Abstract
Cytolethal distending toxin (CDT) induces apoptosis using the caspase-dependent classical pathway in the majority of human leukemic T cells (MOLT-4). However, we found the process to cell death is only partially inhibited by pretreatment of the cells with a general caspase inhibitor, z-VAD-fmk. Flow cytometric analysis using annexin V and propidium iodide showed that a 48-h CDT treatment decreased the living cell population by 35% even in the presence of z-VAD-fmk. z-VAD-fmk completely inhibited caspase activity in 24 h CDT-intoxicated cells. Further, CDT with z-VAD-fmk treatment clearly increased the cell population that had a low level of intracellular reactive oxygen. This is a characteristic opposite to that of caspase-dependent apoptosis. Overexpression of bcl2 almost completely inhibited cell death using CDT treatment in the presence of z-VAD-fmk. The data suggest there are at least two different pathways used in CDT-induced cell death: conventional caspase-dependent (early) apoptotic cell death and caspase-independent (late) death. Both occur via the mitochondrial membrane disruption pathway.
Programmed cell death is critical for organ development and homeostasis in eukaryotes (24, 49, 52). In the past, the caspases were considered essential proteases for apoptosis. However, accumulating data suggest that caspase-independent cell death occurs in programmed cell death (4, 23) and, in certain conditions, the caspase-independent pathway is an important mechanism to protect organs when caspase-dependent cell death does not occur (4).
Viral or bacterial infection and cancer often influence programmed cell death pathways. This is true of death induced by the cytolethal distending toxin (CDT), one of the bacterial toxins produced by Aggregatibacter actinomycetemcomitans, a gram-negative pathogen implicated in the pathogenesis of juvenile and adult periodontitis (39, 42). CDT was demonstrated to induce apoptosis in various cell types, including the T-lymphocytic leukemia cell lines, Jurkat cells, and MOLT-4 cells (31, 35, 50).
CDT belongs to a family of toxins with cell cycle specific inhibitory activity blocking the G2 to M phase through inactivation of the CDC2/cyclin B complex (for recent reviews, see references 17, 30, and 32). CDT is a complex of three subunits: CdtA, CdtB, and CdtC. CdtB induces double-strand breaks, acting as a DNase that triggers the CDT intoxication (9, 25). CdtA and CdtC have homologies to lectin-like domains that can bind to surface molecules on the target cells (7). CDT internalizes through a receptor-mediated endocytosis and subsequently reaches the nucleus by retrograde transport and active nuclear pore transport (15, 29). In the nucleus, the CDT-induced chromatin injury is found as double-strand breaks that may recruit a large protein complex, the PIDDosome, in which caspase-2 activation occurs (44). Previously, we demonstrated that CDT induces apoptosis and activates caspase-2 in two T-cell leukemia cell lines, Jurkat and MOLT-4 (31). Activated caspase-2 acts as an upstream initiator of mitochondrial membrane permeability (22). Increased permeability of the mitochondrial membrane releases proapoptotic molecules, including cytochrome c, to activate executive caspases, and this loss of the mitochondrial membrane potential leads to the production of reactive oxygen species (ROS) (34). In the presence of a caspase inhibitor, CDT-induced apoptosis was completely blocked for 16 h in Jurkat cells, suggesting that CDT-induced cell death was dependent on caspase activation (31). However, we found that some of the cells, after 24 h of CDT intoxication, undergo death in a manner different from conventional apoptosis using caspase activation. Here, we report the detailed features of this cell death and discuss the importance of caspase-independent cell death during late-stage CDT-intoxication.
MATERIALS AND METHODS
Purification of A. actinomycetemcomitans CDT.
CDT holotoxin was purified by using a Ni-chelated agarose resin column as described previously where the C-terminal His6-tagged CdtC was expressed using the pQE 60 expression vector in M15 Escherichia coli (Qiagen, Tokyo, Japan) that carried the A. actinomycetemcomitans cdtA, cdtB, and cdtC genes downstream of the T5 promoter (31). A mutant CDT with CdtB His274Ala (cdtB 274 histidine changed to alanine) was purified by using the same method. The mutant was constructed by using site-directed mutagenesis of the 274th histidine residue to an alanine in the cdtB gene of pQEcdtABC and was performed by using the overlap extension method (46). The primers used were 5′-ACA TCC GAT gcT TTT CCT GTT-3′ and 5′-AAC AGG AAA Agc ATC GGA TGT-3′ (mutated sites are shown as lowercase letters). The mutant DNA containing cdtAB(H274A)C was subcloned into the pQE60 vector (Qiagen).
Preparation of cells and culture conditions.
The thymic T-cell leukemia cell line, MOLT-4, and the peripheral T-cell leukemia cell line, Jurkat, were cultured in RPMI 1640 with 10% fetal calf serum (FCS), 100 U of penicillin G/ml, and 100 μg of streptomycin/ml and incubated at 37°C using 5% CO2 incubator. Cells (106 cells/ml) were treated with or without CDT (100 ng/ml) and cultured under similar conditions. In some experiment, cells were X-irradiated. Irradiation of cells was performed by an X-ray generator (Shimadzu HF-320; 220 kVp, 8 mA) with a 0.5-mm aluminum and 0.3-mm copper filter at a dose of ∼0.8 Gy/min. Cells were irradiated in a plastic dish at room temperature. z-VAD-fmk, a general caspase inhibitor (MBL Nagoya, Japan), was used at 100 μM and was added 30 min before CDT treatment.
Establishing MOLT-4 cells stably overexpressing bcl-2.
The complete coding sequence of bcl-2 (19), the gene governing antiapoptotic mitochondrial outer membrane permeabilization (MOMP), was amplified by using the PCR and subsequently cloned into an SFFV-neo vector (14). MOLT-4 cells were stably transfected with the plasmid SFFV-human bcl-2 or a control plasmid, SFFV-neo, using electroporation at 350 V with a capacitance of 960 μF with a GenePulser (Bio-Rad, Richmond, CA). Transfected cells were selected in medium containing 0.5 mg of G418/ml for 30 days. bcl-2 transfectants were found by using fluorescence-activated cell sorting (FACS) and Western analysis with Bcl-2 monoclonal antibody, 6C8 (BD Pharmingen, San Jose, CA), where we demonstrated Bcl-2 levels increased 10- to 20-fold greater than Bcl-2 present in untransfected or SFFV-neo-transfected MOLT-4 cells. The cells stably expressing Bcl-2 are referred to as MOLT-4bcl2 cells. The cells transfected with the control plasmid SFFV-neo served as a control and are referred to as MOLT-4neo cells.
Flow cytometry.
Conformational change in the membrane by phosphatidylserine translocation and membrane hole formation was observed by counting the cell population stained with fluorescein isothiocyanate (FITC)-labeled annexin V and propidium iodide (PI) as described previously (31). Briefly, CDT-treated cells (5 × 105 to 10 × 105) were centrifuged at 350 × g for 2 min and washed three times with 500 μl of phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4 [pH 7.3]) with 1% FCS. The washed cells were resuspended in 180 μl of PBS containing 1% FCS, 0.5 μl of FITC-labeled annexin V, and 1 μl of PI using a MEBCYTO apoptosis kit (MBL, Nagoya, Japan). After 5 min at room temperature, 10,000 cells were scanned by using a FACScan (BD Biosciences, San Jose, CA). We performed a quadrant population analysis using CellQuest software (BD Biosciences). The live cell population was negative for both annexin V and PI (shown in the lower left quadrant).
Hydroethidine (HE) was used to measure the intracellular ROS, the superoxide anion (O2·−) (16). HE (5 mM) was added to the PBS-washed cells (5 × 105 cells in 500 μl of PBS with 1% FCS). Cells were incubated for 20 min at 37°C. After a wash with PBS using centrifugation at 350 × g for 5 min, the cells were resuspended in 200 μl of PBS containing 1% FCS and scanned using the FACScan. A gated population analysis was performed by using CellQuest software (BD Biosciences).
The cell cycle was determined as follows. CDT-treated cells were washed twice with PBS and fixed with 70% ethanol for 2 h at 4°C. The fixed cells were washed twice with PBS and incubated with 0.25 mg of RNase A/ml for 15 to 60 min at 37°C. DNA in the RNase-digested cells was stained with 50 μg of PI/ml for 30 min at 4°C and analyzed by using a FACSCalibur flow cytometer (BD Biosciences).
Caspase assay.
CDT-treated cells were harvested and washed with PBS. PBS-washed cells were lysed with lysis buffer (10 mM Tris-Cl [pH 7.4], 25 mM NaCl, 0.25% Triton X-100, 1 mM EDTA) and centrifuged at 15,000 × g for 10 min. The supernatant was diluted with lysis buffer at a protein concentration adjusted to 1 mg/ml. Then, 5 μg of total protein was incubated with 200 μl of caspase buffer (50 mM Tris-Cl [pH 7.2], 100 mM NaCl, 1 mM EDTA, 10% sucrose, 0.1% CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate}, 5 mM dithiothreitol) using 50 μM concentrations of the various fluorigenic substrate peptides. The peptides used were Ac-DEVD-7-amino-4-methyl cumarine (AMC) for caspase-3, caspase-7, and caspase-8; Ac-IETD-AMC for caspase-8, caspase-6, and Granzyme; Ac-LEHD-AMC for caspase-9; and Ac-VDVAD-AMC for caspase-2 (Peptide Institute, Inc., Osaka, Japan). The reaction mixture was incubated at 37°C for 60 min where the release of 7-amino-4-methylcumarin was measured by using a fluorometer (Shimazu RF-540) with an excitation at 380 nm and an emission at 460 nm.
RESULTS
Early effect of CDT in MOLT-4 cells in the presence of the general caspase inhibitor, z-VAD-fmk.
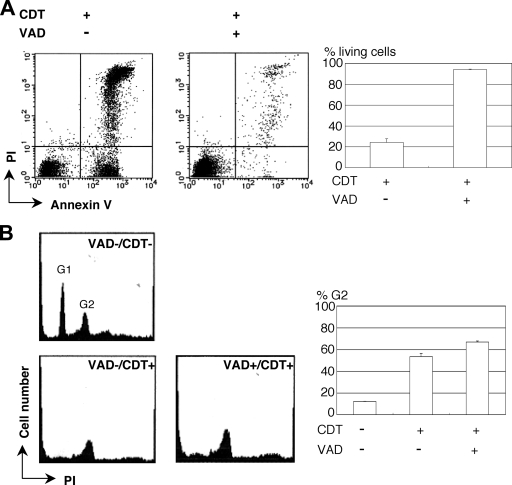
The effect of the general caspase inhibitor, z-VAD-fmk, on MOLT-4 cells was examined for 16 h after the cells were treated with 100 ng of CDT/ml. As shown in Fig. 1, when the cells were treated with z-VAD-fmk (100 μM) most cells retained the phenotype of living cells (annexin V negative, PI negative). This indicated the caspase inhibitor almost completely blocked CDT-induced apoptosis for at least 16 h but did not block the G2/M arrest (Fig. 1B). We investigated DNA ladder formation in the presence of z-VAD-fmk in the CDT-treated MOLT-4 cells. z-VAD-fmk completely inhibited internucleosomal DNA fragmentation (data not shown). These results are consistent with our previous study using Jurkat cells (31).
FIG. 1.
Early effects of CDT intoxication on MOLT-4 cells in the presence of the general caspase inhibitor, z-VAD-fmk. The effects of CDT on MOLT-4 cells were examined 16 h after pretreatment of the cells with or without the general caspase inhibitor, z-VAD-fmk (100 μM), for 30 min. (A) MOLT-4 cells treated with CDT (100 ng/ml) for 16 h were observed after staining with PI and FITC-labeled annexin V for fluorescence by using a FACSCalibur. The percentages of living (annexin V-negative, PI-negative) cells are shown in the graphs along with the standard deviations. (B) The cell cycle was determined by staining the cells with PI after fixing the cells with 70% ethanol and RNase treatment. The percentages of the cells in the G2 phase are shown in the graph, along with the standard deviations.
Late effect of CDT in MOLT-4 cells in the presence or absence of z-VAD-fmk.
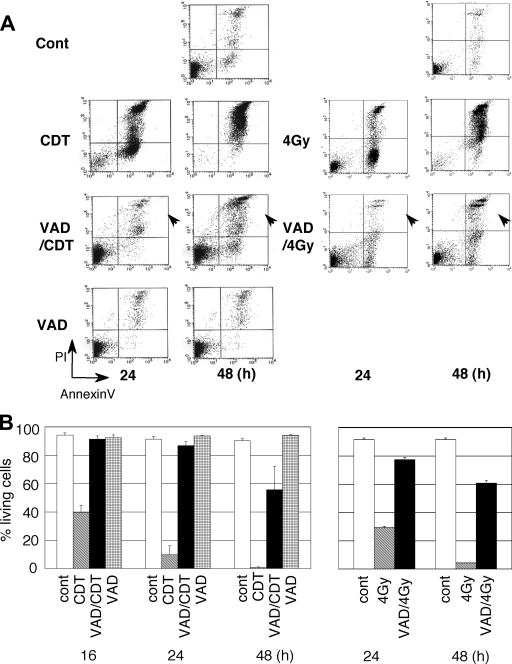
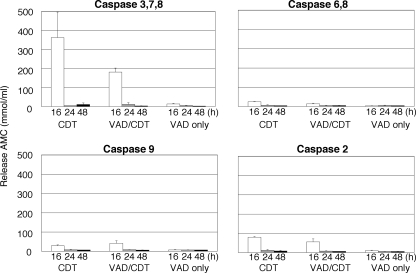
We then assessed the effect of long-term exposure to CDT on MOLT-4 cells in the presence or absence of z-VAD-fmk. A 24-h exposure to CDT on MOLT-4 cells increased the population of annexin V-positive and PI-negative cells, as well as a population of annexin V-positive and PI-positive (dead) cells (Fig. 2A). Pretreatment with z-VAD-fmk significantly inhibited the increase of these populations at 24 h (Fig. 2A). At 48 h, however, a population of annexin V-positive and PI-negative cells and also a population of annexin V-positive and PI-positive cells increased compared to the numbers at 16 h (Fig. 1 and 2). At 48 h after CDT exposure in the presence of z-VAD-fmk, living cells defined as annexin V-negative and PI-negative cells decreased by 35% compared to nontreated cells (90.18% living) (Fig. 2B). This is very similar to the observation when MOLT-4 cells were X-irradiated (5). Ionizing radiation on MOLT-4 cells induced G2/M arrest (37). Moreover, pretreatment of z-VAD-fmk inhibited the increase of dead cells at 24 h (Fig. 2A) (5). However, at 48 h after X-irradiation in the presence of z-VAD-fmk, the number of living cells decreased. With CDT treatment, caspase activities increased after 16 h (Fig. 3). This was apparent for caspase-3, caspase-7, and caspase-8. However, pretreatment with z-VAD-fmk significantly suppressed caspase activity, and this was likewise observed in Jurkat cells (31). Further, there was almost no caspase activity in MOLT-4 cells at 24 h after CDT exposure in the presence of z-VAD-fmk, even though 85% of the cells were alive (Fig. 2B and 3). This was also found at 48 h after CDT exposure in the presence of z-VAD-fmk. This suggests that CDT is able to induce cell death in the late stages of cell intoxication without caspase activation.
FIG. 2.
Late effects of CDT in MOLT-4 cells in the presence of z-VAD-fmk. CDT-induced cell death was monitored in MOLT-4 cells at 24 h or 48 h after CDT treatment (100 ng/ml) in the presence or absence of z-VAD-fmk (100 μM). (A) Cell death was observed by using a FACScan after staining with PI and FITC-labeled annexin V. (B) Percentages of living (annexin V-negative, PI-negative) cells at 16, 24, and 48 h. As a control, radiation-induced cell death was monitored in MOLT-4 cells at 24 and 48 h with or without z-VAD-fmk. Arrowheads show that dead cell populations increased at 48 h even in the presence of z-VAD-fmk after treatment with CDT or radiation.
FIG. 3.
Caspase activities in CDT-treated MOLT-4 cells in the presence of z-VAD-fmk. Caspase activities were monitored in MOLT-4 cells at 24 or 48 h after CDT treatment (100 ng/ml) in the presence or absence of z-VAD-fmk (100 μM). CDT-treated cell extracts (5 μg of total protein) were incubated with 50 μM concentrations of the various fluorigenic substrate peptides: AMC for caspase-3, caspase-7, and caspase-8; Ac-DQTD-AMC for caspase-7 and caspase-3; Ac-IETD-AMC for caspase-8, caspase-6, and Granzyme; Ac-LEHD-AMC for caspase-9; and Ac-VDVAD-AMC for caspase-2 (Peptide Institute, Inc., Osaka, Japan). The reaction mixture was incubated at 37°C for 60 min, where the release of AMC was measured by using a fluorometer (Shimazu RF-540) with an excitation at 380 nm and an emission at 460 nm.
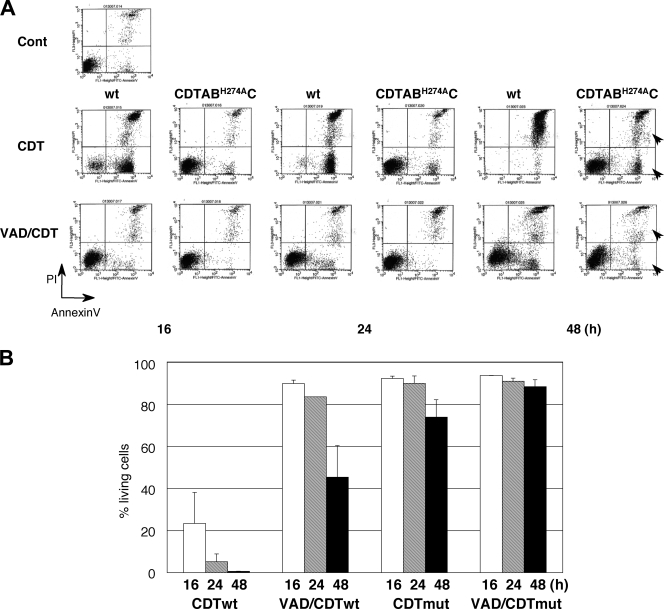
Comparison of the late effects of CDT to the mutant CdtB His274Ala CDT.
Previous studies suggest CdtB shows sequence similarity to DNase I, where it shares conserved amino acids essential for Mg binding (9, 25). The 274th histidine residue in the CdtB subunit is one of the active sites where a mutation at 274th His to Ala abolishes CDT cytodistending and cell cycle arrest activities (28). This strongly suggests that CdtB acts as a nuclease. To determine whether the CdtB nuclease activity is responsible for CDT-induced late cell death, mutant CDT containing CdtB H274A was generated and added to the medium of MOLT-4 cells. Mutated CDT did not show cytokilling activity until 24 h, as expected (Fig. 4). By 48 h, ca. 20% of the cells treated with the mutant CDT were dead, and this late cell death was almost completely inhibited by pretreatment with z-VAD-fmk. This shows that the possible nuclease activity of CdtB is also important for late cell death and was not inhibited by the general caspase inhibitor, z-VAD-fmk.
FIG. 4.
Effects of mutated CDT on MOLT-4 cells in the presence or absence of z-VAD-fmk. CDT-induced cell death was monitored at 24 or 48 h in MOLT-4 cells after CDT treatment (100 ng/ml) or mutated CdtB His274Ala CDT. (A) Cell death was analyzed by using a FACScan after staining with PI and FITC-labeled annexin V. (B) Percentages of living (annexin V-negative, PI-negative) cells at16, 24, and 48 h. Arrowheads show that dead cell populations increased at 48 h after treatment with CDT but was not seen in the presence of z-VAD-fmk.
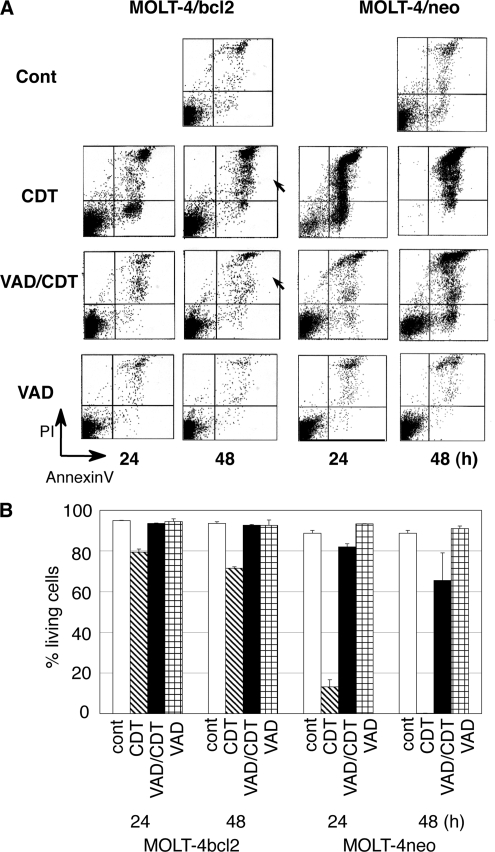
CDT-induced cell death in MOLT-4 cells overexpressing the bcl2 gene.
We previously demonstrated that CDT induces mitochondrial membrane permeability, resulting in an apoptotic cell death (early cell death) (31). To determine the role of mitochondria in late-stage CDT-induced cell death, we examined MOLT-4 cells forced to overexpress the bcl2 gene (MOLT-4/bcl2). As shown in Fig. 5A, Bcl2 overexpression showed an inhibitory effect on the increase of the population of annexin V-positive, PI-positive cells using CDT intoxication. After 48 h of CDT intoxication, a 23% decrease in the living cell population was observed in CDT-treated MOLT-4/bcl2 cells (72% alive) compared to nontreated cells (95% alive) (Fig. 5B). This shows that CDT-induced cell death was significantly attenuated by the overexpression of bcl2 in MOLT-4 cells. Further, pretreatment of MOLT-4/bcl2 with z-VAD-fmk completely inhibited CDT-induced cell death for 48 h (Fig. 5A [arrowheads] and B). This finding suggests that increased permeability of the mitochondrial membrane is a central factor in the overall cell death by CDT intoxication.
FIG. 5.
Late effects of CDT in MOLT-4 cells overexpressing the bcl2 gene in the presence or absence of z-VAD-fmk. CDT-induced cell death was monitored at 24 or 48 h after the treatment in MOLT-4 cells that were forced to overexpress the bcl2 gene (MOLT-4/bcl2), MOLT-4 cells transfected with pSFFV-neo vector only (MOLT-4/neo), and parental MOLT-4 cells. (A) Cell death was analyzed by using a FACScan after staining with PI and FITC-labeled annexin V. (B) Percentages of living (annexin V-negative, PI-negative) cells at 48 h. Arrowheads show that dead cell populations increased at 48 h after treatment with CDT but was not seen in the presence of z-VAD-fmk.
Appearance of a cell fraction with a low level of superoxide anion in CDT-treated MOLT-4 in the presence of z-VAD-fmk.
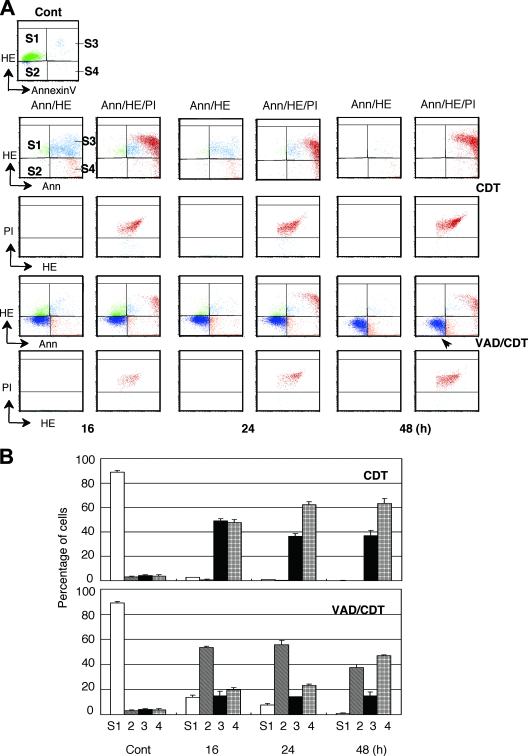
Caspase-dependent apoptosis is characterized by an increase in the intracellular superoxide anion and is related to mitochondrial membrane activity. We examined the levels of superoxide anion in CDT-treated cells using HE that reacts with superoxide anion. Concomitantly, FITC-labeled annexin V and PI were used to detect apoptotic and dead cell populations, respectively. We divided the cell population obtained by FACS into four groups (Fig. 6A): (i) S1 with normal levels of superoxide anion and nonapoptotic annexin V-negative, (ii) S2 with low levels of superoxide anion and annexin V negative, (iii) S3 with high level levels of superoxide anion and apoptotic annexin V positive, and (iv) S4 with low levels of superoxide anion (low HE) or at a normal level and apoptotic annexin V positive. Compensation between HE and PI was adjusted to separate the locations for the PI-positive and PI-negative (dead/live) populations in the quadrant analysis, where the PI-positive population is shown as dark red. As shown in Fig. 6, CDT treatment increased the S3 population to a maximum at 16 h, together with the appearance of a PI-positive dead cell population. Longer exposure increased the PI-positive population, as well as the S4 population. This shows that the CDT-induced early apoptosis was followed by cell death. We then assessed the population change in MOLT-4 cells exposed to CDT in the presence of z-VAD-fmk. z-VAD-fmk induced the appearance of an S2 population in CDT-treated MOLT-4 cells at 16 h, at which point the S3 population did not increase. At 48 h, the cells in the S2 population decreased and PI-positive population, as well as the S4 population, increased. MOLT-4/bcl2 cells exposed to CDT in the presence of z-VAD-fmk showed most cells remained in the S1 population even after 48 h of intoxication (data not shown). This, together with the caspase assay, clearly shows that CDT intoxication in the presence of z-VAD-fmk induced MOLT-4 cells into a caspase-independent nonapoptotic cell death, where MOMP played a significant role.
FIG. 6.
ROS and phosphatidylserine translocation in CDT-treated MOLT-4 cells in the presence or absence of z-VAD-fmk. (A) The levels of ROS and phosphatidylserine translocation in the cytoplasmic membrane in the CDT-treated MOLT-4 cells were measured at 24 and 48 h by using a FACScan. To monitor the dead cells, the cells were subjected to double (Ann/HE) and triple (Ann/HE/PI) staining. Compensation between HE and PI was adjusted to separate the locations of PI-positive and PI-negative populations in the quadrant analysis. PI-positive populations were gated and are shown in the quadrant analysis of HE and annexin V double staining as a dark red color. S1, region of cells with normal levels of HE and annexinV; S2, cells with low levels of HE; S3, cells with high levels of HE and annexin V binding; S4, cells with low levels of HE and high levels of annexin V binding. The arrowhead shows an increased population in S2 by pretreatment with z-VAD-fmk, i.e., cells with a low level of ROS. (B) Percentages of cells distributed into the fractions at 16, 24, and 48 h, where CDT indicates the cells treated with 100 ng of CDT alone/ml and VAD/CDT indicates CDT-treated cells after pretreatment with z-VAD-fmk (100 μM).
DISCUSSION
We show here that long exposure to CDT killed MOLT-4 cells even if caspase activation was inhibited by z-VAD-fmk. A similar observation was reported when MOLT-4 cells were X-irradiated in the presence of z-VAD-fmk (5). X-irradiation induces caspase-3-dependent apoptosis, but treatment of inhibitor of caspase-3 was not accompanied by any persistent increase in cell survival. Instead, irradiated cells treated by the inhibitor exhibited characteristics of a necrotic cell death. Similarly, our results show that caspase-dependent and caspase-independent pathways are involved in the CDT-induced MOLT-4 cell deaths. Similar responses of CDT-intoxicated MOLT-4 cells and X-irradiated MOLT-4 cells further support the idea that CDT intoxication triggers DNA damage.
Preincubation with z-VAD-fmk almost completely inhibited CDT-induced cell death in MOLT-4 cells overexpressing bcl2 that increases the stability of the mitochondrial membrane. The data shows CDT-induced cell death is mitochondrion dependent and suggests the caspase-dependent and caspase-independent death pathways converge at the mitochondria (45). Reports demonstrate death signaling through the mitochondrial pathways induces MOMP to release several mitochondrial proteins including cytochrome c (13, 47, 48), where the Bcl-2 family of proteins are considered to be important regulatory factors for MOMP (8, 41). A possible reason why general caspase inhibitors fail to prevent cell death is because these inhibitors lack the ability to inhibit MOMP (8, 10, 21).
CDT is not the only bacterial toxin that induces cell death through multiple pathways. Staphylococcus aureus alpha-toxin, the major hemolysin, induces massive cell necrosis by forming pores in lipid bilayers at high doses (>6 μg/ml). At low doses, this toxin can induce DNA fragmentation and caspase activation, the typical classical apoptosis pathway, and also caspase-independent cell death (11). Streptococcus pneumoniae induces a rapid caspase-independent cell death in cultured bone marrow-derived dendritic cells (6). This induction is dependent on the expression of pneumolysin, one of the major cytotoxins of S. pneumoniae. This is followed by the delayed onset of caspase-dependent cell death associated with the terminal maturation of dendritic cells. Escherichia coli heat-labile enterotoxin is composed of a single catalytically active A subunit and a pentameric B subunit that interacts with a receptor that mediates the uptake of the holotoxin into the target cells (38). Interestingly, the nontoxic B subunit induces both caspase-dependent and -independent cell death in CD8 T cells. The enterotoxin B subunit induces a rapid loss of mitochondrial membrane potential where cell viability is not affected by caspase inhibitors, suggesting some other intracellular signaling pathways are involved following interaction with the B subunit receptor. Another example is Clostridium difficile toxin B (33). C. difficile toxin B inactivates small GTPases, Rho, Rac, and Cdc42, which lead to caspase-3 activation in HeLa cells. Caspase inhibitors delayed cell death but did not alter the consequence.
Several classifications are proposed to differentiate types of cell death (1, 4, 12, 23). For example, Kroemer et al. (23) proposes four types: (i) classical apoptosis showing programmed cell death through caspase activation; (ii) apoptosis-like cell death resembling apoptosis but lacking total chromatin condensation, karyorhexis, and oligonucleosomal DNA fragmentation (20, 43); (iii) autophagic cell death with an accompanying accumulation of autophagic vacuoles in the cells (36, 51); and (iv) necrosis exhibiting pronounced swelling of the cytoplasmic organelles (18, 48).
Caspases, a group of cysteine proteases, normally act only during classical apoptosis; however, the activation of caspases is also observed in apoptosis-like autophagic cell death, and necrosis as well (36, 43, 48, 51). Further, caspases may be activated not only during the lethal process but also in nonlethal signal transduction (27). Paradoxically, accumulating evidence suggests several types of programmed cell deaths occur without caspase activation in parallel to caspase-dependent cell death as found in apoptosis-like cell death, autophagic cell death, and necrosis (2, 3, 13, 40). Fink et al. (12) propose four types of dying cells caused by infection with microorganisms: apoptosis, autophagy, oncosis, and pyroptosis. Apoptosis is a form of caspase-mediated cell death with particular morphological features, e.g., the apoptotic body, without inflammation. Oncosis is a prelethal process that occurs in ATP-depleted cells concomitant with morphological swelling and eventual membrane permeability. Autophagic cell death involves degradation of intracellular components using autophagic vacuoles. Pyroptosis is a pathway to cell death that involves interleukin-1-mediated inflammation.
Although several morphotypes have been proposed, a definitive classification of the types of cell death pathways has not been established. This is probably because there may be some signaling pathways overlapping and sharing the different death programs (4, 26). It has also been proposed that a dominant cell death morphotype may be determined by comparing the rapidity of the available death programs, i.e., the fastest and most effective pathway among them is dominant (4). In the case of CDT intoxication, the caspase-2-related classical pathway may be the fastest and most efficient pathway. Identification of molecule(s) involved in the CDT-induced caspase-independent and the MOMP-dependent pathway is required to further characterize this death pathway.
Acknowledgments
We thank the Research Facilities of the Hiroshima University School of Dentistry and School of Medicine for the use of their facilities. We are grateful to Jim Nelson for editorial assistance.
This study was supported in part by a grant for development of highly advanced medical technology (type A) and a grant-in-aid for research (type C) from the Ministry of Education, Science, Sports, and Culture of Japan.
Editor: V. J. DiRita
Footnotes
Published ahead of print on 21 July 2008.
REFERENCES
- 1.Abraham, M. C., and S. Shaham. 2004. Death without caspases, caspases without death. Trends Cell Biol. 14184-193. [DOI] [PubMed] [Google Scholar]
- 2.Borner, C., and L. Monney. 1999. Apoptosis without caspases: an inefficient molecular guillotine? Cell Death Differ. 6497-507. [DOI] [PubMed] [Google Scholar]
- 3.Broker, L. E., C. Huisman, S. W. Span, J. A. Rodriguez, F. A. Kruyt, and G. Giaccone. 2004. Cathepsin B mediated caspase-independent cell death induced by microtubule stabilizing agents in non-small cell lung cancer cells. Cancer Res. 6427-30. [DOI] [PubMed] [Google Scholar]
- 4.Broker, L. E., F. A. Kruyt, and G. Giaccone. 2005. Cell death independent of caspases: a review. Clin. Cancer Res. 113155-3162. [DOI] [PubMed] [Google Scholar]
- 5.Coelho, D., V. Holl, D. Weltin, T. Lacornerie, P. Magnenet, P. Dufour, and P. Bischoff. 2000. Capase-3-like activity determines the type of cell death following ionizing radiation in MOLT-4 human leukaemia cells. Br. J. Cancer 83642-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colino, J., and C. M. Snapper. 2003. Two distinct mechanisms for induction of dendritic cell apoptosis in response to intact Streptococcus pneumoniae. J. Immunol. 1712345-2365. [DOI] [PubMed] [Google Scholar]
- 7.Cortes-Bratti, X., C. Karlsson, T. Lagergard, M. Thelestam, and T. Frisan. 2001. The Haemophilus ducreyi cytolethal distending toxin induces cell cycle arrest and apoptosis via the DNA damage checkpoint pathways. J. Biol. Chem. 2765296-5302. [DOI] [PubMed] [Google Scholar]
- 8.Danial, N., and S. Korsmeyer. 2004. Cell death: critical control points. Cell 116205-219. [DOI] [PubMed] [Google Scholar]
- 9.Elwell, C. A., and L. A. Dreyfus. 2000. DNase I homologous residues in CdtB are critical for cytolethal distending toxin-mediated cell cycle arrest. Mol. Microbiol. 37952-963. [DOI] [PubMed] [Google Scholar]
- 10.Enoksson, M., J. D. Robertson, V. Gogvadze, P. Bu, A. Kropotov, B. Zhivotovsky, and S. Orrenius. 2004. Caspase-2 permeabilizes the outer mitochondrial membrane and disrupts the binding of cytochrome c to anionic phospholipids. J. Biol. Chem. 27949575-49578. [DOI] [PubMed] [Google Scholar]
- 11.Essmann, F., H. Bantel, G. Totzke, I. H. Engels, B. Sinha, K. Schulze-Osthoff, and R. U. Janicke. 2003. Staphylococcus aureus alpha-toxin-induced cell death: predominant necrosis despite apoptotic caspase activation. Cell Death Differ. 101260-1272. [DOI] [PubMed] [Google Scholar]
- 12.Fink, S. L., and B. T. Cookson. 2005. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect. Immun. 731907-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foghsgaard, L., D. Wissing, D. Mauch, U. Lademann, L. Bastholm, M. Boes, F. Elling, M. Leist, and M. Jaattela. 2001. Cathepsin B acts as a dominant execution protease in tumor cell apoptosis induced by tumor necrosis factor. J. Cell Biol. 153999-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuhlbrigge, R. C., S. M. Fine, E. R. Unanue, and D. D. Chaplin. 1988. Expression of membrane interleukin 1 by fibroblasts transfected with murine pro-interleukin 1 alpha cDNA. Proc. Natl. Acad. Sci. USA 855649-5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guerra, L., K. Teter, B. N. Lilley, B. Sternerlow, R. K. Holmes, H. L. Ploegh, K. Sandvig, M. Thelestam, and T. Frisan. 2005. Cellular internalization of cytolethal distending toxin: a new end to a known pathway. Cell. Microbiol. 7921-934. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi, T., I. Hayashi, T. Shinohara, Y. Morishita, H. Nagamura, Y. Kusunoki, K. Kyoizumi, T. Seyama, and K. Nakachi. 2004. Radiation-induced apoptosis of stem/progenitor cells in human umbilical cord blood is associated with alterations in reactive oxygen and intracellular pH. Mutat. Res. 55683-91. [DOI] [PubMed] [Google Scholar]
- 17.Heywood, W., B. Henderson, and S. P. Nair. 2005. Cytolethal distending toxin: creating a gap in the cell cycle. J. Med. Microbiol. 54207-216. [DOI] [PubMed] [Google Scholar]
- 18.Hirsch, T., P. Marchetti, S. A. Susin, B. Dallaporta, N. Zamzami, I. Marzo, M. Geuskens, and G. Kroemer. 1997. The apoptosis-necrosis paradox: apoptogenic proteases activated after mitochondrial permeability transition determine the mode of cell death. Oncogene 151573-1582. [DOI] [PubMed] [Google Scholar]
- 19.Hockenbery, D., G. Nunez, C. Milliman, R. D. Schreiber, and S. J. Korsmeyer. 1990. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature 348334-336. [DOI] [PubMed] [Google Scholar]
- 20.Jaattela, M., and J. Tschopp. 2003. Capspase-independent cell death in T lymphocytes. Nat. Immunol. 4416-423. [DOI] [PubMed] [Google Scholar]
- 21.Jiang, X., and X. Wang. 2004. Cytochrome c-mediated apoptosis. Annu. Rev. Biochem. 7387-106. [DOI] [PubMed] [Google Scholar]
- 22.Kadenbach, B., S. Arnold, I. Lee, and M. Huttermann. 2003. The possible role of cytochrome c oxidase in stress-induced apoptosis and degenerative diseases. Biochim. Biophys. Acta 1655400-408. [DOI] [PubMed] [Google Scholar]
- 23.Kroemer, G., and S. J. Martin. 2005. Caspase-independent cell death. Nat. Med. 11725-730. [DOI] [PubMed] [Google Scholar]
- 24.Kuida, K., T. S. Zheng, S. Na, C. Kuan, D. Yang, H. Karasuyama, P. Rakic, and R. A. Flavell. 1996. Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature 384368-372. [DOI] [PubMed] [Google Scholar]
- 25.Lara-Tejero, M., and J. E. Galan. 2000. A bacterial toxin that controls cell cycle progression as a deoxyribonuclease I-like protein. Science 290354-357. [DOI] [PubMed] [Google Scholar]
- 26.Leist, M., and M. Jaattela. 2001. Four deaths and a funeral: from caspases to alternative mechanisms. Nat. Rev. Mol. Cell. Biol. 2589-598. [DOI] [PubMed] [Google Scholar]
- 27.Martinon, F., and J. Tschopp. 2004. Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell 117561-574. [DOI] [PubMed] [Google Scholar]
- 28.Nishikubo, S., M. Ohara, M. Ikura, K. Katayanagi, T. Fujiwara, H. Komatsuzawa, H. Kurihara, and M. Sugai. 2006. Single nucleotide polymorphism in the cytolethal distending toxin B gene confers heterogeneity in the cytotoxicity of A. actinomycetemcomitans. Infect. Immun. 747014-7020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishikubo, S., M. Ohara, Y. Ueno, M. Ikura, H. Kurihara, H. Komatsuzawa, E. Oswald, and M. Sugai. 2003. An N-terminal segment of the active component of the bacterial genotoxin cytolethal distending toxin B (CDTB) directs CDTB into the nucleus. J. Biol. Chem. 27850671-50681. [DOI] [PubMed] [Google Scholar]
- 30.Nougayrede, J. P., F. Taieb, J. De Rycke, and E. Oswald. 2005. Cyclomodulins: bacterial effectors that modulate the eukaryotic cell cycle. Trend Microbiol. 13103-110. [DOI] [PubMed] [Google Scholar]
- 31.Ohara, M., T. Hayashi, Y. Kusunoki, M. Miyauchi, T. Takata, and M. Sugai. 2004. Caspase-2 and caspase-7 are involved in cytolethal distending toxin-induced apoptosis in Jurkat and MOLT-4 T-cell lines. Infect. Immun. 72871-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohara, M., E. Oswald, and M. Sugai. 2004. Cytolethal distending toxin: a bacterial bullet targeted to nucleus. J. Biochem. 136409-413. [DOI] [PubMed] [Google Scholar]
- 33.Qa'Dan, M., M. Ramsey, J. Daniel, L. M. Spyres, B. Safiejko-Mroczka, W. Ortiz-Leduc, and J. D. Ballard. 2002. Clostridium difficile toxin B activates dual caspase-dependent and caspase-independent apoptosis in intoxicated cells. Cell. Microbiol. 4425-434. [DOI] [PubMed] [Google Scholar]
- 34.Ricci, J. E., R. A. Gottlieb, and D. R. Green. 2003. Caspase-mediated loss of mitochondrial function and generation of reactive oxygen species during apoptosis. J. Cell Biol. 16065-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shenker, B. J., R. H. Hoffmaster, A. Zekavat, N. Yamaguchi, E. T. Lally, and D. Demuth. 2001. Induction of apoptosis in human T cells by Actinobacillus actinomycetemcomitans cytolethal distending toxin is a consequence of G2 arrest of the cell cycle. J. Immunol. 167435-441. [DOI] [PubMed] [Google Scholar]
- 36.Shimizu, S., T. Kanaseki, N. Mizushima, T. Mizuta, S. Arakawa-Kobayashi, C. B. Thompson, and Y. Tsujimoto. 2004. A role of Bcl-2 family of proteins in non-apoptotic programmed cell death dependent on autophagy genes. Nat. Cell Biol. 61221-1228. [DOI] [PubMed] [Google Scholar]
- 37.Shinohara, K., and H. Nakano. 1993. Interphase death and reproductive death in X-irradiated MOLT-4 cells. Radiat. Res. 135197-205. [PubMed] [Google Scholar]
- 38.Slmond, R. J., R. Williams, T. R. Hirst, and N. A. Williams. 2004. The B subunit of Escherichia coli heat-labile enterotoxin induced both caspase-dependent and -independent cell death pathways in CD8+ T cells. Infect. Immun. 725850-5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slots, J., H. S. Reynolds, and R. J. Genco. 29. Actinobacillus actinomycetemcomitans in human periodontal disease: a cross-sectional microbiological investigation. Infect. Immun. 291013-1020. [DOI] [PMC free article] [PubMed]
- 40.Stoka, V., B. Turk, S. L. Schendel, T. H. Kim, T. Cirman, S. J. Snipas, L. M. Ellerby, D. Bredesen, H. Freeze, M. Abrahamson, D. Bromme, S. Krajewski, J. C. Reed, X. M. Yin, V. Turk, and G. S. Salvesen. 2001. Lysosomal protease pathways to apoptosis: cleavage of bid, non-pro-caspases, is the most likely route. J. Biol. Chem. 2763149-3157. [DOI] [PubMed] [Google Scholar]
- 41.Strasser, A. 2005. The role of BH-3only proteins in the immune system. Nat. Rev. Immunol. 5189-200. [DOI] [PubMed] [Google Scholar]
- 42.Sugai, M., T. Kawamoto, S. Y. Peres, Y. Ueno, H. Komatsuzawa, T. Fujiwara, H. Kurihara, H. Suginaka, and E. Oswald. 1998. The cell cycle-specific growth-inhibitory factor produced by Actinobacillus actinomycetemcomitans is a cytolethal distending toxin. Infect. Immun. 665008-5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Susin, S. A., E. Daugas, L. Ravagnan, K. Samejima, N. Zamzami, M. Loeffler, P. Costantini, K. F. Ferri, T. Irinopoulou, M. C. Prevost, G. Brothers, T. W. Mak, J. Penninger, W. C. Earnshaw, and G. Kroemer. 2000. Two distinct pathways leading to nuclear apoptosis. J. Exp. Med. 192571-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tinel, A., and J. Tschopp. 2004. The PIDDosome, a protein complex implicated in activation of caspase-2 in response to genotoxic stress. Science 304843-846. [DOI] [PubMed] [Google Scholar]
- 45.Tsujimoto, Y. 2003. Cell death regulation by the Bcl-2 protein family in the mitochondria. J. Cell Physiol. 195158-167. [DOI] [PubMed] [Google Scholar]
- 46.Ueno, Y., M. Ohara, T. Kawamoto, T. Fujiwara, H. Komatsuzawa, E. Oswald, and M. Sugai. 2006. Biogenesis of the Actinobacillus actinomycetemcomitans cytolethal distending toxin holotoxin. Infect. Immun. 743480-3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vanden Berghe, T., G. van Loo, X. Saelens, M. Van Gurp, G. Brouckaert, M. Kalai, W. Declercq, and P. Vandenabeele. 2004. Differential signaling to apoptotic and necrotic cell death by FAS-associated death domain protein FADD. J. Biol. Chem. 2797925-7933. [DOI] [PubMed] [Google Scholar]
- 48.Vercammen, D., R. Beyaert, G. Denecker, V. Goossens, G. Van Loo, W. Declercq, J. Grooten, W. Fiers, and P. Vandenabeele. 1998. Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J. Exp. Med. 1871477-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woo, M., R. Hakem, M. S. Soengas, G. S. Duncan, A. Shahinian, D. Kagi, A. Hakem, M. McCurrach, W. Khoo, S. A. Kaufman, G. Senaldi, T. Howard, S. W. Lowe, and T. W. Mak. 1998. Essential contribution of caspase 3/CPP32 to apoptosis and its associated nuclear changes. Genes Dev. 12806-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamamoto, K., K. Tominaga, M. Sukedai, T. Okinaga, K. Iwanaga, T. Nishihara, and J. Fukuda. 2004. Delivery of cytolethal distending toxin B induces cell cycle arrest and apoptosis in gingival squamous cell carcinoma in vitro. Eur. J. Oral Sci. 112445-451. [DOI] [PubMed] [Google Scholar]
- 51.Yu, L., A. Alva, H. Su, P. Dutt, E. Freundt, S. Welsh, E. H. Baehrecke, and M. J. Lenardo. 2004. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science 3041500-1502. [DOI] [PubMed] [Google Scholar]
- 52.Yuan, J. Y., and H. R. Horvitz. 1990. The Caenorhabditis elegans genes ced-3 and ced-4 act cell autonomously to cause programmed cell death. Dev. Biol. 13833-41. [DOI] [PubMed] [Google Scholar]