Abstract
Genetically regulated mechanisms of host defense against Cryptococcus neoformans infection are not well understood. In this study, pulmonary infection with the moderately virulent C. neoformans strain 24067 was used to compare the host resistance phenotype of C57BL/6J with that of inbred mouse strain SJL/J. At 7 days or later after infection, C57BL/6J mice exhibited a significantly greater fungal burden in the lungs than SJL/J mice. Characterization of the pulmonary innate immune response at 3 h after cryptococcal infection revealed that resistant SJL/J mice exhibited significantly higher neutrophilia, with elevated levels of inflammatory cytokine tumor necrosis factor alpha (TNF-α) and keratinocyte-derived chemokine (KC)/CXCL1 in the airways, as well as increased whole-lung mRNA expression of chemokines KC/CXCL1, MIP-1α/CCL3, MIP-1β/CCL4, MIP-2/CXCL2, and MCP-1/CCL2 and cytokines interleukin 1β (IL-1β) and IL-1Ra. At 7 and 14 days after infection, SJL/J mice maintained significantly higher levels of TNF-α and KC/CXCL1 in the airways and exhibited a Th1 response characterized by elevated levels of lung gamma interferon (IFN-γ) and IL-12/IL-23p40, while C57BL/6J mice exhibited Th2 immunity as defined by eosinophilia and IL-4 production. Alveolar and resident peritoneal macrophages from SJL/J mice also secreted significantly greater amounts of TNF-α and KC/CXCL1 following in vitro stimulation with C. neoformans. Intracellular signaling analysis demonstrated that TNF-α and KC/CXCL1 production was regulated by NF-κB and phosphatidylinositol 3 kinase in both strains; however, SJL/J macrophages exhibited heightened and prolonged activation in response to C. neoformans infection compared to that of C57BL/6J. Taken together, these data demonstrate that an enhanced innate immune response against pulmonary C. neoformans infection in SJL/J mice is associated with natural resistance to progressive infection.
Cryptococcus neoformans infection may cause severe and potentially life-threatening cases of pneumonia, meningitis, and disseminated disease in the immunocompromised host (36, 40). C. neoformans infection disease has been associated with several virulence factors including growth at 37°C (37), synthesis of a polysaccharide capsule (8), melanin (37), and mannitol (10), as well as the expression of urease (13), laccase (41), and extracellular phospholipase (12). Despite the organism's potential to subvert a broad range of host defenses (36, 52), epidemiologic studies indicate that asymptomatic exposure or mild infection is much more common than severe cryptococcal disease (1, 19). Therefore, the progression and outcome of human cryptococcal infection appears to be determined, to a large extent, by the competence of the host immune response.
Significant insight into the immunopathogenesis of cryptococcal infection has been acquired from experimental animal models. The fundamental importance of T-lymphocyte-mediated immunity in the clearance of experimental pulmonary infection was demonstrated through the depletion of CD4+ and CD8+ T-lymphocyte subsets (34, 47), as well as by infection of congenitally lymphocyte-deficient SCID and athymic mutant mouse strains (7, 24, 35, 58). Additional studies have shown that Th1 polarization of the immune response is protective against C. neoformans infection (28, 49). Macrophages and pulmonary dendritic cells rapidly internalize C. neoformans organisms in murine models of pneumonia (17, 67); however, in vivo depletion of neutrophils unexpectedly enhanced the host defense (45). B-cell-mediated humoral immunity participates in host resistance against cryptococcal infection (2, 57, 65), although its protective efficacy depends on the genetic background of the host (56, 69, 70). A clear role for cytokines such as tumor necrosis factor alpha (TNF-α), gamma interferon (IFN-γ), interleukin 12 (IL-12), and IL-18, as well as chemokines such as monocyte chemoattractant protein-1 (MCP-1/C-C motif ligand 2 [CCL2]) and macrophage inflammatory protein-1-alpha (MIP-1α/CCL3) in leukocyte recruitment and effective host defense (33) has been demonstrated. Conversely, the microbial pattern recognition receptor Toll-like receptor 2 (TLR2) or the cytoplasmic adaptor molecule MyD88 may have a limited role in host resistance to C. neoformans challenge (5, 50, 68). Thus, a complex cellular and molecular network mediates an effective host immune response against C. neoformans infection.
Infection of inbred mouse strains with C. neoformans has revealed significant variations in host susceptibility that are regulated by genetic factors (11, 26, 55, 71). For example, experimental intratracheal infection with 104 CFU of a moderately virulent serotype D isolate of C. neoformans revealed that C57BL/6J mice had natural susceptibility, whereas those of the BALB/c and CBA/J inbred strains did not (26). In this study, C57BL/6J mice developed an allergic bronchopulmonary mycosis (ABPM) characterized by chronic IL-5-dependent eosinophilia and intracellular, as well as extracellular, deposition of Charcot-Leyden-like crystals. Interestingly, C57BL/6J and CBA/J mice were equally susceptible to high-dose (106 CFU) intravenous infection with the same cryptococcal isolate, demonstrating that host resistance depends on the dose and route of infection (71). In another report that used an intravenous challenge with 5 × 106 CFU of a serotype D C. neoformans isolate, inbred strains lacking the fifth component of complement (C5) had a mean survival time of 4 days, compared to 13 days for C5-sufficient strains (55). Among the 16 inbred strains that were tested in this study, SJL/J mice had the longest mean survival time (mean ± standard error of the mean, 31.3 ± 2.0 days) (55).
Characterizing the genetic basis of host resistance is an important and proven approach to advancing the understanding of microbe-host interactions and disease pathogenesis (6, 64). Despite the importance of host factors in controlling the development and outcome of human cryptococcal disease and the clear evidence for substantial variations in host resistance shown by experimental mouse models of C. neoformans infection, the cellular and molecular bases of these traits remain largely undefined (11). In this study, we hypothesized that genetically controlled variation of the innate response to pulmonary cryptococcal infection is an important mechanism of host resistance. To test this possibility, we compared the early inflammatory response and pathogen burden of the resistant SJL/J mouse with that of susceptible C57BL/6J inbred mouse strains, using a clinically relevant lung infection model (29). Our findings demonstrate that the SJL/J strain generates a significantly stronger lung innate immune response than the innate immune response of C57BL/6J mice, following intratracheal C. neoformans challenge. The heightened innate response of SJL/J mice precedes and instructs the development of Th1 immunity in the lung that is associated with natural resistance to progressive pulmonary and extrapulmonary cryptococcal infection.
MATERIALS AND METHODS
Reagents and media.
RPMI 1640 medium, l-glutamine, and penicillin-streptomycin were from Gibco (Grand Island, NY). Heat-inactivated fetal calf serum (FCS) was from HyClone (Logan, UT). Complete RPMI denotes RPMI 1640 medium supplemented with 2 mM glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% FCS (vol/vol). [γ-33P]UTP was from Perkin-Elmer (Woodbridge, ON, CA). Protease and phosphatase inhibitors (leupeptin, aprotinin, soybean trypsin inhibitor, phenylmethylsulfonyl fluoride, benzamidine, N-ethylmaleimide, and sodium orthovanadate) were from Sigma (Oakville, ON). The NF-κB inhibitor (BAY11-7082) was obtained from Calbiochem (La Jolla, CA). The phospho- and total-NF-κB p65, phospho- and total-Akt, and α-tubulin antibodies, as well as the phosphatidylinositol 3 kinase (PI3K) inhibitor (LY294002), were obtained from Cell Signaling technology (Beverly, MA).
Animals.
Six-week-old male C57BL/6J and SJL/J mice were obtained from Harlan Laboratories (Indianapolis, IN) and maintained in our animal facilities under conditions specified by the Canadian Council on Animal Care. All protocols were reviewed and approved by the McGill University Animal Care Committee.
C. neoformans culture.
C. neoformans 24067 (ATCC, Manassas, VA) was grown and maintained on Sabouraud dextrose agar (SDA) (BD, Sparks, MD) (26). For infection, a single colony suspension in Sabouraud dextrose broth (BD, Sparks, MD) was prepared and grown to early stationary phase (48 h) at room temperature with continuous rotation. The stationary culture was then washed with phosphate-buffered saline (PBS), counted on a hemacytometer, and diluted to the desired fungal concentration in sterile PBS. Confirmation of the fungus dose was done before and after experimental infection by plating a diluted aliquot on SDA and counting the CFU after 72 h of incubation at room temperature.
Intratracheal and intranasal administration of C. neoformans.
Mice underwent intratracheal infection as described elsewhere (26). Briefly, mice were anesthetized by intraperitoneal injection of ketamine (10 mg/kg of body weight; Ayerst Veterinary Laboratories, Guelph, ON, Canada) and xylazine (125 mg/kg; Bayer Inc., Pittsburgh, PA) in 0.9% sterile saline. A small midline skin incision was made over the trachea, and the underlying tissue was retracted using a two-pronged blunt retractor (Fine Scientific Tools, North Vancouver, BC, Canada). The smooth muscle surrounding the trachea was then dissected to allow for the insertion of a 22-gauge catheter. A dose of 104 CFU of C. neoformans 24067 in a 50-μl volume (2 × 105 CFU/ml) was then administered through the catheter via a 1-ml tuberculin syringe (BD, Sparks, MD), followed immediately by 50 μl of air. The incision was closed using a 9-mm EZ Clip wound closing kit (Stoelting, Wood Dale, IL). For intranasal infection, mice were lightly anesthetized with 5% isoflurane and placed in a vertical position, and 106 CFU of C. neoformans in a 50-μl volume (2 × 107 CFU/ml) was administered via the nares.
Lung isolation and CFU assay.
After mice were euthanized by anesthetic overdose, their infected lungs were excised and placed in 2 ml of sterile, ice-cold PBS. Individual lungs were then weighed and homogenized using an autoclaved glass tube and pestle attached to a tissue homogenizer (Glas-Col, Terre Haute, IN) at 0.02 × g. Organ homogenates were serially diluted using sterile PBS, plated in duplicate on SDA, and incubated at 37°C for 72 h prior to enumeration of C. neoformans CFU. To detect bacterial contamination or concomitant infection, each homogenate was simultaneously plated on Columbia blood agar and incubated for 72 h at 37°C with 5% CO2.
Collection of BALF.
Mice were killed by CO2 exposure at various times, and bronchoalveolar lavage fluid (BALF) was collected from the airways four times with 0.5 ml of ice-cold, sterile PBS. The total cell count for each BALF sample was determined with a hemacytometer, and differential cell counts were determined with 200 cells after cells were subjected to Cytospin centrifugation and staining with Diff-Quick (Dade Behring, Newark, DE). Each BALF supernatant was collected and stored at −20°C for subsequent quantification of cytokines and chemokines after it was centrifuged at 306 × g for 10 min at 4°C.
Histological analyses.
Whole lungs were inflated to a fixed pressure of 25 cm of H2O with 10% buffered formalin acetate (Fischer Scientific, Fair Lawn, NJ), embedded in paraffin, and sectioned at 5-μm thicknesses. The sections were stained with periodic acid-Schiff (PAS) to identify mucus-secreting goblet cells. Slide image were captured with a coolSNAP-Pro cf digital capture kit (Media Cybernetics, Bethesda, MD) using an Olympus BX51 light microscope (Olympus Canada, Inc., Markham, ON, Canada).
Alveolar and peritoneal macrophage isolation and stimulation.
Naïve mice were killed by CO2 exposure, and resident alveolar macrophages were obtained by lung bronchoalveolar lavage with 5 ml (5 × 1 ml) of ice-cold, sterile PBS. Cells were centrifuged at 306 × g for 10 min at 4°C, followed by treatment with red blood cell lysis buffer that was quenched by the addition of 10 volumes of complete RPMI. After cells underwent another centrifugation, they were resuspended in complete medium and plated at 1 × 105 cells/well into 96-well tissue culture plates (Sarstedt, Montreal, QC, Canada). After samples were incubated for 1 h at 37°C in 5% CO2, nonadherent cells were removed by one wash with warm complete RPMI and incubated overnight until stimulation was performed. Resident peritoneal macrophages were obtained from individual mice by serial peritoneal lavages with 10 ml of ice-cold RPMI medium that were pooled and centrifuged at 306 × g for 10 min at 4°C. Peritoneal macrophages were subsequently prepared as described for alveolar macrophages. In both cases, adherent cells were stimulated after overnight incubation with various multiplicities of infection (MOI) of C. neoformans 24067. Cell supernatants were collected 24 h later, immediately frozen at −20°C, and subsequently assayed for cytokine and chemokine secretion. For pharmacologic inhibition experiments, macrophages were pretreated for 45 min with individual compounds and then stimulated for 24 h with C. neoformans.
Cytokine and chemokine measurements.
Cell culture supernatants were assayed for mouse TNF-α and IL-6, using an OptEIA instrument (detection sensitivity, 15.6 pg/ml; BD Biosciences, San Diego, CA). IL-12/IL-23p40 (detection sensitivity, 62.5 pg/ml), keratinocyte-derived chemokine (KC)/CXCL1 (detection sensitivity, 15.6 pg/ml), IFN-γ (detection sensitivity, 15.6 pg/ml), and IL-4 (detection sensitivity, 15.6 pg/ml) concentrations were determined with a Duo-Set (R&D Systems, Minneapolis, MN) enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer's instructions.
Immunoblotting.
Macrophage cell extracts were prepared from 5 × 105 cells and solubilized as described previously (21). An equal amount of protein (10 μg) from each sample was size separated on a 10% sodium dodecyl sulfate-polyacrylamide gel and electrotransferred to a nitrocellulose membrane (Bio-Rad). Immunodetection was performed with antibodies specific for the total and phosphorylated forms of Akt and for the p65 subunit of NF-κB, as well as total α-tubulin. Bound antibodies were detected using an ECL-plus detection system (GE Healthcare/Amersham, Piscataway, NJ) according to the manufacturer's instructions. Between successive probes, membranes were treated with Western restore blot stripping reagent (Pierce, Rockford, IL). Molecular masses were determined using the biotinylated calibration standards included in each gel (Cell Signaling Technology). Images were recorded with a Gene Genius bioimaging system (Syngene, Frederick, MD).
RT-PCR.
Reverse transcription (RT) was performed with 1 μg of total RNA that had been extracted using an ABI high-capacity cDNA archive kit (ABI, Foster City, CA). PCR was performed for mouse IFN-γ, using the specific primers sense (5′-CAT TGA AAG CCT AGA AAG TCT G-3′) and antisense (5′-CTC ATG AAT GCA TCC TTT TTC G-3′) (amplicon size, 267 bp) (43). Primers for the detection of the internal control mouse β-actin were sense (5′-GGT ACC ACC ATG TAC CCA GG-3′) and antisense (5′-ACA TCT GCT TGT GGA AGG TGG AC-3′) (amplicon size, 163 bp). PCR amplifications were performed in a Peltier thermal cycler apparatus (MJ Research, Watertown, MA) with AmpliTaq polymerase (ABI, Foster City, CA). The thermocycling protocol was 94°C for 1 min, followed by 36 cycles at 94°C for 1 min, 60°C for 1 min, and 72°C for 2 min, with a final extension at 72°C for 10 min. Amplification products were resolved on a 1.5% agarose gel containing 0.5 μg/ml ethidium bromide and recorded with a Gene Genius bioimaging system.
RNase protection assay (RPA).
Total lung RNA was extracted using an RNeasy mini-kit (Qiagen, Mississauga), and the integrity of all samples was confirmed on a denaturing agarose gel. 33P-labeled riboprobes were synthesized from custom-made mouse multiprobe kits (BD Bioscience, Pharmingen, San Diego, CA) for the chemokines lymphotactin (LTN)/XCL1, RANTES/CCL5, KC/CXCL1, MIP-1α/CCL3, MIP-1β/CCL4, MIP-2/CXCL2, IP-10/CXCL10, MCP-1/CCL2, and eotaxin/CCL11; and for the cytokines IL-12p35, IL-12p40, TNF-α, IL-1α, IL-1β, IL-1Ra, IL-6, IL-18, IFN-γ and MIF. To quantify mRNA expression levels, the riboprobes were hybridized with each RNA sample (10 μg) overnight at 56°C according to the manufacturer's instructions. The protected RNA fragments were separated using a 5% polyacrylamide gel. The quantity of each mRNA was determined by measuring signal intensity with a phosphorimager (Molecular Dynamics, Sunnyvale, CA), followed by analysis with ImageJ 1.34S software. The signals were normalized to the L32 housekeeping gene to control for loading of the samples and expressed in arbitrary units.
Statistical analysis.
All data are expressed as the means ± standard errors of the means, unless otherwise indicated. Data were analyzed by using the unpaired Student t test for single comparisons. Welch's correction was used for comparisons with unequal variation.
RESULTS
Restriction of progressive pulmonary infection in SJL/J mice infected with C. neoformans.
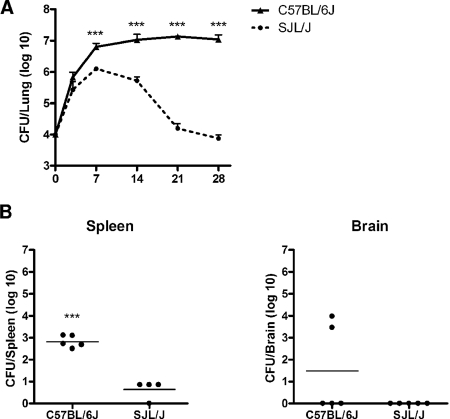
To determine whether SJL/J mice exhibit heritable resistance to pulmonary cryptococcal infection, the lung fungal burdens of C57BL/6J and SJL/J mice were determined starting at day 3 after an intratracheal challenge with 104 CFU of C. neoformans 24067 until day 28 (Fig. 1A). As early as day 7 after challenge, the lung fungal burden was significantly lower in SJL/J mice than that in C57BL/6J mice. At all subsequent time points, a progressive decrease in the lung CFU of SJL/J mice was observed, while the lung fungal burden of C57BL/6J remained stable during the course of experimental infection, as previously reported (25). By day 28, quantitative analysis of fungal growth in the lungs of these two inbred strains demonstrated clearly dichotomous phenotypes, with C57BL/6J mice having a significantly higher fungal burden (log CFU = 6.93 ± 0.32) than SJL/J mice (log CFU = 3.84 ± 0.15) (P ≤ 0.001). To determine whether this difference was specific to the initial infecting dose, intranasal infection of the C57BL/6J and SJL/J strains was also performed with 106 CFU of C. neoformans 24067. Four weeks following intranasal infection, a similar significant strain-dependent divergence in lung fungal burden was observed (data not shown; P ≤ 0.001). As disseminated cryptococcal infection has been shown to correlate with survival (71), spleen and brain fungal burdens were also measured at day 28 following intratracheal infection (Fig. 1B). C57BL/6J mice had a significantly higher spleen fungal burden than SJL/J mice (log CFU = 2.82 ± 0.12 versus log CFU = 0.64 ± 0.21, respectively) (P ≤ 0.001). Two of five C57BL/6J mice also had cryptococcal infection of the brain (log CFU = 3.71 ± 0.26), while fungal growth was not observed with any of the five SJL/J mouse brains.
FIG. 1.
Fungal burden following C. neoformans infection. Inbred male C57BL/6J and SJL/J mice underwent intratracheal infection with 104 CFU of a serotype D C. neoformans isolate. (A) Fungal burden in the lung was determined at 0, 3, 7, 14, and 28 days after infection. Each point represents the mean ± standard error of the mean of CFU (n = 5; ***, P ≤ 0.001). (B) Fungal burden in the spleen and brain was determined 28 days after infection. Bars represent the means of CFU (n ≥ 4; ***, P ≤ 0.001).
C. neoformans-infected SJL/J lungs demonstrate enhanced inflammation without ABPM.
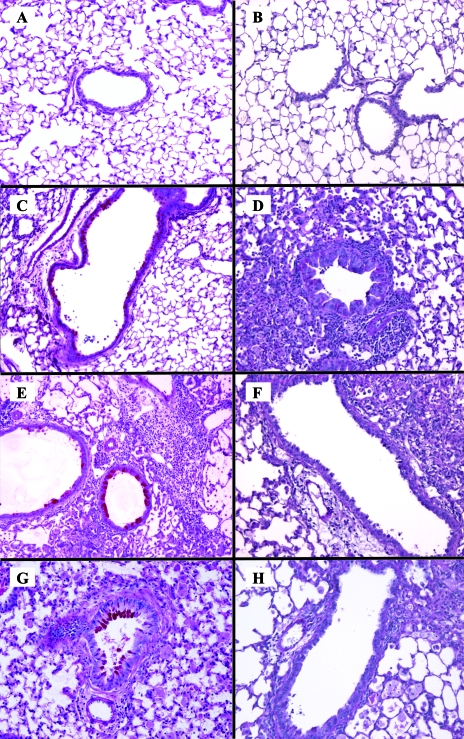
The significant difference in pulmonary fungal burden between SJL/J and C57BL/6J mice throughout the duration of experimental infection raised the prospect that these two inbred strains develop distinct lung tissue pathology. To evaluate this possibility, formalin-fixed lung sections from each strain were prepared at days 0, 7, 14, and 28 following intratracheal infection with 104 CFU of C. neoformans 24067 (Fig. 2). The tissue sections were stained with PAS to detect the presence of mucus-secreting goblet cells, a feature that is characteristic of ABPM. An intense leukocyte infiltration was observed for both inbred strains, although no true granulomas were present. Consistent with previous reports (3, 23), the airways of C57BL/6J mice exhibited prominent airway epithelial mucus accumulation (dark pink stain) at all time points following infection (Fig. 2C, E, and G) that was absent from SJL/J mice airways. This histologic pattern suggests that the pulmonary response of SJL/J mice naturally polarizes to a Th1-cell-mediated pattern following experimental cryptococcal infection, in contrast to the well-described allergic bronchopulmonary response of C57BL/6J mice.
FIG. 2.
Inflammatory response in the lungs of resistant and susceptible mice following intratracheal C. neoformans infection. Photomicroscopy of PAS-stained lung sections from individual C57BL/6J (A, C, E, and G) or SJL/J (B, D, F, H) inbred mice that were uninfected (A and B) or underwent intratracheal infection with 104 CFU of a serotype D C. neoformans isolate for 7 (C and D), 14 (E and F), and 28 (G and H) days. Goblet cells and mucus stain dark pink (magnification, ×100). Each image is representative of n = 3 mice/group/time point.
The resistant phenotype of SJL/J mice is associated with an increased proinflammatory innate host immune response that leads to a Th1-cell-polarized adaptive immune response.
To determine whether the marked differences between the fungal burdens observed at 1 week postinfection for C57BL/6J mice and SJL/J mice were associated with heritable variations of the innate immune response, the lung inflammatory profiles of these two inbred strains were examined following an intranasal infection with 106 CFU of C. neoformans, with sterile PBS administration used as a control. The total and differential cell counts in the BALF were examined at early (3 h and 24 h) and late (7 days and 14 days) time points after mice were infected (Table 1). After receiving PBS treatment, the C57BL/6J and SJL/J strains had similar total airway cell numbers (≈5 × 104) and morphology consisting almost exclusively of macrophages. After mice received C. neoformans infection, both strains showed a progressive increase in the percentage of neutrophils in the BALF. At 3 h postinfection, SJL/J mice had a significantly higher percentage of neutrophils than the C57BL/6J mice (32.03% ± 5.33% versus 7.53% ± 1.79%, respectively) (P ≤ 0.01), while the C57BL/6J mice maintained a significantly higher percentage of macrophages (92.13% ± 1.77% versus 59.21% ± 7.68%, respectively) (P ≤ 0.01). By 24 h, no significant differences in the BALF neutrophil or macrophage populations were demonstrable between the two inbred strains. Mild though progressive eosinophil recruitment was demonstrable in the BALF of C57BL/6J mice at 3 h (0.29% ± 0.12%) and at 24 h (1.15% ± 0.56%) but was undetectable in SJL/J mice at either time point, indicating that an allergic phenotype begins during the very early stages of the innate immune response in this strain (26).
TABLE 1.
Airway cell recruitment following lung infection with C. neoformansa
| Time | BALF cell | % of BALF cells ± SEM (relative to total)
|
|
|---|---|---|---|
| C57BL/6J | SJL/J | ||
| Uninfected | Macrophage | 97.89 ± 1.41 | 98.82 ± 0.21 |
| PMN | 2.11 ± 1.41 | 1.176 ± 0.21 | |
| Eosinophil | 0 | 0 | |
| Lymphocyte | 0 | 0 | |
| 3 h | Macrophage | 92.13 ± 1.77 | 59.21 ± 7.68** |
| PMN | 7.53 ± 1.79 | 32.03 ± 5.33** | |
| Eosinophil | 0.29 ± 0.12 | 0 | |
| Lymphocyte | 0 | 0 | |
| 24 h | Macrophage | 44.90 ± 4.25 | 35.72 ± 8.01 |
| PMN | 54.00 ± 3.76 | 64.28 ± 8.01 | |
| Eosinophil | 1.15 ± 0.56 | 0 | |
| Lymphocyte | 0 | 0 | |
| 7 days | Macrophage | 18.06 ± 1.21 | 13.12 ± 2.88 |
| PMN | 57.95 ± 3.01 | 83.91 ± 2.45*** | |
| Eosinophil | 21.96 ± 2.84 | 0.30 ± 0.30** | |
| Lymphocyte | 2.04 ± 1.35 | 2.67 ± 0.34 | |
| 14 days | Macrophage | 15.31 ± 3.17 | 23.76 ± 2.95 |
| PMN | 10.64 ± 3.67 | 67.77 ± 2.69*** | |
| Eosinophil | 70.96 ± 6.39 | 0 | |
| Lymphocyte | 3.09 ± 0.435 | 8.59 ± 1.31 | |
Total and differential cell counts were obtained from the BALF of uninfected C57BL/6J and SJL/J mice at 3 h, 24 h, 7 days, and 14 days after mice received intranasal administration of 106 CFU of a serotype D C. neoformans isolate. Cell counts are reported as the percentage (mean ± standard error of the mean [SEM]) of total cells in the BALF (n ≥ 4 mice/group/time point). PMN, polymorphonuclear leukocyte. **, P ≤ 0.01; ***, P ≤ 0.001.
C57BL/6J mice are well known to develop a Th2 pattern of lung adaptive immunity to C. neoformans, while relatively resistant strains generate a protective Th1 response (26). To determine whether the stronger early inflammatory response in SJL/J mice leads to a more robust Th1 pattern of adaptive immune response, we compared the profiles of cellular and soluble mediators in the airways of SJL/J with those of C57BL/6J mice at 7 and 14 days after infection (Table 1).
At day 7 after infection, SJL/J mice had significantly higher airway neutrophilia than C57BL6/J mice (83.91% ± 2.45% versus 57.95% ± 3.01%, respectively) (P ≤ 0.001). By day 14, this difference was even more pronounced: SJL/J mice had 67.77% ± 2.69% BALF neutrophils compared to 10.64% ± 3.67% BALF neutrophils for C57BL/6J mice (P ≤ 0.001). Conversely, at day 7, C57BL/6J mice had significantly higher airway eosinophilia than SJL/J mice (21.96% ± 2.84% versus 0.30% ± 0.30%, respectively) (P ≤ 0.01). At day 14, airway eosinophilia was not detectable in SJL/J mice, while eosinophils represented 70.96% ± 6.39% of the total cells in the airways of C57BL/6J mice. At day 14, SJL/J mice also had a significantly higher percentage of lymphocytes in the BALF than C57BL/6J mice (8.59% ± 1.31% versus 3.09% ± 0.44%, respectively) (P ≤ 0.01).
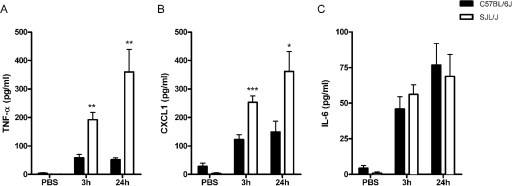
To identify molecular signals that regulate early cell recruitment to the airways, the expression of mediators known to trigger rapid inflammatory responses was then characterized. Cytokine and chemokine analyses of the BALF at 3 h and 24 h postinfection demonstrated significantly higher levels of TNF-α and KC/CXCL1, but not IL-6, in the airways of SJL/J mice than in those of C57BL/6J mice (Fig. 3A, B, and C, respectively). To determine whether the increased response observed in the airways of SJL/J mice also correlated with inflammatory changes in lung tissue, quantitative mRNA expression of a panel of cytokines and chemokines was examined by RPA at 3 h after infection. Compared to uninfected mice, mice of both the C57BL/6J and SJL/J strains exhibited transcriptional upregulation for the cytokines TNF-α, IL-1α, IL-1β and IL-1Ra (see Fig. S1A in the supplemental material and Table 2) and the chemokines RANTES/CCL5, KC/CXCL1, MIP-1α/CCL3, MIP1-β/CCL4, MIP2/CXCL2, and MCP-1/CCL2 (see Fig. S1B in the supplemental material and Table 2) following the C. neoformans challenge. Relative to the expression levels determined for C57BL/6J mice, infected SJL/J mice exhibited a more than 3-fold increase in expression for all of these mediators except for RANTES/CCL5. Taken together, these results indicate that SJL/J mice naturally mount a stronger early airway and lung tissue inflammatory response to C. neoformans.
FIG. 3.
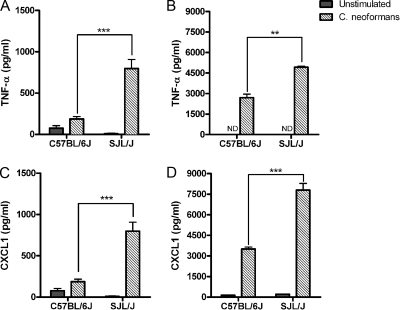
Early release of airway cytokines and chemokines following C. neoformans lung infection. The secretion of TNF-α (A), KC/CXCL1 (B), and IL-6 (C) in the BALF of C57BL/6J and SJL/J mice was measured at 3 h and 24 h after intranasal administration of 106 CFU of a serotype D C. neoformans isolate. Each bar represents the mean ± standard error of the mean (n ≥ 8 mice/group/time point; *, P < 0.05; **, P ≤ 0.01; ***, P ≤ 0.001).
TABLE 2.
Lung cytokine and chemokine mRNA expression following C. neoformans infectiona
| Immune response | Mediator | Mean RPA signal intensity ± SD
|
Fold change SJL/J versus C57BL/6 | |||
|---|---|---|---|---|---|---|
| C57BL/6J plus PBS | C57BL/6J plus C. neoformans | SJL/J plus PBS | SJL/J plus C. neoformans | |||
| Cytokines | TNF-α | 1.4 ± 1.6 | 4.5 ± 3.3 | 0.4 ± 0.1 | 9.9 ± 1.1 | 3.0 |
| IL-1α | 2.4 ± 3.2 | 4.3 ± 0.6 | 1.3 | 12.8 ± 0.1 | 1.3 | |
| IL-1β | 0.1 ± 0.1 | 1.5 ± 1 | 11.0 | 94.7 ± 15.4 | 11.0 | |
| IL-1Ra | 2.4 ± 2.8 | 3.8 ± 0.5 | 5.3 | 33.3 ± 9.6 | 5.3 | |
| IL-18 | 3.1 ± 3.9 | 5.2 ± 1.1 | 8.8 ± 0.6 | 9.3 ± 1 | 0.2 | |
| MIF | 15.7 ± 5.1 | 18.3 ± 2.8 | 12.5 ± 2.7 | 17.5 ± 2.8 | 1.9 | |
| Chemokines | CCL5 | 18.5 ± 5.6 | 46.4 ± 0.01 | 30.2 ± 0.1 | 63.8 ± 1.1 | 1.2 |
| CXCL1 | ND | 17.0 ± 4 | 2.5 ± 3.5 | 109.0 ± 3 | 6.3 | |
| CCL4 | ND | 1.8 ± 1.7 | ND | 35.6 ± 15.1 | 19.8 | |
| CCL3 | ND | 0.7 ± 1.0 | ND | 20.9 ± 9.7 | 29.9 | |
| CXCL2 | ND | 9.3 ± 6.6 | ND | 78.6 ± 8.5 | 8.5 | |
| CCL2 | ND | 2.0 ± 2.6 | ND | 43.1 ± 17.4 | 21.6 | |
The intensity of the individual cytokine or chemokine band that was measured by RPA (see Fig. S1 in the supplemental material) was quantified using digital analysis software. Data expressed in the table are the means ± standard deviations (SD) of the signal obtained for each group. ND, not detected. The fold change value is the signal intensity of SJL/J (C. neoformans minus PBS)/C57BL/6J (C. neoformans minus PBS).
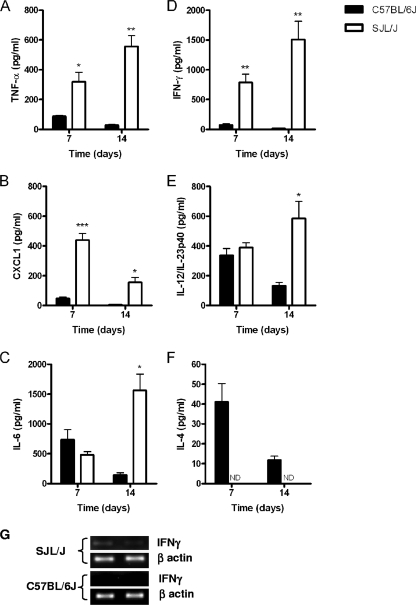
Significant differences in the BALF inflammatory cytokine and chemokine levels were also observed between C57BL/6J and SJL/J mice at days 7 and 14 after C. neoformans infection. Indeed, as observed for the early host response, SJL/J mice exhibited significantly higher levels of both TNF-α (Fig. 4A) and KC/CXCL1 (Fig. 4B) at both time points than C57BL/6J. In SJL/J mice, a progressive increase of the TNF-α level was observed from day 7 to day 14, while the highest level of KC/CXCL1 was observed at day 7. Finally, although there were no significant differences between the BALF IL-6 levels of C57BL/6J mice and those of SJL/J mice at day 7 after infection, the level of IL-6 was significantly higher in SJL/J mice BALF at day 14 postinfection. (P < 0.05) (Fig. 4C).
FIG. 4.
Analysis of airway cytokine/chemokine expression in C57BL/6J and SJL/J mice at 7 and 14 days after C. neoformans lung infection. The levels of TNF-α (A), KC/CXCL1 (B), IL-6 (C), IFN-γ (D), IL-12/IL-23p40 (E), and IL-4 (F) were measured in the BALF of C57BL6J and SJL/J mice that had been infected with a serotype D C. neoformans isolate for 7 or 14 days. Each bar represents the mean ± standard error of the mean (n ≥ 5 mice/group/time point; *, P < 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ND, not detected). (G) RT-PCR analysis of lung IFN-γ mRNA expression in C57BL/6J and SJL/J mice at 7 days after infection with a serotype D C. neoformans isolate. Amplification of mouse β-actin was performed as a control.
To compare the adaptive immune response in the airways of SJL/J mice with that in the airways of C57BL/6J mice that had been infected with C. neoformans, classical markers of Th1 (IL-12, IFN-γ)- and Th2 (IL-4)-associated immunity were measured in the BALF. SJL/J mice produced increasing amounts of IFN-γ that were significantly higher at day 7 (P ≤ 0.01) and day 14 (P ≤ 0.01) than those of C57BL/6J mice (Fig. 4D). At 1 week after infection, IFN-γ mRNA was also detectable in the infected lung tissue of SJL/J mice but not in that of C57BL/6J mice (see Fig. 7G). The level of IL-12/IL23p40 (Fig. 4E) was also significantly higher in SJL/J airways at day 14 (P < 0.05). In contrast, IL-4 expression was detectable only in the airways of C57BL/6J mice (Fig. 4F). Collectively, these data demonstrate that C57BL/6J and SJL/J mice develop differential Th1/Th2 polarization of the adaptive immune response following C. neoformans infection.
FIG. 7.
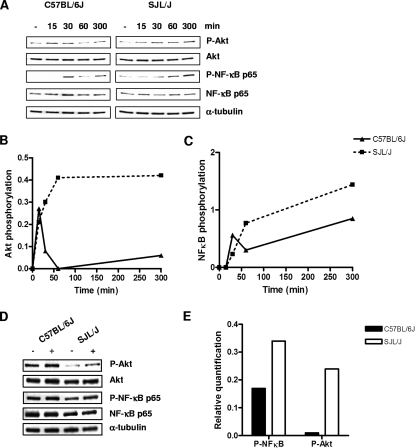
Macrophage signal transduction induced by C. neoformans infection. Activation of NF-κB and the PI3K-dependent kinase Akt. (A) Resident peritoneal macrophages from C57BL/6J and SJL/J mice were stimulated or not with a serotype D C. neoformans isolate at an MOI of 5:1 for increasing durations (0, 15, 30, 60, and 300 min). Cells were lysed, and the total and phosphorylated forms of Akt and NF-κB were determined by immunoblotting with specific antibodies. To confirm equal protein loading, the membranes were reprobed with anti-α-tubulin antibody. (B and C) Relative quantification of Akt and NF-κB phosphorylation. Bands were digitized, quantified, and expressed in arbitrary units as the ratio between the phosphorylated and total forms of each protein. (D) Alveolar macrophages from C57BL/6J and SJL/J mice were stimulated (+) or not stimulated (−) with a serotype D C. neoformans isolate at an MOI of 5:1 for 1 hour. Cells were lysed and analyzed for phospho-Akt and the phospho-p65 subunit of NF-κB. (E) Relative quantification of phospho-Akt and phospho-p65 NF-κB was performed as described above.
Increased proinflammatory responses of SJL/J macrophages stimulated with C. neoformans infection are regulated by NF-κB and PI3K/Akt signaling pathways.
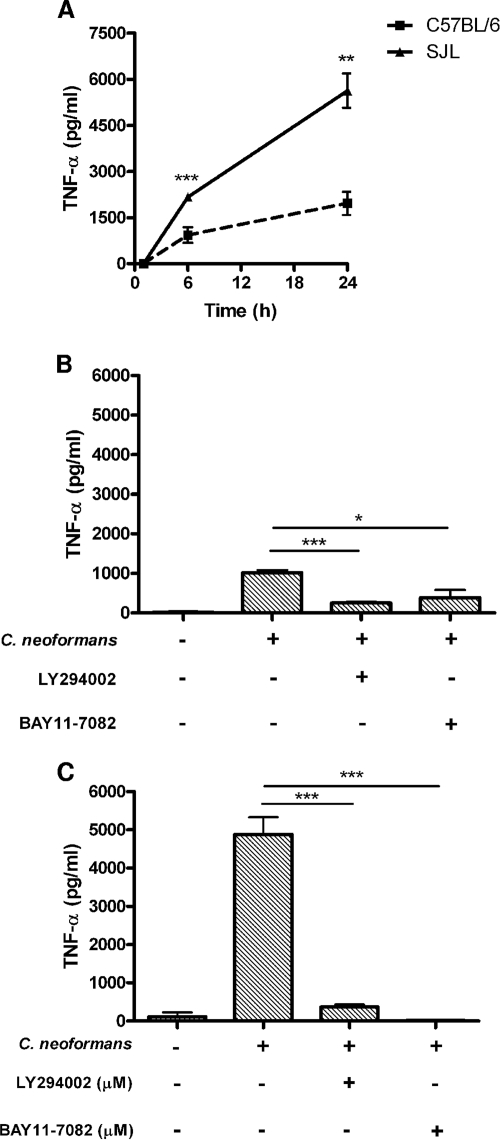
To investigate the cellular mediators that underlie the differential innate immune responsiveness of C57BL/6J and SJL/J mice to C. neoformans, we studied macrophage responses following in vitro stimulation for 24 h. Differences in macrophage function have been related to susceptibility or resistance to cryptococcal infection (59). Alveolar macrophages from the resistant SJL/J inbred strain secreted significantly higher levels of TNF-α (P ≤ 0.001) and KC/CXCL1 (P ≤ 0.001) than that from the C57BL/6J strain (Fig. 5A and C, respectively). Peritoneal macrophages from SJL/J mice also secreted higher levels of TNF-α (P ≤ 0.01) and KC/CXCL1 (P ≤ 0.001) under the same conditions (Fig. 5B and D). Comparative kinetic analysis between the TNF-α production of the resistant strain and that of the susceptible inbred strain showed significantly increased production by SJL/J macrophages at 6 h (P ≤ 0.001) and 24 h (P ≤ 0.01) after stimulation (Fig. 6A).
FIG. 5.
Macrophage inflammatory mediator release following in vitro stimulation with C. neoformans. Alveolar (A and C) and resident peritoneal macrophages (B and D) isolated from C57BL/6J and SJL/J mice were stimulated or not with a serotype D C. neoformans isolate at an MOI of 5:1. Supernatants were collected 24 h later and assayed by ELISA for TNF-α (A and B) and KC/CXCL1 (C and D) production. Each bar represents the mean ± standard error of the mean of triplicate determinations (**, P ≤ 0.01; ***, P ≤ 0.001; ND, not detected). Results shown are representative of three independent experiments.
FIG. 6.
Regulation of macrophage TNF-α production in response to stimulation with C. neoformans by PI3K and NF-κB. (A) TNF-α production by resident peritoneal macrophages from C57BL/6J and SJL/J mice stimulated with a serotype D C. neoformans isolate at an MOI of 5:1 for 6 and 24 h. (B and C) Roles of NF-κB and PI3K/Akt-dependent signaling in the activation of resident peritoneal macrophages by C. neoformans. Macrophages from C57BL/6J (B) and SJL/J (C) mice were pretreated for 45 min with inhibitors of PI3K (LY294002, 50 μM) and NF-κB (BAY11-7082, 10 μM) and then stimulated with C. neoformans at an MOI of 5:1 for 24 h. Supernatants were collected and assayed for TNF-α production by ELISA. Data are the means ± standard error of the means of triplicate determinations (*, P < 0.05; **, P ≤ 0.01; ***, P ≤ 0.001). Results are representative of three independent experiments.
To identify potential mechanisms that underlie the strain-dependent variations of macrophage cytokine and chemokine responses following C. neoformans stimulation, we analyzed intracellular signaling activity. Induction of the transcription factor NF-κB and activation of PI3K are both central processes during the innate immune response to infection (14, 22). Involvement of NF-κB and PI3K in C. neoformans FcγR signaling has been shown in human microglial cells (61) and NK cells (66), yet the role of these pathways in C. neoformans infection-associated macrophage signaling has not been well studied. Therefore, as a first step in defining the role of these intermediates in the production of TNF-α, we treated resident peritoneal macrophages with pharmacologic inhibitors of NF-κB (BAY11-7082) and PI3K (LY294002) prior to stimulation with C. neoformans infection. Pretreatment with either of these compounds significantly reduced the C. neoformans-induced production of TNF-α in both C57BL/6J and SJL/J mouse macrophages (Fig. 6B and C).
To confirm the genetic regulation of differential macrophage NF-κB and PI3K signaling between resistant and susceptible mouse strains, we directly determined the activation of these pathways by specific immunoblotting techniques. Treatment of peritoneal macrophages from both strains with C. neoformans induced the phosphorylation of the serine/threonine kinase Akt, a downstream target of PI3K (Fig. 7A and B). Kinetic analysis showed that Akt phosphorylation was observable by 15 min after stimulation with C. neoformans in both the C57BL/6J and the SJL/J macrophages. Interestingly, the duration of Akt phosphorylation in the C57BL/6J macrophages was relatively brief, lasting for approximately 30 min, while in the SJL/J macrophages, Akt phosphorylation peaked at 1 h and remained visible at 5 h (Fig. 7A and B). For both strains, the total amount of Akt protein remained constant throughout the period of observation (Fig. 7A). At 30 min after C. neoformans stimulation, phosphorylation of the p65 subunit of NF-κB was detectable in both strains and appeared to be slightly higher in C57BL/6J macrophages (Fig. 7A and C); subsequent measurements at 1 h and 5 h after stimulation also demonstrated stronger NF-κB p65 phosphorylation in SJL/J macrophages. During this time, the total amount of NF-κB p65 subunit remained constant in both strains (Fig. 7A). Finally, alveolar macrophages were stimulated with C. neoformans to determine whether resident cells from the lung also exhibit a distinct strain-dependent signaling profile (Fig. 7D and E). Stimulation of C57BL/6J and SJL/J alveolar macrophages for 1 h resulted in the phosphorylation of Akt and NF-κB p65 in both strains. Consistent with the results obtained using peritoneal macrophages, alveolar macrophages from SJL/J mice exhibited stronger phosphorylation of both signaling molecules than those obtained from the C57BL/6J inbred strain.
DISCUSSION
The host immune response is a major determinant of the outcome of cryptococcal infection; however, the underlying basis for the differences in susceptibility is poorly understood (11, 71). To elucidate the mechanisms of host resistance against C. neoformans infection, we have compared the innate and adaptive pulmonary responses using C57BL/6J and SJL/J mice, two strains that have been reported to be susceptible and resistant to experimental pulmonary (26) and systemic (55) cryptococcal infection, respectively. The major findings of our study include the following: (i) SJL/J mice are highly resistant to pulmonary and extrapulmonary C. neoformans infection compared to the C57BL/6J inbred strain; (ii) SJL/J mice mount a significantly stronger inflammatory response in the airways, as well as in lung tissue, than C57BL/6J mice within 3 h of administration and maintain this differential response at days 7 and 14 after infection; and (iii) alveolar and peritoneal macrophages from SJL/J mice exhibit heightened responsiveness to in vitro stimulation with C. neoformans infection through differential activation of intracellular signaling cascades. Collectively, these observations clearly demonstrate that genetically regulated innate immune responsiveness in the murine lung is associated with host resistance to progressive cryptococcal infection.
In this report, a moderately virulent clinical isolate of C. neoformans was chosen for administration via the mouse respiratory tract to model the development of human cryptococcal infection. Under these conditions, SJL/J mice were naturally resistant to progressive pulmonary infection as well as to dissemination to the spleen and brain compared to C57BL/6J mice. Consistent with previous studies of inbred mouse strains, SJL/J mice developed a Th1 response characterized by significantly higher IL-12 and IFN-γ expression, while susceptible C57BL/6J mice had increased IL-4 expression in the BALF, indicative of Th2 polarization. Previously, SJL/J mice were also shown to be resistant to C. neoformans infection, using a very-high-dose (5 × 106 CFU) intravenous challenge model with a mean survival time of 31.3 ± 2.0 days (55). In that report, resistance and susceptibility were linked to the Hc locus on chromosome 2 that encodes the wild-type (Hc1) and the mutated (Hc0) alleles of the fifth component of complement (C5), respectively (54, 55). Although SJL/J male mice have been reported to have a significantly higher serum C5 level than other strains (42), it is important to note that the C57BL/6J mice used in this study also carry the wild-type Hc1 allele and are C5 sufficient. The highly resistant phenotype of SJL/J mice strongly suggests that genetic factors, in addition to a potential contribution from C5, control host defense against pulmonary C. neoformans infection.
It is well established that a Th1 pattern of adaptive immunity is protective against progressive cryptococcal infection (27). Adaptive immunity requires clonal expansion of lymphocytes, a process that typically develops by 7 days in the naïve host. In the current study, the fungal burden of previously unexposed mice began to diverge at 3 days after infection, implicating innate immune mechanisms in differential host resistance to pulmonary cryptococcal infection. To characterize the initial events that precede and instruct the development of a protective immune response against C. neoformans infection, we compared the expression levels of the inflammatory cytokine TNF-α and the neutrophil chemokine KC/CXCL1 in the airways of C57BL/6J mice with those of SJL/J mice at 3 h after pulmonary cryptococcal infection and demonstrated a significant elevation of both proteins in the resistant SJL/J mice. This finding is consistent with a previous study that demonstrated an essential role for TNF-α during the afferent phase of the immune response to C. neoformans infection (32). More recently, intratracheal delivery of a TNF-α-expressing adenoviral vector switched the natural allergic bronchopulmonary/Th2 response of C57BL/6J mice to C. neoformans toward Th1, resulting in the control of infection (46). Therefore, it is likely that the higher TNF-α protein expression during the innate immune response to C. neoformans infection contributes to the naturally resistant SJL/J phenotype. On the other hand, the precise role of the neutrophil chemokine KC/CXCL1 in host defense against cryptococcal infection is less well characterized. Recent reports have shown that both C. neoformans stimulation of human bronchial epithelial cells in vitro and adenoviral overexpression of TNF-α in the mouse lung induce the expression of KC/CXCL1 (20, 46); however, further studies will be required to determine its contribution to host defense.
IL-1β and IL-Ra were also differentially upregulated in SJL/J mouse lungs 3 h after C. neoformans infection. Cryptococcal capsular polysaccharide has been shown to induce IL-1β release by human neutrophils (53), and a recent report described a functional defect of TNF-α, IL-1β, and nitric oxide production by monocytes and neutrophils in an apparently immunocompetent patient with pulmonary cryptococcosis (44). Together, these observations suggest a potential role for the IL-1β-IL-1Ra axis in the host innate immune response against C. neoformans infection that also warrants further investigation.
Three hours after they received C. neoformans infection, differential upregulation of the chemokines MIP-1α/CCL3, MIP-1β/CCL4, MIP-2/CXCL2, and MCP-1/CCL2 was demonstrable in the lungs of C57BL/6J and SJL/J mouse strains, while RANTES/CCL5 was upregulated in both strains. Chemokines play an important role during the innate immune response to cryptococcal infection by recruiting and activating cells and influencing subsequent adaptive immunity. Crucial roles for MCP-1/CCL2 and its receptor CCR2 in the development of pulmonary Th1 immunity and cryptococcal clearance have been reported (31, 33, 62, 63). Similarly, depletion of MIP-1α/CCL3 during the efferent phase of pulmonary cell-mediated immunity reduced macrophage/monocyte and neutrophil recruitment and resulted in a threefold higher burden of the moderately virulent C. neoformans isolate in the lung (30), while infection of CCL3 knockout mice with a highly virulent cryptococcal strain resulted in a Th2 phenotype with dramatically decreased survival at 12 weeks (51). The specific contributions of MIP-1β/CCL4 and MIP-2/CXCL2 to cryptococcal host defense have not been reported; however, based on existing data, it appears that multiple chemokines are required to orchestrate an effective Th1 response against pulmonary cryptococcal infection. Therefore, it seems very likely that the rapid and heightened innate chemokine response in the SJL/J inbred strain also contributes to a resistant phenotype.
Consistent with differential chemokine expression levels following C. neoformans infection, resistant SJL/J mice also exhibited significantly greater airway neutrophilia than C57BL/6J mice at 3 h after challenge. Neutrophils are important in host defense against infection by certain fungi including Candida spp. and Aspergillus spp.; however, their contribution to host resistance against cryptococci infection is uncertain. Human neutrophils have been shown to kill cryptococci in vitro through oxidative and nonoxidative mechanisms (10, 15, 39), yet, somewhat unexpectedly, transient depletion of neutrophils in BALB/c mice, using a monoclonal antibody, enhanced survival following a high-dose (5 × 106 CFU) cryptococcal challenge without altering the fungal burden, possibly through modulation of the adaptive immune response (45). Due to the important differences between the experimental designs in that study and the current investigation, a potential contribution of neutrophils to the resistant SJL/J phenotype described here cannot be excluded, particularly since differential neutrophilia were also observed at 7 and 14 days after infection in association with a progressive decline in the fungal burden. In contrast, C57BL/6J mice developed a mild eosinophilia during the first 24 h after cryptococcal infection that progressively increased during the phase of adaptive immunity. C57BL/6J mice are known to develop an ABPM associated with chronic cryptococcal infection (4, 23, 26). The rapid development of airway eosinophilia in C57BL/6J mice exposed to C. neoformans points to uncharacterized innate mechanisms of eosinophil recruitment to the lung that precede the development of ABPM. It is possible that the rapid recruitment of eosinophils causes significant lung tissue destruction that may contribute to chronic fungal infection in C57BL/6J mice.
To establish a cellular mechanism for the differential innate immune responsiveness between C57BL/6J and SJL/J mice, analysis of macrophage activation was performed. Resident peritoneal and alveolar macrophages from SJL/J mice secreted significantly greater ΤNF-α and KC/CXCL1 than C57BL/6J mice, following in vitro stimulation with C. neoformans. Macrophages play an important role in host defense against cryptococci through various mechanisms including phagocytosis, killing, cytokine and chemokine production, and antigen presentation (38). During chronic pulmonary infection in mice, C. neoformans may survive as a facultative intracellular pathogen in alveolar macrophages and may cause cytotoxicity and disruption in association with intracellular polysaccharide production (17, 18). Consistent with the finding that human monocyte-derived macrophages express cellular receptors including TLR4 (60), CD14 (68), and CD18 (16) that are involved in the uptake of GXM (48), no specific opsonin or priming stimulus was required to induce cytokine and chemokine secretion in mouse macrophages. Both NF-κB and PI3K/Akt are well-recognized elements of the innate immune response against infection (14, 22). TLR4 stimulation by cryptococcal GXM has been shown to activate NF-κB (60), and PI3K/Akt has been implicated in the killing of C. neoformans organisms by human NK cells (66). Therefore, pharmacologic inhibitors and immunoblotting were used to study and confirm the differential activation of NF-κB and PI3K/Akt in SJL/J macrophages following cryptococcal stimulation. As relatively little is known about the signaling pathways that are activated by cryptococci, identification of the receptors and mechanisms of macrophage activation by cryptococci during the innate immune response is an important area for future investigation (9).
In summary, natural variations between the host resistance to cryptococcal infection of the C57BL/6J inbred mouse and that of the SJL/J inbred mouse strain are associated with the differential regulation of immune responsiveness in the airways, lung tissue, and macrophages that is demonstrable within hours of infection. Heightened pulmonary innate immunity precedes and instructs the development of definitive Th1 adaptive responses that lead to a progressive reduction in fungal burden.
Supplementary Material
Acknowledgments
We thank Qutayba Hamid for providing microscopy facilities and Markus Schnare for critical reading of the manuscript.
This study was supported by the Research Institute of the McGill University Health Centre, an operating grant from the Canadian Institutes of Health Research (MOP-81259), a Canada research chair and a career award in the biomedical sciences from the Burroughs Wellcome Fund (S.Q.), and postdoctoral training awards from the McGill McLaughlin fellowship and McGill University Health Centre (L.G.).
We have no competing financial interests.
Editor: A. Casadevall
Footnotes
Published ahead of print on 4 August 2008.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Abadi, J., and L. Pirofski. 1999. Antibodies reactive with the cryptococcal capsular polysaccharide glucuronoxylomannan are present in sera from children with and without human immunodeficiency virus infection. J. Infect. Dis. 180915-919. [DOI] [PubMed] [Google Scholar]
- 2.Aguirre, K. M., and L. L. Johnson. 1997. A role for B cells in resistance to Cryptococcus neoformans in mice. Infect. Immun. 65525-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arora, S., Y. Hernandez, J. R. Erb-Downward, R. A. McDonald, G. B. Toews, and G. B. Huffnagle. 2005. Role of IFN-gamma in regulating T2 immunity and the development of alternatively activated macrophages during allergic bronchopulmonary mycosis. J. Immunol. 1746346-6356. [DOI] [PubMed] [Google Scholar]
- 4.Arora, S., R. A. McDonald, G. B. Toews, and G. B. Huffnagle. 2006. Effect of a CD4-depleting antibody on the development of Cryptococcus neoformans-induced allergic bronchopulmonary mycosis in mice. Infect. Immun. 744339-4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biondo, C., A. Midiri, L. Messina, F. Tomasello, G. Garufi, M. R. Catania, M. Bombaci, C. Beninati, G. Teti, and G. Mancuso. 2005. MyD88 and TLR2, but not TLR4, are required for host defense against Cryptococcus neoformans. Eur. J. Immunol. 35870-878. [DOI] [PubMed] [Google Scholar]
- 6.Casanova, J. L., and L. Abel. 2007. Human genetics of infectious diseases: a unified theory. EMBO J. 26915-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cauley, L. K., and J. W. Murphy. 1979. Response of congenitally athymic (nude) and phenotypically normal mice to Cryptococcus neoformans infection. Infect. Immun. 23644-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang, Y. C., and K. J. Kwon-Chung. 1994. Complementation of a capsule-deficient mutation of Cryptococcus neoformans restores its virulence. Mol. Cell. Biol. 144912-4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang, Z. L., D. Netski, P. Thorkildson, and T. R. Kozel. 2006. Binding and internalization of glucuronoxylomannan, the major capsular polysaccharide of Cryptococcus neoformans, by murine peritoneal macrophages. Infect. Immun. 74144-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaturvedi, V., B. Wong, and S. L. Newman. 1996. Oxidative killing of Cryptococcus neoformans by human neutrophils. Evidence that fungal mannitol protects by scavenging reactive oxygen intermediates. J. Immunol. 1563836-3840. [PubMed] [Google Scholar]
- 11.Chen, G. H., D. A. McNamara, Y. Hernandez, G. B. Huffnagle, G. B. Toews, and M. A. Olszewski. 2008. Inheritance of immune polarization patterns is linked to resistance versus susceptibility to Cryptococcus neoformans in a mouse model. Infect. Immun. 762379-2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cox, G. M., H. C. McDade, S. C. Chen, S. C. Tucker, M. Gottfredsson, L. C. Wright, T. C. Sorrell, S. D. Leidich, A. Casadevall, M. A. Ghannoum, and J. R. Perfect. 2001. Extracellular phospholipase activity is a virulence factor for Cryptococcus neoformans. Mol. Microbiol. 39166-175. [DOI] [PubMed] [Google Scholar]
- 13.Cox, G. M., J. Mukherjee, G. T. Cole, A. Casadevall, and J. R. Perfect. 2000. Urease as a virulence factor in experimental cryptococcosis. Infect. Immun. 68443-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deane, J. A., and D. A. Fruman. 2004. Phosphoinositide 3-kinase: diverse roles in immune cell activation. Annu. Rev. Immunol. 22563-598. [DOI] [PubMed] [Google Scholar]
- 15.Diamond, R. D., R. K. Root, and J. E. Bennett. 1972. Factors influencing killing of Cryptococcus neoformans by human leukocytes in vitro. J. Infect. Dis. 125367-376. [DOI] [PubMed] [Google Scholar]
- 16.Dong, Z. M., and J. W. Murphy. 1997. Cryptococcal polysaccharides bind to CD18 on human neutrophils. Infect. Immun. 65557-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feldmesser, M., Y. Kress, P. Novikoff, and A. Casadevall. 2000. Cryptococcus neoformans is a facultative intracellular pathogen in murine pulmonary infection. Infect. Immun. 684225-4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feldmesser, M., S. Tucker, and A. Casadevall. 2001. Intracellular parasitism of macrophages by Cryptococcus neoformans. Trends Microbiol. 9273-278. [DOI] [PubMed] [Google Scholar]
- 19.Goldman, D. L., H. Khine, J. Abadi, D. J. Lindenberg, L. Pirofski, R. Niang, and A. Casadevall. 2001. Serologic evidence for Cryptococcus neoformans infection in early childhood. Pediatrics 107E66. [DOI] [PubMed] [Google Scholar]
- 20.Guillot, L., S. F. Carroll, M. Badawy, and S. T. Qureshi. 2008. Cryptococcus neoformans induces IL-8 secretion and CXCL1 expression by human bronchial epithelial cells. Respir. Res. 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guillot, L., S. Medjane, K. Le-Barillec, V. Balloy, C. Danel, M. Chignard, and M. Si-Tahar. 2004. Response of human pulmonary epithelial cells to lipopolysaccharide involves Toll-like receptor 4 (TLR4)-dependent signaling pathways: evidence for an intracellular compartmentalization of TLR4. J. Biol. Chem. 2792712-2718. [DOI] [PubMed] [Google Scholar]
- 22.Hayden, M. S., and S. Ghosh. 2008. Shared principles in NF-kappaB signaling. Cell 132344-362. [DOI] [PubMed] [Google Scholar]
- 23.Hernandez, Y., S. Arora, J. R. Erb-Downward, R. A. McDonald, G. B. Toews, and G. B. Huffnagle. 2005. Distinct roles for IL-4 and IL-10 in regulating T2 immunity during allergic bronchopulmonary mycosis. J. Immunol. 1741027-1036. [DOI] [PubMed] [Google Scholar]
- 24.Hill, J. O., and A. G. Harmsen. 1991. Intrapulmonary growth and dissemination of an avirulent strain of Cryptococcus neoformans in mice depleted of CD4+ or CD8+ T cells. J. Exp. Med. 173755-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoag, K. A., N. E. Street, G. B. Huffnagle, and M. F. Lipscomb. 1995. Early cytokine production in pulmonary Cryptococcus neoformans infections distinguishes susceptible and resistant mice. Am. J. Respir. Cell Mol. Biol. 13487-495. [DOI] [PubMed] [Google Scholar]
- 26.Huffnagle, G. B., M. B. Boyd, N. E. Street, and M. F. Lipscomb. 1998. IL-5 is required for eosinophil recruitment, crystal deposition, and mononuclear cell recruitment during a pulmonary Cryptococcus neoformans infection in genetically susceptible mice (C57BL/6). J. Immunol. 1602393-2400. [PubMed] [Google Scholar]
- 27.Huffnagle, G. B., and G. S. Deepe. 2003. Innate and adaptive determinants of host susceptibility to medically important fungi. Curr. Opin. Microbiol. 6344-350. [DOI] [PubMed] [Google Scholar]
- 28.Huffnagle, G. B., and M. F. Lipscomb. 1998. Cells and cytokines in pulmonary cryptococcosis. Res. Immunol. 149387-396. [DOI] [PubMed] [Google Scholar]
- 29.Huffnagle, G. B., and M. F. Lipscomb. 1992. Pulmonary cryptococcosis. Am. J. Pathol. 1411517-1520. [PMC free article] [PubMed] [Google Scholar]
- 30.Huffnagle, G. B., R. M. Strieter, L. K. McNeil, R. A. McDonald, M. D. Burdick, S. L. Kunkel, and G. B. Toews. 1997. Macrophage inflammatory protein-1alpha (MIP-1alpha) is required for the efferent phase of pulmonary cell-mediated immunity to a Cryptococcus neoformans infection. J. Immunol. 159318-327. [PubMed] [Google Scholar]
- 31.Huffnagle, G. B., R. M. Strieter, T. J. Standiford, R. A. McDonald, M. D. Burdick, S. L. Kunkel, and G. B. Toews. 1995. The role of monocyte chemotactic protein-1 (MCP-1) in the recruitment of monocytes and CD4+ T cells during a pulmonary Cryptococcus neoformans infection. J. Immunol. 1554790-4797. [PubMed] [Google Scholar]
- 32.Huffnagle, G. B., G. B. Toews, M. D. Burdick, M. B. Boyd, K. S. McAllister, R. A. McDonald, S. L. Kunkel, and R. M. Strieter. 1996. Afferent phase production of TNF-alpha is required for the development of protective T cell immunity to Cryptococcus neoformans. J. Immunol. 1574529-4536. [PubMed] [Google Scholar]
- 33.Huffnagle, G. B., T. R. Traynor, R. A. McDonald, M. A. Olszewski, D. M. Lindell, A. C. Herring, and G. B. Toews. 2000. Leukocyte recruitment during pulmonary Cryptococcus neoformans infection. Immunopharmacology 48231-236. [DOI] [PubMed] [Google Scholar]
- 34.Huffnagle, G. B., J. L. Yates, and M. F. Lipscomb. 1991. Immunity to a pulmonary Cryptococcus neoformans infection requires both CD4+ and CD8+ T cells. J. Exp. Med. 173793-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huffnagle, G. B., J. L. Yates, and M. F. Lipscomb. 1991. T cell-mediated immunity in the lung: a Cryptococcus neoformans pulmonary infection model using SCID and athymic nude mice. Infect. Immun. 591423-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Idnurm, A., Y. S. Bahn, K. Nielsen, X. Lin, J. A. Fraser, and J. Heitman. 2005. Deciphering the model pathogenic fungus Cryptococcus neoformans. Nat. Rev. Microbiol. 3753-764. [DOI] [PubMed] [Google Scholar]
- 37.Kwon-Chung, K. J., I. Polacheck, and T. J. Popkin. 1982. Melanin-lacking mutants of Cryptococcus neoformans and their virulence for mice. J. Bacteriol. 1501414-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levitz, S. M. 1994. Macrophage-cryptococcus interactions. Immunol. Ser. 60533-543. [PubMed] [Google Scholar]
- 39.Levitz, S. M., D. J. DiBenedetto, and R. D. Diamond. 1990. Inhibition and killing of fungi by the polyamine oxidase-polyamine system. Antifungal activity of the PAO-polyamine system. Antonie Van Leeuwenhoek 58107-114. [DOI] [PubMed] [Google Scholar]
- 40.Lin, X., and J. Heitman. 2006. The biology of the cryptococcus neoformans species complex. Annu. Rev. Microbiol. 6069-105. [DOI] [PubMed] [Google Scholar]
- 41.Liu, L., R. P. Tewari, and P. R. Williamson. 1999. Laccase protects Cryptococcus neoformans from antifungal activity of alveolar macrophages. Infect. Immun. 676034-6039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lynch, D. M., and P. H. Kay. 1995. Studies on the polymorphism of the fifth component of complement in laboratory mice. Exp. Clin. Immunogenet. 12253-260. [PubMed] [Google Scholar]
- 43.Maffei, C. M., L. F. Mirels, R. A. Sobel, K. V. Clemons, and D. A. Stevens. 2004. Cytokine and inducible nitric oxide synthase mRNA expression during experimental murine cryptococcal meningoencephalitis. Infect. Immun. 722338-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marroni, M., E. Pericolini, E. Cenci, F. Bistoni, and A. Vecchiarelli. 2007. Functional defect of natural immune system in an apparent immunocompetent patient with pulmonary cryptococcosis. J. Infect. 54e5-e8. [DOI] [PubMed] [Google Scholar]
- 45.Mednick, A. J., M. Feldmesser, J. Rivera, and A. Casadevall. 2003. Neutropenia alters lung cytokine production in mice and reduces their susceptibility to pulmonary cryptococcosis. Eur. J. Immunol. 331744-1753. [DOI] [PubMed] [Google Scholar]
- 46.Milam, J. E., A. C. Herring-Palmer, R. Pandrangi, R. A. McDonald, G. B. Huffnagle, and G. B. Toews. 2007. Modulation of the pulmonary T2 response to Cryptococcus neoformans by intratracheal delivery of a TNF alpha-expressing adenoviral vector. Infect. Immun. 754951-4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mody, C. H., M. F. Lipscomb, N. E. Street, and G. B. Toews. 1990. Depletion of CD4+ (L3T4+) lymphocytes in vivo impairs murine host defense to Cryptococcus neoformans. J. Immunol. 1441472-1477. [PubMed] [Google Scholar]
- 48.Monari, C., F. Bistoni, A. Casadevall, E. Pericolini, D. Pietrella, T. R. Kozel, and A. Vecchiarelli. 2005. Glucuronoxylomannan, a microbial compound, regulates expression of costimulatory molecules and production of cytokines in macrophages. J. Infect. Dis. 191127-137. [DOI] [PubMed] [Google Scholar]
- 49.Murphy, J. W. 1998. Protective cell-mediated immunity against Cryptococcus neoformans. Res. Immunol. 149373-386. [DOI] [PubMed] [Google Scholar]
- 50.Nakamura, K., K. Miyagi, Y. Koguchi, Y. Kinjo, K. Uezu, T. Kinjo, M. Akamine, J. Fujita, I. Kawamura, M. Mitsuyama, Y. Adachi, N. Ohno, K. Takeda, S. Akira, A. Miyazato, M. Kaku, and K. Kawakami. 2006. Limited contribution of Toll-like receptor 2 and 4 to the host response to a fungal infectious pathogen, Cryptococcus neoformans. FEMS Immunol. Med. Microbiol. 47148-154. [DOI] [PubMed] [Google Scholar]
- 51.Olszewski, M. A., G. B. Huffnagle, R. A. McDonald, D. M. Lindell, B. B. Moore, D. N. Cook, and G. B. Toews. 2000. The role of macrophage inflammatory protein-1 alpha/CCL3 in regulation of T cell-mediated immunity to Cryptococcus neoformans infection. J. Immunol. 1656429-6436. [DOI] [PubMed] [Google Scholar]
- 52.Perfect, J. R. 2005. Cryptococcus neoformans: a sugar-coated killer with designer genes. FEMS Immunol. Med. Microbiol. 45395-404. [DOI] [PubMed] [Google Scholar]
- 53.Retini, C., A. Vecchiarelli, C. Monari, C. Tascini, F. Bistoni, and T. R. Kozel. 1996. Capsular polysaccharide of Cryptococcus neoformans induces proinflammatory cytokine release by human neutrophils. Infect. Immun. 642897-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rhodes, J. C. 1985. Contribution of complement component C5 to the pathogenesis of experimental murine cryptococcosis. Sabouraudia 23225-234. [DOI] [PubMed] [Google Scholar]
- 55.Rhodes, J. C., L. S. Wicker, and W. J. Urba. 1980. Genetic control of susceptibility to Cryptococcus neoformans in mice. Infect. Immun. 29494-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rivera, J., and A. Casadevall. 2005. Mouse genetic background is a major determinant of isotype-related differences for antibody-mediated protective efficacy against Cryptococcus neoformans. J. Immunol. 1748017-8026. [DOI] [PubMed] [Google Scholar]
- 57.Rivera, J., O. Zaragoza, and A. Casadevall. 2005. Antibody-mediated protection against Cryptococcus neoformans pulmonary infection is dependent on B cells. Infect. Immun. 731141-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salkowski, C. A., and E. Balish. 1991. Cutaneous cryptococcosis in athymic and beige-athymic mice. Infect. Immun. 591785-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shao, X., A. Mednick, M. Alvarez, N. van Rooijen, A. Casadevall, and D. L. Goldman. 2005. An innate immune system cell is a major determinant of species-related susceptibility differences to fungal pneumonia. J. Immunol. 1753244-3251. [DOI] [PubMed] [Google Scholar]
- 60.Shoham, S., C. Huang, J. M. Chen, D. T. Golenbock, and S. M. Levitz. 2001. Toll-like receptor 4 mediates intracellular signaling without TNF-alpha release in response to Cryptococcus neoformans polysaccharide capsule. J. Immunol. 1664620-4626. [DOI] [PubMed] [Google Scholar]
- 61.Song, X., S. Tanaka, D. Cox, and S. C. Lee. 2004. Fc gamma receptor signaling in primary human microglia: differential roles of PI-3K and Ras/ERK MAPK pathways in phagocytosis and chemokine induction. J. Leukoc. Biol. 751147-1155. [DOI] [PubMed] [Google Scholar]
- 62.Traynor, T. R., A. C. Herring, M. E. Dorf, W. A. Kuziel, G. B. Toews, and G. B. Huffnagle. 2002. Differential roles of CC chemokine ligand 2/monocyte chemotactic protein-1 and CCR2 in the development of T1 immunity. J. Immunol. 1684659-4666. [DOI] [PubMed] [Google Scholar]
- 63.Traynor, T. R., W. A. Kuziel, G. B. Toews, and G. B. Huffnagle. 2000. CCR2 expression determines T1 versus T2 polarization during pulmonary Cryptococcus neoformans infection. J. Immunol. 1642021-2027. [DOI] [PubMed] [Google Scholar]
- 64.Tuite, A., and P. Gros. 2006. The impact of genomics on the analysis of host resistance to infectious disease. Microbes Infect. 81647-1653. [DOI] [PubMed] [Google Scholar]
- 65.Vecchiarelli, A., and A. Casadevall. 1998. Antibody-mediated effects against Cryptococcus neoformans: evidence for interdependency and collaboration between humoral and cellular immunity. Res. Immunol. 149321-333. [DOI] [PubMed] [Google Scholar]
- 66.Wiseman, J. C., L. L. Ma, K. J. Marr, G. J. Jones, and C. H. Mody. 2007. Perforin-dependent cryptococcal microbicidal activity in NK cells requires PI3K-dependent ERK1/2 signaling. J. Immunol. 1786456-6464. [DOI] [PubMed] [Google Scholar]
- 67.Wozniak, K. L., J. M. Vyas, and S. M. Levitz. 2006. In vivo role of dendritic cells in a murine model of pulmonary cryptococcosis. Infect. Immun. 743817-3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yauch, L. E., M. K. Mansour, S. Shoham, J. B. Rottman, and S. M. Levitz. 2004. Involvement of CD14, Toll-like receptors 2 and 4, and MyD88 in the host response to the fungal pathogen Cryptococcus neoformans in vivo. Infect. Immun. 725373-5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yuan, R., A. Casadevall, G. Spira, and M. D. Scharff. 1995. Isotype switching from IgG3 to IgG1 converts a nonprotective murine antibody to Cryptococcus neoformans into a protective antibody. J. Immunol. 1541810-1816. [PubMed] [Google Scholar]
- 70.Yuan, R. R., G. Spira, J. Oh, M. Paizi, A. Casadevall, and M. D. Scharff. 1998. Isotype switching increases efficacy of antibody protection against Cryptococcus neoformans infection in mice. Infect. Immun. 661057-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zaragoza, O., M. Alvarez, A. Telzak, J. Rivera, and A. Casadevall. 2007. The relative susceptibility of mouse strains to pulmonary Cryptococcus neoformans infection is associated with pleiotropic differences in the immune response. Infect. Immun. 752729-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.