Abstract
Type I interferons (IFNs) induced during in vitro chlamydial infection exert bactericidal and immunomodulatory functions. To determine the precise role of type I IFNs during in vivo chlamydial genital infection, we examined the course and outcome of Chlamydia muridarum genital infection in mice genetically deficient in the receptor for type I IFNs (IFNAR−/− mice). A significant reduction in chlamydial shedding and duration of lower genital tract infection was observed in IFNAR−/− mice in comparison to the level of chlamydial shedding and duration of infection in wild-type (WT) mice. Furthermore, IFNAR−/− mice developed less chronic oviduct pathology in comparison to that in WT mice. Compared to the WT, IFNAR−/− mice had a greater number of chlamydial-specific T cells in their iliac lymph nodes 21 days postinfection. IFNAR−/− mice also exhibited earlier and enhanced CD4 T-cell recruitment to the cervical tissues, which was associated with increased expression of CXCL9 in the genital secretions of IFNAR−/− mice, but not with expression of CXCL10, which was reduced in the genital secretions of IFNAR−/− mice. These data suggest that type I IFNs exacerbate C. muridarum genital infection through an inhibition of the chlamydial-specific CD4 T-cell response.
Type I interferons (alpha/beta interferon [IFN-α/β]) are produced in response to a number of viral infections and act as antiviral and immunomodulatory cytokines (21). Intracellular bacterial infections also induce type I IFNs, though they are not always bactericidal (reviewed in reference 9). Chlamydia spp. have been shown to induce type I IFNs in multiple cell types, including a murine oviduct epithelial-cell line and murine macrophages (11, 14, 22, 32). The documented effects of type I IFNs during in vitro chlamydial infection include direct chlamydial killing (31) and induction of IFN-γ (32), nitric oxide synthase (11, 41), and CXCL10 (22).
Unlike the well-defined role of type I IFNs during viral infection (21), the contribution of type I IFNs to innate immunity during intracellular bacterial infection in vivo varies with different microorganisms and the site of infection (reviewed in reference 9). IFNAR−/− mice exhibited resistance to Listeria monocytogenes infection that correlated with elevated serum interleukin-12 (IL-12) p70 levels during infection (1) and with the proapoptotic function of type I IFNs (24, 36). In contrast, a protective role for type I IFNs has been observed with Streptococcus pneumoniae infection (38). A study of mice infected with a clinical Mycobacterium tuberculosis isolate showed that virulence was associated with the ability to induce type I IFNs (18). However, an independent examination found that mice deficient for IFN-α/β signaling exhibited a slight increase in M. tuberculosis bacterial burden compared with that in WT controls (5). Considering the pleiotropic function of type I IFNs (reviewed in reference 9), the variation reported in the role for type I IFNs during specific bacterial infections could reflect differences in tissue-specific effector mechanisms that operate against individual bacterial species.
CD4 T cells play a dominant role in the clearance of Chlamydia muridarum genital infection in the mouse model (20, 27, 28, 37), though some contribution by the CD8 cells has been suggested (17, 30). Cytokines and chemokines generated as part of the innate response to infection are the stimuli for recruitment of inflammatory cells, including T cells, to the site of infection. Chlamydial infection generates cytokine/chemokine responses both by direct infection of host epithelial cells (29) and by interaction with cells of the innate immune system (12). The innate cytokine and chemokine responses generated during chlamydial infection may also contribute directly or indirectly to oviduct pathology by eliciting the influx of inflammatory cells, particularly neutrophils (35). Therefore, a determination of the specific contribution of early cytokines induced during infection is essential to understanding chlamydial disease pathogenesis.
Type I IFNs, and specifically IFN-β, are induced as early as 3 h after in vitro infection of mouse macrophages with C. muridarum and result in the expression of IFN response genes (22). However, the specific role of type I IFNs and type I IFN signaling during chlamydial genital infection is not known. In the present study, we determined the role of type I IFNs in chlamydial genital infection by examining the course of infection, immune responses, and pathology after resolution of C. muridarum infection in mice deficient for type I IFN signaling. We show that IFNAR−/− mice clear genital infection faster and exhibit reduced oviduct pathology in comparison to the clearance of infection and oviduct pathology in WT mice. The data indicate that the improved outcome of infection in IFNAR−/− mice is secondary to an enhanced chlamydial-specific CD4 T-cell response in the absence of type I IFN signaling.
MATERIALS AND METHODS
Chlamydial stocks.
Chlamydia muridarum strain Nigg was grown in mycoplasma-free McCoy cells as described previously (4, 8). Elementary bodies were harvested from infected cells, resuspended in SPG buffer (250 mM sucrose, 10 mM sodium phosphate, and 5 mM l-glutamic acid, pH 7.2), and quantified as inclusion-forming units (IFU) on McCoy cell monolayers as described previously (4).
Animals.
IFNAR−/− mice deficient in the α subunit of the type I IFN receptor (necessary for signaling by both IFN-α and IFN-β) were originally generated by Muller et al. (21) in the background strain 129Sv/Ev. These mice and their congenic WT controls (129Sv/Ev) were purchased from B&K Universal Limited (United Kingdom). IFNAR mice backcrossed six times to the C57BL/6J background (IFNAR−/−:C57BL/6J) were a kind gift from Egil Lien and bred in house. Control C57BL/6J mice were purchased from Jackson Laboratory. Age-matched (8 to 12 weeks) female mice were used for all experiments. Mice were given food and water ad libitum in an environmentally controlled room with 12-h light and dark cycles. All animal experiments were preapproved by the Institutional Animal Care and Use Committee of the University of Arkansas for Medical Sciences.
Murine genital infection with Chlamydia cells and analysis of cytokines in genital secretions.
Mice received 2.5 mg of Depo-Provera (medroxy-progesterone acetate; Upjohn) in 0.1 ml of saline subcutaneously 7 days before vaginal infection. Mice were anesthetized by using sodium pentobarbital and infected by placing C. muridarum cells (3 × 105 IFU in 30 μl SPG buffer) into the vaginal vault. Mice were monitored by swabbing the vaginal vault and cervix with a calcium alginate swab (Spectrum Medical Industries) at various times following infection, and bacterial numbers were determined by enumerating IFU using McCoy cells (4). Mice were infected in groups of 5 or 10, and each experiment was repeated at least once. Genital tract secretions were collected as described previously (8), and cytokine levels in the individual genital tract sponge eluates were determined by using enzyme-linked immunosorbent assay (ELISA) kits from R&D Systems or a Luminex bead array (BioSource).
Histopathology.
Mice were sacrificed at day 50 postinfection, and the entire genital tract was removed en bloc, fixed in 10% buffered formalin, and embedded in paraffin. Longitudinal sections (4 μm) were stained with hematoxylin and eosin stain and evaluated by a pathologist blinded to the experimental design, as described previously (6).
Antigen-specific T-cell proliferation and serum chlamydial-immunoglobulin G (IgG) response.
The iliac lymph nodes were processed for chlamydial-specific T-cell proliferation as described previously (6), with a few modifications. The iliac lymph nodes were excised from mice at day 7, 14, or 21 postinfection and passed through nylon mesh to yield single-cell suspensions. Cells (2 × 105 cells/well) were seeded in a 96-well flat-bottomed tissue culture plate in complete medium (RPMI containing 10% fetal bovine serum, 2 mM glutamine, 10 mM HEPES, pH 7.4, 100 μM nonessential amino acids, 1 mM sodium pyruvate, 50 μM β-mercaptoethanol, and 50 μg/ml gentamicin). C. muridarum bacteria grown in HeLa cells and purified on a renograffin gradient (4) were UV inactivated and used as chlamydial antigen (5 μg/well) in a final volume of 200 μl. Cells treated with concanavalin A (ConA) (0.5 μg/well) and cells that received no treatment were used as positive and negative controls, respectively. A parallel proliferation assay was carried out in the presence of anti-CD4 monoclonal antibody (mAb) (1 μg/well, clone RM4-5; BD Biosciences) that can sterically block antigen-specific T-cell proliferation due to CD4-T-cell receptor coclustering (15). Cells were treated with 20 μl of Alamar blue (Biosource) 24 h before the end of a five-day culture, and the proliferation responses were read at 530-nm excitation/590-nm emission by using a Biotek fluorescence microplate reader. Chlamydial-specific Ab responses were measured in sera collected from mice at days 23, 35, and 42 postinfection, as previously described (6).
Flow cytometric analysis of iliac node and genital tract cells.
Single-cell suspensions were generated from iliac lymph nodes as described above. The cervix was excised from the genital tract of an individual mouse and incubated separately with 3 ml of collagenase I (5 mg/ml) for 30 min at 37°C. Cells resuspended in 1 ml of RPMI medium containing 1% fetal bovine serum were further treated with 0.1 ml of DNase/RNase mixture (at 2/18 mg/ml) to obtain a single-cell suspension. After the enzymes were neutralized with EDTA (10 mM), the cells were washed and resuspended in fluorescence-activated cell sorter (FACS) buffer (phosphate-buffered saline, 1% bovine serum albumin, and 1 mM EDTA). Cells (2 ×105 to 5 ×105 cells/25 μl) were incubated in Fc block (5 μg/ml) for 10 min, followed by staining for individual cell surface markers or isotype controls (5 μg/ml) for 20 min on ice. Cells were stained by using the following combinations of mAbs (BD Biosciences): CD3-fluorescein isothiocyanate, CD4-phycoerythrin, and CD8-allophycocyanin. Cells were washed with FACS buffer and treated with 4′,6′-diamidino-2-phenylindole (DAPI; 2 μg/ml) to stain for dead cells. Data were acquired in a FACSAria (BD Biosciences) and analyzed using Cell Quest software (BD Biosciences) after excluding the DAPI-positive dead cells.
Statistics.
Statistical comparisons between the WT and IFNAR−/− mice for levels of infection and cytokine production over the course of infection were made by using a two-factor (days and murine strain) analysis of variance (ANOVA). A post hoc Tukey test was used as a multiple-comparison procedure. The Wilcoxon rank sum test was used to compare the duration of infection in the respective strains over time. The Kruskal-Wallis one-way ANOVA on ranks was used to determine significant differences in the pathological data between groups. Comparison of pathological data within groups was made by using the Student-Newman-Keuls method of all-pairwise multiple comparisons. ELISA, T-cell proliferation, and FACS data are provided as the means ± standard deviations of the results of triplicate samples, and one-way ANOVA or the Student t test was used to determine significance. Statistical tests were performed by using SigmaStat or Instat software.
RESULTS
IFNAR−/− mice exhibit a more-rapid resolution of infection and less pathology than WT mice.
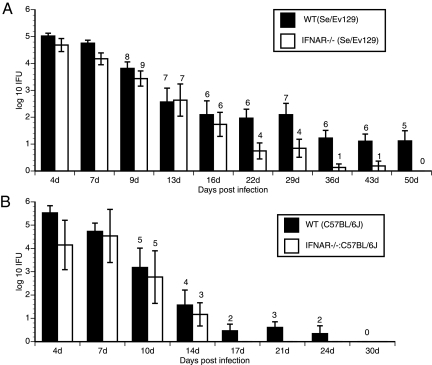
To determine the functional significance of type I IFNs during genital chlamydial infection in vivo, IFNAR−/− and WT Sv/Ev129 female mice were infected vaginally with C. muridarum. The intensity and duration of infection were monitored by enumerating IFU from cervicovaginal swabs. A 10-fold reduction in chlamydial shedding was observed in IFNAR−/− mice from day 17 onwards in comparison to the level in WT mice (Fig. 1A). Furthermore, the IFNAR−/− mice cleared infection at a faster rate than WT mice (P value of <0.001 by two-way repeated-measures [RM] ANOVA) (Fig. 1A). Interestingly, the infection course in the lower genital tract was similar in WT and IFNAR−/− mice until day 14, but subsequently, the IFNAR−/− mice displayed a more-rapid clearance (Fig. 1A). By day 50, all the mice in the IFNAR−/− group had cleared their infections, whereas 60% of the WT mice remained infected (Fig. 1A). In an independent experiment, we determined that the WT mice cleared their infections by day 60 to 65 (data not shown). No gross hydrosalpinx was observed in the oviducts harvested from IFNAR−/− mice 50 days postinfection. However, hydrosalpinx was seen in 6 of 16 oviducts from WT mice (P value of 0.004 by z-test for sample proportions). In addition, inflammation was not observed in the majority of the oviducts from IFNAR−/− mice (only 3 of 18 were scored as >0 for acute, chronic, and plasma-cell infiltration inflammation measures) in comparison to inflammation in oviducts from WT mice (9 of 16 were scored as >0 for inflammatory cells; P < 0.001).
FIG. 1.
Course and intensity of C. muridarum infection in WT and IFNAR−/− mice. (A) Genital infection of WT Se/Ev129 (n = 8) and IFNAR−/− (n = 9) mice with C. muridarum was carried out as described in Materials and Methods. Genital swabs were analyzed for IFU on a McCoy cell monolayer. Data are represented as averages of IFU obtained from all mice in each group (P value of <0.001 by two-way RM ANOVA). (B) Genital infection of WT C57BL/6J (n = 5) and IFNAR−/−:C57BL/6J (n = 5) mice with C. muridarum was carried out as described in Materials and Methods. Genital swabs were analyzed for IFU on a McCoy cell monolayer (P value of <0.001 by two-way RM ANOVA). The number of mice that remained infected at each time point is indicated above the histograms. Data are representative of the results of two experiments. Error bars show standard deviations.
As the infection course in the Se/Ev129 mice was found to be much longer than in C57BL/6J or BALB/c mice (7), we examined the infection course in IFNAR mice backcrossed in the C57BL/6J background (IFNAR−/−:C57BL6/J) (Fig. 1B). Again, the IFNAR−/−:C57BL/6J mice cleared their infections at a faster rate than the control C57BL/6J mice (Fig. 1B) (P value of <0.001 by two-way RM ANOVA), suggesting that type I IFN signaling affects the course of infection independent of the mouse strain. All of the IFNAR−/−:C57BL/6J mice cleared their infections by day 17, at which point 60% of C57BL6/J mice remained infected (Fig. 1B). Further, 8 of 10 oviducts from infected C57BL/6J mice exhibited hydrosalpinx, while only 1 of 10 oviducts from infected IFNAR−/−:C57BL/6J mice exhibited hydrosalpinx, confirming that a lack of IFN-α/β signaling protects against the development of chronic oviduct pathology. Further experiments described below were carried out using IFNAR−/− (Se/Ev129) mice and their corresponding controls.
Chlamydia-specific CD4 T-cell responses are enhanced in iliac lymph nodes of IFNAR−/− mice.
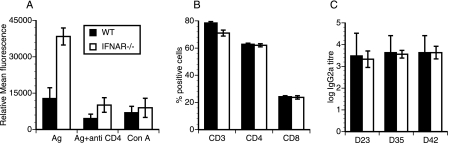
CD4 T cells play a dominant role in the clearance of Chlamydia muridarum genital infection in the mouse model (20, 27, 28, 37), though some contribution by the CD8 cells has been suggested (17, 30). Thus, we sought to determine if the improved clearance of chlamydial infection in IFNAR−/− mice was associated with an enhanced chlamydial-specific CD4 T-cell response. T-cell proliferation assays were carried out by using iliac lymph nodes harvested from WT and IFNAR−/− mice 14 and 21 days postinfection. A slight increase (1.5-fold) in chlamydial-specific T-cell proliferation was observed in the iliac lymph nodes of IFNAR−/− mice 14 days postinfection (data not shown). A significant increase (threefold) in chlamydial-specific T-cell proliferation was observed at 21 days postinfection (P < 0.001) (Fig. 2A), with no difference in background T-cell proliferation (medium control) between the two groups. The addition of an anti-CD4 mAb that sterically blocks antigen-specific T-cell proliferation inhibited 80% of the total response (Fig. 2A). Mitogen (ConA)-induced T-cell proliferation was not different between the two groups (Fig. 2A), suggesting that the enhanced proliferation detected in IFNAR−/− mouse iliac lymph nodes was chlamydial specific. Further, the total numbers of CD3, CD4, and CD8 lymphocytes in the iliac lymph nodes were similar between the two groups (Fig. 2B). Thus, the increase in chlamydial-specific T-cell proliferation observed in IFNAR−/− mouse iliac lymph nodes was not a result of increased numbers of total T cells. Despite the detection of an enhanced chlamydial-specific CD4 T-cell response in the iliac lymph nodes of IFNAR−/− mice, their serum antichlamydial IgG2a levels were not different than those of WT mice (Fig. 2C). However, the CD4 T-cell proliferation data indicate that an absence of type I IFN signaling significantly augments the chlamydial-specific T-cell immune response.
FIG. 2.
Chlamydial-specific T-cell proliferation in IFNAR−/− mouse iliac lymph nodes and chlamydial-specific IgG2a response. (A) Iliac lymph nodes obtained from infected mice at day 21 postinfection (n = 3/group) were subjected to T-cell proliferation assay as described in Materials and Methods. Anti-CD4 mAb was used to block antigen (Ag)-specific proliferation. ConA was used as a control mitogen. Proliferation was measured by using Alamar blue fluorescence, and data presented are values after subtraction of values for medium wells. Data are represented as means and standard deviations of values obtained from individual animals from a single experiment, representative of two experiments. (B) Results of flow cytometric analysis of iliac lymph nodes used for the proliferation experiment. Cells were gated for CD3 and analyzed for CD4 and CD8 populations. Bars represent percentages of CD3, CD4, and CD8 cells of total live-cell population. (C) Chlamydial-specific IgG2a responses in sera collected from infected mice on days 23, 35, and 42 (D23, D35, and D42). Titers represent highest dilutions of sera (log) that gave a reading of an optical density of 0.1 or greater at 405 nm. Error bars show standard deviations.
Earlier T-cell recruitment occurs in the endocervix of IFNAR−/− mice.
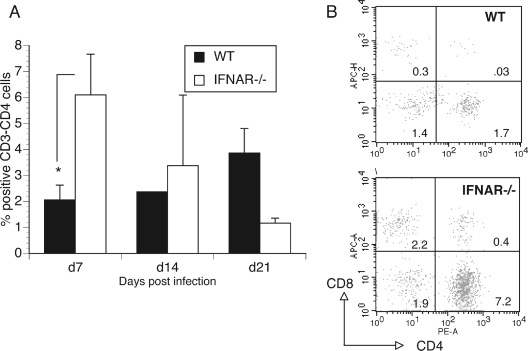
Since there were more chlamydial-specific CD4 T cells in the iliac lymph nodes of the IFNAR−/− mice than in those of WT mice 21 days postinfection, it was important to address whether this corresponded to earlier and/or increased recruitment of CD4 T cells to the genital tract of IFNAR−/− mice. We investigated the kinetics of CD4 T-cell recruitment into the cervical and oviduct tissues of IFNAR−/− and WT mice after infection. At day 7 postinfection, a three- to fourfold increase (P value <0.05 by one-way ANOVA) in the total number of CD3+ T cells was observed in the cervix of IFNAR−/− mice compared to the number in WT mice (Fig. 3). When the gated CD3 cells were analyzed for CD4 and CD8 cells, a similar increase was proportionally observed in both CD4 and CD8 populations (Fig. 3). Examinations performed on cervical tissues harvested on days 14 and 21 postinfection revealed interesting T-cell kinetics (Fig. 3). In WT mice, the peak recruitment of CD4 T cells to the cervix was not observed until day 21. In contrast, in the IFNAR−/− mice, there was a rapid increase in CD4+ T cells that was early (day 7) compared to that in WT mice (P value of <0.05 by one-way ANOVA). However, the number of T cells in IFNAR−/− mice decreased over time, with significantly lower numbers of CD4+ T cells (P value of <0.01 by one-way ANOVA) being found in the cervix at 21 days postinfection than at 7 days postinfection (Fig. 3).
FIG. 3.
T-cell recruitment to cervix of WT and IFNAR−/− mice. (A) Flow cytometric analysis of cells in cervix at days 7, 14, and 21 postinfection (n = 3/group). Cells were gated for CD3 and analyzed for CD4+ lymphocytes. Data are percentages of CD4+ cells from total live-cell populations. Error bars show standard deviations. *, P value was <0.05 for WT versus IFNAR−/− on day 7 by one-way ANOVA. (B) FACS data showing cells gated for CD3 from a representative mouse from each group, at day 7 postinfection. The numbers in the quadrants represent the percent positive cells from the total population.
Association of CXCL9 (MIG) with enhanced T-cell response in the genital tract of Chlamydia-infected IFNAR−/− mice.
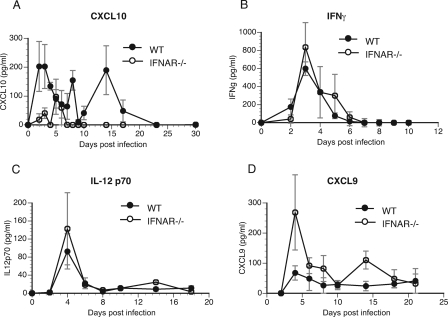
We examined the possibility that an absence of type I IFN signaling led to an enhanced production of T-cell chemokines, promoting improved recruitment of CD4 T cells to the cervix of IFNAR−/− mice. Recently, we reported a role in vitro for type I IFN in the induction of CXCL10 (IP-10), a T-cell chemokine (6). Concurrent with this in vitro observation, CXCL10 levels were significantly reduced in the genital secretions of IFNAR−/− mice in comparison to their levels in the genital secretions of WT mice (P value of 0.017 by two-way ANOVA) (Fig. 4A). The detection of low levels of CXCL10 in IFNAR−/− mice suggests that it plays a minimal role in T-cell recruitment in response to chlamydial genital infection in vivo. CXCL10 is inducible by both type I and type II IFNs (10, 26). However, the IFN-γ levels detected in genital tract secretions were not different in WT and IFNAR−/− mice (Fig. 4B). Thus, in chlamydial genital infection, it appears that CXCL10 production is primarily controlled by type I IFNs.
FIG. 4.
Cytokine levels in genital secretions. (A and B) Genital secretions obtained at different time points postinfection were analyzed for CXCL10 (A) and IFN-γ (B) protein by ELISA. Two-way ANOVA was performed to compare the groups over time (for CXCL10, the P value was 0.017 by two-way ANOVA). (C and D) Secretions were analyzed for IL-12 p70 (C) and CXCL9 (D) by Luminex-based assay using a BioSource chemokine bead array kit (P value of <0.05 by two-way ANOVA). Error bars show standard deviations.
To determine if other T-cell chemokines/cytokines might be responsible for the improved recruitment of CD4 T cells in IFNAR−/− mice, a Luminex-based chemokine/cytokine array was carried out on genital tract secretions. It has been shown that the IL-12 p70 levels in serum were elevated in IFNAR−/− mice following L. monocytogenes infection (1). However, analysis of genital secretions following C. muridarum infection showed that the levels of IL-12 p70 were not significantly different between WT and IFNAR−/− mice (Fig. 4C). The levels of tumor necrosis factor alpha, IL-6, CXCL1 (KC), and CCL3 (MIP-1α) were also similar in secretions from infected WT and IFNAR−/− mice (data not shown). Interestingly, CXCL9 (MIG) was increased significantly on day 4 postinfection (P value of 0.019 by one-way ANOVA) in the genital secretions of IFNAR−/− mice, and the overall difference in mean counts between the two groups was significant (P value of <0.05 by two-way ANOVA) (Fig. 4D). These data suggest a possible role for CXCL9 in enhanced clearance of C. muridarum infection in the absence of type I IFN signaling.
DISCUSSION
Type I IFNs are well known for their antiviral activity (21). However, their role in bacterial infection is variable depending on the nature of the bacterial pathogen and the effector mechanisms that function in its clearance (reviewed in reference 9). The results of our study show that during genital chlamydial infection, type I IFN signaling actually benefits the pathogen rather than the host. The IFNAR−/− mice exhibited a reduced bacterial burden compared to WT mice and also cleared infection faster than WT mice. The improved bacterial clearance in IFNAR−/− mice is associated with increased chlamydial-specific CD4 T-cell proliferation in the iliac lymph nodes and enhanced CD4 T-cell recruitment to the genital tract. The enhanced clearance is also reflected in the observation of less chronic oviduct pathology in the IFNAR−/− mice.
It is interesting to note that the function of type I IFNs during genital C. muridarum infection is not consistent with its role observed in a mouse model of lung infection using C. pneumoniae. In the lung model, Rothfuchs et al. (33) found no difference in bacterial load between WT and IFNAR−/− (IFN-α/β receptor deficient) mice. However, clearance of infection was delayed in IFN-γR−/− and IFNAR−/− double-knockout mice in comparison to that in IFN-γR−/− mice, suggesting that type I IFNs cooperate with IFN-γ to effect the resolution of C. pneumoniae lung infection (33). It is likely that tissue tropism influences the role of type I IFNs during chlamydial infection, since the distribution and nature of innate immune cells that respond to infection are unique to each tissue.
The course of infection in the WT 129Sv/Ev mice was unusually long with, surprisingly, less pathology than previously observed with C57BL/6J or BALB/c mice (7). However, the course of infection in F1 progeny from C57BL/6J and 129Sv/Ev mice has been shown to be prolonged with respect to that in C57BL6/J mice (13). Nevertheless, the absence of type I IFN signaling led to a more-rapid rate of clearance and decreased bacterial load independent of the mouse strain. Further, decreased incidence of hydrosalpinx and decreased numbers of inflammatory cells were observed in the oviducts harvested 50 days postinfection from the IFNAR−/− mice on both genetic backgrounds in comparison to the incidence of hydrosalpinx and numbers of inflammatory cells in their corresponding WT controls. The reduction in pathology in the IFNAR−/− mice is associated with earlier clearance of infection in these mice, though a direct correlation between bacterial load and inflammation in the oviducts still needs to be established.
Our data suggest an important role for T cells in improved chlamydial clearance in IFNAR−/− mice, as there were clearly more chlamydial-specific T cells in the lymph nodes of IFNAR−/− mice. Additionally, increased numbers of T cells were observed in the cervix of IFNAR−/− mice at day 7 postinfection in comparison to the numbers in WT mice. However, since the differences in chlamydial shedding are not evident until after day 17, it is not clear if the earlier recruitment is directly responsible for the improved clearance. The increased number of chlamydial-specific T cells in the lymph nodes or the overall early increase in T cells in the cervix could be due to reduced apoptosis of T cells, as observed during Listeria monocytogenes infection in IFNAR−/− mice (24). However, during C. muridarum genital infection, no significant differences between WT and IFNAR−/− mice were observed in numbers of dead effector (T) cells in the lymph nodes or cervix by FACS (data not shown).
An important question is, what are the molecular mechanisms by which type I IFNs delay chlamydial clearance? IL-12 p70 can significantly impact chlamydial infection and is necessary for chlamydial clearance, by a mechanism independent of IFN-γ induction (25). IL-12 p70 has been implicated as the cause of improved clearance of systemic L. monocytogenes infection in IFNAR−/− mice (1). However, IL-12 p70 levels in the genital secretions during chlamydial infection were no different between IFNAR−/− and WT mice. The appearance of Th1 cells expressing CXCR3 in the genital tract has been found to be synchronous with the kinetics of immune induction and clearance of C. muridarum infection (2). CXCR3 is a receptor for the T-cell chemokines CXCL10, CXCL9, and CXCL11 (I-TAC) (reviewed in reference 16). The T-cell chemokine CXCL10 is unlikely to play a dominant role in chlamydial clearance since its levels were significantly reduced in the genital secretions of IFNAR−/− mice. Interestingly, CXCL9 levels were increased in genital secretions from infected IFNAR−/− mice, suggesting that it could be responsible for the improved outcome in IFNAR−/− mice. CXCL9 has been shown to be a predictive marker for antigen-specific T-cell recruitment (3) and has also been shown to induce T-cell proliferation (39) in other infection models. The inverse relationship observed between CXCL9 and CXCL10 levels is suggestive of their nonredundant regulation and function (42). CXCL9 is induced by both type I and type II IFNs (10, 26). Since the levels of IFN-γ in genital secretions were not significantly different between the two groups, it is not clear how type I IFN signaling inversely controls CXCL9 levels during chlamydial infection. It is likely that the IFN-γ levels in the genital secretions do not reflect the subtle differences occurring in the target tissues. Alternatively, IFN-γ signaling could be enhanced in the host epithelial cells of IFNAR−/− mice by downregulation of SOCS-1 (34), as observed during in vitro C. pneumoniae infection (40). However, the exact mechanism through which type I IFN influences chlamydial clearance needs further investigation.
At this point it is not clear evolutionarily why host cells secrete type I IFNs during chlamydial infection if it is not beneficial to the host. An interesting possibility is that a successful pathogen, such as C. trachomatis, induces type I IFNs for its own benefit, enhancing its longevity in a single host and thus increasing its transmissibility from one host to another. In addition, it may also provide an opportunity for C. trachomatis to survive and even thrive in the presence of a genital viral infection that induces a strong type I IFN response. Incidentally, an increased prevalence of chlamydial infection has been observed in women coinfected with human papilloma virus and C. trachomatis (19). Similarly, increased susceptibility to L. monocytogenes has been observed following lymphocytic choriomeningitis virus infection and this was shown to be due to type I IFN-induced apoptosis in bone marrow granulocytes, which leads to higher bacterial titers in solid organs (23). From the host immune system's perspective, the induction of type I IFNs during chlamydial genital infection could be a case of an efficient antiviral system being exploited by an intracellular mucosal bacterium.
Acknowledgments
This study was supported by Public Health Service Grants AI067678 (to U.M.N.) and AI054624 (to T.D.) from the National Institutes of Health and, in part, by funding from Children's University Medical Group of Arkansas Children's Hospital Research Institute and Arkansas BioScience Institute to U.M.N.
We thank Roger Rank and Richard Morrison (University of Arkansas for Medical Sciences) for critical reading of the manuscript. We gratefully acknowledge the receipt of IFNAR−/− mice backcrossed in the C57BL/6J background from Egil Lien (University of Massachusetts Medical School).
All authors have no financial disclosures.
Editor: B. A. McCormick
Footnotes
Published ahead of print on 28 July 2008.
REFERENCES
- 1.Auerbuch, V., D. G. Brockstedt, N. Meyer-Morse, M. O'Riordan, and D. A. Portnoy. 2004. Mice lacking the type I interferon receptor are resistant to Listeria monocytogenes. J. Exp. Med. 200527-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belay, T., F. O. Eko, G. A. Ananaba, S. Bowers, T. Moore, D. Lyn, and J. U. Igietseme. 2002. Chemokine and chemokine receptor dynamics during genital chlamydial infection. Infect. Immun. 70844-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brice, G. T., N. L. Graber, S. L. Hoffman, and D. L. Doolan. 2001. Expression of the chemokine MIG is a sensitive and predictive marker for antigen-specific, genetically restricted IFN-gamma production and IFN-gamma-secreting cells. J. Immunol. Methods 25755-69. [DOI] [PubMed] [Google Scholar]
- 4.Caldwell, H. D., J. Kromhout, and J. Schachter. 1981. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect. Immun. 311161-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper, A. M., J. E. Pearl, J. V. Brooks, S. Ehlers, and I. M. Orme. 2000. Expression of the nitric oxide synthase 2 gene is not essential for early control of Mycobacterium tuberculosis in the murine lung. Infect. Immun. 686879-6882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darville, T., C. W. Andrews, Jr., K. K. Laffoon, W. Shymasani, L. R. Kishen, and R. G. Rank. 1997. Mouse strain-dependent variation in the course and outcome of chlamydial genital tract infection is associated with differences in host response. Infect. Immun. 653065-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darville, T., C. W. Andrews, Jr., J. D. Sikes, P. L. Fraley, and R. G. Rank. 2001. Early local cytokine profiles in strains of mice with different outcomes from chlamydial genital tract infection. Infect. Immun. 693556-3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darville, T., J. M. O'Neill, C. W. Andrews, Jr., U. M. Nagarajan, L. Stahl, and D. M. Ojcius. 2003. Toll-like receptor-2, but not toll-like receptor-4, is essential for development of oviduct pathology in chlamydial genital tract infection. J. Immunol. 1716187-6197. [DOI] [PubMed] [Google Scholar]
- 9.Decker, T., M. Muller, and S. Stockinger. 2005. The yin and yang of type I interferon activity in bacterial infection. Nat. Rev. Immunol. 5675-687. [DOI] [PubMed] [Google Scholar]
- 10.de Veer, M. J., M. Holko, M. Frevel, E. Walker, S. Der, J. M. Paranjape, R. H. Silverman, and B. R. Williams. 2001. Functional classification of interferon-stimulated genes identified using microarrays. J. Leukoc. Biol. 69912-920. [PubMed] [Google Scholar]
- 11.Devitt, A., P. A. Lund, A. G. Morris, and J. H. Pearce. 1996. Induction of alpha/beta interferon and dependent nitric oxide synthesis during Chlamydia trachomatis infection of McCoy cells in the absence of exogenous cytokine. Infect. Immun. 643951-3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fitzpatrick, D. R., J. Wie, D. Webb, R. Bonfiglioli, I. D. Gardner, J. D. Mathews, and H. Bielefeldt-Ohmann. 1991. Preferential binding of Chlamydia trachomatis to subsets of human lymphocytes and induction of interleukin-6 and interferon-gamma. Immunol. Cell Biol. 69337-348. [DOI] [PubMed] [Google Scholar]
- 13.Imtiaz, M. T., J. T. Distelhorst, J. H. Schripsema, I. M. Sigar, J. N. Kasimos, S. R. Lacy, and K. H. Ramsey. 2007. A role for matrix metalloproteinase-9 in pathogenesis of urogenital Chlamydia muridarum infection in mice. Microbes Infect. 91561-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson, R. M. 2004. Murine oviduct epithelial cell cytokine responses to Chlamydia muridarum infection include interleukin-12-p70 secretion. Infect. Immun. 723951-3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kupfer, A., S. J. Singer, C. A. Janeway, Jr., and S. L. Swain. 1987. Coclustering of CD4 (L3T4) molecule with the T-cell receptor is induced by specific direct interaction of helper T cells and antigen-presenting cells. Proc. Natl. Acad. Sci. USA 845888-5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu, L., M. K. Callahan, D. Huang, and R. M. Ransohoff. 2005. Chemokine receptor CXCR3: an unexpected enigma. Curr. Top. Dev. Biol. 68149-181. [DOI] [PubMed] [Google Scholar]
- 17.Magee, D. M., D. M. Williams, J. G. Smith, C. A. Bleicker, B. G. Grubbs, J. Schachter, and R. G. Rank. 1995. Role of CD8 T cells in primary chlamydia infection. Infect. Immun. 63516-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manca, C., L. Tsenova, A. Bergtold, S. Freeman, M. Tovey, J. M. Musser, C. E. Barry III, V. H. Freedman, and G. Kaplan. 2001. Virulence of a Mycobacterium tuberculosis clinical isolate in mice is determined by failure to induce Th1 type immunity and is associated with induction of IFN-alpha /beta. Proc. Natl. Acad. Sci. USA 985752-5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molano, M., E. Weiderpass, H. Posso, S. A. Morre, M. Ronderos, S. Franceschi, A. Arslan, C. J. Meijer, N. Munoz, and A. J. van den Brule. 2003. Prevalence and determinants of Chlamydia trachomatis infections in women from Bogota, Colombia. Sex. Transm. Infect. 79474-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morrison, R. P., K. Feilzer, and D. B. Tumas. 1995. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infect. Immun. 634661-4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muller, U., U. Steinhoff, L. F. Reis, S. Hemmi, J. Pavlovic, R. M. Zinkernagel, and M. Aguet. 1994. Functional role of type I and type II interferons in antiviral defense. Science 2641918-1921. [DOI] [PubMed] [Google Scholar]
- 22.Nagarajan, U. M., D. M. Ojcius, L. Stahl, R. G. Rank, and T. Darville. 2005. Chlamydia trachomatis induces expression of IFN-gamma-inducible protein 10 and IFN-beta independent of TLR2 and TLR4, but largely dependent on MyD88. J. Immunol. 175450-460. [DOI] [PubMed] [Google Scholar]
- 23.Navarini, A. A., M. Recher, K. S. Lang, P. Georgiev, S. Meury, A. Bergthaler, L. Flatz, J. Bille, R. Landmann, B. Odermatt, H. Hengartner, and R. M. Zinkernagel. 2006. Increased susceptibility to bacterial superinfection as a consequence of innate antiviral responses. Proc. Natl. Acad. Sci. USA 10315535-15539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Connell, R. M., S. K. Saha, S. A. Vaidya, K. W. Bruhn, G. A. Miranda, B. Zarnegar, A. K. Perry, B. O. Nguyen, T. F. Lane, T. Taniguchi, J. F. Miller, and G. Cheng. 2004. Type I interferon production enhances susceptibility to Listeria monocytogenes infection. J. Exp. Med. 200437-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perry, L. L., K. Feilzer, and H. D. Caldwell. 1997. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-gamma-dependent and -independent pathways. J. Immunol. 1583344-3352. [PubMed] [Google Scholar]
- 26.Petry, H., L. Cashion, P. Szymanski, O. Ast, A. Orme, C. Gross, M. Bauzon, A. Brooks, C. Schaefer, H. Gibson, H. Qian, G. M. Rubanyi, and R. N. Harkins. 2006. Mx1 and IP-10: biomarkers to measure IFN-beta activity in mice following gene-based delivery. J. Interferon Cytokine Res. 26699-705. [DOI] [PubMed] [Google Scholar]
- 27.Rank, R. G., K. H. Ramsey, E. A. Pack, and D. M. Williams. 1992. Effect of gamma interferon on resolution of murine chlamydial genital infection. Infect. Immun. 604427-4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rank, R. G., L. S. Soderberg, and A. L. Barron. 1985. Chronic chlamydial genital infection in congenitally athymic nude mice. Infect. Immun. 48847-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rasmussen, S. J., L. Eckmann, A. J. Quayle, L. Shen, Y. X. Zhang, D. J. Anderson, J. Fierer, R. S. Stephens, and M. F. Kagnoff. 1997. Secretion of proinflammatory cytokines by epithelial cells in response to chlamydia infection suggests a central role for epithelial cells in chlamydial pathogenesis. J. Clin. Investig. 9977-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roan, N. R., and M. N. Starnbach. 2006. Antigen-specific CD8+ T cells respond to Chlamydia trachomatis in the genital mucosa. J. Immunol. 1777974-7979. [DOI] [PubMed] [Google Scholar]
- 31.Rothermel, C. D., G. I. Byrne, and E. A. Havell. 1983. Effect of interferon on the growth of Chlamydia trachomatis in mouse fibroblasts (L cells). Infect. Immun. 39362-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rothfuchs, A. G., D. Gigliotti, K. Palmblad, U. Andersson, H. Wigzell, and M. E. Rottenberg. 2001. IFN-alpha beta-dependent, IFN-gamma secretion by bone marrow-derived macrophages controls an intracellular bacterial infection. J. Immunol. 1676453-6461. [DOI] [PubMed] [Google Scholar]
- 33.Rothfuchs, A. G., C. Trumstedt, F. Mattei, G. Schiavoni, A. Hidmark, H. Wigzell, and M. E. Rottenberg. 2006. STAT1 regulates IFN-alphabeta- and IFN-gamma-dependent control of infection with Chlamydia pneumoniae by nonhemopoietic cells. J. Immunol. 1766982-6990. [DOI] [PubMed] [Google Scholar]
- 34.Rothlin, C. V., S. Ghosh, E. I. Zuniga, M. B. Oldstone, and G. Lemke. 2007. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell 1311124-1136. [DOI] [PubMed] [Google Scholar]
- 35.Shah, A. A., J. H. Schripsema, M. T. Imtiaz, I. M. Sigar, J. Kasimos, P. G. Matos, S. Inouye, and K. H. Ramsey. 2005. Histopathologic changes related to fibrotic oviduct occlusion after genital tract infection of mice with Chlamydia muridarum. Sex. Transm. Dis. 3249-56. [DOI] [PubMed] [Google Scholar]
- 36.Stockinger, S., T. Materna, D. Stoiber, L. Bayr, R. Steinborn, T. Kolbe, H. Unger, T. Chakraborty, D. E. Levy, M. Muller, and T. Decker. 2002. Production of type I IFN sensitizes macrophages to cell death induced by Listeria monocytogenes. J. Immunol. 1696522-6529. [DOI] [PubMed] [Google Scholar]
- 37.Su, H., and H. D. Caldwell. 1995. CD4+ T cells play a significant role in adoptive immunity to Chlamydia trachomatis infection of the mouse genital tract. Infect. Immun. 633302-3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weigent, D. A., T. L. Huff, J. W. Peterson, G. J. Stanton, and S. Baron. 1986. Role of interferon in streptococcal infection in the mouse. Microb. Pathog. 1399-407. [DOI] [PubMed] [Google Scholar]
- 39.Whiting, D., G. Hsieh, J. J. Yun, A. Banerji, W. Yao, M. C. Fishbein, J. Belperio, R. M. Strieter, B. Bonavida, and A. Ardehali. 2004. Chemokine monokine induced by IFN-gamma/CXC chemokine ligand 9 stimulates T lymphocyte proliferation and effector cytokine production. J. Immunol. 1727417-7424. [DOI] [PubMed] [Google Scholar]
- 40.Yang, T., P. Stark, K. Janik, H. Wigzell, and M. E. Rottenberg. 2008. SOCS-1 protects against Chlamydia pneumoniae-induced lethal inflammation but hampers effective bacterial clearance. J. Immunol. 1804040-4049. [DOI] [PubMed] [Google Scholar]
- 41.Yao, S. Y., A. Ljunggren-Rose, C. W. Stratton, W. M. Mitchell, and S. Sriram. 2001. Regulation by IFN-beta of inducible nitric oxide synthase and interleukin-12/p40 in murine macrophages cultured in the presence of Chlamydia pneumoniae antigens. J. Interferon Cytokine Res. 21137-146. [DOI] [PubMed] [Google Scholar]
- 42.Zeng, X., T. A. Moore, M. W. Newstead, J. C. Deng, S. L. Kunkel, A. D. Luster, and T. J. Standiford. 2005. Interferon-inducible protein 10, but not monokine induced by gamma interferon, promotes protective type 1 immunity in murine Klebsiella pneumoniae pneumonia. Infect. Immun. 738226-8236. [DOI] [PMC free article] [PubMed] [Google Scholar]