, 5, 26). We have previously described a fully human immunoglobulin G1 (IgG1) MAb specific to the alginate capsule of P. aeruginosa (18) that protected mice against lung infection with both nonmucoid P. aeruginosa strains and alginate-overproducing mucoid P. aeruginosa strains. Protection could even be achieved against the sequenced P. aeruginosa strains PAO1 and PA14, which do not elaborate alginate under typical in vitro, aerobic growth conditions (27), but rapidly elaborate alginate in the lung within 1 to 24 h of infection (2, 18). Prior work has also shown that active immunization of mice with P. aeruginosa lipopolysaccharide (LPS), as well as systemic administration of murine MAbs to P. aeruginosa LPS O-side chains, can protect against keratitis (20), indicating that antibody-mediated immunity to P. aeruginosa can be effective in the infected eye. However, due to the serologic variability in the LPS O antigens (17) and the toxicity associated with vaccination with LPS (16), alternative vaccine targets such as the conserved alginate molecule represent attractive candidates for immunotherapy. In addition, it is not known whether systemic injection or topical application of a MAb to alginate would be efficacious in the setting of keratitis. Therefore, we evaluated the prophylactic and therapeutic efficacy of the human IgG1 MAb F429 in the setting of P. aeruginosa keratitis, using six different P. aeruginosa strains as challenge organisms to determine the potential capacity of this MAb to ameliorate the consequences of infection in the setting of P. aeruginosa eye infection.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Three P. aeruginosa strains positive for expression of the ExoS toxin and designated as invasive strains were used. One of these was strain PAO1V (serogroup O5), a previously described variant of strain PAO1 (19) that has an intact pilC gene unlike the sequenced variant (21) and does not overexpress the MexEF-OprN efflux pump associated with some variants of strain PAO1 with decreased virulence factor production (10) and decreased virulence in the murine model of microbial keratitis (19). The other two, strains 6294 (serogroup O6) and 6354 (serogroup O7), were clinical isolates from cases of microbial keratitis (7). Three isolates expressing the ExoU toxin and designated as cytotoxic strains were also used. One was strain PAO1 ExoU+, containing the cloned P. aeruginosa exoU gene and related chaperone (1); the other two, strains 6077 and 6206, were both serogroup O11 and were isolated from cases of keratitis (7). An additional strain, P. aeruginosa PAO1V with the algD gene interrupted by insertion of a tetracycline resistance gene (PAO1V algD::tet) (25) and thus unable to synthesize alginate, was also used as a specificity control in confocal microscopic analysis, opsonic killing, and protection assays (18). P. aeruginosa PAO1V algD::tet complemented with the intact algD gene on plasmid pSS359 was used in the confocal microscopic analysis. P. aeruginosa was grown overnight at 37°C on a Trypticase soy agar plate for experimental infections.
Human MAb production.
The fully human IgG1 variant of MAb F429 has been previously described (18). Chinese hamster ovary (CHO) cells stably transfected with the MAb F429 IgG1 construct in vector TCAE 6.2 were grown as suspensions in 500 to 1,000 ml of CHO-SFMII medium (Invitrogen Corp., Carlsbad, CA) containing l-glutamine, HEPES buffer, 500 nM methotrexate, and antibiotic supplements. Supernatants were recovered by removing cells via centrifugation and filtration, and the pH was adjusted to 6.5 then applied directly to a protein G column (Invitrogen) to capture the IgG1 antibody. The recommended running buffer was adjusted to pH 6.5 to wash the column, and bound MAb was detached by using a 0.1 M glycine (pH 2.6) buffer that eluted into tubes containing 1 M phosphate buffer (pH 6.4). Fractions containing protein were pooled and dialyzed against phosphate-buffered saline (pH 6.5) and filter sterilized, and the concentration of the MAb was ascertained by analysis of protein concentration using the BCA protein assay reagent (Pierce, Rockford, IL).
Determination of alginate production by P. aeruginosa strains in vitro and in vivo.
Since all of the strains used were phenotypically nonmucoid, we evaluated alginate production following in vitro microaerophilic/anaerobic growth at 37°C in tightly sealed screw-cap tubes filled completely with tryptic soy broth (TSB) medium. Cells were recovered, washed, and then stained for alginate production by immunofluorescence using MAb F429 for detection. After the bacterial cells were suspended in 100 μl of either MAb F429 at 1 mg/ml or a control solution lacking the primary antibody, an incubation period of 60 min at 37°C was carried out, followed by washing the bacterial cells three times in phosphate-buffered saline and resuspending them in a 1:100 dilution of protein A conjugated to Alexa Fluor 546 (Molecular Probes/Invitrogen, Carlsbad, CA). After another 60 min at 37°C, the cells were washed and placed on microscope slides for visualization of alginate elaboration using confocal microscopy. Controls lacked primary MAb.
Opsonic killing assay.
Determination of the in vitro opsonophagocytic killing activity of MAb F429 against the target P. aeruginosa strains was measured by using previously published methods (8) except that infant rabbit serum was used as a source of complement (18, 22). Also, for this assay, the P. aeruginosa strains were grown under the microaerophilic/anaerobic conditions described above in tubes filled with TSB medium.
Murine model of P. aeruginosa keratitis.
The induction of infection on scratch-injured mouse eyes was done as described previously (19) using inocula delivered in 5-μl volumes to mice anesthetized with ketamine (35 mg/kg) and xylazine (5 mg/kg). Corneal pathology was determined by an observer unaware of the infections or treatments given to the animals and graded on a scale of 0 to 4 as follows: 0, eye macroscopically identical to the uninfected contralateral control eye; 1, faint opacity partially covering the pupil; 2, dense opacity covering the pupil; 3, dense opacity covering the entire anterior segment; and 4, perforation of the cornea, phthisis bulbi (shrinkage of the globe after inflammatory disease), or both. The maximal pathology was always observed by 48 h postinfection, and thus comparisons among groups were made at this time point. To evaluate CFU of P. aeruginosa present in infected eyes, mice were euthanized by inhalation of CO2 48 h postinfection, eyes were removed, and corneas were excised as described previously (29). Extracellular P. aeruginosa corneal counts were determined after vortexing the cornea in Dulbecco modified Eagle medium (DMEM) with 1% fetal bovine serum (FBS) and diluting the solution in DMEM with 1% FBS for bacterial enumeration. The corneas were then placed in a solution of DMEM with 1% FBS and 300 μg of gentamicin/ml for 1 h at 37°C to kill any remaining extracellular bacteria. The corneal tissue was removed, washed three times in 5 ml of DMEM, and then homogenized in 0.5% Triton X-100 in TSB to release intracellular P. aeruginosa. The solution was then diluted in DMEM with 1% FBS and plated for bacterial enumeration.
Prophylactic and therapeutic administration of human MAbs.
For evaluation of prophylactic protection prior to infection, 100 μg of human MAb F429 or 100 μg of a control human IgG1 MAb (Sigma Chemical Co., St. Louis, MO) was injected intraperitoneally (i.p.) 18 h prior to infection of the eyes. Bacterial levels and corneal pathology were evaluated at 48 h postinfection. For evaluation of therapeutic efficacy, 100 μg of MAb F429 or control human IgG1 MAb was injected i.p. 8, 24, and 32 h after initiation of infection, and the levels of bacteria and final corneal pathology were evaluated at 48 h postinfection. For evaluation of the therapeutic efficacy of topical application of MAb F429, 10 μg of MAb or control human IgG1 MAb were applied to the infected eyes in a 10-μl volume 8, 24, and 32 h postinfection, and bacterial levels and corneal pathology were determined at 48 h postinfection. Topical application was done after induction of anesthesia by inhalation of isofluorane.
Statistical analysis.
Pairwise differences in the corneal pathology scores and differences in the CFU of P. aeruginosa in the eyes (both extracellular and intracellular) were evaluated with the Mann-Whitney U test. Categorical differences in the level of pathology scores measured between groups were analyzed by the Fisher exact test or chi-square tests. All P values reported are based on two-tailed comparisons. When exact P values were obtained, they are reported using an equal (“=”) sign; when P values were estimated by Gaussian approximations, they are reported using a less than (“<”) sign.
RESULTS
Analysis of alginate elaboration by P. aeruginosa strains.
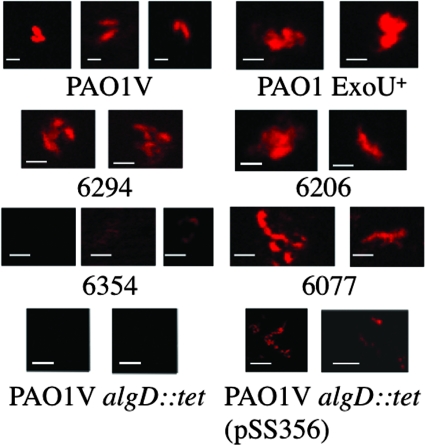
Production of alginate by nonmucoid strains of P. aeruginosa in vitro is variable, although microaerophilic to anaerobic growth conditions have been reported to enhance alginate biosynthesis (2). Furthermore, in the P. aeruginosa murine corneal infection model the majority of the bacterial cells are intracellular, indicating that they are also causing infections and growing under low-oxygen tension in vivo. We used immunofluorescence to detect alginate production among a number of laboratory and clinical isolates of P. aeruginosa after overnight growth in tubes filled with TSB medium to minimize the dissolved oxygen level and analyzed six of these strains of P. aeruginosa for the present study. Five of the six (Fig. 1) strongly expressed alginate, while the sixth, strain 6354, had a minimal staining, indicating little to no alginate was made by this strain, suggesting it could serve as a specificity control in subsequent experiments. We also could not detect uronic acid-containing polymers on this organism's surface using a specific assay (11; data not shown). Strain PAO1V algD::tet did not produce detectable alginate, whereas the complemented strain, PAO1V algD::tet(pSS356), produced a clear reaction with the MAb to alginate (Fig. 1). Controls lacking primary antibody were uniformly negative for immunofluorescence (not shown).
FIG. 1.
Analysis of alginate expression among clinical isolates and alginate mutant and complemented P. aeruginosa strains grown under low-oxygen conditions. Confocal microscopic images of bacterial cells treated with MAb F429 and a secondary reagent of protein A conjugated to Alexa Fluor 546 are shown. The specific P. aeruginosa strain is indicated below each panel of micrographs. On the left-hand side, the first three rows show invasive strains (ExoS+, ExoU−); on the right-hand side, the first three rows show cytotoxic strains (ExoU+; strains 6206 and 6077 are ExoS−). The bottom row shows control strains [alginate-negative PAO1V algD::tet and complemented strain PAO1V algD::tet(pSS356)]. Controls lacking the primary MAb showed no detectable immunofluorescence (not shown). White scale bar, 1 μm.
Opsonic killing of nonmucoid P. aeruginosa corneal isolates.
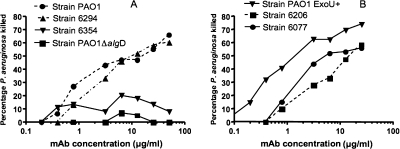
We have previously shown that MAb F429 mediates opsonic killing of nonmucoid strains of P. aeruginosa obtained from sputum samples of cystic fibrosis patients or from the blood of bacteremic patients (18). This was not true, however, for strain PAO1, which does express alginate in vivo following infection (18) but only expresses alginate in vitro when grown under microaerophilic/anaerobic conditions (2). To asses if alginate was a target for opsonic killing of P. aeruginosa corneal isolates, as well as for strain PAO1 grown under low oxygen, we evaluated killing with this MAb using strains grown overnight at 37°C in tubes filled with TSB. As shown in Fig. 2A, MAb F429 readily mediated opsonic killing of strains PAO1 and 6294 but had no activity on the non-alginate producing strains 6354 or PAO1ΔalgD. Cytotoxic strains PAO1 ExoU+, 6077, and 6206 were all killed in the opsonic assay (Fig. 2B). Thus, when alginate is expressed on nonmucoid P. aeruginosa grown under low-oxygen conditions, there is a sufficient amount to serve as a target for opsonic killing by MAb F429.
FIG. 2.
Opsonic killing of P. aeruginosa isolates by MAb F429. Growth of nonmucoid isolates in a low-oxygen environment promotes expression of alginate and under these conditions two of three invasive strains (A) and all three cytotoxic strains (B) are effectively killed by MAb F429 in the presence of polymorphonuclear leukocyte and complement. P. aeruginosa strain PAO1ΔalgD, unable to produce alginate due to disruption of the algD gene, is not killed (A). Points indicate averages of duplicate samples.
Prophylactic administration of MAb F429 reduces infection and pathology.
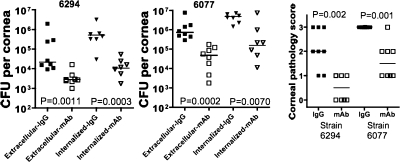
We first evaluated whether prophylactic administration of MAb F429 would be efficacious in reducing bacterial levels and corneal pathology in eyes that were scratched and then infected 18 h after injection of the human MAb. This type of therapy could potentially be used in cases of eye injury, eye surgery, or other circumstances where there is a high potential for subsequent P. aeruginosa corneal infection following injury or surgery. It was also important to determine whether systemic administration of the MAb resulted in a positive effect in terms of pathology in the cornea. We found that MAb F429 reduced both extracellular and internalized levels of invasive P. aeruginosa strain 6294 and cytotoxic strain 6077 by >90% in the corneas of mice given this reagent compared to controls given irrelevant human IgG1 (Fig. 3). Of note, in the controls 87% or more of the total bacteria were inside of corneal cells, and in the MAb-treated group 70 to 75% of the recovered P. aeruginosa cells were internalized. Consistent with the findings for bacterial levels, MAb F429 treatment reduced corneal pathology significantly for mice infected with either P. aeruginosa strain, with six of eight mice infected with strain 6294 having pathology scores ≥2 compared to none of eight MAb-treated mice (P = 0.0070, Fisher exact test). For the more virulent cytotoxic strain 6077 all eight mice had pathology scores of 3 compared to only one of eight mice given MAb F429 (P = 0.0014, Fisher exact test). Since the bacteria were not grown in low-oxygen conditions prior to challenge of the mice, it also appears that in the infected mouse eye P. aeruginosa expresses sufficient alginate for it to serve as a target for bacterial clearance mediated by MAb F429.
FIG. 3.
Prophylactic administration of MAb F429 18 h prior to infection reduces bacterial levels and pathology. Both extracellular and internalized CFU per eye and corneal pathology were reduced after challenge with either invasive strain 6294 or cytotoxic strain 6077. Points indicate values for an individual mouse, bars indicate medians, and P values were calculated by the Mann-Whitney U test. Infectious inocula in a 5-μl volume for both strains: strain 6294, 106 CFU/eye; and strain 6077, 9.0 × 105 CFU/eye.
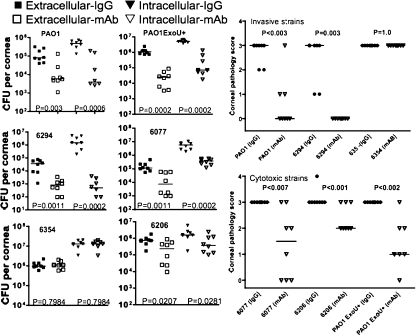
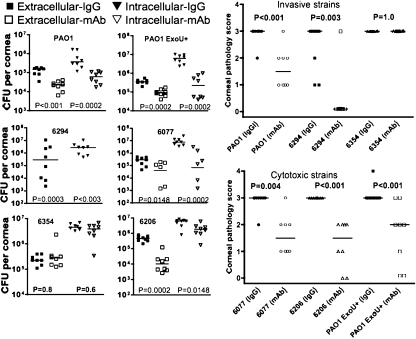
Therapeutic systemic administration of MAb F429 reduces infection and corneal pathology.
A more likely scenario for use of a MAb in the setting of keratitis is as adjunctive therapy for established infection, so we next evaluated whether MAb F429 could be given systemically to mice starting 8 h after infection and produce an efficacious outcome. Three i.p. injections at 8, 24, and 32 h postinfection of MAb F429 resulted in a significant decrease in the extracellular and internalized levels of five of six P. aeruginosa strains (Fig. 4). The only strain for which no effect was found was the alginate-negative 6354 isolate. The levels of bacteria internalized into the corneal cells of mice treated with the IgG1 control human MAb were much higher than the extracellular levels in comparably treated mice. In the MAb F429-treated animals, this difference was more variable, with some P. aeruginosa strains yielding higher levels of internalized versus extracellular bacteria, whereas for others these levels were more-or-less comparable (Fig. 4). As expected, corneal pathology was also significantly reduced by MAb F429 treatment (Fig. 4), except for strain 6354. Notably, all of the mice infected with invasive strains PAO1 or 6294 and treated with MAb F429 had corneal pathology scores of 0, while 12 of 16 irrelevant IgG1-treated control mice had pathology scores of ≥2. Among the cytotoxic strains, more overall corneal pathology was seen in the MAb F429-treated groups compared to the pathology in the animals infected with invasive strains, but still the MAb F429 significantly reduced pathology engendered by the cytotoxic strains. Of the 24 mice in either the control or MAb F429-treated groups and infected with one of the three cytotoxic strains, all 24 control mice had pathology scores of 3 to 4, whereas 18 of the 24 mice treated with MAb F429 had pathology scores of ≤2 (P < 0.0001, chi-square test).
FIG. 4.
Therapeutic administration of MAb F429 starting 8 h after infection and repeated at 24 and 32 h after infection reduces bacterial levels in the eyes. Both extracellular and internalized CFU per eye were reduced after challenge with two of three invasive strains (PAO1 and 6294 but not 6354) or all three cytotoxic strains (PAO1 ExoU+, 6077, and 6206), as was corneal pathology. Points indicate values for an individual mouse, bars indicate medians, and P values were calculated by the Mann-Whitney U test. Infectious inocula (CFU per eye given in a 5-μl volume): PAO1, 106; 6294, 2.5 × 106; 6354, 8.5 × 105; PAO1 ExoU+, 8 × 105; 6077, 9.0 × 105; 6206, 7.5 × 105.
Efficacy of topical application of MAb F429 on P. aeruginosa keratitis.
Topical application of a therapeutic drug for keratitis has clear advantages in terms of delivery and cost of the drug, since small volumes and amounts could be used, although it may have a drawback of requiring frequent administration due to a short half-life on the corneal surface. We thus evaluated whether delivery of 10 μg of MAb F429 in a 5-μl volume onto the eye 8, 24, and 32 h postinfection with P. aeruginosa was efficacious in reducing bacterial levels and pathology. As with the systemic therapeutic administration of MAb F429, topical application significantly reduced bacterial levels in the eye 48 h after infection for the five strains expressing alginate, but not for the alginate-negative strain 6354 (Fig. 5). Comparable reductions in corneal pathology were also achieved (Fig. 5) for the alginate-producing strains. Except with invasive strain PAO1, the topical application of MAb F429 was as efficacious in reducing corneal pathology as was the systemic administration of this MAb (Fig. 5 versus Fig. 4).
FIG. 5.
Topical application of MAb F429 onto the eyes of mice starting 8 h after infection and repeated at 24 and 32 h after infection reduces bacterial levels and associated corneal pathology. Both extracellular and internalized CFU per eye were reduced after challenge with two of three invasive strains (PAO1 and 6294 but not 6354) or all three cytotoxic strains (PAO1 ExoU+, 6077, and 6206), as was corneal pathology. Points indicate values for an individual mouse, bars indicate medians, and P values were calculated by the Mann-Whitney U test. Infectious inocula (CFU per eye given in a 5-μl volume): PAO1, 106; 6294, 2.2 × 106; 6354, 106; PAO1 ExoU+, 9 × 105; 6077, 8.5 × 105; 6206, 8.5 × 105.
DISCUSSION
In the past 15 years more than 20 MAbs have been licensed for clinical use for a variety of medical conditions, but to date only one, palivizumab (Synagis), for prevention of respiratory syncytial virus infection, is for an infectious agent. Although the technology for producing MAbs for clinical use is now robust, applications to infectious agents likely lag because of the difficulty of identifying conserved target antigens against which a useful MAb could be made. Here we show that among nonmucoid strains of P. aeruginosa that express only low levels of alginate, a fully human IgG1 MAb targeting this antigen can reduce bacterial numbers in an infected cornea and reduce the associated corneal pathology when used in either a prophylactic or a therapeutic manner. There was no protection against one clinical isolate that did not produce alginate, indicating the specificity of the MAb for this antigen. Of note, both systemic administration and topical application of the MAb onto the cornea resulted in significant reductions in bacterial levels and pathology, suggesting that a combination of both modes might optimize the outcomes from treating P. aeruginosa keratitis.
The efficacy of MAb F429 against nonmucoid, low-alginate-producing P. aeruginosa strains indicates that this antigen acts much like a capsule in regard to its utility as a protective antigen. Thus, antibody, complement, and phagocytes are all needed for opsonic killing and protective efficacy (18). However, it does not appear as if alginate has the property of being an essential virulence factor for P. aeruginosa in the setting of keratitis, since the non-alginate-producing strain 6354, purposefully chosen as a specificity control for these studies, was still virulent. This raises the question as to what proportion of clinical isolates from keratitis are alginate negative but nonetheless virulent strains, or whether MAb treatment could select out for alginate-negative strains as antibiotic treatment selects out for drug-resistant strains, without a loss in the strain's virulence. Among a small sample of 25 clinical isolates from keratitis, strain 6354 was the only one negative for alginate (T. S. Zaidi and G. B. Pier, unpublished data), suggesting that this is a rare occurrence. Loss of alginate production during treatment for P. aeruginosa keratitis could occur, and this might not compromise virulence and thus could represent a means of bacterial escape from immunotherapy. However, whether such rare variant strains could then resist the immune effectors such as phagocytes and complement present in the eye during infection and emerge as a MAb-resistant mutant able to grow to the levels needed to sustain infection is unlikely. Also, current antibiotic treatment regimens for P. aeruginosa keratitis would undoubtedly be used along with any future MAb-based therapy, providing another line of defense against emergence of MAb-resistant variants during therapy. Finally, unlike drug-resistant P. aeruginosa variants that can spread within a hospital environment and are associated primarily with ventilator use and catheter infections, keratitis is most often initiated outside the hospital by environmental strains. Thus, alginate-negative variants that might emerge during MAb therapy are unlikely to be a threat to others at risk for P. aeruginosa keratitis.
MAb F429 has previously shown excellent efficacy in mice against pneumonia and sepsis due to nonmucoid P. aeruginosa (18), but the MAb had to be delivered into the lung to observe this effect. We expected that we might encounter a similar situation in therapeutically treating experimental murine keratitis but found that both i.p. and topical application of the MAb resulted in a protective effect on infection and pathology. Presumably, the antibody injected i.p.is able to enter the circulation of the mouse and from there gain access to the cornea through transudation, whereas in the lung of a mouse, getting enough of a human MAb into the lung during pneumonia was not feasible via serum delivery. Once in the eye the MAb likely uses both complement factors that have been locally produced (6) or transudated from the serum along with polymorphonuclear leukocytes derived from the blood to phagocytose and kill P. aeruginosa cells This reduction in bacterial levels likely accounts for most, if not all, of the reduced pathology since there is a clear correlation between bacterial levels and inflammatory pathology in keratitis. Any reductions in pathology related to reduced production of P. aeruginosa virulence factors is likely secondary to the reduced bacterial levels, inasmuch as the MAb to alginate is unlikely to affect virulence factor production by an means other than by reducing bacterial levels in the infected tissue.
We did not attempt to see whether using both systemic delivery and topical application had an additive effect because each treatment alone was highly effective in reducing bacterial levels and pathology. We would have had to use suboptimal levels of the MAb to observe any additive or synergistic effects from combining treatment routes, but this would not be reflective of how the MAbs might be used in clinical practice and would thus represent too artificial an experimental situation to yield practical information. Synergistic or additive effects from delivering the MAb by multiple routes likely will need to be evaluated in humans with P. aeruginosa keratitis.
Overall, these results show that the fully human IgG1 MAb F429 has potential as part of the treatment regimen for a serious P. aeruginosa infection, ulcerative keratitis. Extension of the potential clinical utility of the MAb based on results from experimental lung infections (18) to the setting of keratitis is important in terms of showing the potential value for full-fledged clinical development of this reagent. In addition, if use of the MAb in this clinical setting reduces the need for the frequent administration of antibiotic eye drops often initiated after a diagnosis of P. aeruginosa keratitis is made, or the need to inject antibiotics intravitreally if progression of infection to endophthalmitis is suspected (14), it will have clear benefits to patients. Although MAb-based therapies are expensive and likely to remain so, topical use of small amounts of MAb F429 in the setting of keratitis could be quite cost-effective. This situation might be analogous to the use of the anti-vascular endothelial growth factor MAb bevacizumab (Avastin) for the treatment of wet macular degeneration (3), wherein reformulation of the drug approved for use in cancer therapy to a smaller dose that could be used in ophthalmic practice resulted in a much reduced cost of this therapy. Topical administration of small doses of MAbs are unlikely to be prohibitively costly, particularly if dosing schedules do not require application more than once ever 8 to 12 h. Thus, our overall results in treating experimental keratitis in mice suggest that MAb F429 could be a useful addition to current therapies for the potential devastating consequences of vision loss as a consequence of P. aeruginosa keratitis.
Acknowledgments
This study was supported by NIH grant EY016144.
We thank Lisa Cavacini and Marshall Posner from the Beth Israel/Deaconnes Medical Center, Harvard Medical School, for deriving the original hybridomas used to produce MAb F429 and Martha Grout and Taqueeer Zaidi for technical assistance.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 21 July 2008.
REFERENCES
- 1.Allewelt, M., F. T. Coleman, M. Grout, G. P. Priebe, and G. B. Pier. 2000. Acquisition of expression of the Pseudomonas aeruginosa ExoU cytotoxin leads to increased bacterial virulence in a murine model of acute pneumonia and systemic spread. Infect. Immun. 683998-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bragonzi, A., D. Worlitzsch, G. B. Pier, P. Timpert, M. Ulrich, M. Hentzer, J. B. Andersen, M. Givskov, M. Conese, and G. Doring. 2005. Nonmucoid Pseudomonas aeruginosa expresses alginate in the lungs of patients with cystic fibrosis and in a mouse model. J. Infect. Dis. 192410-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown, D. M., and C. D. Regillo. 2007. Anti-VEGF agents in the treatment of neovascular age-related macular degeneration: applying clinical trial results to the treatment of everyday patients. Am. J. Ophthalmol. 144627-637. [DOI] [PubMed] [Google Scholar]
- 4.Chusid, M. J., D. B. Nelson, and L. A. Meyer. 1986. The role of the polymorphonuclear leukocyte in the induction of corneal edema. Investig. Ophthalmol. Vis. Sci. 271466-1469. [PubMed] [Google Scholar]
- 5.Cole, N., E. Hume, S. Khan, M. Madigan, A. J. Husband, L. Garthwaite, and M. Willcox. 2005. Contribution of the cornea to cytokine levels in the whole eye induced during the early phase of Pseudomonas aeruginosa challenge. Immunol. Cell Biol. 83301-306. [DOI] [PubMed] [Google Scholar]
- 6.Diehn, J. J., M. Diehn, M. F. Marmor, and P. O. Brown. 2005. Differential gene expression in anatomical compartments of the human eye. Genome Biol. 6R74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleiszig, S. M. J., T. S. Zaidi, R. Ramphal, and G. B. Pier. 1994. Modulation of Pseudomonas aeruginosa adherence to the corneal surface by mucus. Infect. Immun. 621799-1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garner, C. V., D. DesJardins, and G. B. Pier. 1990. Immunogenic properties of Pseudomonas aeruginosa mucoid exopolysaccharide. Infect. Immun. 581835-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsiao, C. H., L. Yeung, D. H. Ma, Y. F. Chen, H. C. Lin, H. Y. Tan, S. C. Huang, and K. K. Lin. 2007. Pediatric microbial keratitis in Taiwanese children: a review of hospital cases. Arch. Ophthalmol. 125603-609. [DOI] [PubMed] [Google Scholar]
- 10.Kohler, T., C. van Delden, L. K. Curty, M. M. Hamzehpour, and J. C. Pechere. 2001. Overexpression of the MexEF-OprN multidrug efflux system affects cell-to-cell signaling in Pseudomonas aeruginosa. J. Bacteriol. 1835213-5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kosakai, M., and Z. Yosizawa. 1979. A partial modification of the carbazole method of Bitter and Muir for quantitation of hexuronic acids. Anal. Biochem. 93295-298. [DOI] [PubMed] [Google Scholar]
- 12.Mah-Sadorra, J. H., S. G. Yavuz, D. M. Najjar, P. R. Laibson, C. J. Rapuano, and E. J. Cohen. 2005. Trends in contact lens-related corneal ulcers. Cornea 2451-58. [DOI] [PubMed] [Google Scholar]
- 13.Mela, E. K., I. P. Giannelou, K. X. John, and G. P. Sotirios. 2003. Ulcerative keratitis in contact lens wearers. Eye Contact Lens 29207-209. [DOI] [PubMed] [Google Scholar]
- 14.Ohl, C. A., and M. Pollack. 2005. Infections due to Pseudomonas species and related organisms, p. 889-897. In D. L. Kasper, E. Braunwald, A. S. Fauci, S. L. Hauser, D. L. Longo, and J. L. Jameson (ed.), Harrison's principles of internal medicine, 16th ed. McGraw-Hill Book Co., New York, NY.
- 15.Pachigolla, G., P. Blomquist, and H. D. Cavanagh. 2007. Microbial keratitis pathogens and antibiotic susceptibilities: a 5-year review of cases at an urban county hospital in north Texas. Eye Contact Lens 3345-49. [DOI] [PubMed] [Google Scholar]
- 16.Pennington, J. E., W. F. Hickey, L. L. Blackwood, and M. A. Arnaut. 1981. Active immunization with lipopolysaccharide Pseudomonas antigen for chronic Pseudomonas bronchopneumonia in guinea pigs. J. Clin. Investig. 681140-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pier, G. B. 2007. Pseudomonas aeruginosa lipopolysaccharide: a major virulence factor, initiator of inflammation and target for effective immunity. Int. J. Med. Microbiol. 297277-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pier, G. B., D. Boyer, M. Preston, F. T. Coleman, N. Llosa, S. Mueschenborn-Koglin, C. Theilacker, H. Goldenberg, J. Uchin, G. P. Priebe, M. Grout, M. Posner, and L. Cavacini. 2004. Human monoclonal antibodies to Pseudomonas aeruginosa alginate that protect against infection by both mucoid and nonmucoid strains. J. Immunol. 1735671-5678. [DOI] [PubMed] [Google Scholar]
- 19.Preston, M. J., S. M. J. Fleiszig, T. S. Zaidi, J. B. Goldberg, V. D. Shortridge, M. L. Vasil, and G. B. Pier. 1995. Rapid and sensitive method for evaluating Pseudomonas aeruginosa virulence factors during corneal infections in mice. Infect. Immun. 633497-3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Preston, M. J., A. A. Gerceker, N. L. Koles, M. Pollack, and G. B. Pier. 1997. Prophylactic and therapeutic efficacy of immunoglobulin G antibodies to Pseudomonas aeruginosa lipopolysaccharide against murine experimental corneal infection. Investig. Ophthalmol. Vis. Sci. 381418-1425. [PubMed] [Google Scholar]
- 21.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. Lim, K. Smith, D. Spencer, G. K. Wong, Z. Wu, I. T. Paulsen, J. Reizer, M. H. Saier, R. E. Hancock, S. Lory, and M. V. Olson. 2000. Complete genome sequence of Pseudomonas aeruginosa PA01, an opportunistic pathogen. Nature 406959-964. [DOI] [PubMed] [Google Scholar]
- 22.Theilacker, C., F. Coleman, S. Mueschenborn, M. Grout, and G. B. Pier. 2003. Construction and characterization of a Pseudomonas aeruginosa mucoid exopolysaccharide/alginate conjugate vaccine. Infect. Immun. 713875-3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomas, P. A., and P. Geraldine. 2007. Infectious keratitis. Curr. Opin. Infect. Dis. 20129-141. [DOI] [PubMed] [Google Scholar]
- 24.Verhelst, D., C. Koppen, J. Van Looveren, A. Meheus, and M. J. Tassignon. 2006. Contact lens-related corneal ulcers requiring hospitalization: a 7-year retrospective study in Belgium. Acta Ophthalmol. Scand. 84522-526. [DOI] [PubMed] [Google Scholar]
- 25.Whitchurch, C. B., T. E. Erova, J. A. Emery, J. L. Sargent, J. M. Harris, A. B. T. Semmler, M. D. Young, J. S. Mattick, and D. J. Wozniak. 2002. Phosphorylation of the Pseudomonas aeruginosa response regulator AlgR is essential for type IV fimbria-mediated twitching motility. J. Bacteriol. 1844544-4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Willcox, M. D. 2007. Pseudomonas aeruginosa infection and inflammation during contact lens wear: a review. Optom. Vis. Sci. 84273-278. [DOI] [PubMed] [Google Scholar]
- 27.Wozniak, D. J., T. J. Wyckoff, M. Starkey, R. Keyser, P. Azadi, G. A. O'Toole, and M. R. Parsek. 2003. Alginate is not a significant component of the extracellular polysaccharide matrix of PA14 and PAO1 Pseudomonas aeruginosa biofilms. Proc. Natl. Acad. Sci. USA 1007907-7912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Young, A. L., A. T. Leung, E. Y. Cheung, L. L. Cheng, A. K. Wong, and D. S. Lam. 2003. Orthokeratology lens-related Pseudomonas aeruginosa infectious keratitis. Cornea 22265-266. [DOI] [PubMed] [Google Scholar]
- 29.Zaidi, T. S., G. P. Priebe, and G. B. Pier. 2006. A Live-attenuated Pseudomonas aeruginosa vaccine elicits outer membrane protein-specific active and passive protection against corneal infection. Infect. Immun. 74975-983. [DOI] [PMC free article] [PubMed] [Google Scholar]