Abstract
Hospital-acquired bacterial pneumonia is a common and serious complication of modern medical care. Many aspects of such infections remain unclear, including the mechanisms by which invading pathogens resist clearance by the innate immune response and the tendency of the infections to be polymicrobial. Here, we used a mouse model of infection to show that Pseudomonas aeruginosa, a leading cause of hospital-acquired pneumonia, interferes with the ability of recruited phagocytic cells to eradicate bacteria from the lung. Early in infection, phagocytic cells, predominantly neutrophils, are recruited to the lungs but are incapacitated when they enter the airways by the P. aeruginosa toxin ExoU. The resulting paucity of functioning phagocytes allows P. aeruginosa to persist within the lungs and results in local immunosuppression that facilitates superinfection with less-pathogenic bacteria. Together, our results provide explanations for previous reports linking ExoU-secreting P. aeruginosa with more severe pulmonary infections and for the tendency of hospital-acquired pneumonia to be polymicrobial.
Hospital-acquired pneumonia (HAP) is a common and frequently lethal complication of admission to an acute medical care facility. HAP infections occur in 0.5 to 2% of hospitalized patients (30, 33) and are associated with mortality rates of approximately 30% (6, 13, 41, 54). HAP is usually caused by bacterial pathogens, and in 26 to 67% of cases the etiology is polymicrobial (3, 8, 39). While the reasons for this are unclear, the coexistence of multiple species of bacteria within the lungs of patients may contribute to the high mortality associated with this disease.
The gram-negative bacterium Pseudomonas aeruginosa is the leading cause of HAP in patients undergoing mechanical ventilation (referred to as ventilator-associated pneumonia [VAP]) (40). Interestingly, infection with P. aeruginosa, as opposed to infection with most other bacterial species, is an independent risk factor for death in patients with VAP (23). Although a high incidence of antibiotic resistance among P. aeruginosa strains contributes to the excess mortality associated with this bacterium, its intrinsic virulence also likely plays a role. Even VAP patients treated with antimicrobial agents to which their P. aeruginosa isolates were susceptible had a relapse rate of 18% (42). This suggests that P. aeruginosa elaborates potent virulence determinants that are adept at neutralizing the host immune response, resulting in persistent bacterial infections with poor outcomes.
Despite the severity of HAP caused by P. aeruginosa, much remains unknown about the mechanisms by which this bacterium persists in the lungs and causes the tissue damage and inflammation associated with pneumonia. P. aeruginosa elaborates a number of virulence factors that may augment the disease process (12, 45), including a type III secretion system, which has been associated with more-severe disease in patients with HAP (18, 44). Via a type III secretion system, some strains of P. aeruginosa inject the potent toxin ExoU directly into host cells. Following injection ExoU is activated and causes cytolytic death of an intoxicated cell via its phospholipase A2 activity (16, 20, 36, 38, 48). Strains of P. aeruginosa that secrete ExoU cause increased mortality in animal models of pneumonia (50) and are associated with poorer clinical outcomes in human patients (18, 44). Although it has been shown that ExoU plays an important role in the virulence of P. aeruginosa, the exact mechanisms by which ExoU contributes to the development of severe pneumonia remain unclear.
In the current study, we used a mouse model of acute pneumonia to demonstrate that P. aeruginosa persists in the lungs of acutely infected mice by an ExoU- and neutrophil-dependent mechanism. Our findings suggest that recruited phagocytes are a major target of ExoU intoxication in the lung, resulting in inhibition of the ability of these cells to eradicate P. aeruginosa. Furthermore, the impairment of phagocyte-mediated clearance by ExoU results in localized immunosuppression within the lungs of infected animals, rendering them susceptible to coinfection with otherwise nonpathogenic bacteria. These observations suggest that ExoU-induced immunosuppression in the lung contributes to both the high mortality and the frequency of polymicrobial infections associated with P. aeruginosa HAP.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
PA99 is a P. aeruginosa clinical isolate that naturally contains the exoU, exoS, and exoT genes but lacks the exoY gene (14). Previously, genes encoding ExoS and ExoT were disrupted to eliminate confounding effects of these effector proteins on outcomes (52). The resulting strain was designated PA99U. As previously described, PA99null was generated from PA99U by replacement of a large portion of the exoU gene with an aacC1 gentamicin resistance cassette, allowing this mutant to be distinguished from PA99U by growth on gentamicin-supplemented agar (52). Complementation restored the mutants to an expected level of virulence, and neither mutant displayed a growth defect on laboratory medium (52). The absence of the other type III effectors secreted by parental strain PA99 (ExoS and ExoT) does not significantly affect the in vitro cytotoxicity or in vivo virulence of ExoU (51).
PA103 is a P. aeruginosa laboratory isolate that was originally cultured from the respiratory tract of a patient (28) and secretes ExoT and ExoU. PA103(mutant 1) is a PA103 strain defective in the secretion of PopB and PopD (21); this mutant secretes ExoU and ExoT but is unable to translocate these effector proteins into host cells (21). PA103ΔUT contains disruptions in both the exoT and exoU genes and does not secrete any type III effector proteins (38). Escherichia coli strain XL1-Blue (Stratagene Corp., La Jolla, CA) was used for cloning experiments and in mixed infections, and E. coli strain S17.1 (53) was used for conjugation experiments.
Bacterial strains were streaked from frozen cultures onto Luria-Bertani (LB) agar or Vogel-Bonner minimal (VBM) agar (57). For infection, overnight cultures of P. aeruginosa grown in 5 ml MINS medium (32) at 37°C were diluted into fresh medium and regrown to exponential phase. E. coli strain XL1-Blue was grown in 5 ml LB medium without antibiotics prior to animal infection. Antibiotics were used when necessary at the following concentrations: ampicillin, 50 μg/ml; carbenicillin, 500 μg/ml; gentamicin, 100 μg/ml; and tetracycline, 100 μg/ml.
Construction of tagged and mutant strains.
To generate luciferase-expressing PA99null, the constitutive promoter npt2 was amplified from plasmid pnptgfp, a generous gift from Barbara Kazmierczak, using primers pnpt2XhoI-F (5′-AAAAAACTCCTCGAGGCAGGTAGCTTGCAGTGGGCTTACATGGCG) and pnpt2EcoRI-R (5′-AAAAAACTCGAATTCGCGCCATCAGATCCTTGGCGGCAAGAAA GC). The amplified DNA fragment was digested with XhoI and EcoRI and inserted into the multiple cloning site of the integration-proficient vector miniCTX-lux, a generous gift from Herbert Schweizer and Matthew Parsek (4). The resulting construct (miniCTXnpt2lux) was transformed into E. coli strain S17.1 (53) and, following conjugation, was introduced into the attB site of the PA99null chromosome via integrase-mediated recombination using previously described approaches (22) to generate luciferase-tagged PA99null. Competition assays indicated that luciferase-tagged PA99null did not have a virulence disadvantage relative to untagged PA99null; following infection of mice with an inoculum containing these strains at a ratio of 1:1 (a total of 1.2 ×106 CFU), approximately equal numbers of tagged and untagged PA99null cells were recovered from the lungs of infected mice at 18 h postinfection (data not shown).
A PA99 strain that secreted catalytically inactive ExoU was constructed by ligating into plasmid miniCTX (a generous gift from Herbert Schweizer) the exoU allele from plasmid pExoU-S142A, as previously described (38). This exoU allele encodes a toxin containing a serine-to-alanine substitution at residue 142 (S142A), which eliminates ExoU's phospholipase activity (36, 38, 47). The resulting construct was transformed into E. coli strain S17.1 and, following conjugation, was inserted into the attB site of the PA99null chromosome using previously described approaches (22). The attB site is located in an intergenic region such that insertion into it does not disrupt existing genes (22). The resulting strain was designated PA99null+U(S142A).
PA103ΔT, a mutant that secretes only ExoU, was constructed by conjugation of PA103 with E. coli strain S17.1 carrying pCM104, a plasmid containing an in-frame deletion allele of the exoT gene, as previously described (38).
Mouse model of acute pneumonia.
Studies of acute pneumonia were conducted using the aspiration mouse model described by Comolli et al. (9). Briefly, bacteria were collected by centrifugation and resuspended at the appropriate concentration in phosphate-buffered saline (PBS). Six- to eight-week-old female BALB/c mice were anesthetized by intraperitoneal injection of a mixture of ketamine (100 mg/ml) and xylazine (20 mg/ml). Mice were intranasally inoculated with 1.2 × 106 CFU of bacteria in 50 μl PBS, as determined by optical density. Inocula were confirmed by plating serial dilutions. For each experiment, at least four mice per strain were infected for each time point. At various time points after infection, lungs were aseptically removed and individually homogenized in PBS. The bacterial load in each organ was determined following plating of serial dilutions on VBM agar and incubation at 37°C for 24 h.
For mixed infections, inocula were prepared as described above prior to mixing of PA99U with PA99null or E. coli at different dilutions. The total inoculum was kept constant at 1.2 × 106 CFU. Twenty-five percent of this inoculum (3.0 × 105 CFU) was luciferase-tagged PA99null or E. coli XL1-Blue, while the remainder (75%) was comprised of various proportions of PA99U and untagged PA99null (0, 33, 66, or 100% PA99U). Inocula and homogenized lungs were then plated on VBM agar (for PA99U-PA99null infections) or on LB medium and LB medium containing ampicillin (for PA99-E. coli infections), and colonies were counted. Luciferase-tagged PA99null colonies were differentiated from untagged P. aeruginosa colonies by luminescence using an Alpha Imager FC 8000 (AlphaInnotech) with 2-min exposures.
Animals were purchased from Harlan and housed in the containment ward of the Center for Comparative Medicine at Northwestern University. All experiments were performed in accordance with the guidelines of the Northwestern University Animal Care and Use Committee.
Analysis of inflammatory response by flow cytometry.
At the appropriate time point, mice were anesthetized and sacrificed by cervical dislocation and thoracotomy. The lungs were perfused and flushed by injection of 2 ml PBS into the right side of the heart to remove circulating blood cells. Lungs were excised and mashed through 40-μm filters (Falcon). The filters were rinsed repeatedly with PBS. For bronchoalveolar lavage (BAL) experiments, mouse lungs were lavaged by instilling and withdrawing 1 ml of PBS three times. Recovered cells were gently pelleted by centrifugation at 200 × g for 5 min. The supernatant was removed, and red blood cells were lysed by addition of 1 ml of cold sterile water for 10 s. One milliliter of 2× saline (1.8% NaCl) was quickly added to prevent additional cell lysis. The remaining cells were pelleted and resuspended in PBS, and trypan blue-excluding cells were quantified using a hemacytometer.
A total of 106 cells were placed in each well of a 96-well V-bottom plate (Nunc, Roskilde, Denmark). Surface Fc receptors and nonspecific binding sites were blocked by incubation in 10% rat serum (Sigma) and anti-CD16/32 in fluorescence-activated cell sorting (FACS) buffer (1% bovine serum albumin, 0.1% NaN3 in PBS) for 5 to 15 min on ice. Cell discriminatory antibodies were added at the appropriate dilutions in FACS buffer, and the final volume in each well was adjusted to 125 μl. Antibodies and cells were incubated for 15 to 30 min on ice. Cells were pelleted by centrifugation at 200 × g for 5 min and resuspended in 2% paraformaldehyde in PBS for 2 min. An equal volume of FACS buffer was added, and cells were pelleted again. Cells were resuspended in FACS buffer and transferred into Falcon 2052 tubes after passage through 70-μm Nitex filters. Antibodies were used at the following final dilutions: anti-CD16/32, 1:50; anti-CD45, 1:1,250; anti-Gr1, 1:1,250; anti-F4/80, 1:50; and isotype controls, 1:25 each. All antibodies were purchased from eBioscience.
Cells were analyzed by flow cytometry using either a Becton Dickinson FACSCalibur or FACSort machine. Instrument settings were determined each day using appropriate positive and negative control samples. After data collection, cell debris was removed from the analysis by gating for forward and side scatter. The remaining cells were considered to represent the total number of cells extracted from the lung. Next, the number of CD45+ cells, which represented the total inflammatory cell population, was determined. Cells were gated as follows: CD45+ Gr1+ F4/80− cells as neutrophils, CD45+ Gr1lo F4/80+ cells as macrophages, and CD45+ Gr1− F4/80− cells as lymphocytes. The total number of viable inflammatory cells per mouse lung was determined by equating the number of total cells with normal scatter characteristics measured by flow cytometry to the number of trypan blue-negative cells counted with the hemacytometer.
Histological examination.
For histopathological examination, lung tissue was fixed in 4% paraformaldehyde and embedded in paraffin. Sections were stained with hematoxylin and eosin (H&E) at the Pathology Core Facility of the Robert H. Lurie Comprehensive Cancer Center at Northwestern University. Light microscopy examination was conducted by a trained pathologist blinded to the experimental conditions associated with the samples. Pictures were taken with a Leica DMR upright microscope at magnifications of ×100 and ×200 using a Zeiss AxioCam camera and AxioVision 4.5 software.
Depletion of circulating phagocytic cells.
Affinity-purified anti-mouse anti-Gr1 (Ly6-G) antibodies (clone RB6-8C5) were purchased from eBioscience (San Diego, CA). For in vivo depletion of systemic neutrophils and monocytes, 200 μl (100 μg) of antibodies was injected intraperitoneally into each mouse. Depletion lasts up to 5 days (55). Animals were infected 24 to 30 h following antibody injection. Anti-Gr1-treated mice became severely ill quite rapidly, so 12 h rather than 18 h was chosen as the time point for dissection in these experiments. Depletion of the cells was confirmed by examination of May-Grunwald Giemsa-stained peripheral blood smears by a trained pathologist blinded to the experimental conditions associated with the samples.
Cytotoxicity assays.
Release of lactate dehydrogenase (LDH) was measured to quantify the death of neutrophils in vitro. Human neutrophils were isolated from blood from healthy human donors by dextran sedimentation followed by density centrifugation using Histopaque 1077 (Sigma Aldrich, St. Louis, MO). Red blood cells were lysed by addition of 1 ml of cold sterile water for 10 s, followed by 1 ml of cold 2× saline to prevent further lysis. Neutrophils were enumerated with a hemacytometer using trypan blue exclusion as an indicator of viability. A total of 1.0 × 106 neutrophils were incubated with 1.0 × 108 CFU of PA99U or PA99null. At the appropriate time points, a 50-μl aliquot of supernatant was removed and assayed for release of LDH (CytoTox 96 nonradioactive cytotoxicity assay; Promega). Cell lysis was quantified by determining the absorbance at 490 nm and normalizing the results using the total cell lysis that occurred when 0.05% Triton X-100 was added. The Northwestern University Institutional Review Board approved the study, and informed consent was obtained from all subjects.
Fluorescence microscopy.
Human neutrophils were isolated from blood from healthy human donors as described above. Approximately 2.7 × 106 neutrophils were allowed to adhere to a poly-l-lysine-coated coverglass (Nalge Nunc, Naperville, IL) in the presence of 2.5 μM ethidium homodimer-1 (Invitrogen) for 30 min in modified HEPES-buffered saline. Cells were then incubated with 2.7 × 108 CFU PA99U and viewed with a Leica DMIREZ microscope equipped with a 100-W mercury lamp and a Hamamatsu ORCA-ER camera. Pictures were taken at a magnification of ×1,000 using the Openlab 5.0.1 software.
Video microscopy.
Neutrophil infection was performed as described above for the fluorescence microscopy analysis. A suspension of neutrophils (2.7 × 106 cells) was loaded into a poly-l-lysine-coated chamber slide in the presence of 2.5 μM ethidium homodimer-1 and allowed to adhere at 37°C in a 5% CO2 atmosphere for 30 min. Time-lapse video microscopy was performed following addition of PA99U to the wells. Infected neutrophils were viewed with a Leica DMIREZ microscope equipped with a 100-W mercury lamp and a Hamamatsu ORCA-ER camera. Pictures were taken every 2 min at a magnification of ×1,000 using the Openlab 5.0.2 software. The temperature was maintained at 37°C throughout the experiment by use of a temperature-controlled microscope stage. Differential interfering contrast and CY3-filtered images were collected and merged. Video editing was performed using Openlab 5.0.2 software.
Statistical methods.
Analyses of bacterial load differences were performed by using analysis of variance (ANOVA). For ANOVA comparisons with a P value of <0.05, adjustment for multiple unplanned comparisons was performed using the Tukey-Kramer honestly significant difference (HSD) test with an α value of 0.05. Prior to analysis, all colonization data were natural log transformed so that they fit a normal distribution. The use of parametric tests with transformed colonization data was justified by analysis of a large set of control data that confirmed that colonization data were log normally distributed. Analyses of differences in LDH release were performed by using Student's t test for two-sample comparisons and ANOVA with the Tukey-Kramer honestly significant difference test for three-sample comparisons.
RESULTS
ExoU promotes bacterial survival in the lung over time.
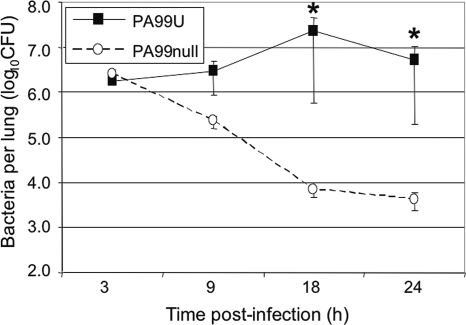
In a mouse model of acute pneumonia, ExoU has been associated with increased virulence of P. aeruginosa and has been shown to cause extensive tissue damage in the lung (1, 16, 20, 24, 25, 34). To understand the mechanism by which ExoU causes increased disease severity, we first examined the ability of P. aeruginosa to persist in the lungs during early infection. For this purpose, we utilized two isogenic strains of P. aeruginosa, PA99U (ExoU+) and PA99null (ExoU−), which differ only in the production of ExoU (52). Mice were infected by intranasal aspiration with 1.2 × 106 CFU of either PA99U or PA99null, and the bacterial burdens in the lungs were measured at several times after infection (Fig. 1). Equal numbers of bacteria were recovered from lungs 3 h after inoculation with either PA99U or PA99null, suggesting that ExoU is not required to establish infection in the lung (Fig. 1). However, at subsequent time points, the infections caused by these two strains differed significantly. By 9 h postinfection, PA99null was already being efficiently cleared from the lung, and after 24 h over 99% of the inoculum had been eradicated. In contrast, the numbers of PA99U CFU in the lung increased and remained stable through 24 h (Fig. 1). These results are consistent with previous observations of impaired clearance of ExoU+ P. aeruginosa from the lung (27) and suggest that ExoU promotes bacterial survival in the lung during early infection.
FIG. 1.
Persistence of ExoU+ and ExoU− strains of P. aeruginosa in the lungs over the first 24 h of infection. Mice were infected with PA99U or PA99null and sacrificed at the indicated time points following infection. The data are means ± standard errors of the means (n ≥ 5) for a representative experiment. Similar results were obtained in at least three separate experiments. *, P < 0.01.
ExoU induces a profound inflammatory response.
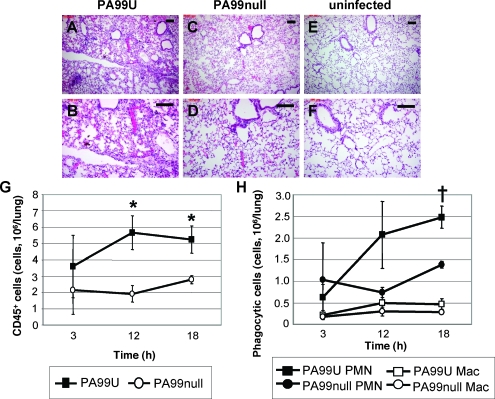
We hypothesized that ExoU facilitated bacterial persistence by interfering with the innate immune response in the lung. To test this idea, we examined tissue sections from the lungs of mice infected with PA99U or PA99null (Fig. 2A to F). Although the degree of inflammation varied throughout the lung tissue, H&E staining revealed overall markedly increased inflammation in the pulmonary tissues of mice infected with PA99U compared to the pulmonary tissues of uninfected controls (Fig. 2A, B, E, and F). In contrast, infection with PA99null resulted in more moderate inflammatory infiltration in the lung parenchyma (Fig. 2C and D). Morphological quantification of inflammatory cells in H&E-stained tissue sections from mice infected with ExoU+ or ExoU− bacteria indicated that the majority of the inflammatory cells were neutrophils (data not shown). Quantification of the inflammatory infiltrate in the lung by flow cytometry confirmed that there was increased recruitment of inflammatory cells (CD45+ leukocytes) to the lungs of mice infected with PA99U (Fig. 2G). The majority of these cells were neutrophils, while relatively few were macrophages or lymphocytes (Fig. 2H and data not shown). Thus, the persistence of PA99U within the lungs of infected mice was not due to the absence of or reduction in inflammation at the site of infection. Rather, ExoU secretion appeared to allow P. aeruginosa bacteria to persist despite the induction of a robust inflammatory response consisting primarily of neutrophils.
FIG. 2.
Inflammation in the lungs of mice infected with an ExoU+ or ExoU− strain of P. aeruginosa. (A to F) H&E staining of lung sections from mice inoculated with PA99U (A and B), PA99null (C and D), or PBS alone (E and F) at 18 h postinfection. Scale bars, 100 μm. (G and H) Flow cytometry analysis of inflammatory cells in the lungs of mice infected with an ExoU+ or ExoU− strain at 18 h postinfection. (G) Number of CD45+ cells (leukocytes) per lung for mice infected with PA99U or PA99null. (H) Number of neutrophils per lung for mice infected with PA99U or PA99null and number of macrophages per lung for mice infected with PA99U or PA99null. The data are means ± standard errors of the means for at least three independent experiments. *, P < 0.05; †, P < 0.01. PMN, neutrophils; Mac, macrophages.
ExoU does not enhance bacterial survival in the lungs in the absence of recruited phagocytes.
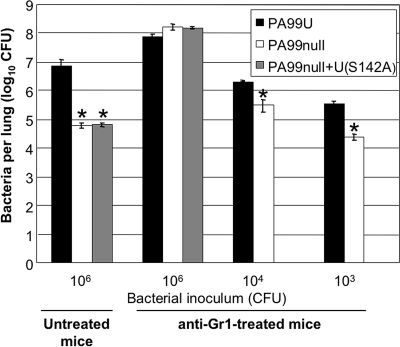
The inability of high numbers of recruited phagocytes to eradicate ExoU+ P. aeruginosa from the lung during infection suggested that ExoU in some way impaired phagocyte function to prevent bacterial clearance during pneumonia. If this were the case, ExoU's ability to augment bacterial numbers in the lungs relative to the bacterial numbers observed with an ExoU− strain should diminish in the absence of neutrophils and monocytes. To test this hypothesis, we compared PA99U infections to PA99null infections in normal mice and mice depleted of neutrophils and monocytes (Fig. 3). Recruited phagocytic cells, the vast majority of which were neutrophils, were depleted by treatment with anti-mouse anti-Gr1 (Ly6-G) antibodies. This treatment depleted 93 to 99% of the peripheral blood neutrophils and 95% of the peripheral blood monocytes within 24 h (data not shown). At 12 h after infection of untreated mice, 7.5 × 106 CFU of PA99U per lung were present, compared to only 6.0 × 104 CFU of PA99null per lung (P < 0.05), confirming ExoU's ability to enhance bacterial persistence in the presence of recruited phagocytes. As expected, depletion of circulating phagocytic cells was accompanied by increased numbers of bacteria and decreased inflammation in the lungs, showing the importance of these cells in containing P. aeruginosa (Fig. 3 and data not shown). However, in contrast to the results for untreated mice, similar numbers of bacteria were recovered after 12 h of infection with PA99U (8.1 × 107 CFU) and after 12 h of infection with PA99null (1.4 × 108 CFU) in mice treated with anti-Gr1 (Fig. 3). Thus, secretion of ExoU resulted in a 125-fold increase in the number of bacteria in the lungs of untreated mice but no increase in the number of bacteria in the lungs of mice depleted of circulating phagocytic cells, indicating that the effects of ExoU occur only in the presence of these recruited cells. However, it is possible that the dose of bacteria used simply overwhelmed the immunocompromised animals, thus obviating the need for ExoU. To determine whether this was indeed the case, lower doses of bacteria were used to infect anti-Gr1-treated animals, and lung colonization was measured at 12 h postinfection. As the bacterial inoculum given to these mice was decreased, so that the number of remaining recruited phagocytic cells (approximately 5% of the recruited phagocytic cells found in untreated mice) relative to the number of bacteria became greater, ExoU-mediated enhancement of bacterial numbers again became apparent, although the levels never reached the levels observed in untreated mice (Fig. 3). This observation suggests that the small number of residual neutrophils and monocytes or the contribution of other cell populations in the lungs of the mice may have been sufficient to exert a measurable antibacterial effect and thus constitute an immunologically relevant target for ExoU with the small inocula used. Furthermore, PA99null+U(S142A), which secretes a catalytically inactive form of ExoU, was recovered at numbers similar to the numbers of PA99null in both untreated and anti-Gr1-treated mice, indicating that the phospholipase activity of the ExoU toxin is necessary for its ability to counteract phagocyte-mediated clearance (Fig. 3). Together, these data indicate that ExoU causes the persistence of P. aeruginosa during early pneumonia by directly or indirectly targeting recruited phagocytes, the vast majority of which are neutrophils, in a phospholipase-dependent manner.
FIG. 3.
Persistence of ExoU+, ExoU−, and catalytically inactive ExoU strains in untreated and anti-Gr1-treated mice. Mice were infected with PA99U, PA99null, or PA99null+U(S142A) using inocula containing 106, 104, or 103 CFU and were sacrificed at 12 h postinfection, after which the numbers of viable bacteria in the lungs were determined. The data are means ± standard errors of the means (n ≥ 5) for two independent experiments. Similar results were obtained in two separate experiments. *, P < 0.05 for a comparison to PA99U at the same dose.
ExoU causes lysis of neutrophils.
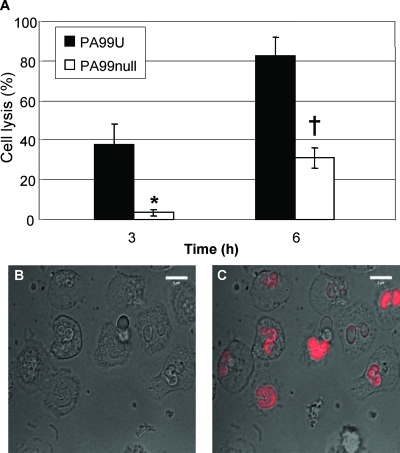
The phospholipase activity of ExoU results in the lysis of many cell types in vitro, including macrophages (7, 16). Since ExoU's phospholipase activity was necessary for P. aeruginosa to resist eradication by a predominantly neutrophilic inflammatory response, we hypothesized that ExoU intoxication also resulted in killing of neutrophils. We therefore coincubated P. aeruginosa strains with purified human neutrophils in vitro and quantified neutrophil lysis by measuring LDH release. Coincubation with PA99U resulted in lysis of 38% of the neutrophils after 3 h and 80% lysis after 6 h (Fig. 4A). In contrast, PA99null lysed only 4 and 34% of the cells at these time points, respectively (P < 0.01 at 3 h and P < 0.001 at 6 h). Similar patterns of lysis were observed by microscopic evaluation of primary human neutrophils incubated with PA99U or PA99null in the presence of ethidium homodimer-1, a dead cell stain that labels the nuclei of membrane-permeable cells (Fig. 4B and 4C). Loss of cell volume coincided with nuclear accumulation of ethidium homodimer-1 (see the video in the supplemental material). Lysis of neutrophils by PA99null at later times was likely due to other factors produced by P. aeruginosa that have been shown to kill neutrophils or have been hypothesized to have such activity (5, 37). Ethidium homodimer-1 staining was not observed in uninfected cells (data not shown). A distinct P. aeruginosa strain, PA103, was used to show that ExoU-mediated killing of neutrophils is not specific to strain PA99 (data not shown). PA103(mutant 1), a translocation-defective PA103 mutant that does not secrete the translocon proteins PopB and PopD (21), exhibited minimal cytotoxicity with neutrophils, indicating that ExoU must be injected directly into neutrophils to induce cell death (data not shown). From these data, it is clear that ExoU is able to rapidly lyse human neutrophils.
FIG. 4.
Lysis of primary human neutrophils by ExoU+ and ExoU− strains of P. aeruginosa. (A) Cell death was assessed by measuring the release of LDH. Each experiment was performed at least in triplicate. The background lysis in the absence of bacteria was subtracted from all values. The data are means ± standard errors of the means for four separate experiments. *, P < 0.01; †, P < 0.0001. (B and C) Cell death was assessed by microscopic examination of cells infected with PA99U in the presence of ethidium homodimer-1, which stains the nuclei red in cells that have lost membrane integrity, at 60 min (B) and 90 min (C) postinfection. Similar results were obtained in two separate experiments. Scale bars, 5 μm.
Based upon these results, we postulated that ExoU killed phagocytes, particularly neutrophils, recruited to the lungs of infected mice. Attempts to directly measure neutrophil killing in vivo were unsuccessful, most likely because alterations in the morphology of dying neutrophils and nonspecific binding of antibodies to membrane-permeable cells precluded detection by flow cytometry (see the video in the supplemental material). We therefore used an alternate approach to detect neutrophil killing in vivo. Our rationale was that if neutrophils are killed by ExoU, killing would require direct contact between bacteria and neutrophils, a precondition for type III secretion (17). Since the majority of P. aeruginosa bacteria are found in the airways and alveoli during pneumonia (11, 29), we reasoned that there should be a relative paucity of viable neutrophils in these lung compartments relative to other lung compartments due to ExoU-mediated cell destruction. We therefore compared the ratio of the number of viable neutrophils in BAL fluid, which included cells recovered from the alveoli, bronchioles, and bronchi, to the total number of viable neutrophils in whole-lung suspensions, which included cells in the airways, as well as the pulmonary interstitium and vascular compartments. Viable neutrophils were identified by trypan blue exclusion and forward and side scatter characteristics measured by flow cytometry. In mice infected with PA99null, the viable neutrophils in the BAL fluid comprised 63% of the total number of neutrophils in the whole lungs (792,000/1,250,000). In contrast, the BAL-derived viable neutrophils comprised only 23% of the total number of viable neutrophils in the lungs of PA99U-infected mice (668,000/2,880,000), indicating that there was preferential reduction of viable neutrophils in BAL fluid in the presence of PA99U (P < 0.01, determined using least nine animals per group and with the experiment repeated three times). This observation indicates that while the total number of neutrophils in the lungs of PA99U-infected mice increases, the proportion of viable neutrophils in the airways decreases significantly, and it suggests that neutrophils are killed after exposure to ExoU-secreting bacteria in the airways.
ExoU+ P. aeruginosa promotes persistence of ExoU− P. aeruginosa in the lung.
We next determined whether infection by ExoU+ strains of P. aeruginosa facilitated the persistence of coinfecting ExoU− P. aeruginosa strains, as would be expected if ExoU were indeed killing neutrophils. To examine this possibility, we developed a mixed-infection model in which the persistence of a set PA99null inoculum could be tracked in the presence of various levels of ExoU intoxication within the lungs. All mice were infected by intranasal aspiration with 3.0 × 105 CFU of PA99null tagged with luciferase, and the fate of the bacteria was followed during the first 18 h of infection. To vary the degree of ExoU intoxication in the lung, mice were coinfected with 9.0 × 105 CFU of P. aeruginosa comprised of PA99U and untagged PA99null at different ratios. Thus, all mice received the same total dose of bacteria (1.2 × 106 CFU), 25% of which was luciferase-tagged PA99null, but were infected with various proportions of ExoU-secreting P. aeruginosa. As expected, infection with increasing numbers of ExoU+ bacteria resulted in recovery of increasing numbers of untagged P. aeruginosa bacteria after 18 h of infection (Fig. 5A). Importantly, we also observed that as the proportion of PA99U in the inoculum increased, the number of luciferase-tagged PA99null recovered from the lung increased in a dose-dependent manner (Fig. 5A). This effect was dependent upon recruited phagocytes, as no enhanced recovery of luciferase-tagged PA99null was observed when the experiments were repeated with mice treated with anti-Gr1 (Fig. 5B). Thus, ExoU was associated with a failure to eradicate not only ExoU-secreting P. aeruginosa but also nonsecreting bacteria infecting the same lung.
FIG. 5.
Recovery of bacteria from the lungs of mice following mixed infections. The x axis indicates the percentage of the untagged P. aeruginosa inoculum consisting of PA99U. (A) Recovery of total untagged P. aeruginosa (left panel) and luciferase-tagged PA99null* (PA99null*) (right panel) from untreated mice after 18 h of infection. (B) Recovery of total untagged P. aeruginosa (left panel) and luciferase-tagged PA99null (right panel) from anti-Gr1-treated mice after 12 h of infection. (C) Recovery of total P. aeruginosa (left panel) and E. coli XL1-Blue (right panel) after 18 h of coinfection. The data are means ± standard errors of the means (n ≥ 4) for a typical experiment. Similar results were obtained in at least three separate experiments. *, P < 0.01; **, P < 0.05.
ExoU enhances infection by nonpathogenic organisms.
The results described above suggested that ExoU intoxication of recruited phagocytes created an environment of localized immunosuppression within the lungs. We hypothesized that such immunosuppression would facilitate infection by less-pathogenic bacterial species. To test this hypothesis, we used mixed infections with combinations of P. aeruginosa and a highly attenuated laboratory strain of E. coli, XL1-Blue. E. coli XL1-Blue is quite avirulent in this model; when a large dose of E. coli (1.2 × 106 bacteria) was given alone to mice, 99.99% of the infecting inoculum was cleared by 18 h (data not shown). In the mixed infections, all mice received a fixed total inoculum containing 1.2 × 106 bacteria, 25% of which was E. coli and 75% of which was P. aeruginosa with various ratios of PA99U to PA99null. Coinfection with an increasing proportion of PA99U resulted in increasing recovery of E. coli from the lungs of infected mice (Fig. 5C). During infection with inocula containing predominantly PA99U, E. coli replicated to a level above the original input level, 3.0 × 105 CFU (Fig. 5C). In other words, as ExoU intoxication in the lung increased, coexisting avirulent bacteria were able to survive and contribute to the infection. Similar results were obtained when this mixed-infection experiment was repeated using ExoU+ and ExoU− strains derived from laboratory strain PA103, indicating that this effect is not unique to strain PA99 (data not shown). Overall, these data suggest that ExoU creates a local environment of immunosuppression in the lung that predisposes the host to infection by other bacteria, including bacteria that are not normally pathogenic.
DISCUSSION
P. aeruginosa as an etiology is an independent risk factor for death in patients with HAP (23), and strains that secrete ExoU are associated with particularly poor outcomes (1, 18, 34, 44, 50). In the current study, we found that ExoU contributes to the development of severe pneumonia by inhibiting the host's ability to contain and clear bacterial infection of the lung. By comparing infections in untreated and anti-Gr1-treated mice, we obtained evidence that phagocytes, particularly neutrophils, normally play a prominent role in eradicating P. aeruginosa from the lungs during pneumonia. Secretion of ExoU, however, blocks phagocyte-mediated clearance at the site of infection, most likely by killing cells of this type. This localized impairment of an essential component of the innate immune response generates an environment of immunosuppression in the lung. The importance of this is twofold; first, it allows P. aeruginosa itself to persist in the lung, and second, it allows other low-pathogenicity bacteria to establish a superinfection. Together, these two effects contribute to the severity of P. aeruginosa pneumonia and explain in part the high frequency of polymicrobial HAP.
We determined that a phagocytic infiltrate comprised predominantly of neutrophils is rapidly recruited to the lungs in response to P. aeruginosa infection. In fact, infection with an ExoU-secreting strain induced an even more intense inflammatory response than infection with an ExoU− strain, supporting previous results showing that ExoU has potent proinflammatory effects (10, 31, 46). Furthermore, we found that in the absence of ExoU, phagocytes are required for clearance of P. aeruginosa from the lung over the first 12 h of infection. These data are consistent with reports indicating that neutrophils are an essential component of the host response to P. aeruginosa (35, 49, 58, 59) and with clinical observations of increased susceptibility to P. aeruginosa infection in patients with neutropenia (43). Overall, our data provide further validation of the importance of phagocytes in the host response to P. aeruginosa.
Since phagocytes are crucial in combating P. aeruginosa, it is not surprising that this pathogen has developed mechanisms to counteract these cells. In the presence of ExoU secretion, phagocytes have little success in clearing bacteria from the lung, indicating that ExoU abrogates this component of the innate immune response. In fact, the enhanced virulence associated with ExoU during early infection is virtually absent in the absence of phagocytes. These results indicate that phagocytes are essential for the pathogenic effects of ExoU and suggest that cells of this type are directly or indirectly targeted for intoxication during acute pneumonia. In turn, the impairment of phagocytes by ExoU contributes to the enhanced severity of pneumonia associated with strains that secrete this toxin (50). However, the effects of ExoU on other cell types in the lung, including lymphocytes and epithelial cells, may also contribute to bacterial survival and disease severity.
Our findings provide insight into the consequences for neutrophils of ExoU intoxication. ExoU has been shown to cause rapid lysis of numerous cell types in vitro (2, 15, 16, 19, 25, 49, 56), and this lysis is dependent on the phospholipase activity of ExoU (36, 38, 48). In agreement with Lee and colleagues, we found that human neutrophils are also susceptible to killing by ExoU in vitro (26). Furthermore, examination of BAL fluid and whole-lung homogenate indicated that neutrophils were robustly recruited to the lungs but that the proportion of viable neutrophils within the airspace, where the majority of bacteria reside, was reduced in the presence of ExoU secretion. Taken together, these data suggest that ExoU directly kills neutrophils in the lung. However, we cannot exclude the possibility that the observed defect in bacterial clearance may be a result of sublethal effects of ExoU on neutrophils, such as inhibition of phagocytosis or bacterial killing mechanisms. Alternatively, ExoU may act indirectly on this cell type. Specifically, altered production of cytokines and chemokines or antimicrobial products, differential activation, or defective local migration due to gross tissue damage in the lung may explain the observed inability of neutrophils to eradicate bacteria from the lung. Further investigation is needed to more precisely define the mechanism by which ExoU prevents neutrophils from eradicating bacteria from the lungs.
The consequences of ExoU's effects on phagocytes for the outcome of an infection are profound. Despite the fact that the host had an otherwise intact immune system, secretion of ExoU created an environment of localized immunosuppression in the lung that allowed P. aeruginosa to avoid clearance. This effect was not limited to bacteria that secreted this toxin. Without functional phagocytes at the site of infection, P. aeruginosa bacteria that did not secrete ExoU were also able to avoid clearance. Local immunosuppression due to ExoU even rendered an avirulent laboratory strain of E. coli pathogenic. Based on these data, we propose that secretion of ExoU may allow development of polymicrobial infections by inactivating phagocytes in the lung. As these phagocytic cells become intoxicated with ExoU, coinfecting bacteria that are normally effectively controlled by the host become capable of establishing infection.
Our findings and those of others suggest the following model for ExoU's role in the pathogenesis of acute pneumonia. Early in infection, ExoU targets phagocytes, which prevents clearance of P. aeruginosa from the lungs. The large numbers of P. aeruginosa cells that persist produce a variety of virulence factors, which exert their action to bring about the pathophysiological consequences of severe pneumonia. ExoU itself may play an important second role in this process by disrupting the epithelial barrier of the lungs and allowing large amounts of proinflammatory cytokines to escape into the circulation, causing septic shock (25). For these reasons, therapeutic targeting of ExoU may provide important benefits to patients with HAP. Since P. aeruginosa strains that do not secrete ExoU often secrete ExoS, studies to determine whether this type III effector protein plays a similar role in pathogenesis are under way.
Supplementary Material
Acknowledgments
This work was supported by the NIH (grants AI053674 and AI065615 [A.R.H.], AI07476 [C.M.S.], T32 GM008061 [M.H.D.], and ES013082 [C.M.S. and A.R.H.]) and by Philip Morris (J.A.K.).
We thank Alison Criss, Yi Lu, Geoff Kansas, Warren Tourtelotte, John Carter, Laurie Tudor, Cheryl Olson, and Susan Winandy for their advice and technical assistance with the experiments.
Editor: B. A. McCormick
Footnotes
Published ahead of print on 28 July 2008.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Allewelt, M., F. T. Coleman, M. Grout, G. P. Priebe, and G. B. Pier. 2000. Acquisition of expression of the Pseudomonas aeruginosa ExoU cytotoxin leads to increased bacterial virulence in a murine model of acute pneumonia and systemic spread. Infect. Immun. 683998-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apodaca, G., M. Bomsel, R. Lindstedt, J. Engel, D. Frank, K. Mostov, and J. Wiener-Kronish. 1995. Characterization of Pseudomonas aeruginosa-induced MDCK cell injury: glycosylation defective host cells are resistant to bacterial killing. Infect. Immun. 631541-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker, A. M., J. W. Meredith, and E. F. Haponik. 1996. Pneumonia in intubated trauma patients. Microbiology and outcomes. Am. J. Respir. Crit. Care Med. 153343-349. [DOI] [PubMed] [Google Scholar]
- 4.Becher, A., and H. P. Schweizer. 2000. Integration-proficient Pseudomonas aeruginosa vectors for isolation of single-copy chromosomal lacZ and lux gene fusions. BioTechniques 29948-952. [DOI] [PubMed] [Google Scholar]
- 5.Bishop, M. B., A. L. Baltch, L. A. Hill, R. P. Smith, F. Lutz, and M. Pollack. 1987. The effect of Pseudomonas aeruginosa cytotoxin and toxin A on human polymorphonuclear leukocytes. J. Med. Microbiol. 24315-324. [DOI] [PubMed] [Google Scholar]
- 6.Chastre, J., J. L. Trouillet, A. Vuagnat, M. L. Joly-Guillou, H. Clavier, M. C. Dombret, and C. Gibert. 1998. Nosocomial pneumonia in patients with acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 1571165-1172. [DOI] [PubMed] [Google Scholar]
- 7.Coburn, J., and D. Frank. 1999. Macrophages and epithelial cells respond differently to the Pseudomonas aeruginosa type III secretion system. Infect. Immun. 673151-3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Combes, A., C. Figliolini, J. L. Trouillet, N. Kassis, M. Wolff, C. Gibert, and J. Chastre. 2002. Incidence and outcome of polymicrobial ventilator-associated pneumonia. Chest 1211618-1623. [DOI] [PubMed] [Google Scholar]
- 9.Comolli, J. C., A. R. Hauser, L. Waite, C. B. Whitchurch, J. S. Mattick, and J. N. Engel. 1999. Pseudomonas aeruginosa gene products PilT and PilU are required for cytotoxicity in vitro and virulence in a mouse model of acute pneumonia. Infect. Immun. 673625-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuzick, A., F. R. Stirling, S. L. Lindsay, and T. J. Evans. 2006. The type III pseudomonal exotoxin U activates the c-Jun NH2-terminal kinase pathway and increases human epithelial interleukin-8 production. Infect. Immun. 744104-4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiMango, E., H. Zar, R. Bryan, and A. Prince. 1995. Diverse Pseudomonas aeruginosa gene products stimulate respiratory epithelial cells to produce interleukin-8. J. Clin. Investig. 962204-2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engel, J. N. 2003. Molecular pathogenesis of acute Pseudomonas aeruginosa infections, p. 201-229. In A. R. Hauser and J. Rello (ed.), Severe infections caused by Pseudomonas aeruginosa, vol. 7. Kluwer Academic Publishers, Boston, MA. [Google Scholar]
- 13.Fagon, J.-Y., J. Chastre, A. J. Hance, P. Montravers, A. Novara, and C. Gibert. 1993. Nosocomial pneumonia in ventilated patients: a cohort study evaluating attributable mortality and hospital stay. Am. J. Med. 94281-288. [DOI] [PubMed] [Google Scholar]
- 14.Feltman, H., G. Schulert, S. Khan, M. Jain, L. Peterson, and A. R. Hauser. 2001. Prevalence of type III secretion genes in clinical and environmental isolates of Pseudomonas aeruginosa. Microbiology 1472659-2669. [DOI] [PubMed] [Google Scholar]
- 15.Finck-Barbançon, V., and D. W. Frank. 2001. Multiple domains are required for the toxic activity of Pseudomonas aeruginosa ExoU. J. Bacteriol. 1834330-4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finck-Barbançon, V., J. Goranson, L. Zhu, T. Sawa, J. P. Wiener-Kronish, S. M. J. Fleiszig, C. Wu, L. Mende-Mueller, and D. Frank. 1997. ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol. Microbiol. 25547-557. [DOI] [PubMed] [Google Scholar]
- 17.Frank, D. W. 1997. The exoenzyme S regulon of Pseudomonas aeruginosa. Mol. Microbiol. 26621-629. [DOI] [PubMed] [Google Scholar]
- 18.Hauser, A. R., E. Cobb, M. Bodí, D. Mariscal, J. Vallés, J. N. Engel, and J. Rello. 2002. Type III protein secretion is associated with poor clinical outcomes in patients with ventilator-associated pneumonia caused by Pseudomonas aeruginosa. Crit. Care Med. 30521-528. [DOI] [PubMed] [Google Scholar]
- 19.Hauser, A. R., and J. N. Engel. 1999. Pseudomonas aeruginosa induces type III secretion-mediated apoptosis of macrophages and epithelial cells. Infect. Immun. 675530-5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hauser, A. R., P. J. Kang, and J. Engel. 1998. PepA, a novel secreted protein of Pseudomonas aeruginosa, is necessary for cytotoxicity and virulence. Mol. Microbiol. 27807-818. [DOI] [PubMed] [Google Scholar]
- 21.Hauser, A. R., P. J. Kang, S. J. M. Fleiszig, K. Mostov, and J. Engel. 1998. Defects in type III secretion correlate with internalization of Pseudomonas aeruginosa by epithelial cells. Infect. Immun. 661413-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoang, T. T., A. J. Kutchma, A. Becher, and H. P. Schweizer. 2000. Integration-proficient plasmids for Pseudomonas aeruginosa: site-specific integration and use for engineering of reporter and expression strains. Plasmid 4359-72. [DOI] [PubMed] [Google Scholar]
- 23.Kollef, M., P. Silver, D. Murphy, and E. Trovillion. 1995. The effect of late-onset ventilator-associated pneumonia in determining patient mortality. Chest 1081655-1662. [DOI] [PubMed] [Google Scholar]
- 24.Kudoh, I., J. P. Wiener-Kronish, S. Hashimoto, J.-F. Pittet, and D. Frank. 1994. Exoproduct secretions of P. aeruginosa strains influence severity of alveolar epithelial injury. Am. J. Physiol. 267L551-L556. [DOI] [PubMed] [Google Scholar]
- 25.Kurahashi, K., O. Kajikawa, T. Sawa, M. Ohara, M. A. Gropper, D. W. Frank, T. R. Martin, and J. P. Wiener-Kronish. 1999. Pathogenesis of septic shock in Pseudomonas aeruginosa pneumonia. J. Clin. Investig. 104743-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee, V. T., S. Pukatzki, H. Sato, E. Kikawada, A. A. Kazimirova, J. Huang, X. Li, J. P. Arm, D. W. Frank, and S. Lory. 2007. Pseudolipasin A is a specific inhibitor for phospholipase A2 activity of Pseudomonas aeruginosa cytotoxin ExoU. Infect. Immun. 751089-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee, V. T., R. S. Smith, B. Tummler, and S. Lory. 2005. Activities of Pseudomonas aeruginosa effectors secreted by the type III secretion system in vitro and during infection. Infect. Immun. 731695-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu, P. V. 1966. The roles of various fractions of Pseudomonas aeruginosa in its pathogenesis. II. Effects of lecithinase and protease. J. Infect. Dis. 116112-116. [DOI] [PubMed] [Google Scholar]
- 29.Lyczak, J. B., C. L. Cannon, and G. B. Pier. 2002. Lung infections associated with cystic fibrosis. Clin. Microbiol. Rev. 15194-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayhall, C. G. 1997. Nosocomial pneumonia. Diagnosis and prevention. Infect. Dis. Clin. N. Am. 11427-457. [DOI] [PubMed] [Google Scholar]
- 31.McMorran, B., L. Town, E. Costelloe, J. Palmer, J. Engel, D. Hume, and B. Wainwright. 2003. Effector ExoU from the type III secretion system is an important modulator of gene expression in lung epithelial cells in response to Pseudomonas aeruginosa infection. Infect. Immun. 716035-6044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nicas, T. I., and B. H. Iglewski. 1984. Isolation and characterization of transposon-induced mutants of Pseudomonas aeruginosa deficient in production of exoenzyme S. Infect. Immun. 45470-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niederman, M. S., D. E. Craven, M. J. Bonten, J. Chastre, W. A. Craig, J. Y. Fagon, J. Hall, G. A. Jacoby, M. H. Kollef, C. M. Luna, L. A. Mandell, A. Torres, and R. G. Wunderink. 2005. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 171388-416. [DOI] [PubMed] [Google Scholar]
- 34.Pankhaniya, R. R., M. Tamura, L. R. Allmond, K. Moriyama, T. Ajayi, J. P. Wiener-Kronish, and T. Sawa. 2004. Pseudomonas aeruginosa causes acute lung injury via the catalytic activity of the patatin-like phospholipase domain of ExoU. Crit. Care Med. 322293-2299. [DOI] [PubMed] [Google Scholar]
- 35.Pennington, J. E., M. G. Ehrie, and W. F. Hickey. 1984. Host defense mechanisms against pneumonia due to Pseudomonas aeruginosa. Rev. Infect. Dis. 6S657-S666. [DOI] [PubMed] [Google Scholar]
- 36.Phillips, R. M., D. A. Six, E. A. Dennis, and P. Ghosh. 2003. In vivo phospholipase activity of the Pseudomonas aeruginosa cytotoxin ExoU and protection of mammalian cells with phospholipase A2 inhibitors. J. Biol. Chem. 27841326-41332. [DOI] [PubMed] [Google Scholar]
- 37.Prince, L. R., S. M. Bianchi, K. M. Vaughan, M. A. Bewley, H. M. Marriott, S. R. Walmsley, G. W. Taylor, D. J. Buttle, I. Sabroe, D. H. Dockrell, and M. K. Whyte. 2008. Subversion of a lysosomal pathway regulating neutrophil apoptosis by a major bacterial toxin, pyocyanin. J. Immunol. 1803502-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rabin, S. D. P., and A. R. Hauser. 2005. Functional regions of the Pseudomonas aeruginosa cytotoxin ExoU. Infect. Immun. 73573-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rello, J., V. Ausina, M. Ricart, J. Castella, and G. Prats. 1993. Impact of previous antimicrobial therapy on the etiology and outcome of ventilator-associated pneumonia. Chest 1041230-1235. [DOI] [PubMed] [Google Scholar]
- 40.Rello, J., E. Diaz, and A. Rodriguez. 2005. Etiology of ventilator-associated pneumonia. Clin. Chest Med. 2687-95. [DOI] [PubMed] [Google Scholar]
- 41.Rello, J., P. Jubert, J. Valles, A. Artigas, M. Rue, and M. S. Niederman. 1996. Evaluation of outcome for intubated patients with pneumonia due to Pseudomonas aeruginosa. Clin. Infect. Dis. 23973-978. [DOI] [PubMed] [Google Scholar]
- 42.Rello, J., D. Mariscal, F. March, P. Jubert, F. Sanchez, J. Valles, and P. Coll. 1998. Recurrent Pseudomonas aeruginosa pneumonia in ventilated patients: relapse or reinfection? Am. J. Respir. Crit. Care Med. 157912-916. [DOI] [PubMed] [Google Scholar]
- 43.Rolston, K. V. I. 2003. Pseudomonas aeruginosa infections in cancer patients, p. 113-125. In A. Hauser and J. Rello (ed.), Severe infections caused by Pseudomonas aeruginosa, vol. 7. Kluwer Academic Publishers, Boston, MA. [Google Scholar]
- 44.Roy-Burman, A., R. H. Savel, S. Racine, B. L. Swanson, N. S. Revadigar, J. Fujimoto, T. Sawa, D. W. Frank, and J. P. Wiener-Kronish. 2001. Type III protein secretion is associated with death in lower respiratory and systemic Pseudomonas aeruginosa infections. J. Infect. Dis. 1831767-1774. [DOI] [PubMed] [Google Scholar]
- 45.Sadikot, R. T., T. S. Blackwell, J. W. Christman, and A. S. Prince. 2005. Pathogen-host interactions in Pseudomonas aeruginosa pneumonia. Am. J. Respir. Crit. Care Med. 1711209-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saliba, A. M., D. O. Nascimento, M. C. Silva, M. C. Assis, C. R. Gayer, B. Raymond, M. G. Coelho, E. A. Marques, L. Touqui, R. M. Albano, U. G. Lopes, D. D. Paiva, P. T. Bozza, and M. C. Plotkowski. 2005. Eicosanoid-mediated proinflammatory activity of Pseudomonas aeruginosa ExoU. Cell. Microbiol. 71811-1822. [DOI] [PubMed] [Google Scholar]
- 47.Sato, H., and D. W. Frank. 2004. ExoU is a potent intracellular phospholipase. Mol. Microbiol. 531279-1290. [DOI] [PubMed] [Google Scholar]
- 48.Sato, H., D. W. Frank, C. J. Hillard, J. B. Feix, R. R. Pankhaniya, K. Moriyama, V. Finck-Barbancon, A. Buchaklian, M. Lei, R. M. Long, J. Wiener-Kronish, and T. Sawa. 2003. The mechanism of action of the Pseudomonas aeruginosa-encoded type III cytotoxin, ExoU. EMBO J. 222959-2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sawa, T., M. Ohara, K. Kurahashi, S. S. Twining, D. Frank, D. B. Doroques, T. Long, M. A. Gropper, and J. P. Wiener-Kronish. 1998. In vitro cellular toxicity predicts Pseudomonas aeruginosa virulence in lung infections. Infect. Immun. 663242-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schulert, G. S., H. Feltman, S. D. P. Rabin, C. G. Martin, S. E. Battle, J. Rello, and A. R. Hauser. 2003. Secretion of the toxin ExoU is a marker for highly virulent Pseudomonas aeruginosa isolates obtained from patients with hospital-acquired pneumonia. J. Infect. Dis. 1881695-1706. [DOI] [PubMed] [Google Scholar]
- 51.Shaver, C. M., and A. R. Hauser. 2006. Interactions between effector proteins of the Pseudomonas aeruginosa type III secretion system do not significantly affect several measures of disease severity in mammals. Microbiology 152143-152. [DOI] [PubMed] [Google Scholar]
- 52.Shaver, C. M., and A. R. Hauser. 2004. Relative contributions of Pseudomonas aeruginosa ExoU, ExoS, and ExoT to virulence in the lung. Infect. Immun. 726969-6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simon, R., U. Priefer, and A. Puhler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Bio/Technology 1784-791. [Google Scholar]
- 54.Timsit, J. F., S. Chevret, J. Valcke, B. Misset, B. Renaud, F. W. Goldstein, P. Vaury, and J. Carlet. 1996. Mortality of nosocomial pneumonia in ventilated patients: influence of diagnostic tools. Am. J. Respir. Crit. Care Med. 154116-123. [DOI] [PubMed] [Google Scholar]
- 55.Tsai, W. C., R. M. Strieter, B. Mehrad, M. W. Newstead, X. Zeng, and T. J. Standiford. 2000. CXC chemokine receptor CXCR2 is essential for protective innate host response in murine Pseudomonas aeruginosa pneumonia. Infect. Immun. 684289-4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vallis, A. J., V. Finck-Barbancon, T. L. Yahr, and D. W. Frank. 1999. Biological effects of Pseudomonas aeruginosa type III-secreted proteins on CHO cells. Infect. Immun. 672040-2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vogel, H. J., and D. M. Bonner. 1956. Acetylornithinase of Escherichia coli partial purification and some properties. J. Biol. Chem. 21897-106. [PubMed] [Google Scholar]
- 58.Wieland, C. W., B. Siegmund, G. Senaldi, M. L. Vasil, C. A. Dinarello, and G. Fantuzzi. 2002. Pulmonary inflammation induced by Pseudomonas aeruginosa lipopolysaccharide, phospholipase C, and exotoxin A: role of interferon regulatory factor 1. Infect. Immun. 701352-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilson, N. R., M. L. Dunkley, A. Buret, B. Young, and A. W. Cripps. 1995. Histopathology of the lung following intratracheal challenge with live Pseudomonas aeruginosa in intestinally immunized rats. Immunol. Cell Biol. 73440-445. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.