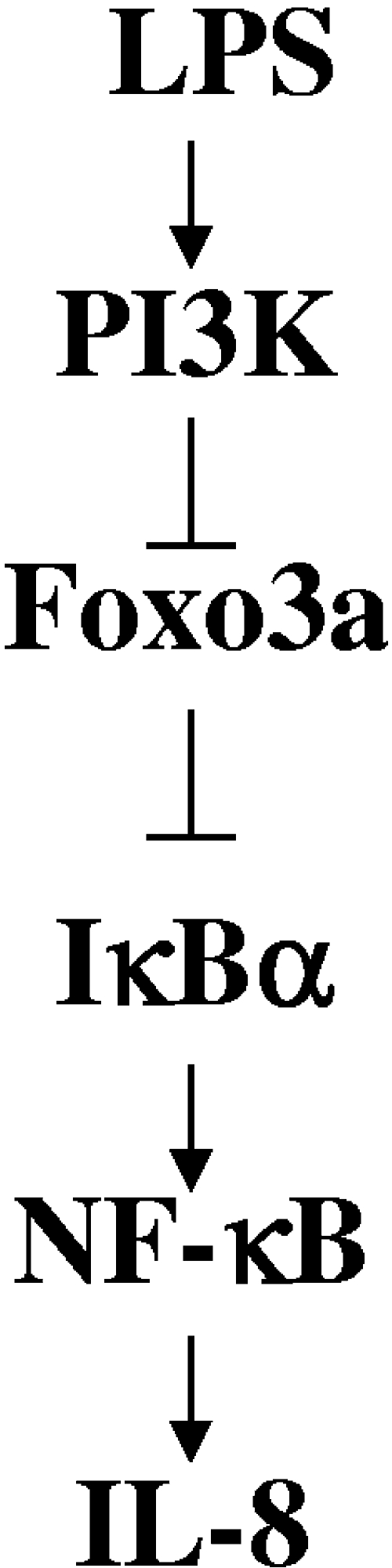Abstract
Enteric bacteria and their products play an important role in intestinal inflammation; however, the complete mechanisms are not elucidated yet. Tumor suppressor Foxo3a regulates gene expression in the nucleus, and its translocation to the cytosol leads to inactivation. Proximally, Foxo3a is regulated by different pathways including the phosphoinositide 3-kinase (PI3K) pathway. The aim of this study was to determine the effect of bacterial infection on Foxo3a in intestinal epithelial cells and to examine the contribution of Foxo3a in intestinal inflammation. Bacterial lipopolysaccharide (LPS) and infection with mouse pathogen Citrobacter rodentium induce translocation of the nuclear Foxo3a into the cytosol, where it degrades in human HT-29 and mouse CMT-93 cells. In colonic epithelia of healthy mice, Foxo3a is localized in the epithelia at the bottom of the crypts in both the nucleus and the cytosol, while in C. rodentium-infected colon Foxo3a is expressed along the crypts and located mainly in the cytosol, suggesting its inactivation. LPS utilized the PI3K pathway to inhibit Foxo3a. Additionally, inhibition of PI3K attenuated LPS-induced proinflammatory interleukin-8 (IL-8). LPS-induced IL-8 is increased in HT-29 cells with silenced Foxo3a. Moreover, in HT-29 cells with silenced Foxo3a, the amount of IκBα, an NF-κB inhibitor, is decreased. In conclusion, LPS and bacterial infection inactivate Foxo3a in intestinal epithelia via the PI3K pathway and inactivated Foxo3a leads to the upregulation of IL-8 by suppressing inhibitory IκBα.
Inflammatory bowel disease, including Crohn's disease and ulcerative colitis, is characterized by chronic mucosal injury and infiltration of inflammatory cells. In the pathogenesis of intestinal inflammation, luminal bacteria play an important role (14, 49). Most commonly, bacteria trigger an epithelial response through toll-like receptors (TLR). TLR generate signals that activate a specific pattern of genes that elicit an inflammatory response (1, 2). Downstream of TLR, the inflammatory response is controlled by NF-κB, a transcriptional factor involved in the regulation of cytokines, chemokines, and adhesion molecules. In unstimulated cells, NF-κB is localized in the cytoplasm and associated with inhibitory proteins known as IκBs (48). Proinflammatory stimuli induce the phosphorylation of IκB proteins, which are mediated by the proximal IκB kinase (IKK) complex. The phosphorylated IκB degrades, and NF-κB is released and subsequently translocates to the nucleus (48). Other molecules that are involved in the regulation of the NF-κB pathway also play an important role in controlling inflammation.
Tumor suppressor Foxo3a belongs to the Foxo family of Forkhead transcriptional factors together with Foxo1, Foxo4, and Foxo6 (10). In the absence of stimuli, Foxo3a is retained in the nucleus, bound to the DNA or to other transcriptional factors (7). Foxo3a regulates the expression of specific target genes that modulate the metabolic state, control cell cycle progression, regulate the mitotic program, and induce cellular apoptosis (3, 10). Foxo proteins are regulated by several pathways including phosphoinositide 3-kinase (PI3K), casein kinase 1, DYRK1A, and IKK (4, 7, 20). These pathways phosphorylate Foxo3a, which leads to its translocation to the cytoplasm and attachment to 14-3-3 proteins (7, 29, 54). Cytoplasmic Foxo3a may also be regulated by the proteasome system (4, 20).
Foxo3a-deficient mice develop a spontaneous, multisystemic inflammatory syndrome, accompanied with increased cytokine production, increased NF-κB activation, and hyperactivation of T cells (35); therefore, we assessed the role of Foxo3a in intestinal inflammation. We have shown in this paper that bacterial lipopolysaccharide (LPS) and infection with Citrobacter rodentium inactivate Foxo3a in intestinal epithelia, in vitro and in vivo. LPS-dependent Foxo3a inactivation in intestinal HT-29 cells is controlled by the PI3K pathway. We further demonstrated that blocking PI3K leads to attenuation of LPS-induced interleukin-8 (IL-8) in intestinal HT-29 cells. Additionally, our data revealed that LPS-induced IL-8 is increased in HT-29 cells with silenced Foxo3a. Also, in HT-29 cells with silenced Foxo3a, the amount of IκBα, an inhibitor of NF-κB, is decreased. Altogether, our results suggest that bacterial infection inactivates the tumor suppressor Foxo3a, which additionally increases IL-8 by downregulating inhibitory IκBα (see Fig. 7 for model).
FIG. 7.
Schematic representation of Foxo3a regulation by LPS and its contribution in inflammation. The data presented here suggest that LPS activation of PI3K leads to an inactivation of Foxo3a in intestinal epithelial cells. Inactivated Foxo3a downregulates inhibitory IκBα, which leads to increased NF-κB activity and increased IL-8 expression. This provides a new insight into how bacteria regulate tumor suppressor Foxo3a and inflammation in intestinal epithelial cells.
MATERIALS AND METHODS
Cell culture.
Human intestinal epithelial HT-29 cells and mouse intestinal epithelial CMT-93 cells (American Type Culture Collection, Manassas, VA) from passages 14 to 25 were used in these studies. HT-29 cells were propagated in McCoy's 5A medium (Sigma-Aldrich, Saint Louis, MO), and CMT-93 cells were propagated in Dulbecco-Vogt modified Eagle medium (Gibco-Invitrogen, Carlsbad, CA) with 10% fetal bovine serum (Gibco). For protein analysis, cells were plated in six-well plates and used when 60 to 70% confluent, while for cytokine analysis, cells were plated in 12-well plates and used as 50% confluent monolayers. Monolayers were serum deprived overnight before use in experiments.
LPS treatment and bacterial infection.
Monolayers of human HT-29 cells were treated with LPS purified from Escherichia coli serotype O111:B4 (Sigma) at a concentration of 100 μg/ml.
Monolayers of mouse CMT-93 cells were infected with C. rodentium DBS100 (American Type Culture Collection, Manassas, VA). C. rodentium cultures were grown overnight with shaking in Luria-Bertani broth (LB) at 37°C, diluted (1:33) in serum-free and antibiotic-free tissue culture medium containing 0.5% mannose, and grown at 37°C with aeration to mid-log growth phase (5 × 108 cells/ml). Bacteria were spun down and resuspended in fresh serum-free medium; monolayers of CMT-93 cells were then infected with ∼100 bacteria/cell (37°C in 5% CO2) for designated time periods. For cytokine analysis, C. rodentium culture grown in serum-free, antibiotic-free tissue culture medium was spun down and the supernatant was sterilized by filtration through 0.22-μm filters.
Treatment with PI3K inhibitors.
For inhibitor studies, HT-29 cells were pretreated for 1 h with 200 nM wortmannin or 30 μM LY294002 (Calbiochem, San Diego, CA) and then treated with LPS in the presence of an inhibitor for various time periods. These concentrations of inhibitors were based on the most effective inhibition of PI3K in other cell lines (19, 37, 38, 56).
Immunofluorescent staining.
Monolayers of cells grown on coverslips were LPS treated, washed with phosphate-buffered saline (PBS), fixed with 3.7% paraformaldehyde, and permeabilized with 0.2% Triton X-100 in PBS. Following permeabilization, monolayers were blocked in 2.5% bovine serum albumin and incubated with anti-Foxo3a antibody (1:200; Upstate Biotechnology, Temecula, CA). After being washed with PBS, monolayers were incubated with secondary anti-rabbit immunoglobulin G antibody conjugated with Alexa 488 (Molecular Probes-Invitrogen). Monolayers were mounted with Prolong Gold antifade reagent (Molecular Probes) and assessed using a Nikon Opti-Photo microscope. Images were captured using a Spot RT-slider camera (Diagnostic Instruments, Sterling Heights, MI), and images were managed with Image Pro software (Media Cybernetics, San Diego, CA).
Protein extraction and immunoblot assays.
Total proteins were extracted with lysis buffer (Cell Signaling, Beverly, MA), in the presence of protease inhibitor cocktail (Sigma).
Proteins (40 μg) were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred electrophoretically to nitrocellulose membranes (Bio-Rad, Hercules, CA). Blots were incubated in blocking solution and sequentially with a primary antibody against total Foxo3a or phosphorylated Foxo3a (both from Upstate Biotechnology), actin (Santa Cruz Biotechnology, Santa Cruz, CA), or IκBα (Cell Signaling) according to the manufacturer's protocols for antibodies. The blots were washed and incubated with appropriate dilutions of secondary antibodies conjugated with horseradish peroxidase for 1 h, and detection was achieved with ECL Plus Western blotting detection reagents (GE Healthcare, Buckinghamshire, United Kingdom).
siRNA of Foxo3a.
Two different sequences of Foxo3a small interfering RNA (siRNA; 30 nM) or equal amounts of negative-control oligonucleotides were incubated in 50 μl of Opti-MEM containing Lipofectamine RNAiMax (Invitrogen) for 20 min at room temperature. The complexes were then added to ∼90,000 HT-29 cells plated in 12-well plates and kept at 37°C for 24, 48, or 72 h. The initial experiment confirmed that a period of 48 h leads to optimal silencing of Foxo3a; thus, this time point was further used for experiments.
Overexpression of Foxo3a.
To create CMT-93 cells that overexpress Foxo3a, we employed pMX-Foxo3a-IRES-GFP and pMX-IRES-GFP retroviral vectors kindly provided by Stanford Peng (Roche Palo Alto LLC, Palo Alto, CA). Purified plasmids (10 μg) were used to transfect Phoenix packaging cell lines (Clontech, Mountain View, CA) by Lipofectamine RNAiMax (Invitrogen). These packaging cells are able to assemble the amphotropic viral particles with a genome containing pMX constructs. Retroviral particles were harvested from the medium of the packaging cells after 48 h and used to infect mouse intestinal CMT-93 cells. Infected CMT-93 cells were allowed to express Foxo3a for a period of 48 h.
Cytokine quantification.
The amounts of IL-8 and macrophage inflammatory protein 2 (MIP-2) were determined using enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN) according to the manufacturer's protocol.
Statistical analysis.
All data are represented as the means ± standard errors. Data comparisons were made with Student's t test. Differences were considered significant when the P values were ≤0.05.
Mouse infection.
Mice of strain C57BL/J6, aged 4 to 6 weeks, were obtained from the Jackson Laboratory (Bar Harbor, ME) and were maintained in the Biological Resources Laboratory at the University of Illinois at Chicago. Maintenance and experiments were performed according to standard animal care protocols. Approximately 2 × 108 C. rodentium bacteria in 200 μl of PBS were introduced to animals by gavage using a 4-cm-long curved needle with a steel ball at the tip. Control animals received 200 μl of PBS. Mice were returned to microisolator cages with free access to food and water. After 14 days of infection, animals were euthanized and the intestinal tissue was used for further analysis.
Histological analysis.
For histological analysis, colon tissues of experimental mice were washed with PBS, fixed in formalin, processed by a Tissue-Tek VIP 5 processor (Sakura Finetek, Torrance, CA), and embedded in paraffin. Tissue sections 5 μm thick were stained with hematoxylin and eosin (H&E) solutions. Images were acquired by use of a DMLB microscope equipped with Fluotar objectives (Leica Microsystems Inc., Bannockburn, IL) and a Micropublisher 5.0 camera (Q Imaging, Burnaby, British Columbia, Canada). Images were collected by use of QCapture software 2.6.
Immunohistostaining.
Antigen retrieval was performed in a pressure cooker using citrate buffer (Millipore, Bedford, United Kingdom). Immunohistochemistry was done on a Dako Autostainer universal staining system using a two-step indirect immunoperoxidase technique. Foxo3a primary antibody was applied (1:150, as determined by antibody titration) for 1 h at room temperature. Next, labeled polymer rabbit horseradish peroxidase was added for 1 h, followed by incubation with diaminobenzidine-positive chromogen, and then counterstained with hematoxylin. All tissues were then dehydrated in graded alcohol and xylene and coverslipped using Permount (Biomeda, Foster City, CA).
RESULTS
LPS induces Foxo3a translocation and degradation in human intestinal epithelial cells.
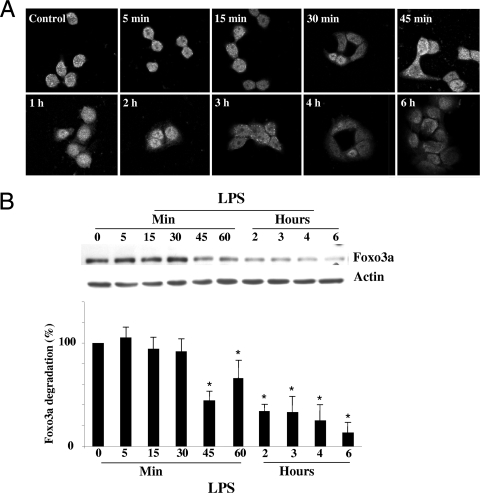
Nuclear Foxo3a, as a transcription factor, is actively involved in the regulation of gene expression (3, 10, 12). Upon stimulation with growth factors, nuclear Foxo3a translocates to the cytosol and becomes inactive (7, 29, 54). The role of Foxo3a in inflammation in colonic epithelia is not yet defined. To address whether bacteria and their products affect Foxo3a, human colonic epithelial HT-29 cells were treated with LPS and Foxo3a localization was examined by immunofluorescent staining. In untreated HT-29 cells, Foxo3a is localized in the nucleus as is expected (Fig. 1A). During LPS treatment, Foxo3a translocates from the nucleus to the cytosol (at 30 min), and the immunofluorescent signal appears dim during the course of the LPS treatment, suggesting a decreased amount of Foxo3a. Therefore, we examined the effect of LPS on the amount of Foxo3a in HT-29 cells. The total amount of Foxo3a remained the same in the first 30 min of LPS treatment (Fig. 1B). However, after 45 min of LPS treatment, the amount of Foxo3a decreased, implying degradation of Foxo3a in HT-29 cells during LPS treatment. These data show that bacterial LPS induces Foxo3a translocation and degradation in colonic HT-29 cells, which suggests its inactivation.
FIG. 1.
LPS induces Foxo3a translocation and degradation in human HT-29 intestinal epithelial cells. (A) HT-29 cells treated with LPS were fixed and immunofluorescently stained for Foxo3a (magnification, ×60). This experiment was repeated three independent times in triplicate. (B) Total proteins from HT-29 cells (control cells and cells treated with LPS) were separated by SDS-PAGE and immunoblotted for Foxo3a and actin. Each experiment was performed three times, and three samples were used per experimental group. Densitometric analysis shows significant (*) degradation of Foxo3a during the course of LPS treatment compared to untreated cells (P < 0.05).
Infection with C. rodentium induces translocation and degradation of Foxo3a in mouse intestinal epithelial cells.
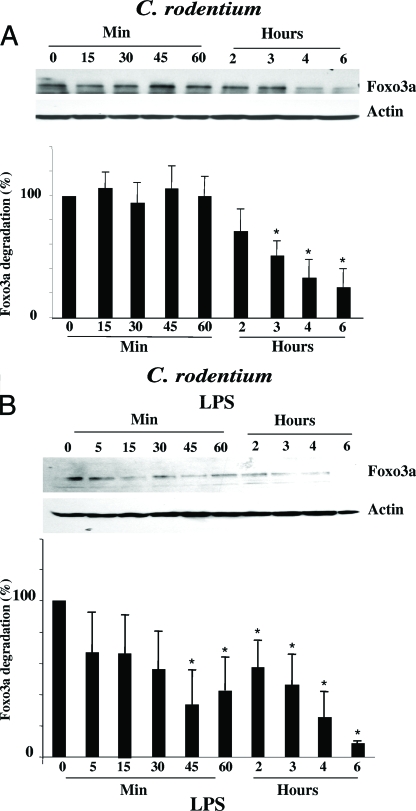
To further examine the effect of bacterial infection on Foxo3a, we utilized in vitro and in vivo infection with the mouse enteric pathogen Citrobacter rodentium, known to induce colonic inflammation (8). In mouse colonic CMT-93 cells, infected with C. rodentium, Foxo3a starts to degrade after 2 h of infection, which further progressed during the course of infection (Fig. 2A). This delayed Foxo3a degradation upon C. rodentium infection, compared to LPS treatment, might be the result of LPS treatment versus infection or a difference in response between HT-29 and CMT-93 cells. To address this, we treated mouse CMT-93 cells with LPS. Figure 2B shows that LPS induces Foxo3a degradation in mouse CMT-93 cells with kinetics similar to the pattern seen in human HT-29 cells. Hence, we speculate that the delayed response to C. rodentium infection is due to the fact that bacterial infection takes a longer time to elicit a response from the host cells.
FIG. 2.
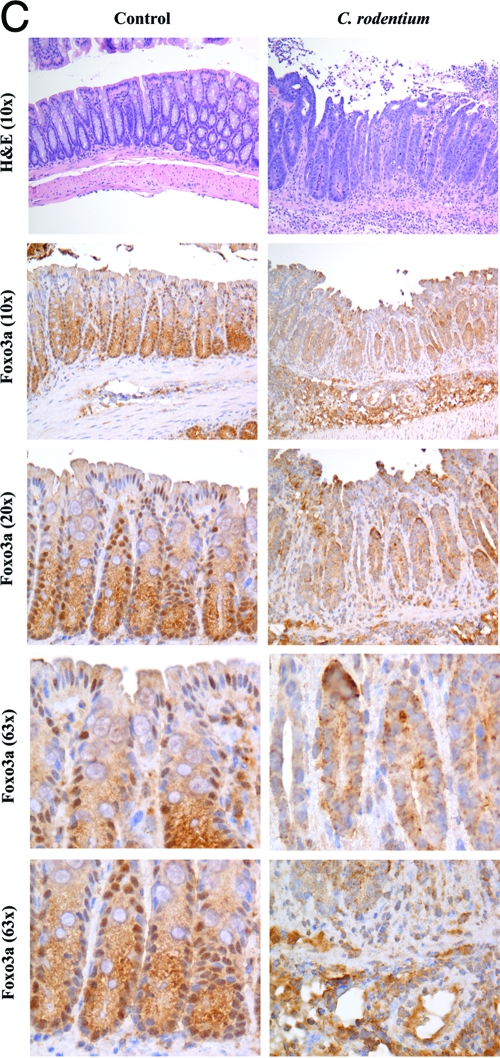
Infection with C. rodentium alters Foxo3a status in colonic epithelia. (A and B) Mouse colonic CMT-93 cells were infected with C. rodentium (A) or treated with LPS (B), and total proteins were separated by SDS-PAGE. Proteins were immunoblotted with Foxo3a and actin antibodies. These experiments were repeated three times (three samples were used per experimental group), and densitometric analysis reveals significant (*) Foxo3a degradation during treatment compared with control (P < 0.05). (C) Colonic tissue from C57BL/J6 mice, both control mice and those infected with C. rodentium, was initially H&E stained (upper panel). Tissue sections were further immunohistostained for Foxo3a (lower panels). Magnifications, ×10, ×20, and ×63.
We examined whether Foxo3a distribution is affected in colonic epithelia of mice after 14 days of C. rodentium infection. H&E staining revealed severely active inflammation in the colonic mucosa of C. rodentium-infected mice (Fig. 2C), compared to control lamina propria; inflammatory cells, including granulocytes and lymphoplasmatic cells, were also increased. In control, immunohistostaining revealed that nuclear Foxo3a is present in crypts and surface epithelial cells. However, there was more cytoplasmic staining in crypt epithelial cells than in apical epithelial cells (Fig. 2C). In contrast, in C. rodentium-infected colon Foxo3a is expressed along the entire crypt-villous axis and is primarily cytosolic with no nuclear staining. We noticed that the infiltrated immune cells in the lamina propria of the C. rodentium-infected colon also stained positive for Foxo3a (Fig. 2C, 63×). These data demonstrate that C. rodentium infection alters Foxo3a localization in mouse colonic epithelia in vitro and in vivo, suggesting its inactivation.
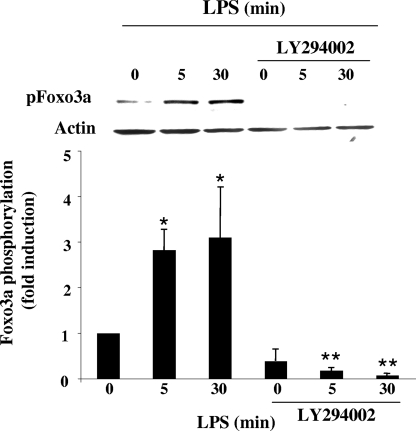
LPS-induced Foxo3a phosphorylation is PI3K dependent.
Prior to Foxo3a translocation from the nucleus to the cytosol, Foxo3a must be phosphorylated (7, 20). Several upstream signaling pathways, including the PI3K pathway, regulate Foxo3a phosphorylation (7). Consequently, we assessed the role of PI3K in the phosphorylation of Foxo3a in intestinal epithelial cells treated with LPS. We employed an antibody that recognizes Foxo3a phosphorylation on the Thr32 site, known to be phosphorylated by the PI3K pathway (7). Figure 3 shows the basal level of Foxo3a phosphorylation in untreated HT-29 cells. We speculated that because HT-29 cells are colon cancer cells and PI3K is activated in colon cancer (42), it is more likely to have a basal level of PI3K activity in these cells, which contributes to the basal level of Foxo3a phosphorylation. Furthermore, LPS increases Foxo3a phosphorylation on the Thr32 site threefold in the first 5 min of treatment. C. rodentium infection also increased Thr32 phosphorylation of Foxo3a in mouse CMT-93 cells (data not shown). We utilized a PI3K pharmacological inhibitor, wortmannin or LY294002, to assess the contribution of the PI3K pathway in LPS-induced Foxo3a phosphorylation. Inhibition of the PI3K pathway with both inhibitors blocked LPS-induced Foxo3a phosphorylation and decreased the basal level of Foxo3a phosphorylation in HT-29 cells (Fig. 3 shows inhibition with LY294002). These data show that LPS-induced Foxo3a phosphorylation in HT-29 cells is controlled proximally by the PI3K pathway.
FIG. 3.
LPS-induced Foxo3a phosphorylation is PI3K dependent. HT-29 cells, with or without pretreatment with a PI3K inhibitor (LY294002), were incubated with LPS. Total proteins were separated by SDS-PAGE and immunoprobed with an antibody against phosphorylated Foxo3a and actin. This immunoblot is representative of three independent experiments (three samples were used per experimental group). Densitometric analysis shows a significant increase (*) in phosphorylated Foxo3a after LPS treatment, which is completely abrogated in the presence of LY294002 (**, P < 0.05).
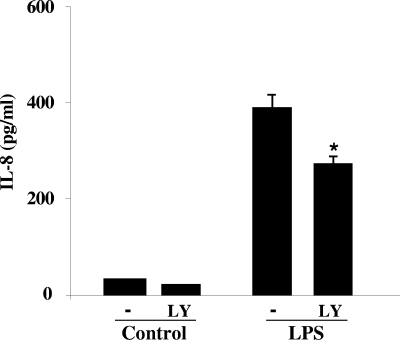
Effect of PI3K on IL-8 expression.
IL-8 is a proinflammatory chemokine that is a strong chemoattractant for neutrophils and T lymphocytes (24, 31, 51). It has been reported that IL-8 expression is in part controlled by the PI3K pathway (18, 43, 57). To study the contribution of the PI3K pathway in LPS-induced IL-8 regulation in HT-29 cells, we utilized the more specific PI3K inhibitor LY294002, since wortmannin increases NF-κB interactions with DNA, a critical regulator of cytokine expression (52). LY294002, at a concentration of 30 μM, significantly attenuated LPS-induced Akt phosphorylation, a target of PI3K (data not shown); we therefore applied this concentration for further studies. We showed that IL-8 is dramatically increased (12-fold, P < 0.001) after HT-29 monolayers were treated for 6 h with LPS (Fig. 4), which corresponds with data shown by others (33). Inhibition of the PI3K pathway with LY294002 significantly decreased (30%, P < 0.05) LPS-induced IL-8 expression (Fig. 4). These data showed that active PI3K is involved in the regulation of IL-8 induced by LPS.
FIG. 4.
Effect of PI3K inhibitors on LPS-induced IL-8 expression. HT-29 monolayers were pretreated with 30 μM LY294002 (LY) for 1 h and then incubated with LPS for 6 h. Medium was collected, and IL-8 was quantified by enzyme-linked immunosorbent assay. This experiment was repeated three independent times in triplicate, and the graph represents one experiment. LY294002 significantly attenuated (*) the LPS-induced IL-8 response (P < 0.05).
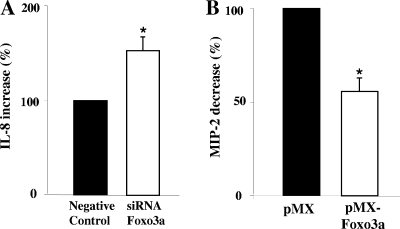
Foxo3a is involved in the regulation of LPS-induced IL-8 expression.
To examine the contribution of Foxo3a in the regulation of LPS-induced IL-8 expression in intestinal HT-29 cells, we performed siRNA experiments. The efficiency of silencing Foxo3a was greater than 90% (data not shown). Monolayers, both control layers and those with silent Foxo3a, were stimulated with LPS for 6 h, and the medium from the different experimental groups was collected and assayed for IL-8. All monolayers that were exposed to Lipofectamine produced lower levels of IL-8 in response to LPS than did the control monolayers exposed to LPS (2.5-fold). We hypothesize that Lipofectamine rearranges receptors on the surface of the cells and that the cells then become less responsive to LPS. Silenced Foxo3a insignificantly increased the basal level of IL-8 in HT-29 monolayers. However, LPS-induced IL-8 levels were increased on average by 63% (P < 0.05) in monolayers with silenced Foxo3a compared with the negative control (Fig. 5A). These data support a negative regulation of IL-8 by Foxo3a in HT-29 cells.
FIG. 5.
Effect of Foxo3a on cytokine expression. (A) HT-29 cells, with knocked-down Foxo3a, were treated with LPS for 6 h. Medium was collected for IL-8 quantification. The graph represents the average IL-8 ratio of three independent experiments performed in triplicate. The asterisk represents a significant difference between LPS-treated monolayers with Foxo3a and those without Foxo3a (P < 0.05). (B) CMT-93 cells transfected with a retrovirus vector that overexpressed Foxo3a were treated with sterile SN from C. rodentium culture, and medium was collected after 4 h and assayed for MIP-2. The asterisk represents a significant difference between MIP-2 produced by monolayers with normal Foxo3a and that produced by monolayers with overexpressed Foxo3a (n = 4, P < 0.05).
To further confirm the role of Foxo3a in the regulation of expression of proinflammatory cytokines, mouse CMT-93 cells were transfected with an adenovirus vector that overexpressed Foxo3a. In these monolayers, Foxo3a expression was increased by 40% (data not shown). CMT-93 monolayers, both control layers and those with overexpressed Foxo3a, were treated with sterile supernatant (SN) from C. rodentium culture; medium was collected and assayed for MIP-2 (mouse analog of human IL-8). Treatment with sterile SN from C. rodentium culture induced a 6.5-fold (P < 0.001) increase in MIP-2 compared with untreated CMT-93 monolayers (data not shown). Overexpressed Foxo3a did not alter the basal level of MIP-2 in untreated CMT-93 monolayers. However, overexpression of Foxo3a attenuated C. rodentium-induced MIP-2 expression by 54% (P < 0.05) (Fig. 5B). These data suggest that overexpressed Foxo3a negatively regulates MIP-2 expression induced by C. rodentium in mouse CMT-93 cells. Altogether, these experiments show that active Foxo3a repressed cytokine expression induced by LPS or bacterial infection in colonic epithelial cells.
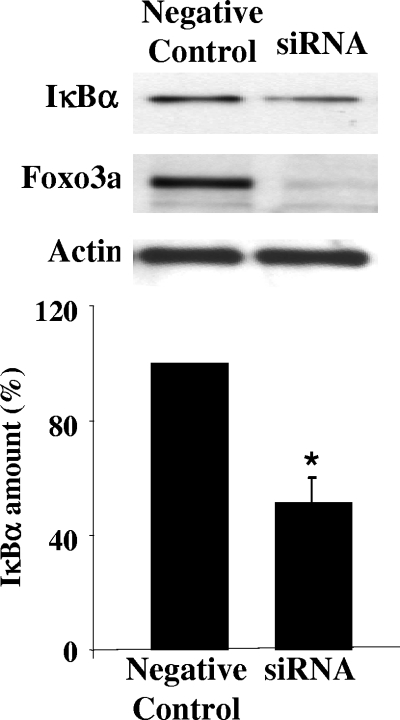
Foxo3a is involved in regulation of IκBα.
Jobin et al. demonstrated that in intestinal HT-29 cells IL-8 expression and NF-κB activity are controlled by inhibitory IκBα molecules (23). Since the IL-8 promoter did not show any putative Foxo3a binding sites (9, 50), we assessed the effect of Foxo3a deficiency on IκBα. Figure 6 shows that silencing Foxo3a leads to a ∼40% (P < 0.05) decrease of IκBα in HT-29 cells. These data correspond with the data of Lin et al. for immune cells from Foxo3a-deficient mice with decreased amounts of other IκB molecules (35). It is unclear at this point whether Foxo3a regulates IκBs directly or indirectly, but its effect on IκBα is obvious.
FIG. 6.
Foxo3a regulates IκBα in HT-29 cells. Total proteins from HT-29 cells with silent Foxo3a were immunoblotted for IκBα, Foxo3a, and actin. A representative immunoblot of four independent experiments shows a decreased amount of IκBα in Foxo3a-silenced cells compared with negative controls. Densitometric analysis shows significant (*) differences in the amounts of IκBα (P < 0.05).
DISCUSSION
Enteric bacteria and their products play an important role in intestinal inflammation (13, 17, 55). Here we demonstrated for the first time that bacterial LPS and infection with C. rodentium inactivate tumor suppressor Foxo3a in intestinal epithelia. LPS-dependent Foxo3a regulation in intestinal epithelial cells is controlled by the PI3K pathway. Additionally, in HT-29 cells with silent Foxo3a, LPS-induced IL-8 is increased, while the amount of IκBα is decreased. We hypothesized that Foxo3a regulates IL-8 through the inhibitory IκBα molecule. Altogether this suggests that bacterial infections and their products regulate Foxo3a tumor suppressor via the PI3K pathway and that Foxo3a contributes to the regulation of IL-8 expression. Regulation may occur by IκBα either directly or indirectly (Fig. 7).
The importance of intestinal bacteria in triggering and controlling colitis is well established; however, the underlying mechanisms are still unclear. Enteric bacteria affect host intestinal epithelia by using toxins, effector molecules, and/or direct invasion of the host cell (16, 34). On the other hand, host cells are equipped with tools that are able to recognize and respond to bacteria as well. One of the mechanisms that the host utilizes to recognize bacterial product is through TLR. The different members of the TLR family are located on the cell membrane, and when interacting with their ligands, they generate signals by which they activate a specific pattern of gene expression (1, 2). In the pathogenesis of C. rodentium, a model of bacterium-induced colitis (8, 40), TLR signaling plays an important role. TLR4-deficient mice infected with C. rodentium showed delayed mortality compared with infected wild-type mice (27). MyD88-deficient mice (MyD88 is downstream of TLR) showed, upon C. rodentium infection, severe necrotizing colitis, high bacterial counts in the colon, and delayed recruitment of neutrophils (32). Our data suggest that Foxo3a is a downstream target of TLR4 in intestinal HT-29 cells and that LPS binding to TLR4 leads to Foxo3a inactivation.
One of the downstream pathways utilized by TLR to control expression of various genes is the PI3K pathway (5, 45). PI3K activation mediates several critical cellular responses, including cell differentiation, proliferation, and survival (11, 25). The upregulation of PI3K has been suggested to cause a hyperproliferative epithelium, and its upregulation has been observed in a majority of colon cancers (26, 42). Also, PI3K participates in the regulation of cytokines, such as IL-12 in mouse dendritic cells and IL-8 in response to flagellin or LPS in intestinal epithelial cells (43, 57). Rhee et al. showed that blocking PI3K activation substantially inhibits IL-8 induced by flagellin/TLR5 in NCM460 cells (43), while Yu et al. showed opposite data in T84 cells (57). Also, Huang et al. suggested that inhibition of PI3K in T84 cells results in increased Salmonella enterica-induced IL-8 (21). Our data demonstrated that inhibition of PI3K attenuates LPS-induced IL-8 expression in HT-29 cells. Although the mechanisms of these two different responses are not clear, we speculate that pathways proximal to NF-κB might respond differently to various stimuli.
Downstream, PI3K phosphorylates Foxo3a proteins at several sites, which leads to the translocation of Foxo3a to the cytosol and abrogation of its transcriptional activity (6, 9, 28). Cytoplasmic localization of Foxo3a is associated with certain tumor cell lines and also contributes to the pathogenesis and development of tumors (36, 39, 41). Foxo3a is phosphorylated in colon carcinoma cell lines (26), and we have also detected a basal level of Foxo3a phosphorylation in HT-29 cancer cells. Kristof et al. have demonstrated that LPS controls Foxo3a in human lung epithelial cells (30), suggesting that in other epithelial cells Foxo3a also may be regulated by TLR4. Moreover, we have demonstrated a gradient distribution of Foxo3a in proliferating intestinal epithelia in mouse colon. A similar distribution is seen with PCNA, one of the markers of proliferation of colonic epithelia (53). Interestingly, in C. rodentium-infected colon, the distribution of Foxo3a is along the entire crypt and it is mainly cytosolic, suggesting an inactivated state. These data imply that Foxo3a might be involved in the proliferation of normal colonic epithelia while bacterium-induced inflammation affects the function of Foxo3a. Moreover, we have demonstrated that Foxo3a contributes in PI3K-dependent IL-8 regulation. Effective silencing of Foxo3a yields an increase in IL-8. Altogether, this suggests that Foxo3a plays a role in intestinal inflammation induced by bacteria or their products.
Several investigators have shown a regulatory network comprised of Foxo3a and NF-κB in immune cells (15, 35, 47). Lin et al. provided evidence that Foxo3a modulates NF-κB activity in lymphoid cells. They have shown that T cells from Foxo3a-deficient mice displayed markedly increased NF-κB activity accompanied with decreased IκBβ and IκBɛ expression (35). In intestinal HT-29 cells treated with cytokines or LPS, IκBα degradation is incomplete and relatively few molecules of NF-κB are needed in the nuclei of HT-29 cells to induce high levels of IL-8 expression (22, 44). Nevertheless, Jobin et al. demonstrated that inhibition of IκBα degradation completely blocked IL-8-induced expression (23), suggesting that IκBα is an important player in the regulation of NF-κB-dependent genes. Our data showed that in HT-29 cells with silent Foxo3a, the amount of inhibitory IκBα is decreased. We speculate that unstimulated cells with knocked-down Foxo3a equilibrate to a smaller amount of IκBα; thus, IL-8 is not significantly increased. However, we hypothesize that during LPS stimulation de novo synthesis of IκBα is important to keep IL-8 expression down. Lack of Foxo3a in the nucleus in LPS-stimulated cells directly or indirectly inhibits IκBα; thus, IL-8 expression is higher. However, the immediate targets of Foxo3a for repressing IκBα activity remain unspecified. It is known that Foxo members and other Foxs can form complexes together with additional signaling molecules to exert transcriptional effects (46). Thus, it is possible that Foxo3a could cooperatively bind and recruit different coactivators or corepressors to control IκBα.
In summary, bacterial LPS and infection with C. rodentium regulate tumor suppressor Foxo3a in intestinal epithelia and Foxo3a participates in the regulation of inflammation. It will be necessary to further define the role of Foxo3a in the pathogenesis of colon diseases. Understanding how Foxo3a participates in the pathogenesis of colon diseases may help unlock novel therapeutic possibilities.
Acknowledgments
We thank Hemant Roy, Michael Goldberg, and Ramesh Wali for constructive assistance in preparation of the manuscript. The vector that overexpresses Foxo3a was a generous gift from Stanford Peng (Roche Palo Alto LLC, Palo Alto, CA).
This work was in part supported by a Senior Investigator Award from the Crohn's and Colitis Foundation of America (CCFA#1953) and an ENH Pilot Study grant. Lobke Snoeks received a Fulbright scholarship for her visiting fellowship, and Christopher R. Weber received an NIH F32DK082134 fellowship award.
Editor: B. A. McCormick
Footnotes
Published ahead of print on 4 August 2008.
REFERENCES
- 1.Akira, S., and K. Takeda. 2004. Functions of toll-like receptors: lessons from KO mice. C. R. Biol. 327581-589. [DOI] [PubMed] [Google Scholar]
- 2.Akira, S., and K. Takeda. 2004. Toll-like receptor signaling. Nat. Rev. Immunol. 4499-511. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez, B., A. C. Martinez, B. M. Burgering, and A. C. Carrera. 2001. Forkhead transcription factors contribute to execution of the mitotic program in mammals. Nature 413744-747. [DOI] [PubMed] [Google Scholar]
- 4.Aoki, M., H. Jiang, and P. K. Vogt. 2004. Proteasomal degradation of the FoxO1 transcriptional regulator in cells transformed by the P3K and Akt oncoproteins. Proc. Natl. Acad. Sci. USA 10113613-13617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arbibe, L., J. P. Mira, N. Teusch, L. Kline, M. Guha, N. Mackman, P. J. Godowski, R. J. Ulevitch, and U. G. Knaus. 2000. Toll-like receptor 2-mediated NF-κB activation requires a Rac1-dependent pathway. Nat. Immunol. 1533-540. [DOI] [PubMed] [Google Scholar]
- 6.Biggs, W. H., III, J. Meisenhelder, T. Hunter, W. K. Cavenee, and K. C. Arden. 1999. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc. Natl. Acad. Sci. USA 96:7421-7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birkenkamp, K. U., and P. J. Coffer. 2003. FOXO transcription factors as regulators of immune homeostasis: molecules to die for? J. Immunol. 1711623-1629. [DOI] [PubMed] [Google Scholar]
- 8.Borenshtein, D., M. E. McBee, and D. B. Schauer. 2008. Utility of the Citrobacter rodentium infection model in laboratory mice. Curr. Opin. Gastroenterol. 2432-37. [DOI] [PubMed] [Google Scholar]
- 9.Brunet, A., A. Bonni, M. J. Zigmond, M. Z. Lin, P. Juo, L. S. Hu, M. J. Anderson, K. C. Arden, J. Blenis, and M. E. Greenberg. 1999. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96857-868. [DOI] [PubMed] [Google Scholar]
- 10.Burgering, B. M., and G. J. Kops. 2002. Cell cycle and death control: long live Forkheads. Trends Biochem. Sci. 27352-360. [DOI] [PubMed] [Google Scholar]
- 11.Cantley, L. C. 2002. The phosphoinositide 3-kinase pathway. Science 2961655-1657. [DOI] [PubMed] [Google Scholar]
- 12.Carlsson, P., and M. Mahlapuu. 2002. Forkhead transcription factors: key players in development and metabolism. Dev. Biol. 2501-23. [DOI] [PubMed] [Google Scholar]
- 13.Clevers, H. 2004. At the crossroads of inflammation and cancer. Cell 118671-674. [DOI] [PubMed] [Google Scholar]
- 14.Dianda, L., A. M. Hanby, N. A. Wright, A. Sebesteny, A. C. Hayday, and M. J. Owen. 1997. T cell receptor-alpha beta-deficient mice fail to develop colitis in the absence of a microbial environment. Am. J. Pathol. 15091-97. [PMC free article] [PubMed] [Google Scholar]
- 15.Finnberg, N., and W. S. El-Deiry. 2004. Activating FOXO3a, NF-κB and p53 by targeting IKKs: an effective multi-faceted targeting of the tumor-cell phenotype? Cancer Biol. Ther. 3614-616. [DOI] [PubMed] [Google Scholar]
- 16.Frankel, G., A. D. Phillips, I. Rosenshine, G. Dougan, J. B. Kaper, and S. Knutton. 1998. Enteropathogenic and enterohaemorrhagic Escherichia coli: more subversive elements. Mol. Microbiol. 30911-921. [DOI] [PubMed] [Google Scholar]
- 17.Fukata, M., and M. T. Abreu. 2007. TLR4 signaling in the intestine in health and disease. Biochem. Soc. Trans. 351473-1478. [DOI] [PubMed] [Google Scholar]
- 18.Gobert, A. P., M. Vareille, A. L. Glasser, T. Hindre, T. de Sablet, and C. Martin. 2007. Shiga toxin produced by enterohemorrhagic Escherichia coli inhibits PI3K/NF-κB signaling pathway in globotriaosylceramide-3-negative human intestinal epithelial cells. J. Immunol. 178:8168-8174. [DOI] [PubMed] [Google Scholar]
- 19.Guo, S., and G. E. Sonenshein. 2004. Forkhead box transcription factor FOXO3a regulates estrogen receptor alpha expression and is repressed by the Her-2/neu/phosphatidylinositol 3-kinase/Akt signaling pathway. Mol. Cell. Biol. 248681-8690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu, M. C., D. F. Lee, W. Xia, L. S. Golfman, F. Ou-Yang, J. Y. Yang, Y. Zou, S. Bao, N. Hanada, H. Saso, R. Kobayashi, and M. C. Hung. 2004. IκB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell 117225-237. [DOI] [PubMed] [Google Scholar]
- 21.Huang, F. C., Q. Li, and B. J. Cherayil. 2005. A phosphatidyl-inositol-3-kinase-dependent anti-inflammatory pathway activated by Salmonella in epithelial cells. FEMS Microbiol. Lett. 243265-270. [DOI] [PubMed] [Google Scholar]
- 22.Jobin, C., S. Haskill, L. Mayer, A. Panja, and R. B. Sartor. 1997. Evidence for altered regulation of IκBα degradation in human colonic epithelial cells. J. Immunol. 158226-234. [PubMed] [Google Scholar]
- 23.Jobin, C., A. Panja, C. Hellerbrand, Y. Iimuro, J. Didonato, D. A. Brenner, and R. B. Sartor. 1998. Inhibition of proinflammatory molecule production by adenovirus-mediated expression of a nuclear factor κB super-repressor in human intestinal epithelial cells. J. Immunol. 160410-418. [PubMed] [Google Scholar]
- 24.Kasahara, T., N. Mukaida, K. Yamashita, H. Yagisawa, T. Akahoshi, and K. Matsushima. 1991. IL-1 and TNFα induction of IL-8 and monocyte chemotactic and activating factor (MCAF) mRNA expression in a human astrocytoma cell line. Immunology 7460-67. [PMC free article] [PubMed] [Google Scholar]
- 25.Katso, R., K. Okkenhaug, K. Ahmadi, S. White, J. Timms, and M. D. Waterfield. 2001. Cellular function of phosphoinositide 3-kinases: implications for development, homeostasis, and cancer. Annu. Rev. Cell Dev. Biol. 17615-675. [DOI] [PubMed] [Google Scholar]
- 26.Khaleghpour, K., Y. Li, D. Banville, Z. Yu, and S. H. Shen. 2004. Involvement of the PI3-kinase signaling pathway in progression of colon adenocarcinoma. Carcinogenesis 25241-248. [DOI] [PubMed] [Google Scholar]
- 27.Khan, M. A., C. Ma, L. A. Knodler, Y. Valdez, C. M. Rosenberger, W. Deng, B. B. Finlay, and B. A. Vallance. 2006. Toll-like receptor 4 contributes to colitis development but not to host defense during Citrobacter rodentium infection in mice. Infect. Immun. 742522-2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kops, G. J., and B. M. Burgering. 1999. Forkhead transcription factors: new insights into protein kinase B (c-akt) signaling. J. Mol. Med. 77656-665. [DOI] [PubMed] [Google Scholar]
- 29.Kops, G. J., N. D. de Ruiter, A. M. De Vries-Smits, D. R. Powell, J. L. Bos, and B. M. Burgering. 1999. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature 398630-634. [DOI] [PubMed] [Google Scholar]
- 30.Kristof, A. S., J. Fielhaber, A. Triantafillopoulos, S. Nemoto, and J. Moss. 2006. Phosphatidylinositol 3-kinase-dependent suppression of the human inducible nitric-oxide synthase promoter is mediated by FKHRL1. J. Biol. Chem. 28123958-23968. [DOI] [PubMed] [Google Scholar]
- 31.Larsen, C. G., A. O. Anderson, J. J. Oppenheim, and K. Matsushima. 1989. Production of IL-8 by human dermal fibroblasts and keratinocytes in response to IL-1 or TNF. Immunology 6831-36. [PMC free article] [PubMed] [Google Scholar]
- 32.Lebeis, S. L., B. Bommarius, C. A. Parkos, M. A. Sherman, and D. Kalman. 2007. TLR signaling mediated by MyD88 is required for a protective innate immune response by neutrophils to Citrobacter rodentium. J. Immunol. 179566-577. [DOI] [PubMed] [Google Scholar]
- 33.Lee, S. K., T. I. Kim, Y. K. Kim, C. H. Choi, K. M. Yang, B. Chae, and W. H. Kim. 2005. Cellular differentiation-induced attenuation of LPS response in HT-29 cells is related to the down-regulation of TLR4 expression. Biochem. Biophys. Res. Commun. 337457-463. [DOI] [PubMed] [Google Scholar]
- 34.Lilic, M., and C. E. Stebbins. 2004. Re-structuring the host cell: up close with Salmonella's molecular machinery. Microbes Infect. 61205-1211. [DOI] [PubMed] [Google Scholar]
- 35.Lin, L., J. D. Hron, and S. L. Peng. 2004. Regulation of NF-κB, Th activation, and autoinflammation by the forkhead transcription factor Foxo3a. Immunity 21203-213. [DOI] [PubMed] [Google Scholar]
- 36.Medema, R. H., G. J. Kops, J. L. Bos, and B. M. Burgering. 2000. AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature 404782-787. [DOI] [PubMed] [Google Scholar]
- 37.Moller, C., J. Alfredsson, M. Engstrom, H. Wootz, Z. Xiang, J. Lennartsson, J. I. Jonsson, and G. Nilsson. 2005. Stem cell factor promotes mast cell survival via inactivation of FOXO3a-mediated transcriptional induction and MEK-regulated phosphorylation of the proapoptotic protein Bim. Blood 1061330-1336. [DOI] [PubMed] [Google Scholar]
- 38.Nadal, A., P. F. Marrero, and D. Haro. 2002. Down-regulation of the mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase gene by insulin: the role of the forkhead transcription factor FKHRL1. Biochem. J. 366289-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakamura, N., S. Ramaswamy, F. Vazquez, S. Signoretti, M. Loda, and W. R. Sellers. 2000. Forkhead transcription factors are critical effectors of cell death and cell cycle arrest downstream of PTEN. Mol. Cell. Biol. 208969-8982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Newman, J. V., T. Kosaka, B. J. Sheppard, J. G. Fox, and D. B. Schauer. 2001. Bacterial infection promotes colon tumorigenesis in Apc(Min/+) mice. J. Infect. Dis. 184227-230. [DOI] [PubMed] [Google Scholar]
- 41.Nicholson, K. M., and N. G. Anderson. 2002. The protein kinase B/Akt signalling pathway in human malignancy. Cell. Signal. 14381-395. [DOI] [PubMed] [Google Scholar]
- 42.Philp, A. J., I. G. Campbell, C. Leet, E. Vincan, S. P. Rockman, R. H. Whitehead, R. J. Thomas, and W. A. Phillips. 2001. The phosphatidylinositol 3′-kinase p85alpha gene is an oncogene in human ovarian and colon tumors. Cancer Res. 617426-7429. [PubMed] [Google Scholar]
- 43.Rhee, S. H., H. Kim, M. P. Moyer, and C. Pothoulakis. 2006. Role of MyD88 in phosphatidylinositol 3-kinase activation by flagellin/TLR5 engagement in colonic epithelial cells. J. Biol. Chem. 28118560-18568. [DOI] [PubMed] [Google Scholar]
- 44.Russo, M. P., R. F. Schwabe, R. B. Sartor, and C. Jobin. 2004. NF-κB-inducing kinase restores defective IκB kinase activity and NF-κB signaling in intestinal epithelial cells. Cell. Signal. 16741-750. [DOI] [PubMed] [Google Scholar]
- 45.Sarkar, S. N., K. L. Peters, C. P. Elco, S. Sakamoto, S. Pal, and G. C. Sen. 2004. Novel roles of TLR3 tyrosine phosphorylation and PI3 kinase in double-stranded RNA signaling. Nat. Struct. Mol. Biol. 111060-1067. [DOI] [PubMed] [Google Scholar]
- 46.Seoane, J., H. V. Le, L. Shen, S. A. Anderson, and J. Massague. 2004. Integration of Smad and forkhead pathways in the control of neuroepithelial and glioblastoma cell proliferation. Cell 117211-223. [DOI] [PubMed] [Google Scholar]
- 47.Su, H., N. Bidere, and M. Lenardo. 2004. Another fork in the road: Foxo3a regulates NF-κB activation. Immunity 21133-134. [DOI] [PubMed] [Google Scholar]
- 48.Tak, P. P., and G. S. Firestein. 2001. NF-κB: a key role in inflammatory diseases. J. Clin. Investig. 1077-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taurog, J. D., J. A. Richardson, J. T. Croft, W. A. Simmons, M. Zhou, J. L. Fernandez-Sueiro, E. Balish, and R. E. Hammer. 1994. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J. Exp. Med. 1802359-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tran, H., A. Brunet, E. C. Griffith, and M. E. Greenberg. 2003. The many forks in FOXO's road. Sci. STKE 2003RE5. [DOI] [PubMed] [Google Scholar]
- 51.Uguccioni, M., P. Gionchetti, D. F. Robbiani, F. Rizzello, S. Peruzzo, M. Campieri, and M. Baggiolini. 1999. Increased expression of IP-10, IL-8, MCP-1, and MCP-3 in ulcerative colitis. Am. J. Pathol. 155331-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang, Q., S. Kim, X. Wang, and B. M. Evers. 2000. Activation of NF-κB binding in HT-29 colon cancer cells by inhibition of phosphatidylinositol 3-kinase. Biochem. Biophys. Res. Commun. 273853-858. [DOI] [PubMed] [Google Scholar]
- 53.Wilson, M. S., and P. F. Schofield. 1995. Markers to study human colonic cell proliferation. Gut 36152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yaffe, M. B., K. Rittinger, S. Volinia, P. R. Caron, A. Aitken, H. Leffers, S. J. Gamblin, S. J. Smerdon, and L. C. Cantley. 1997. The structural basis for 14-3-3:phosphopeptide binding specificity. Cell 91961-971. [DOI] [PubMed] [Google Scholar]
- 55.Yang, L., and Z. Pei. 2006. Bacteria, inflammation, and colon cancer. World J. Gastroenterol. 126741-6746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang, L., S. Xie, M. S. Jamaluddin, S. Altuwaijri, J. Ni, E. Kim, Y. T. Chen, Y. C. Hu, L. Wang, K. H. Chuang, C. T. Wu, and C. Chang. 2005. Induction of androgen receptor expression by phosphatidylinositol 3-kinase/Akt downstream substrate, FOXO3a, and their roles in apoptosis of LNCaP prostate cancer cells. J. Biol. Chem. 28033558-33565. [DOI] [PubMed] [Google Scholar]
- 57.Yu, Y., S. Nagai, H. Wu, A. S. Neish, S. Koyasu, and A. T. Gewirtz. 2006. TLR5-mediated phosphoinositide 3-kinase activation negatively regulates flagellin-induced proinflammatory gene expression. J. Immunol. 1766194-6201. [DOI] [PubMed] [Google Scholar]