Abstract
The ability of Staphylococcus aureus to invade and survive within host cells is believed to contribute to its propensity to cause persistent and metastatic infections. In addition, S. aureus infections often are associated with atopic diseases such as dermatitis, rhinitis, and asthma. Mast cells, the key cells of allergic diseases, have a pivotal role in innate immunity and have the capacity of phagocytosis, and they can destroy some pathogenic bacteria. However, little is known about the ability of some other bacteria to survive and overcome mast cell phagocytosis. Therefore, we were interested in evaluating the interplay between mast cells and S. aureus. In this study, we show that human cord blood-derived mast cells (CBMC) can be infected by pathogenic S. aureus. S. aureus displayed a high adherence to mast cells as well as invasive and survival abilities within them. However, when infections were performed in the presence of cytochalasin D or when CBMC were preincubated with anti-Toll-like receptor 2 (TLR2) or anti-CD48 antibodies, the invasiveness and the inflammatory response were abrogated, respectively. Furthermore, we observed an increase of TLR2 and CD48 molecules on CBMC after S. aureus infection. The infection of CBMC with S. aureus also caused the release of tumor necrosis factor alpha (TNF-α) and interleukin-8 (IL-8). Both live and killed S. aureus organisms were found to trigger TNF-α and IL-8 release by CBMC in a time-dependent manner. Cumulatively, these findings suggest that S. aureus internalizes and survives in mast cells. This may play an important role in infections and in atopic diseases associated with S. aureus.
Staphylococcus aureus, a gram-positive bacterium colonizing the human skin and nasopharynx, is one of the most important human pathogens, and it causes various superficial, systemic, and nosocomial infections (13). S. aureus infections often are followed by the bacterial invasion of the vascular system, leading to bacteremia and sepsis. The ability of S. aureus to be internalized by and survive within some host cells, such as endothelial cells, keratinocytes, and epithelial cells, may contribute to the development of persistent or chronic infections and may eventually lead to deeper tissue infections or dissemination (14, 15, 23). In particular, the invasion of vascular endothelial cells is thought to be a critical step in the development of metastatic infections in patients with S. aureus bacteremia, since it results in the upregulation of procoagulant activity, the expression of surface adhesion molecules, and the release of proinflammatory cytokines (36, 43). The colonization of human skin by S. aureus is also a characteristic feature of several inflammatory skin diseases, which often are followed by tissue invasion and severe cell damage in a fibronectin-binding protein (FnBP)-dependent manner (15, 27). S. aureus also can survive engulfment by professional phagocytes such as neutrophils and monocytes. In both of these cell types, S. aureus promptly escapes from the endosomes/phagosomes and proliferates within the cytoplasm (44). Furthermore, recent in vitro studies showed that S. aureus survived inside macrophages in a metabolically active form that did not affect the viability of these cells until the intracellular environment became suitable for escape. This finding suggests that the ability of S. aureus to survive phagocytosis in human macrophages contributes to the dissemination of the infection and may be detrimental to the host (16).
An interesting link has been shown between S. aureus and atopic diseases, such as dermatitis, rhinitis, and asthma (5), in which it has been hypothesized that S. aureus can exacerbate the immunoglobulin E (IgE)-mediated reactions. For example, studies have shown greater S. aureus colonization in the skin of patients with atopic eczema/dermatitis syndrome than in the skin of normal healthy subjects (7, 38). Moreover, in chronic rhinosinusitis, S. aureus enterotoxin B shifts the cytokine pattern toward Th2. S. aureus enterotoxin B also stimulates the production of interleukin-5 (IL-5) and induces polyclonal IgE production, which might contribute to severe inflammation via the activation of the mast cells (4, 12). IgE antibodies specific to the S. aureus superantigen are present in nasal polyp tissue, and their levels correlate with markers of eosinophil activation and recruitment (25).
Mast cells are known key effector cells of IgE-mediated hypersensitivity reactions. In addition, more recently it has been recognized that mast cells, being strategically stationed at sites exposed to the external environment, such as lung, skin, gastrointestinal, and urinogenital tracts, play a critical role in host defense as cardinal cells of innate immune response against infectious pathogens, including S. aureus (17, 28). Indeed, mast cells have been found to bind and internalize gram-positive and gram-negative bacteria (2, 18). Mast cells can recognize and attach to a wide variety of opsonized bacteria. For example, Salmonella enterica serovar Typhimurium coated with the iC3b fragment of complement is recognized through complement receptor 3 (CR3) on the mast cell membrane (9). In addition, mast cells express several Fcγ receptors that are involved in the binding of IgG-coated bacteria (9). Recently, various strains of Escherichia coli, Enterobacter cloacae, and Klebsiella pneumoniae were found to bind avidly to mouse bone marrow-derived mast cells in opsonin-dependent conditions, followed by their internalization within vacuoles (19). In vitro and in vivo studies have shown that mast cells release proinflammatory and chemotactic mediators upon contact with pathogens (2, 19, 21). For example, mast cell-derived tumor necrosis factor alpha (TNF-α) modulates neutrophil influx and bacterial clearance in Klebsiella pneumoniae infection (21).
Immune cells, including mast cells, express pattern recognition receptors that recognize pathogen-associated molecular patterns and Toll-like receptors (TLRs), a family of proteins that resemble the antimicrobial Toll proteins of Drosophila (26, 35). The involvement of TLRs has been implicated in the host response to several staphylococcal infection models (8, 41). TLR2, expressed on mast cells, has been found to recognize and respond to several pathogen-associated molecular patterns, including peptidoglycan (PGN), lipoproteins, and lipoteichoic acid (37). PGN from S. aureus stimulates mast cells in a TLR2-dependent manner to produce TNF-α, IL-4, IL-5, IL-6, and IL-13 (40). The intradermal injection of PGN also led to increased vasodilatation and inflammation through the TLR2-dependent activation of mast cells (40). Furthermore, in response to PGN or other TLR2 activators, mast cell degranulation has been attributed a critical role in exacerbating allergic diseases, especially atopic dermatitis (5).
Another important molecule in bacterium-mast cell interaction is CD48, a glycosylphosphatidylinositol-anchored protein (39). The cleavage of CD48 from the mast cell surface with phospholipase C or its neutralization with CD48-specific antibodies prevented subsequent bacterial adherence (22). The engagement of CD48 by FimH-expressing type 1 fimbriated Escherichia coli also was found to trigger TNF-α release by mast cells (22).
In spite of all the aforementioned evidence of an interplay between S. aureus and mast cells and of its importance in the dynamics of an allergic disease concomitant to infection, the direct cross-talk between human mast cells and S. aureus has not been studied in depth. Therefore, we decided in the present study to analyze the interactions between human cord blood-derived mast cells (CBMC) and S. aureus.
We report here that, in vitro, S. aureus adheres to, invades, survives in, and stimulates human mast cells with the involvement of CD48 and TLR2.
MATERIALS AND METHODS
Bacterial culture conditions.
Staphylococcus aureus ATCC 25923 and S. aureus HUH1, a methicillin-susceptible strain isolated from a patient with a bloodstream infection in the Department of Clinical Microbiology and Infectious Diseases at the Hadassah University Hospital, were used. Bacteria maintained at −70°C in skim milk-glycerol (Difco) were subcultured onto blood agar and incubated at 37°C overnight. A sweep of colonies was inoculated into tryptic soy broth (TSB; Difco) and incubated at 37°C for 24 h. The culture density was estimated by the measurement of the optical density at 650 nm. Bacteria were diluted to achieve a multiplicity of infection (MOI) of 10 before use. In some experiments, S. aureus was heat killed (for 30 min at 60°C). After treatment, bacteria were washed three times with phosphate-buffered saline (PBS) prior to use.
Labeling of the bacteria.
Bacteria (109 ml−1) were washed twice with PBS, suspended in fluorescein isothiocyanate (FITC; 1 μg ml−1; Sigma, Israel) dissolved in PBS, and incubated for 30 min under constant shaking at 37°C. FITC-labeled bacteria were washed three times with PBS prior to use. In some experiments, sulfo-N-hydroxysuccinimide-hexanoate linker chain-biotin (Perbio Science, Germany) was dissolved at a final concentration of 1 μg ml−1 in PBS. Identical volumes of bacteria in FITC-PBS and biotin were combined and further incubated under the same conditions as those described above (1).
CBMC.
CBMC were obtained by culturing umbilical cord blood mononuclear cells as previously described (3). Briefly, fresh cord blood was diluted with Hank's balanced salt solution, loaded onto Ficoll-Paque, and centrifuged (350 × g for 25 min). Mononuclear cells were washed twice with Hank's balanced salt solution and resuspended in 100 ml minimal essential medium alpha (MEM-α) containing 10% (vol/vol) fetal calf serum (FCS), penicillin (100 U ml−1), streptomycin (100 μg ml−1), ribonucleosides/deoxyribonucleosides, and stem cell factor (100 ng ml−1) (a gift from Amgen). Culture medium was replaced weekly. CBMC were used after 8 to 12 weeks of culture, when >97% were positive for tryptase as assessed by intracellular flow cytometry. Cord blood was obtained according to the Institutional Helsinki Committee guidelines of Hadassah Hospital, and its use was approved by the committee.
Adherence assay.
For quantitative adhesion assays, bacterial suspensions were incubated with CBMC (2.5 × 105 ml−1) in 48-well tissue culture plates (Nunc, Denmark). After incubation periods of 30, 60, 120, and 180 min at 37°C, S. aureus-associated CBMC were washed three times with PBS, lysed with 0.1% Triton X-100 in PBS, diluted, and cultured onto blood agar at 37°C for 24 h for bacterial viable counting (in CFU per milliliter). Bacterial inocula corresponded to the number of viable bacterial cells in the supernatant at each time point plus the number of viable bacteria associated with CBMC (i.e., intracellular plus extracellular bacteria) (34). In order to differentiate between extracellular and intracellular bacteria, CBMC were grown for 24 h on circular glass coverslips coated with 0.01% poly-l-lysine solution (Sigma, Israel) on 48-well tissue culture plates (Nunc, Denmark). Biotinylated and FITC-labeled S. aureus (MOI of 10) cells were incubated with CBMC (2.5 × 105 ml−1) for 180 min at 37°C. After incubation, infected cells were fixed with 4% paraformaldehyde in PBS, washed three times with PBS, and stained with streptavidin-allophycocyanin (BD Biosciences Pharmingen) diluted 1:200 in PBS. Samples were evaluated using an LSM 510 confocal laser-scanning microscope (Zeiss, Germany) (1).
Invasion and intracellular viability assays.
The ability of S. aureus to invade CBMC was assessed by the gentamicin protection assay (34). Briefly, after 180 min of incubation, S. aureus-associated cells were washed three times with PBS and subsequently incubated with MEM-α containing gentamicin (300 μg ml−1). The wells then were incubated for 30 min at 37°C and washed three times with PBS. Cell lysis was carried out as described for the quantitative adhesion assay, and the lysates (100 μl) from each well were diluted and plated onto blood agar for the determination of viable intracellular bacteria (IC). In preliminary experiments, S. aureus strains were tested for gentamicin sensitivity; no colonies were present in blood agar after 180 min of incubation with 300 μg ml−1 of gentamicin in MEM-α medium. The internalization assay also was carried out by flow cytometric analysis (i.e., fluorescence-activated cell sorting [FACS]) (32), and CBMC were incubated with killed or live FITC-labeled S. aureus. After 180 min, the S. aureus-associated CBMC were washed twice with ice-cold flow buffer (PBS containing 1% FCS) and resuspended in flow buffer. The samples were kept in the dark on ice until the analysis. To eliminate signals from extracellular bacteria, trypan blue solution (0.4%; Sigma, Israel) was added to a final concentration of 0.2% directly before analysis. Samples were analyzed using a Becton-Dickinson FACSCalibur and Cell Quest software. To assess the amount of internalized bacteria, the percentage of FITC-positive cells was multiplied by the mean fluorescence intensity of these cells to obtain the uptake index (u.i.). No toxic effect was detected toward S. aureus strains during FITC or biotin labeling. Furthermore, to check the validity of the quenching approach, FITC-labeled S. aureus alone with or without trypan blue treatment was analyzed by flow cytometry. In previous experiments, the FITC-labeled S. aureus signal was abrogated after trypan blue treatment. To observe intracellular killing or bacterial survival, the experimental design of the protection antibiotic assay was changed. CBMC were infected with S. aureus for 180 min, and at this point the medium was replaced with MEM-α containing 300 μg ml−1 gentamicin. Instead of analyzing all samples after 30 min of gentamicin treatment, samples were taken out after 30, 60, 120, and 180 min. The number of viable bacteria at 30 min was considered 100%.
Cytochalasin D treatment.
To study the role of the cytoskeleton in S. aureus uptake, CBMC were incubated with cytochalasin D (2.5 to 5 μg ml−1; Sigma, Israel) for 60 min at 37°C before S. aureus interaction and also during the entire gentamicin protection assay, as described above. In preliminary experiments, cytochalasin D was assessed for toxicity for both S. aureus strains and CBMC and was found either to have no adverse effects or to change adherence and invasion properties at concentrations of up to 5 μg ml−1.
CD48- and TLR2-mediated inhibition invasion assay.
The involvement of CD48 and TLR2 molecules in CBMC during exposure to S. aureus also was investigated. CBMC were incubated with anti-human CD48 (MEM-102; 10 μg ml−1; Santa Cruz Biotechnology), anti-human TLR2 (clone TL2.1; 10 μg ml−1; Ebioscience), or isotype-matched IgG1 (MP Biomedicals, Germany) at room temperature for 30 min before the adherence and internalization assays were performed.
TLR2 and CD48 expression.
To analyze the expression of TLR2 and CD48 on CBMC surfaces, killed or live S. aureus-associated CBMC were incubated with anti-human TLR2 (clone TL2.1; 10 μg ml−1; Ebioscience), anti-human CD48 (MEM-102; 10 μg ml−1; Santa Cruz Biotechnology), or isotype-matched IgG1 (MP Biomedicals, Germany) and were labeled with FITC-goat anti-mouse IgG (1:100; Santa Cruz Biotechnology). Cells were incubated with the antibodies at room temperature for 30 min. Analysis was performed by FACS.
Evaluation of cytokine concentration.
CBMC suspensions were analyzed for S. aureus-specific IL-8 and TNF-α cytokine production. CBMC were incubated with killed or live S. aureus at different time points at 37°C. The levels of each cytokine in culture supernatants were determined by using specific enzyme-linked immunosorbent assay kits according to the manufacturer's instructions (R&D Systems). The results are expressed as the concentration of cytokine per 106 CBMC, as extrapolated from a standard curve with recombinant cytokine. To define the possible role of S. aureus invasion in IL-8 and TNF-α release, CBMC were treated with cytochalasin D for 60 min before infection. To rule out the possibility that cytochalasin D treatment affected the capacity of CBMC to release IL-8 and TNF-α, cytochalasin D-treated cells (5 μg ml−1) and nontreated cells were incubated with PGN (10 μg m−1; Sigma, Israel), a well-known TLR2 agonist and cell activator. To study the possible role of TLR2 and CD48 molecules on S. aureus-induced IL-8 and TNF-α release, CBMC were treated with anti-human CD48 (10 μg ml−1), anti-human TLR2 (10 μg ml−1), both antibodies, or isotype-matched IgG1 for 60 min before infection.
Statistical analysis.
All assays were performed in triplicate and repeated at least three times. Results are presented as the means ± standard deviations. The Student's t test or analysis of variance followed by the Tukey-Kramer multiple comparison test were used to compare means, with a P ≤ 0.05 considered statistically significant.
RESULTS
S. aureus invades and survives in CBMC.
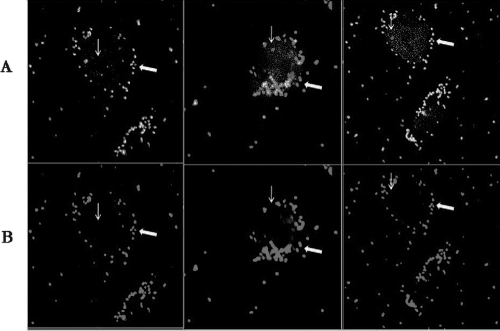
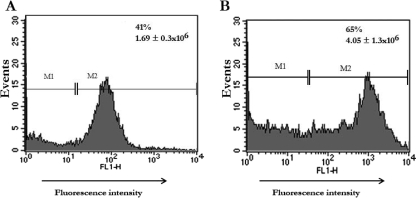
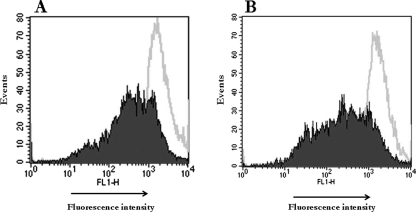
It has been recognized that adherence to and invasion in host cells are important steps in the pathogenesis of many bacteria, including that of S. aureus (15). As shown in Table 1, S. aureus strains quickly adhered to CBMC (within 30 min). The number of adherent bacteria increased steadily with time similarly for both strains. In fact, at 30 min, 9.86 (strain 25923) and 10.66% (HUH1) of the bacteria adhered to CBMC, whereas at 180 min we found 41.14 and 34.29% adherent bacteria, respectively. To check whether bacterial internalization takes place, CBMC were incubated with biotinylated and FITC-labeled S. aureus. Confocal microscopy clearly differentiated between several small clusters of extracellular bacteria adhering to the CBMC surface and small clusters of intracellular bacteria (Fig. 1). In addition, the antibiotic protection assay detected viable internalized bacteria at 30 min postinfection. The percentage of internalized bacteria increased with time, with a mean of 0.09 and 1.49% for the HUH1 strain and 0.06 and 1.36% for strain 25923 after 30 and 180 min, respectively (Table 1). Furthermore, we compared the internalization of live bacteria to that of heat-killed bacteria by CBMC at 180 min. FACS analysis (Fig. 2) revealed that approximately 60% more S. aureus cells were internalized by CBMC when the bacteria were live than when they were heat killed (u.i., 4.05 × 106 ± 1.3 × 106 for live and 1.69 × 106 ± 0.3 × 106 for heat killed [P < 0.001]). Furthermore, FACS analysis detected approximately 65 and 41% of the cell population with a strong fluorescent signal when CBMC were incubated with live or heat-killed S. aureus, respectively. Since fluorescence emitted by extracellular bacteria and CBMC-attached bacteria was quenched by trypan blue, this strong fluorescent signal from CBMC suggests the presence of intracellular bacteria.
TABLE 1.
Staphylococcus aureus viable bacteria associated with and internalized by CBMCa
| Infection period (min) | ATCC 25923
|
HUH1
|
||
|---|---|---|---|---|
| No. (%) of associated cells | No. (%) of internalized cells | No. (%) of associated cells | No. (%) of internalized cells | |
| 30 | 3.38 × 105 ± 1.08 × 105 (9.87) | 2.87 × 103 ± 4.78 × 103 (0.06) | 4.18 × 105 ± 4.76 × 105 (10.66) | 3.25 × 103 ± 2.88 × 103 (0.09) |
| 60 | 1.07 × 106 ± 4.93 × 106 (14.53) | 1.43 × 104 ± 3.69 × 104 (0.23) | 1.78 × 106 ± 4.98 × 106 (19.87) | 1.91 × 104 ± 5.45 × 104 (0.26) |
| 120 | 2.47 × 106 ± 7.02 × 106 (22.12) | 4.31 × 104 ± 1.31 × 104 (0.49) | 4.89 × 106 ± 1.19 × 106 (28.96) | 8.17 × 104 ± 4.84 × 104 (0.66) |
| 180 | 7.69 × 106 ± 1.29 × 106 (41.14) | 1.52 × 105 ± 3.65 × 105 (1.36) | 1.20 × 107 ± 4.08 × 107 (34.29) | 3.48 × 106 ± 1.96 × 106 (1.49) |
S. aureus strains (MOI of 10) were incubated for up to 180 min with CBMC (2.5 × 105 ml−1) at 37°C and analyzed by quantitative adherence and antibiotic protection assays. The numbers are given as absolute values, and numbers in parentheses are the percentages of the recovered bacteria compared to the total number of bacteria in the inocula (i.e., the number of viable bacterial cells in the supernatant at each time point plus the number of viable bacteria associated with CBMC [intracellular plus extracellular] or plus the number of intracellular bacteria only). Data are means ± standard deviations from three experiments performed in triplicate.
FIG. 1.
S. aureus invades CBMC. CBMC were incubated with biotinylated and FITC-labeled S. aureus (MOI of 10) for 180 min at 37°C. After infection, samples were fixed with 4% paraformaldehyde in PBS and stained with streptavidin-allophycocyanin. Samples were evaluated by confocal microscopy. (A) CBMC and small clusters of extracellular bacteria (arrow) plus intracellular bacteria (thin arrow). (B) CBMC and small clusters of extracellular bacteria.
FIG. 2.
FACS analysis of S. aureus internalization. The FACS of CBMC in the presence of trypan blue discriminates cell-associated extracellular bacteria from intracellular bacteria. CBMC were incubated with killed FITC-labeled S. aureus (A) or live FITC-labeled S. aureus (B) for 180 min at 37°C. After the addition of trypan blue, samples were analyzed by FACS. Data are from a representative experiment (n = 3) that depicts the percentage of CBMC intracellular S. aureus as well as the percentage of the u.i. M1 is the marker set at approximately 95% of the total events using nonlabeled S. aureus.
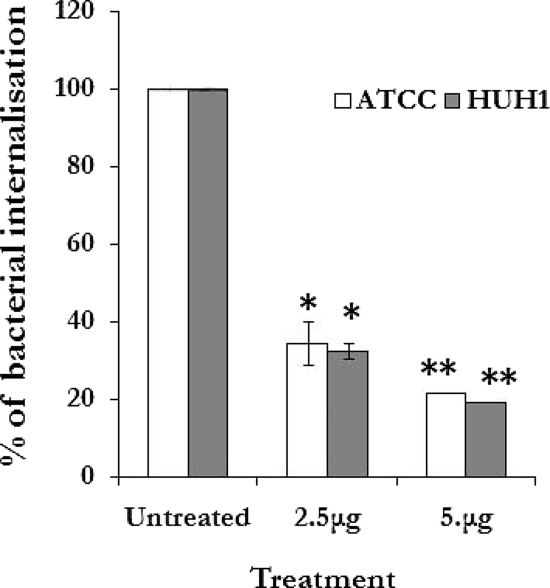
To assess the role of the cytoskeleton on S. aureus internalization by CBMC, the infections were carried out in the presence of cytochalasin D (2.5 and 5 μg ml−1). The data presented in Fig. 3 show that cytochalasin D inhibited S. aureus internalization by up to 80% (P < 0.001).
FIG. 3.
Cytochalasin D inhibits S. aureus invasion of CBMC. CBMC treated with cytochalasin D for 60 min before infection were incubated with S. aureus strains (MOI of 10) for 180 min at 37°C, and the percentage of internalized bacteria was evaluated. Results are the means (± standard deviations) from three independent experiments (* and **, P < 0.001 compared to results for untreated cells).
To evaluate whether intracellular S. aureus is killed by CBMC, after 180 min of bacterium-cell interaction, the CBMC were incubated with gentamicin at different time points. Surprisingly, an increase of bacterial survival was found (∼57% increase) for both strains after 180 min compared to the percentage of recovered bacteria after 30 min (100% of viable bacteria recovery) (Table 2). This is consistent with an increased survival of bacteria after being internalized by CBMC.
TABLE 2.
S. aureus intracellular survival after internalization by CBMCa
| Gentamicin treatment (min) | No. (%) of viable bacteria recovered
|
|
|---|---|---|
| ATCC 25923 | HUH1 | |
| 30 | 1.32 × 105 ± 1.03 × 105 (100) | 2.63 × 105 ± 0.32 × 105 (100) |
| 60 | 1.44 × 105 ± 3.21 × 105 (109) | 2.97 × 105 ± 1.25 × 105 (113) |
| 120 | 1.63 × 105 ± 2.37 × 105 (124) | 3.44 × 105 ± 0.05 × 105 (131) |
| 180 | 1.94 × 105 ± 0.45 × 105 (147) | 4.29 × 105 ± 0.62 × 105 (157) |
CBMC (2.5 × 105 ml−1) were incubated with S. aureus strains (MOI of 10) for 180 min at 37°C. After infection, CBMC were incubated with gentamicin (300 μg ml−1) for the indicated periods of time, and the number of CFU protected from gentamicin killing was counted (calculated as described in footnote a of Table 1). Data are means ± standard deviations from three experiments performed in triplicate.
S. aureus stimulates TLR2 and CD48 expression on CBMC surface.
Uninfected CBMC expressed low levels of TLR2 and CD48 on the cell surface (Fig. 4A, B). However, after infection with S. aureus, an increase of TLR2 and CD48 molecule expression on the CBMC surface was detected. The increase of these molecules was evident at 30 min and was augmented at 180 min after S. aureus invasion and survival (Fig. 4). Infected CBMC incubated with isotype-matched IgG1 expressed low levels of TLR2 and CD48 that were similar to those expressed by uninfected CMBC. These results indicate that S. aureus is capable of upregulating TLR2 and CD48 on CBMC in a time-dependent fashion. To determine whether TLR2 expression induced in response to S. aureus is dependent on bacterial viability, CBMC were incubated with killed S. aureus. Killed S. aureus did not result in the stimulation of TLR2 expression on CBMC (data not shown).
FIG. 4.
Invasive S. aureus upregulates TLR2 and CD48 molecules on CBMC. CBMC were incubated in medium or infected with S. aureus (MOI of 10) for 180 min at 37°C. Cells then were stained with anti-TLR2 (A) or anti-CD48 antibody (B) and analyzed by FACS. Noninfected cells are indicated by the shaded areas, and the infected ones are indicated by the white areas. These results are representative of three independent experiments.
CD48 and TLR2 molecules are involved in S. aureus internalization by CBMC.
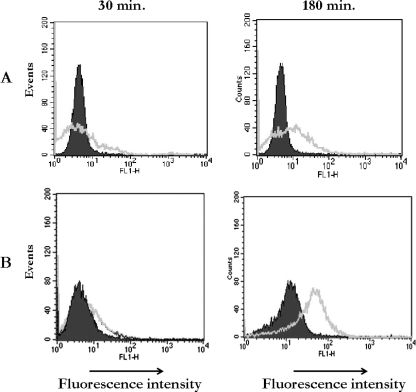
It has been recognized that CD48 and TLR2 are involved in bacterium-mediated cell activation (22, 37). Therefore, CBMC were preincubated with TLR2- and CD48-neutralizing antibodies. As detected by FACS and by the antibiotic protection assay (Fig. 5A, B), anti-TLR2 and anti-CD48 neutralizing antibodies decreased S. aureus internalization by CBMC by approximately 40% compared to S. aureus internalization by nontreated CBMC (for anti-TLR2, 4.38 × 106 ± 1.4 × 106 IC for isotype-treated CBMC and 1.53 × 106 ± 1.7 × 106 IC for pretreated CBMC; for anti-CD48, 3.72 × 106 ± 0.3 × 106 IC for isotype-treated CBMC and 1.62 × 106 ± 1.8 × 106 IC for pretreated CBMC [P < 0.001]). In contrast, S. aureus adherence was not affected by the neutralizing antibody treatment (4.25 × 107 ± 0.2 × 107 adherent bacteria for non-pretreated CBMC and 4.43 × 107 ± 1.6 × 107 adherent bacteria for pretreated CBMC; P > 0.05%) (data not shown).
FIG. 5.
TLR2 and CD48 receptors are involved in S. aureus uptake by CBMC. CBMC were preincubated with anti-human TLR2 (A) or anti-human CD48 (B) neutralizing antibody before incubation with S. aureus (MOI of 10) for 180 min at 37°C. After infection, cells were incubated with gentamicin for 60 min to kill extracellular bacteria, and the samples were analyzed by FACS. Pretreated CBMC are indicated by the shaded areas in the histograms. These results are representative of three independent experiments.
S. aureus induces IL-8 and TNF-α release by CBMC.
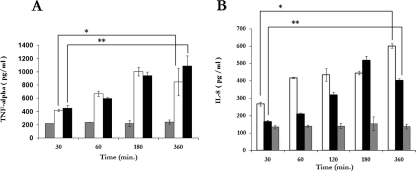
The infection of CBMC with S. aureus induced IL-8 and TNF-α release. Time course experiments showed that there was a positive correlation between IL-8 and TNF-α and the duration of S. aureus-CBMC interaction (for IL-8, 266.67 ± 11.11 pg at 30 min and 600.96 ± 12.40 pg at 360 min; for TNF-α, 420 ± 14.14 pg at 30 min and 1,145.5 ± 265.06 pg at 360 min [P < 0.001]) (Fig. 6). Furthermore, noninfected CBMC released 200.5 ± 33.23 pg of TNF-α (Fig. 6A) and 150.54 ± 14.53 pg of IL-8 (Fig. 6B) after 360 min of incubation.
FIG. 6.
S. aureus induces TNF-α and IL-8 release by CBMC. The kinetics of TNF-α (A) and IL-8 (B) release by uninfected CBMC ( ) and by CBMC following exposure to live (□) and killed (▪) S. aureus strains (MOI of 10) at 37°C for the indicated periods is shown. The values are the means ± standard deviations from three independent experiments (* and **, P < 0.001).
) and by CBMC following exposure to live (□) and killed (▪) S. aureus strains (MOI of 10) at 37°C for the indicated periods is shown. The values are the means ± standard deviations from three independent experiments (* and **, P < 0.001).
To determine whether proinflammatory cytokine release was dependent on bacterial viability, CBMC were incubated with killed S. aureus. Killed S. aureus preserved the ability to adhere to and trigger IL-8 and TNF-α release from CBMC in a time-dependent manner (for IL-8, 164.51 ± 9.49 pg at 30 min and 401.40 ± 11.38 pg at 360 min; for TNF-α, 450.5 ± 42.42 pg at 30 min and 1,060 ± 155.56 pg at 360 min [P < 0.001]) (Fig. 6).
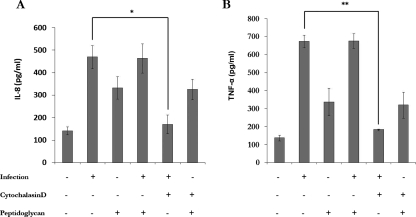
We next evaluated whether proinflammatory cytokine release was dependent on S. aureus uptake. Therefore, the S. aureus-CBMC interaction was performed in the presence of cytochalasin D. The results in Fig. 7 show that in this case, IL-8 (Fig. 7A) and TNF-α (Fig. 7B) release was drastically decreased compared to IL-8 and TNF-α release by infected CBMC without cytochalasin D treatment (P < 0.01 and P < 0.001, respectively). The cytochalasin D treatment did not influence the PGN-induced IL-8 and TNF-α release by CBMC, and no synergistic effect was observed when PGN was incubated with infected CBMC (Fig. 7).
FIG. 7.
IL-8 (A) and TNF-α (B) release by CBMC is dependent on S. aureus internalization. CBMC were left uninfected, infected with S. aureus at 37°C for 180 min in the presence or absence of cytochalasin D, or incubated with PGN (10 μg m−1) at 37°C for 180 min in the presence or absence of cytochalasin D. Supernatants were analyzed for IL-8 and TNF-α. The values are the means ± standard deviations from three independent experiments (*, P < 0.01; **, P < 0.001).
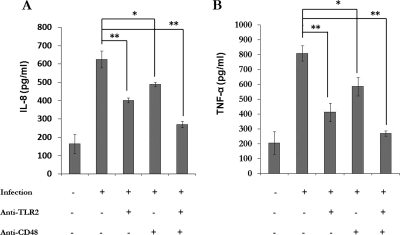
We also investigated the role of TLR2 and CD48 molecules on S. aureus-induced cytokine release. CBMC were incubated with anti-human TLR2 and anti-human CD48 antibodies or both antibodies before infection (Fig. 8). Anti-TLR2 significantly reduced the IL-8 (Fig. 8A) and TNF-α (Fig. 8B) release by CBMC (P < 0.001). Anti-CD48 also decreased the cytokine release (P < 0.01). Furthermore, the combination of these antibodies had a significant synergistic effect (P < 0.001). The amount of IL-8 and TNF-α released by CBMC incubated with isotype-matched IgG was similar to that of infected cells (for IL-8, 602 ± 32.72 pg and 624.94 ± 45.94 pg; for TNF-α, 787.51 ± 56 pg and 808.61 ± 11.95 pg).
FIG. 8.
Role of TLR2 and CD48 in IL-8 (A) and TNF-α (B) release by CBMC. CBMC were left uninfected or were infected with S. aureus at 37°C for 180 min in the presence or absence of anti-TLR2 and CD48 antibodies. Supernatants were analyzed for IL-8 and TNF-α. The values are the means ± standard deviations from three independent experiments. *, P < 0.01; **, P < 0.001.
DISCUSSION
This study provides, for the first time, evidence of a direct in vitro interaction between bacterial pathogens such as S. aureus and human mast cells. In fact, we have demonstrated that human CBMC recognize S. aureus and respond to its infection by releasing proinflammatory cytokines. S aureus displayed a high adherence to, as well as invasive and survival abilities in, the mast cells. FACS analysis showed the percentage of internalized live S. aureus to be higher than that of the antibiotic protection assay at the same time point (30%; P < 0.01) (data not shown). This can be attributable to differences in bioassays. FACS analysis detects both live and killed internalized bacteria, while the antibiotic protection assay detects only live bacteria (32). FACS analysis also detected the uptake of heat-killed S. aureus, suggesting that the mast cells have phagocytic ability. Although there are several previous reports indicating that mast cells have the capacity to recognize, phagocytize, and destroy different bacterial species and, thus, play a potentially crucial role in innate immunity (18, 19, 28), in some cases mast cells can be both infected and eventually destroyed by the pathogenic bacteria. Because the percentage of bacterial recovery after 180 min of gentamicin treatment was higher than that after 30 min, it is suggested that internalized S. aureus not only survived but also multiplied within the mast cells. To the best of our knowledge, this is the first report showing in vitro S. aureus survival and proliferation within human mast cells. In fact, Arock et al. (2) reported an appreciable and time-dependent decrease in the viability of S. aureus (CI127 strain) associated with mast cells. This discrepancy may be due to genetic and virulence differences among S. aureus strains.
The mechanisms underlying bacterial entry, phagosome maturation, and dissemination include strategies, as well as unique tactics, evolved by individual species to establish infection. Invasive bacteria actively induce their own uptake by phagocytosis in normally nonprofessional phagocytic cells and then either establish a protected niche within which they survive and replicate or disseminate from cell to cell by means of an actin-based motility process. Therefore, the uptake of bacteria by mast cells may account for the virulence of S. aureus in diseases, especially those associated with atopy in which there is an increase in mast cell numbers. This organism also may use mast cells to establish inflammatory responses and metastatic foci of infection.
Our data also demonstrate that S. aureus-infected mast cells evoke significant IL-8 and TNF-α release. Mast cell-derived TNF-α and IL-8 have been shown to be responsible for neutrophil recruitment. TNF-α is of particular interest, because mast cells are the only known cells to store this cytokine and, thus, are able to release this mediator immediately upon activation (21). Furthermore, the mast cell enhancement of early neutrophil recruitment is considered a potential mechanism of host defense during bacterial infection (20, 21). Therefore, mast cells also may contribute to immunity against S. aureus infection through this mechanism. Killed S. aureus also was able to trigger proinflammatory cytokine release from the mast cells. There are several reports showing the ability of commercial purified PGN and lipoteichoic acid to induce proinflammatory cytokine release by different types of cells (8, 11, 37, 40). Since heat-killing treatment preserves the integrity of bacteria, it is not surprising that killed S. aureus also preserves this ability. These data also suggest that cytokine release by the mast cells does not require an active bacterial infection. This is particularly interesting because during an infection, bacteria can be killed and release several surface molecules, like PGN and lipoteichoic acid, that can activate mast cells and trigger an inflammatory response (8, 10). We can hypothesize that these data are particularly relevant to atopic dermatitis, in which the interactions of mast cells with products from S. aureus could be critical in the local enhancement of chronic inflammation at specific skin sites (24). In previous works it was reported that S. aureus components may lead to an increase in the release of Th2-related cytokines (4, 42, 48). Specifically, it was shown that PGN may increase the release of IL-5, IL-10, and IL-13 from human mast cells (48). However, the influence of other S. aureus components on human mast cell degranulation and Th2-related cytokine synthesis and release requires further study.
Several glycosylphosphatidylinositol-anchored proteins are located in the lipid rafts of the mast cell plasma membrane. CD48 is one protein that has been implicated specifically in bacterial adherence, invasion, and/or bacterium-mediated cell activation (9, 20, 22). Moreover, we have recently shown a selective increase of the expression of CD48 both in human and in murine allergic asthma. Its selective downregulation by the use of CD48-neutralizing antibodies in murine asthma resulted in an amelioration of the disease (29, 30). Taken together, these data suggest an important link between CD48 and bacterial infection and allergy. TLR2 is another important receptor on the mast cells, and it is involved in the recognition of lipoproteins, lipoteicoic acid, and PGN from gram-positive bacteria (40). Our results show that CD48- and TLR2-neutralizing antibodies highly decrease the S. aureus internalization by the mast cells, indicating the involvement of these two molecules in S. aureus internalization. Furthermore, we found that the S. aureus infection upregulated the expression of TLR2 and CD48 on the mast cell surface. Therefore, it is possible to speculate that, in vivo, invasive S. aureus could prime mast cells to be more responsive to CD48- and TLR2-specific ligands. CD48 also has been implicated in Mycobacterium tuberculosis internalization by rat peritoneal mast cells and the adherence of FimH-expressing type 1 fimbriated E. coli on the mast cell surface (22, 31). In addition, our data are in accordance with recent reports that have demonstrated that Porphyromonas gingivalis, Mycobacterium avium, and Klebsiella pneumoniae invade endothelial cells, macrophages, and airway epithelial cells as well as upregulate TLR expression and elicit an inflammatory response (33, 45, 47). Interestingly, TLR2 could be involved in S. aureus survival in the mast cells. The reason for this speculation comes from two recent reports showing that S. aureus and P. gingivalis were cleared in TLR2-deficient macrophages more rapidly than in the wild type (6, 46). However, it remains to be clarified whether the TLR2-mediated survival of internalized bacteria is accomplished as a part of the host immune response or as a bacterial strategy to evade, survive, and spread in the host organism and whether this mechanism also is observed in mast cells. Although our data suggest that TLR2 and CD48 are involved in S. aureus internalization, neutralizing antibodies to these two receptors did not completely abolish the bacterial internalization. This finding reinforces the complex multifactorial nature of S. aureus interaction with host cells.
In summary, we have demonstrated in vitro that S. aureus has invasive and survival abilities in human mast cells, and together these abilities upregulate its proinflammatory potential. Three observations support this statement: (i) internalized bacteria induced IL-8 and TNF-α release; (ii) the cytochalasin D treatment decreased the bacterial internalization and, consequently, the inflammatory response; and (iii) anti-human TLR2 and anti-human CD48 antibodies inhibited the inflammatory response and S. aureus internalization by the mast cells.
Our data strengthen the previous observations of the worsening of allergic diseases concomitantly with S. aureus infections and indicate novel therapeutical approaches.
Acknowledgments
This project was supported by a grant from the Israel Science Foundation (no. 213105) and of the Aimwell Charitable Trust (United Kingdom). C. M. Rocha-de-Souza's work on this project was supported by the Valazzi-Pikovsky Fellowship Fund (The Hebrew University of Jerusalem). F. Levi-Schaffer is affiliated with the David R. Bloom Center of Pharmacy and the Adolph and Klara Brettler Center for Research in Molecular Pharmacology and Therapeutics at The Hebrew University of Jerusalem.
The authors have no financial conflicts of interest.
Editor: B. A. McCormick
Footnotes
Published ahead of print on 21 July 2008.
REFERENCES
- 1.Agerer, F., S. Waeckerle, and C. R. Hauck. 2004. Microscopic quantification of bacterial invasion by a novel antibody-independent staining method. J. Microbiol. Methods. 5923-32. [DOI] [PubMed] [Google Scholar]
- 2.Arock, M., E. Ross, R. Lai-Kuen, G. Averlant, Z. Gao, and S. N. Abraham. 1998. Phagocytic and tumor necrosis factor alpha response of human mast cells following exposure to gram-negative and gram-positive bacteria. Infect. Immun. 666030-6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachelet, I., A. Munitz, D. Mankuta, and F. Levi-Schaffer. 2006. Mast cell costimulation by CD226/CD112 (DNAM-1/Nectin-2): a novel interface in the allergic process. J. Biol. Chem. 28127190-27196. [DOI] [PubMed] [Google Scholar]
- 4.Bachert, C., N. Zang, J. Patou, T. Van Zele, and P. Gevaert. 2008. Role of staphylococcal superrantigens in upper airway disease. Curr. Opin. Allergy. Clin. Immunol. 834-38. [DOI] [PubMed] [Google Scholar]
- 5.Baker, B. S. 2006. The role of microorganisms in atopic dermatitis. Clin. Exp. Immunol. 1441-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burns, E., G. Bachrach, L. Shapira, and G. Nussbaum. 2006. Cutting edge: TLR2 is required for the innate response to Porphyromonas gingivalis: activation leads to bacterial persistence and TLR2 deficiency attenuates induced alveolar bone resorption. J. Immunol. 1778296-8300. [DOI] [PubMed] [Google Scholar]
- 7.Conley, D. B., A. Tripathi, A. M. Ditto, K. Reid, L. C. Grammer, and R. C. Kern. 2004. Chronic sinusitis with nasal polyps: staphylococcal exotoxin immunoglobulin E and cellular inflammation. Am. J. Rhinol. 18273-278. [PubMed] [Google Scholar]
- 8.Dziarski, R., and D. Gupta. 2005. Staphylococcus aureus peptidoglycan is a toll-like receptor 2 activator: a reevaluation. Infect. Immun. 735212-5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Féger, F., S. Varadaradjalou, Z. Gao, S. N. Abraham, and M. Arock. 2002. The role of mast cells in host defense and their subversion by bacterial pathogens. Trends. Immunol. 23151-158. [DOI] [PubMed] [Google Scholar]
- 10.Feng, B. S., S. H. He, P. Y. Zheng, L. Wu, and P. C. Yang. 2007. Mast cells play a crucial role in Staphylococcus aureus peptidoglycan-induced diarrhea. Am. J. Pathol. 171537-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fournier, B., and D. J. Philpott. 2005. Recognition of Staphylococcus aureus by the innate immune system. Clin. Microbiol. Rev. 18521-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gould, H. J., P. Takhar, H. E. Harries, E. Chevretton, and B. J. Sutton. 2007. The allergic march from Staphylococcus aureus superantigens to immunoglobulin E. Chem. Immunol. Allergy. 93106-136. [DOI] [PubMed] [Google Scholar]
- 13.Greiner, W., A. Rasch, D. Kohler, B. Salzberger, G. Fatkenheuer, and M. Leidig. 2007. Clinical outcome and costs of nosocomial and community-acquired Staphylococcus aureus bloodstream infection in haemodialysis patients. Clin. Microbiol. Infect. 13264-268. [DOI] [PubMed] [Google Scholar]
- 14.Jung, K. Y., J. D. Cha, S. H. Lee, W. H. Woo, D. S. Lim, B. K. Choi, and K. J. Kim. 2001. Involvement of staphylococcal protein A and cytoskeletal actin in Staphylococcus aureus invasion of cultured human oral epithelial cells. J. Med. Microbiol. 5035-41. [DOI] [PubMed] [Google Scholar]
- 15.Kintarak, S., S. A. Whawell, P. M. Speight, S. Packer, and S. P. Nair. 2004. Internalization of Staphylococcus aureus by human keratinocytes. Infect. Immun. 725668-5676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kubica, M., K. Guzik, J. Koziel, M. Zarebski, W. Richter, B. Gajkowska, A. Golda, A. Maciag-Gudowska, K. Brix, L. Shaw, T. Foster, and J. Potempa. 2008. A potential new pathway for Staphylococcus aureus dissemination: the silent survival of S. aureus phagocytosed by human monocyte-derived macrophages. PLoS ONE 31-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin, T. J., R. Garduno, R. T. Boudreau, and A. C. Issekutz. 2002. Pseudomonas aeruginosa activates human mast cells to induce neutrophil transendothelial migration via mast cell-derived IL-1 alpha and beta. J. Immunol. 1694522-4530. [DOI] [PubMed] [Google Scholar]
- 18.Lin, T. J., Z. Gao, M. Arock, and S. N. Abraham. 1999. Internalization of FimH+ Escherichia coli by the human mast cell line (HMC-1 5C6) involves protein kinase C. J. Leukoc. Biol. 661031-1038. [DOI] [PubMed] [Google Scholar]
- 19.Malaviya, R., E. A. Ross, J. I. MacGregor, T. Ikeda, J. R. Little, B. A. Jakschik, and S. N. Abraham. 1994. Mast cell phagocytosis of FimH-expressing enterobacteria. J. Immunol. 1521907-1914. [PubMed] [Google Scholar]
- 20.Malaviya, R., and S. N. Abraham. 2001. Mast cell modulation of immune responses to bacteria. Immunol. Rev. 17916-24. [DOI] [PubMed] [Google Scholar]
- 21.Malaviya, R., T. Ikeda, E. Ross, and S. N. Abraham. 1996. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature 38177-80. [DOI] [PubMed] [Google Scholar]
- 22.Malaviya, R., Z. Gao, K. Thankavel, P. A. van der Merwe, and S. N. Abraham. 1999. The mast cell tumor necrosis factor alpha response to FimH-expressing Escherichia coli is mediated by the glycosylphosphatidylinositol-anchored molecule CD48. Proc. Natl. Acad. Sci. USA 968110-8115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Massey, R. C., M. N. Kantzanou, T. Fowler, N. P. Day, K. Schofield, E. R. Wann, A. R. Berendt, M. Hook, and S. J. Peacock. 2001. Fibronectin-binding protein A of Staphylococcus aureus has multiple, substituting, binding regions that mediate adherence to fibronectin and invasion of endothelial cells. Cell. Microbiol. 3839-851. [DOI] [PubMed] [Google Scholar]
- 24.Matsui, K., and A. Nishikawa. 2006. Percutaneous application of peptidoglycan from Staphylococcus aureus induces mast cell development in mouse spleen. Int. Arch. Allergy Immunol. 139271-278. [DOI] [PubMed] [Google Scholar]
- 25.Matsui, K., M. Wirotessangthong, and A. Nishikawa. 2007. Percutaneous application of peptidoglycan from Staphylococcus aureus induces eosinophil infiltration in mouse skin. Clin. Exp. Allergy 37615-622. [DOI] [PubMed] [Google Scholar]
- 26.Medzhitov, R., P. Preston-Hurlburt, and C. A. Janeway, Jr. 1997. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388394-397. [DOI] [PubMed] [Google Scholar]
- 27.Mempel, M., C. Schnopp, M. Hojka, H. Fesq, S. Sweidinger, M. Schaller, H. C. Korting, J. Ring, and D. Abeck. 2002. Invasion of human keratinocytes by Staphylococcus aureus and intracellular bacterial persistence represent haemolysin-independent virulence mechanisms that are followed by features of necrotic and apoptotic keratinocyte cell death. Br. J. Dermatol. 146943-951. [DOI] [PubMed] [Google Scholar]
- 28.Mielcarek, N., E. H. Hornquist, B. R. Johansson, C. Locht, S. N. Abraham, and J. Holmgren. 2001. Interaction of Bordetella pertussis with mast cells, modulation of cytokine secretion by pertussis toxin. Cell. Microbiol. 3181-188. [DOI] [PubMed] [Google Scholar]
- 29.Munitz, A., I. Bachelet, F. D. Finkelman, M. E. Rothenberg, and F. Levi-Schaffer. 2007. CD48 is critically involved in allergic eosinophilic airway inflammation. Am. J. Respir. Crit. Care. Med. 175911-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munitz, A., I. Bachelet, R. Eliashar, M. Khodoun, F. D. Finkelman, M. E. Rothenberg, and F. Levi-Schaffer. 2006. CD48 is an allergen and IL-3-induced activation molecule on eosinophils. J. Immunol. 17777-83. [DOI] [PubMed] [Google Scholar]
- 31.Muñoz, S., R. Hernandez-Pando, S. N. Abraham, and J. A. Enciso. 2003. Mast cell activation by Mycobacterium tuberculosis: mediator release and role of CD48. J. Immunol. 1705590-5596. [DOI] [PubMed] [Google Scholar]
- 32.Pils, S., T. Schmitter, F. Neske, and C. R. Hauck. 2006. Quantification of bacterial invasion into adherent cells by flow cytometry. J. Microbiol. Methods 65301-310. [DOI] [PubMed] [Google Scholar]
- 33.Regueiro, V., M. A. Campos, J. Pons, S. Alberti, and J. A. Bengoechea. 2006. The uptake of a Klebsiella pneumoniae capsule polysaccharide mutant triggers an inflammatory response by human airway epithelial cells. Microbiology 152555-566. [DOI] [PubMed] [Google Scholar]
- 34.Rocha-De-Souza, C. M., A. L. Mattos-Guaraldi, R. Hirata, Jr., L. O. Moreira, L. H. Monteiro-Leal, A. C. Freitas-Almeida, L. Mendonca-Previato, J. O. Previato, and A. F. Andrade. 2003. Influence of polarisation and differentiation on interaction of 43-kDa outer-membrane protein of Aeromonas caviae with human enterocyte-like Caco-2 cell line. Int. J. Mol. Med. 11661-667. [PubMed] [Google Scholar]
- 35.Rock, F. L., G. Hardiman, J. C. Timans, R. A. Kastelein, and J. F. Bazan. 1998. A family of human receptors structurally related to Drosophila Toll. Proc. Natl. Acad. Sci. USA 95588-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schröder, A., R. Kland, A. Peschel, C. Von Eiff, and M. Aepfelbacher. 2006. Live cell imaging of phagosome maturation in Staphylococcus aureus infected human endothelial cells: small colony variants are able to survive in lysosomes. Med. Microbiol. Immunol. 195185-194. [DOI] [PubMed] [Google Scholar]
- 37.Schwandner, R., R. Dziarski, H. Wesche, M. Rothe, and C. J. Kirschning. 1999. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J. Biol. Chem. 27417406-17409. [DOI] [PubMed] [Google Scholar]
- 38.Seiberling, K. A., D. B. Conley, A. Tripathi, L. C. Grammer, L. Shuh, G. K. Haines III, R. Schleimer, and R. C. Kern. 2005. Superantigens and chronic rhinosinusitis: detection of staphylococcal exotoxins in nasal polyps. Laryngoscope 1151580-1585. [DOI] [PubMed] [Google Scholar]
- 39.Shin, J. S., and S. N. Abraham. 2001. Glycosylphosphatidylinositol-anchored receptor-mediated bacterial endocytosis. FEMS. Microbiol. Lett. 197131-138. [DOI] [PubMed] [Google Scholar]
- 40.Supajatura, V., H. Ushio, A. Nakao, S. Akira, K. Okumura, C. Ra, and H. Ogawa. 2002. Differential responses of mast cell Toll-like receptors 2 and 4 in allergy and innate immunity. J. Clin. Investig. 1091351-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takeuchi, O., K. Hoshino, and S. Akira. 2000. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J. Immunol. 1655392-5396. [DOI] [PubMed] [Google Scholar]
- 42.Varadaradjalou, S., F. Féger, N. Thieblemont, N. B. Hamouda, J. M. Pleau, M. Dy, and M. Arock. 2003. Toll-like receptor 2 (TLR2) and Toll-like receptor 4 (TLR4) differentially activate human mast cells. Eur. J. Immunol. 33899-906. [DOI] [PubMed] [Google Scholar]
- 43.Veltrop, M. H., J. Thompson, and H. Beekhuizen. 2001. Monocytes augment bacterial species- and strain-dependent induction of tissue factor activity in bacterium-infected human vascular endothelial cells. Infect. Immun. 692797-2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Voyich, J. M., K. R. Braughton, D. E. Sturdevant, A. R. Whitney, B. Said-Salim, S. F. Porcella, R. D. Long, D. W. Dorward, D. J. Gardner, B. N. Kreiswirth, G. M. Musser, and F. R. DeLeo. 2005. Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. J. Immunol. 1753907-3919. [DOI] [PubMed] [Google Scholar]
- 45.Wang, T., W. P. Lafuse, and B. S. Zwilling. 2000. Regulation of toll-like receptor 2 expression by macrophages following Mycobacterium avium infection. J. Immunol. 1656308-6313. [DOI] [PubMed] [Google Scholar]
- 46.Watanabe, I., M. Ichiki, A. Shiratsuchi, and Y. Nakanishi. 2007. TLR2-mediated survival of Staphylococcus aureus in macrophages: a novel bacterial strategy against host innate immunity. J. Immunol. 1784917-4925. [DOI] [PubMed] [Google Scholar]
- 47.Yumoto, H., H. H. Chou, Y. Takahashi, M. Davey, F. C. Gibson III, and C. A. Genco. 2005. Sensitization of human aortic endothelial cells to lipopolysaccharide via regulation of Toll-like receptor 4 by bacterial fimbria-dependent invasion. Infect. Immun. 738050-8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang, Q., N. Mousdicas, Q. Yi, Al-Hassani, S. D. Billings, S. M. Perkins, K. M. Howard, S. Shii, T. Shimizu, and J. B. Travers. 2005. Staphylococcal lipoteichoic acid inhibits delayed-type hypersensitivity reactions via the platelet-activating factor receptor. J. Clin. Investig. 1152855-2861. [DOI] [PMC free article] [PubMed] [Google Scholar]