Candida albicans is a normal commensal organism of the oral cavity, gastrointestinal tract, and vagina. Under certain circumstances, C. albicans is capable of causing host damage (or disease) leading to oral, vaginal, cutaneous, or systemic candidiasis. The latter is a serious infection with a high mortality range of 33% to 54% and high morbidity in those who survive (76). In fact, in recent years, systemic candidal infections have become the fourth most frequent cause of nosocomial bloodstream infections in the United States, giving rise to an enormous associated personal and economic cost (79). Systemic candidiasis involves the hematogenous spread of C. albicans to multiple organs, including the brain, kidneys, heart, liver, and lungs (62). Histologically, infection of these organs is characterized by ramifying candidal hyphae and accompanying yeast forms that produce multiple necrotic nodules or abscesses that result in extensive organ damage leading to organ failure. Risk factors for systemic candidiasis include neutropenia, intravascular catheters, hemodialysis, total parenteral nutrition, abdominal surgery, burns, broad-spectrum antibiotics, and corticosteroids (63). Systemic innate immune responses by phagocytic cells, particularly neutrophils and macrophages, appear to play a critical role in the host defense against systemic Candida infections, and consequently, the majority of candidal infections occur in patients with neutropenia or defects in neutrophil or macrophage function (5, 55).
MORPHOGENETIC CONVERSIONS AND C. ALBICANS VIRULENCE
C. albicans is a polymorphic organism that is capable of converting between yeast, pseudohyphal, and hyphal forms. The conventional view was that yeast forms were associated with commensal carriage, whereas hyphal forms were associated with disease. This was based on evidence showing that mutant forms of C. albicans that were locked into the yeast form were avirulent (50). However, this notion was challenged by Braun et al., who found that a tup1-deficient C. albicans strain that was constitutively pseudohyphal was avirulent in a murine model of systemic candidiasis (6, 7). Although the precise nature of the association between fungal morphogenesis and host invasion is a hotly debated topic (30), it is now widely accepted that it is the ability to undergo morphogenetic conversion, rather than the morphological form itself, that is the primary determinant of pathogenicity (71).
The dissemination of fungal organisms in systemic candidiasis starts with their entry into the bloodstream. Given the known risk factors for systemic candidiasis, this is most likely to occur in susceptible individuals by seeding from contaminated intravascular devices, by persorption of C. albicans across the gastrointestinal mucosa, by invasion of epithelially denuded surfaces, or via trauma or surgically related inoculation (4, 11, 34). Exit from the circulation is thought to occur by adhesion and then penetration into the endothelial lining of blood vessels, except possibly in the kidney, where direct adhesion to exposed extracellular matrix components within glomerular regions may occur (45). Animal studies suggest that candidal trafficking from the circulation into the tissues occurs rapidly (1, 38, 52). This review discusses the two critical steps in the migration of C. albicans cells from the circulation into the tissues, which are (i) candidal adhesion to endothelial cells lining the blood vessels and (ii) transmigration of C. albicans across the endothelium into the tissues.
ADHESION OF C. ALBICANS TO ENDOTHELIAL CELLS
During hematogenous dissemination of C. albicans, organisms must first adhere to the endothelial lining of blood vessels before transmigrating across the endothelium to invade the tissues. However, little is known about the mechanisms involved in either process. What is known is complicated further by conflicting evidence concerning the roles played by yeast, pseudohyphal, and hyphal forms of C. albicans and the role of morphogenetic change in the adhesion and transmigration processes.
There are currently two different theories as to how C. albicans adheres to the endothelium. The first theory proposes that cells must first undergo morphogenetic conversion to hyphal forms, which then bind to and damage the endothelial lining of blood vessels before undergoing transmigration from the circulation into the tissues. However, more recent data indicate a second possibility in which morphogenetic change is not necessary for C. albicans invasion of the tissues. In this scenario, yeast cells adhere to the endothelium and then transmigrate into the tissues without undergoing morphogenetic conversion.
The basis of the first theory is morphogenetic conversion of C. albicans to the hyphal form, and there are many lines of evidence to support this hypothesis. These include the observations that germination of C. albicans is necessary for the organism to damage endothelial cells (22, 67) and that substances that inhibit germination block C. albicans-induced endothelial cell damage (27). Moreover, the time course of candidal germination and germ tube elongation on endothelial cells parallels the time course of endothelial cell damage (20). Further evidence has come from experiments using genetically engineered forms of C. albicans with filamentation defects. The ability of these organisms to damage and invade endothelial cells is severely impaired compared to that of wild-type parent strains (64, 67).
Studies showing that germinated C. albicans cells exhibit much greater adherence to epithelial cells than do yeast forms (43) prompted suggestions that C. albicans adherence to endothelial cells might also be hypha dependent. Indeed, there is some evidence to suggest that germinated candidal forms exhibit greater endothelial cell adhesion than do yeast forms of C. albicans (66). However, it is also possible that yeast forms adhere to the endothelial surface, germinate there, and then penetrate and damage the endothelium during transmigration (22) or that yeast forms adhere and are then endocytosed before germinating within the endothelial cell to cause damage (64). Taken together, the data suggest that morphogenetic transformation is involved in endothelial cell adhesion but, more particularly, in the subsequent trans-endothelial cell migration.
Conversely, there is also evidence to suggest that morphogenetic change may not be necessary for C. albicans invasion, and this is the basis for the alternative hypothesis. In animal studies in which mice were intravenously inoculated with different mutant strains, Bendel et al. found that cells from a C. albicans mutant strain locked into the yeast form were able to leave the circulation and enter the tissues in greater numbers than those of the wild-type control (4). However, once cells were in the tissues, the ability of the wild-type strain to undergo hyphal transformation was associated with higher mortality, despite the lower fungal burden in the tissues than that with mutant yeast forms (4). Further evidence to support this theory has come from in vivo experiments investigating tissue invasion and damage, performed by Saville et al. using a genetically engineered strain of C. albicans (SSY50-B) (71). This study demonstrated that yeast cells are capable of extravasating from blood vessels into the tissues without undergoing morphogenetic change. However, once cells were in the tissues, morphogenetic conversion from yeast to hyphal forms was crucial in causing tissue damage leading to death.
Such observations have led to a hypothesis in which circulating yeast cells bind to the endothelium and then transmigrate into the tissues before undergoing the hyphal transformation that results in tissue damage. In support of this, C. albicans migration from the circulation is very rapid (80 to 90% migration within 5 min) (16, 52), whereas hyphal transformation and endothelial cell damage may take several hours (66). Furthermore, because of their more compact shape and size, yeast cells may be better adapted for free dissemination within the circulation (30). In addition, the emergence of C. glabrata and C. parapsilosis as contenders for the second most common cause of disseminated candidiasis, after C. albicans (68), indicates that the ability to form true hyphae may not be essential for tissue adhesion, invasion, and pathogenesis among Candida species (30).
CANDIDATE CANDIDA ADHESINS AND THEIR ENDOTHELIAL LIGANDS
The cell wall of C. albicans is composed primarily of an inner structural layer of β1,3- and β1,6-glucans and chitin (a β1,4-linked polymer of N-acetylglucosamine) and a matrix primarily consisting of proteins (mannoproteins) that are heavily glycosylated with mannose-containing polysaccharides, sometimes called mannans (Fig. 1) (57). These take the form of short, linear, O-linked mannan side chains that stabilize the protein in the cell wall (15) and large, highly branched N-linked mannans (12). It is this outermost layer that represents the first point of contact between C. albicans and the endothelium, although not at bud scars, where the components of the inner layers of the cell wall are exposed (26). Proteins and carbohydrates in these outer layers may have a number of functions, including the ability to act as adhesion molecules, and over recent years several C. albicans cell wall components with the potential to mediate adhesion to the endothelium have been identified (Table 1). These include proteins with integrin-like properties (reviewed in references 32 and 36), Candida agglutinin-like sequence (ALS) gene products (72, 84), and mannans (57).
FIG. 1.
Cartoon of Candida cell wall structure.
TABLE 1.
Candida albicans-endothelial cell adhesion molecules
| C. albicans adhesin | Morphogenetic expressione | Endothelial ligand(s) (potential ligand) | Reference |
|---|---|---|---|
| αMβ2 (Mac-1; also called CD11b/CD18) | Y + PHa | CD54, CD102, CD14 | 9, 32, 48, 82 |
| αvβ3 (CD51/CD61) | Y + Hc | PECAM-1 (CD31) | 70, 73 |
| αvβ5 | Y + Hd | ? | 70, 73 |
| Als1 | Y + Hc | ? | 24, 25, 72, 83 |
| Als2 | Y + Ha | ? | 86 |
| Als3 | H | N-cadherin | 65, 83 |
| Als4 | Y + Ha,d | ? | 86 |
| Als5b | Y | ? | 72, 84 |
| Als6b | Y | ? | 72, 84 |
| Als7b | Y | ? | 84 |
| Als9 | Y | ? | 85 |
| N-linked mannosyl residues | All forms | MR | 58, 81 |
| O-linked mannosyl residues | All forms | TLR-4 | 3, 59, 60, 74 |
| Phospholipomannan | All forms | TLR-2 | 42 |
| β-Mannosides | All forms | Galectin-3 | 41 |
Temperature-dependent expression.
These molecules may be antiadhesive. See the text for further details.
Increased expression on hyphae compared to yeast form.
Decreased expression on hyphae compared to yeast form.
Y, expressed on the yeast form; PH, expressed on the pseudohyphal form; H, expressed on the hyphal form.
Cell wall protein adhesin candidates. (i) Integrin αMβ2-like adhesin.
Integrin analogues first gained interest in 1991, when Gustafson et al. found that adhesion of yeast forms of C. albicans to cultured monolayers of human endothelial cells was mediated in part by a candidal protein antigenically and structurally related to the leukocyte integrin αMβ2 (Mac-1, CD11b/CD18, CR3, or iC3b receptor) (9, 32, 48). They demonstrated the expression of the αMβ2-like molecule on yeast forms of C. albicans and showed that expression was increased by growth in 20 mM d-glucose, as opposed to 20 mM l-glutamine (32, 37). Furthermore, the adhesion of yeast forms of C. albicans to endothelial cells was significantly reduced by anti-αMβ2 antibodies or pretreatment of the Candida cells with purified iC3b. Expression of this ligand may be altered at different temperatures and in different morphogenetic forms of C. albicans (14, 28), and this may affect the ability of C. albicans to adhere to endothelium (80). αMβ2 has many different ligands, including iC3b, fibrinogen, factor X, urokinase receptor, CD14, CD23, CD54 (ICAM-1), CD102 (ICAM-2), CD242 (ICAM-4), heparin, haptoglobin, kininogen, and various microbial proteins (33). Of these molecules, only ICAM-1 and -2 are widely expressed on endothelial cells, although CD14 was recently identified on primary, but not passaged, cultures of human umbilical vein endothelial cells (49). There are no data on the role of CD14, CD102, or CD242 as a possible endothelial ligand for C. albicans adhesion, but Yokomura et al. (82) have shown that anti-CD54 monoclonal antibodies can partially inhibit the adhesion of yeast forms of C. albicans to rat pulmonary artery endothelial cells in vitro and significantly prolong the survival of rats injected intravenously with C. albicans. In certain circumstances, it is also possible that αMβ2 ligands such as fibrinogen, heparin and iC3b could in turn bind to endothelial cells and act as an intermediary in Candida-endothelial cell adhesion.
(ii) Integrin αvβ3- and αvβ5-like adhesins.
Two other integrin-like adhesins that may play a role in candidal adhesion to endothelium have been identified. They are homologs of the vitronectin-binding integrins αvβ3 (CD51/CD61) and αvβ5 (70, 73). Spreghini et al. (73) reported the expression of both of these adhesins on yeast forms of C. albicans, while Santoni et al. (70) showed that transformation to germ tubes was associated with a marked reduction in αvβ5-like integrin expression and an increase in αvβ3-like integrin expression. They also showed that adhesion of C. albicans germ tubes to endothelium was partially inhibited by anti-αvβ3 antibodies or an RGD sequence peptide. Heparin also inhibited germ tube adhesion, and when heparin treatment was combined with either anti-αvβ3 antibody or RGD peptide, the reduction in adhesion was greater still (70). More recently, it was shown that a candidal focal adhesion kinase-like protein may be involved in regulating yeast cell adhesion to endothelium via the αvβ3- or αvβ5-like adhesins (69) or in mediating intracellular signaling following ligand binding, much as focal adhesion kinase proteins are involved in integrin-mediated signaling in mammalian cells (10). Like its human counterpart, the candidal αvβ3-like adhesin has been shown to bind to vitronectin (70, 73), but other ligands for αvβ3 include CD31 (PECAM-1), fibronectin, fibrinogen, thrombospondin, von Willebrand factor, and RGD sequence peptides (33). CD31 is expressed by endothelial cells and could act as a direct ligand for Candida adhesion (39), while in certain circumstances it is possible that other ligands could act as a bridge in Candida-endothelial cell binding. Like its human counterpart, the αvβ5-like adhesin on C. albicans also binds vitronectin and RGD peptides (70, 73), but αvβ5 lacks a known endothelial cell target ligand and thus may not be involved directly with adherence to the endothelium.
(iii) ALS gene family.
The ALS (agglutinin-like sequence) gene family encodes a group of large glycosylphosphatidylinositol-linked cell surface glycoproteins (19). To date, eight ALS genes have been identified, including ALS1 to ALS7 and ALS9, all of which appear to have differing roles in adhesion and transmigration. These genes have gained particular interest recently, and evidence shows that ALS1-transformed Saccharomyces cerevisiae exhibits up to 100-fold greater adherence to endothelial cells (24, 25, 72), while Als1-deficient C. albicans hyphae exhibit reduced adhesion to endothelial cells (83). Similarly, S. cerevisiae transformed with ALS3 shows increased adhesion (72), while Als3-deficient hyphal forms of C. albicans exhibit defective adhesion to endothelial cells (83). The loss of Als9 from yeast forms of C. albicans (85) or the loss of Als4 and decreased expression of Als2 from 1-hour-old germ tubes (86) also inhibit the adhesion of mutant C. albicans strains to endothelial cells. In contrast, mutational analysis has shown that deletion of ALS5, ALS6, or ALS7 results in increased adhesion of yeast forms of C. albicans to endothelial cells, suggesting an antiadhesive role for these proteins (84). On the other hand, the protein Als5 has been found to mediate adhesion, along with Als1, when expressed in S. cerevisiae (72). To date, the only ligand for the ALS gene products that has been found on endothelial cells is N-cadherin, which binds to Als3 on C. albicans hyphae (65).
(iv) C4BP.
The complement protein regulator C4b binding protein (C4BP) is able to bind to both yeast and hyphal forms of C. albicans and is predominantly localized at the tip of the germ tube on hyphae (53). This binding is normally regarded as a survival mechanism that inhibits complement activation and the attachment of opsonins to the microbial surface. However, it may also enhance the adhesion of yeast forms of C. albicans to endothelial cells. It is not clear if this enhancement of adhesion by the C4BP coating occurs by activating other Candida adhesins or by acting as a bridge.
Cell wall carbohydrate adhesin candidates.
The outer cell wall proteins of Candida are heavily glycosylated with N- or O-linked mannosyl residues and have been found to be strongly involved in the recognition of C. albicans by the innate immune system (58). Indeed, some of these sugar residues provide conserved Candida-associated chemical signatures, known as pathogen-associated molecular patterns, by which the host is able to recognize the presence of the pathogen via host pattern recognition receptors (PRRs). In recent years, it has become apparent that specific host PRRs bind to and recognize specific mannosyl residues on C. albicans. For example, the mannose receptor (MR) recognizes and binds to N-linked mannosyl residues (81), while Toll-like receptor 4 (TLR-4) binds O-linked mannosyl residues (3, 59, 60, 74). Similarly, TLR-2 recognizes and binds phospholipomannan (42), and galectin-3 binds β-mannosides (41). As these mannosyl residues are part of the structure of the cell wall, they are expressed on all three different morphological forms of C. albicans. However, there is evidence to suggest that there are differences in the recognition of yeast and hyphae by TLR-2 and TLR-4 (77).
Although these PRRs are principally involved in the recognition of C. albicans by components of the host immune response, it is also possible that they are used by C. albicans to adhere to and transmigrate across the endothelial lining of blood vessels. Indeed, several studies have demonstrated the important role of the TLRs in experimental models of disseminated candidiasis. Netea et al. showed that TLR-4-defective C3H/HeJ mice have an increased susceptibility to disseminated candidiasis (60), and mice deficient in the universal TLR adaptor protein myeloid differentiation factor 88 (MyD88) are extremely susceptible to C. albicans infection (78). However, it has also been shown that TLR-4-deficient mice are more resistant to disseminated Candida infection (3). This is also the case for TLR-2-deficient mice, which have also been shown to be more resistant to disseminated candidiasis (60). However, the majority of the literature on knockout mice and disseminated candidiasis looks at susceptibility to infection and correlates it with the immune response without focusing on receptor expression on endothelial cells. To date, endothelial cells have been shown to express a number of PRRs, including the MR, TLRs, and galectins. The MR was the first receptor on the surfaces of macrophages to be described as a mannan receptor, and it recognizes oligosaccharides that terminate in mannose, fucose, and N-acetylglucosamine. It is also expressed on subtypes of dendritic cells and endothelial cells from certain vascular beds, including human dermal microvascular endothelial cells but not human umbilical vein endothelial cells (31). So far, 10 TLRs have been found, of which 7 or 8 are expressed on unstimulated endothelial cells (23). However, upon stimulation with proinflammatory cytokines, all 10 TLRs are expressed. Perhaps most importantly for interactions with C. albicans, endothelial cells express TLR-2 and TLR-4 (61). TLR-4 is expressed constitutively at a higher level than that of TLR-2 by endothelial cells (17). However, the expression of both is significantly upregulated by stimulation with gamma interferon or bacterial lipopolysaccharide (18). It is also notable that the expression of TLR-2 on endothelial cells is strongly affected by the effects of flow on the endothelial cells (13). The galectins are a family of 15 carbohydrate binding proteins with high affinities for β-galactosides, extracellular glycoproteins, and glycolipids. So far, expression of galectin-1, -3, and -9 has been found on cultured endothelial cells (75), but only galectin-3 has been found to recognize C. albicans (41). Other PRRs that have been found to be involved in the recognition of C. albicans include DC-SIGN, αMβ2, FcγR, and dectin-1, but so far these receptors have not been found to be expressed on endothelial cells.
With so many different cell wall components having the potential to mediate adhesion of C. albicans to the endothelium, it seems that there could be a number of different mechanisms of adhesion. This may have consequences for the development of therapies aimed at blocking adhesion, because with so many molecules potentially playing a role, blocking only one could simply result in its role being taken up by other molecules. However, to investigate this further, more research is needed on the molecules involved in adhesion of C. albicans to the endothelial lining of blood vessels.
STATIC VERSUS FLOW ADHESION ASSAYS
The majority of the above studies that have directly explored candidal adhesion to endothelium were performed by using static in vitro assays where C. albicans was left in prolonged contact with cultured monolayers of endothelial cells. This is very different from the fleeting interactions C. albicans has with endothelial cells under the conditions of shear stress and flow that occur in blood vessels in vivo. Numerous studies with other cells and microorganisms have shown that static assays do not replicate the dynamic interactions that occur with endothelium under conditions of flow and are poor at elucidating the contributions of specific adhesion molecules (29, 47). Only a few studies have attempted to study candidal adhesion to synthetic substrata under conditions of flow. These have shown that there are significant differences in the adhesion of Candida to the same substrata when the assays are performed under static and flow conditions (8, 54). To date, only one study has attempted to examine the adhesion of Candida to endothelium under conditions of flow (29). Glee et al. found that under shear flow, C. albicans formed rapid, tight adhesions in less than 67 ms. This is much quicker than in static assays and is comparable to the rapid adhesion interactions that occur between leukocytes and endothelial cells. In view of this, it is difficult to fully evaluate the contributions of the mechanisms and adhesion molecules discussed above to the adhesion of C. albicans to endothelium in vivo, as none have been studied under conditions of flow.
TRANSMIGRATION
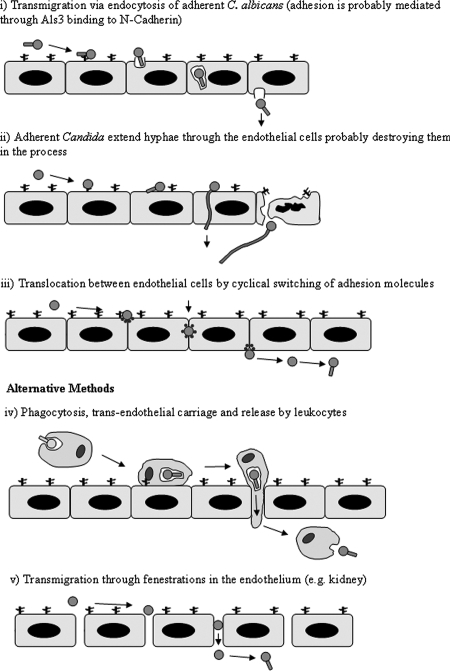
After adhesion of C. albicans to the endothelial lining of blood vessels, the second step in the migration of C. albicans from the circulation into the tissues is transmigration across the endothelial barrier. This step may involve some of the same molecules used for adhesion but could involve others. Transmigration is hard to research in isolation, which explains why there is little information on specific methods of Candida-endothelial cell transmigration. Even so, there are several proposed mechanisms for Candida transmigration across the endothelium (Fig. 2). The first mechanism proposes that endothelial cells endocytose adherent organisms and allow their passage through to the abluminal surface of the endothelial cell layer (Fig. 2, panel i). It is this mechanism that has gained the most interest and for which a model has evolved to explain how candidal hyphae adhere to and then induce endothelial cells to endocytose them (19, 21). In this model, C. albicans hyphae bind to N-cadherin and other, as yet unidentified proteins on the endothelial cell surface via the candidal protein Als3 (65). This adhesive interaction induces tyrosine phosphorylation of unidentified intracellular endothelial cell proteins (2), causing microfilament rearrangement to produce pseudopods, which initiate the endocytosis of adherent hyphal forms of C. albicans (22, 65). However, endothelial endocytosis of C. albicans is not restricted to hyphal forms, and strains that do not undergo hyphal change and cause little endothelial cell damage are endocytosed to a significant degree (40, 51, 64). Since the Als3 protein is predominantly expressed on candidal hyphae (65), this could involve other adhesin-endothelial ligand pairs. There is also evidence that suggests that adherent yeast forms could penetrate endothelial cells, damaging them in the process, without undergoing morphogenetic change allowing them to cross the endothelial barrier (44). Another proposed mechanism of trans-endothelial cell migration of adherent C. albicans involves the extension of penetrating hyphal processes through the endothelial cells, likely destroying them in the process, much as fungal hyphae ramify through other tissues (Fig. 2, panel ii). Alternatively, a further proposal suggests that adherent C. albicans cells may pass between adjacent endothelial cells as a result of translocation and cyclical switching of adhesion molecules at the junction between endothelial cells, in a manner similar to that of leukocyte and tumor cell trans-endothelial cell migration (Fig. 2, panel iii).
FIG. 2.
Possible mechanisms for Candida albicans transmigration of endothelium.
Two alternative methods of transmigration across the endothelium that may not require prior adhesion of C. albicans to the endothelial cell surface have also been proposed. The first mechanism proposes that organisms phagocytosed by leukocytes are transported across the endothelial barrier inside the leukocytes (Fig. 2, panel iv). It is well known that leukocytes are able to cross the endothelium, between adjacent endothelial cells, by diapedesis and cyclical switching of adhesion molecules. Furthermore, there is evidence of C. albicans being found inside circulating leukocytes in systemic candidiasis (56). However, it is unlikely that this represents the only mechanism for candidal transmigration, since neutropenia is a major risk factor for invasive disease (35, 46). The second mechanism, which may or may not require prior adhesion, suggests that circulating Candida cells simply pass through endothelial fenestrations between adjacent endothelial cells in vascular beds such as the kidney (Fig. 2, panel v).
Some of these mechanisms may operate only for the yeast, pseudohyphal, or hyphal form of C. albicans, some may work for all forms, and others may require morphogenetic change for transmigration to occur. As with C. albicans adhesion to endothelial cells, there is clearly much more research required in order to elucidate the precise mechanism by which C. albicans migrates across the endothelium and into the tissues. Additionally, as with leukocyte and tumor cell transmigration, the validity of these mechanisms may become apparent only when transmigration is studied in vivo or in situations where the endothelium is subject to conditions of flow (29, 47).
CONCLUSION
In summary, the interaction of C. albicans with the endothelial lining of blood vessels and its invasion of the tissues involve a complex series of processes that is further complicated by the role played by the morphogenetic conversion of C. albicans. There is still a large amount of work required to clarify these processes. Furthermore, it is important that this work be performed under conditions that replicate the fleeting contacts of C. albicans with the endothelium and the dynamic conditions of flow that occur in vivo. Nonetheless, understanding these mechanisms may be critical in identifying a means for preventing Candida invasion of the tissues and its lethal sequelae in systemic candidiasis.
Acknowledgments
The work presented here was funded in part by grant R21 AI065549-01A1 from the National Institute of Allergy and Infectious Diseases to M.H.T.
The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Editor: J. B. Kaper
Footnotes
Published ahead of print on 23 June 2008.
REFERENCES
- 1.Baine, W. B., M. G. Koenig, and J. S. Goodman. 1974. Clearance of Candida albicans from the bloodstream of rabbits. Infect. Immun. 101420-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belanger, P. H., D. A. Johnston, R. A. Fratti, M. Zhang, and S. G. Filler. 2002. Endocytosis of Candida albicans by vascular endothelial cells is associated with tyrosine phosphorylation of specific host cell proteins. Cell. Microbiol. 4805-812. [DOI] [PubMed] [Google Scholar]
- 3.Bellocchio, S., C. Montagnoli, S. Bozza, R. Gaziano, G. Rossi, S. S. Mambula, A. Vecchi, A. Mantovani, S. M. Levitz, and L. Romani. 2004. The contribution of the Toll-like/IL-1 receptor superfamily to innate and adaptive immunity to fungal pathogens in vivo. J. Immunol. 1723059-3069. [DOI] [PubMed] [Google Scholar]
- 4.Bendel, C. M., D. J. Hess, R. M. Garni, M. Henry-Stanley, and C. L. Wells. 2003. Comparative virulence of Candida albicans yeast and filamentous forms in orally and intravenously inoculated mice. Crit. Care Med. 31501-507. [DOI] [PubMed] [Google Scholar]
- 5.Bodey, G. P., M. Buckley, Y. S. Sathe, and E. J. Freireich. 1966. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann. Intern. Med. 64328-340. [DOI] [PubMed] [Google Scholar]
- 6.Braun, B. R., W. S. Head, M. X. Wang, and A. D. Johnson. 2000. Identification and characterization of TUP1-regulated genes in Candida albicans. Genetics 15631-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braun, B. R., and A. D. Johnson. 1997. Control of filament formation in Candida albicans by the transcriptional repressor TUP1. Science 277105-109. [DOI] [PubMed] [Google Scholar]
- 8.Busscher, H. J., and H. C. van der Mei. 1995. Use of flow chamber devices and image analysis methods to study microbial adhesion. Methods Enzymol. 253455-477. [DOI] [PubMed] [Google Scholar]
- 9.Calderone, R., and P. C. Braun. 1991. Adherence and receptor relationships of Candida albicans. Microbiol. Rev. 551-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark, E. A., and J. S. Brugge. 1995. Integrins and signal transduction pathways: the road taken. Science 268233-239. [DOI] [PubMed] [Google Scholar]
- 11.Cole, G. T., A. A. Halawa, and E. J. Anaissie. 1996. The role of the gastrointestinal tract in hematogenous candidiasis: from the laboratory to the bedside. Clin. Infect. Dis. 22S73-S88. [DOI] [PubMed] [Google Scholar]
- 12.Cutler, J. E. 2001. N-glycosylation of yeast, with emphasis on Candida albicans. Med. Mycol. 3975-86. [PubMed] [Google Scholar]
- 13.Dunzendorfer, S., H. K. Lee, and P. S. Tobias. 2004. Flow-dependent regulation of endothelial Toll-like receptor 2 expression through inhibition of SP1 activity. Circ. Res. 95684-691. [DOI] [PubMed] [Google Scholar]
- 14.Eigentler, A., T. F. Schulz, C. Larcher, E. M. Breitwieser, B. L. Myones, A. L. Petzer, and M. P. Dierich. 1989. C3bi-binding protein on Candida albicans: temperature-dependent expression and relationship to human complement receptor type 3. Infect. Immun. 57616-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ernst, J. F., and S. K. Prill. 2001. O-glycosylation. Med. Mycol. 3967-74. [PubMed] [Google Scholar]
- 16.Evans, Z. A., and D. N. Mardon. 1977. Organ localization in mice challenged with a typical Candida albicans strain and a pseudohyphal variant. Proc. Soc. Exp. Biol. Med. 155234-238. [DOI] [PubMed] [Google Scholar]
- 17.Faure, E., O. Equils, P. A. Sieling, L. Thomas, F. X. Zhang, C. J. Kirschning, N. Polentarutti, M. Muzio, and M. Arditi. 2000. Bacterial lipopolysaccharide activates NF-kappaB through Toll-like receptor 4 (TLR-4) in cultured human dermal endothelial cells. Differential expression of TLR-4 and TLR-2 in endothelial cells. J. Biol. Chem. 27511058-11063. [DOI] [PubMed] [Google Scholar]
- 18.Faure, E., L. Thomas, H. Xu, A. Medvedev, O. Equils, and M. Arditi. 2001. Bacterial lipopolysaccharide and IFN-γ induce Toll-like receptor 2 and Toll-like receptor 4 expression in human endothelial cells: role of NF-κB activation. J. Immunol. 1662018-2024. [DOI] [PubMed] [Google Scholar]
- 19.Filler, S. G. 2006. Candida-host cell receptor-ligand interactions. Curr. Opin. Microbiol. 9333-339. [DOI] [PubMed] [Google Scholar]
- 20.Filler, S. G., B. O. Ibe, P. M. Luckett, J. U. Raj, and J. E. J. Edwards. 1991. Candida albicans stimulates endothelial cell eicosanoid production. J. Infect. Dis. 164928-935. [DOI] [PubMed] [Google Scholar]
- 21.Filler, S. G., and D. C. Sheppard. 2006. Fungal invasion of normally non-phagocytic host cells. PLoS Pathog. 2e129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Filler, S. G., J. N. Swerdloff, C. Hobbs, and P. M. Luckett. 1995. Penetration and damage of endothelial cells by Candida albicans. Infect. Immun. 63976-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fitzner, N., S. Clauberg, F. Essmann, J. Liebmann, and V. Kolb-Bachofen. 2008. Human skin endothelial cells can express all 10 TLR genes and respond to respective ligands. Clin. Vaccine Immunol. 15138-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu, Y., A. S. Ibrahim, D. C. Sheppard, Y. C. Chen, S. W. French, J. E. Cutler, S. G. Filler, and J. E. J. Edwards. 2002. Candida albicans Als1p: an adhesin that is a downstream effector of the EFG1 filamentation pathway. Mol. Microbiol. 4461-72. [DOI] [PubMed] [Google Scholar]
- 25.Fu, Y., G. Rieg, W. A. Fonzi, P. H. Belanger, J. E. J. Edwards, and S. G. Filler. 1998. Expression of the Candida albicans gene ALS1 in Saccharomyces cerevisiae induces adherence to endothelial and epithelial cells. Infect. Immun. 661783-1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gantner, B. N., R. M. Simmons, and D. M. Underhill. 2005. Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. EMBO J. 241277-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghannoum, M. A., S. G. Filler, A. S. Ibrahim, Y. Fu, and J. E. J. Edwards. 1992. Modulation of interactions of Candida albicans and endothelial cells by fluconazole and amphotericin B. Antimicrob. Agents Chemother. 362239-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gilmore, B. J., E. M. Retsinas, J. S. Lorenz, and M. K. Hostetter. 1988. An iC3b receptor on Candida albicans: structure, function, and correlates for pathogenicity. J. Infect. Dis. 15738-46. [DOI] [PubMed] [Google Scholar]
- 29.Glee, P. M., J. E. Cutler, E. E. Benson, R. F. Bargatze, and K. C. Hazen. 2001. Inhibition of hydrophobic protein-mediated Candida albicans attachment to endothelial cells during physiologic shear flow. Infect. Immun. 692815-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gow, N. A., A. J. Brown, and F. C. Odds. 2002. Fungal morphogenesis and host invasion. Curr. Opin. Microbiol. 5366-371. [DOI] [PubMed] [Google Scholar]
- 31.Groger, M., W. Holnthoner, D. Maurere, S. Lechleitner, K. Wolff, B. B. Mayr, W. Lubitz, and P. Petzelbauer. 2000. Dermal microvascular endothelial cells express the 180-kDa macrophage mannose receptor in situ and in vitro. J. Immunol. 1655428-5434. [DOI] [PubMed] [Google Scholar]
- 32.Gustafson, K. S., G. M. Vercellotti, C. M. Bendel, and M. K. Hostetter. 1991. Molecular mimicry in Candida albicans. Role of an integrin analogue in adhesion of the yeast to human endothelium. J. Clin. Investig. 871896-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haas, T. A., and E. F. Plow. 1994. Integrin-ligand interactions: a year in review. Curr. Opin. Cell Biol. 6656-662. [DOI] [PubMed] [Google Scholar]
- 34.Hawser, S. P., and L. J. Douglas. 1994. Biofilm formation by Candida species on the surface of catheter materials in vitro. Infect. Immun. 62915-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hope, W. W., G. L. Drusano, C. B. Moore, A. Sharp, A. Louie, T. J. Walsh, D. W. Denning, and P. A. Warn. 2007. Effect of neutropenia and treatment delay on the response to antifungal agents in experimental disseminated candidiasis. Antimicrob. Agents Chemother. 51285-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hostetter, M. K. 1994. Adhesins and ligands involved in the interaction of Candida spp. with epithelial and endothelial surfaces. Clin. Microbiol. Rev. 729-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hostetter, M. K., J. S. Lorenz, L. Preus, and K. E. Kendrick. 1990. The iC3b receptor on Candida albicans: subcellular localization and modulation of receptor expression by glucose. J. Infect. Dis. 161761-768. [DOI] [PubMed] [Google Scholar]
- 38.Iannini, P. B., G. D. Arai, and F. M. LaForce. 1977. Vascular clearance of blastospore and pseudomycelial phase Candida albicans. Sabouraudia 15201-205. [PubMed] [Google Scholar]
- 39.Jackson, D. E. 2003. The unfolding tale of PECAM-1. FEBS Lett. 5407-14. [DOI] [PubMed] [Google Scholar]
- 40.Jong, A. Y., M. F. Stins, S. H. Huang, S. H. Chen, and K. S. Kim. 2001. Traversal of Candida albicans across human blood-brain barrier in vitro. Infect. Immun. 694536-4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jouault, T., M. El Abed-El Behi, M. Martinez-Esparza, L. Breuih, P. A. Trinel, M. Chamaillard, F. Trottein, and D. Poulain. 2006. Specific recognition of Candida albicans by macrophages requires galectin-3 to discriminate Saccharomyces cerevisiae and needs association with TLR2 for signaling. J. Immunol. 1774679-4687. [DOI] [PubMed] [Google Scholar]
- 42.Jouault, T., S. Ibata-Ombetta, O. Takeuchi, P. A. Trinel, P. Sacchetti, P. Lefebvre, S. Akira, and D. Poulain. 2003. Candida albicans phospholipomannan is sensed through Toll-like receptors. J. Infect. Dis. 188165-172. [DOI] [PubMed] [Google Scholar]
- 43.Kimura, L. H., and N. N. Pearsall. 1980. Relationship between germination of Candida albicans and increased adherence to human buccal epithelial cells. Infect. Immun. 28464-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klotz, S. A., D. J. Drutz, J. L. Harrison, and M. Huppert. 1983. Adherence and penetration of vascular endothelium by Candida yeasts. Infect. Immun. 42374-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klotz, S. A., and R. L. Smith. 1991. A fibronectin receptor on Candida albicans mediates adherence of the fungus to extracellular matrix. J. Infect. Dis. 163604-610. [DOI] [PubMed] [Google Scholar]
- 46.Koh, A. Y., J. R. Kohler, K. T. Coggshall, N. Van Rooijen, and G. B. Pier. 2008. Mucosal damage and neutropenia are required for Candida albicans dissemination. PLoS Pathog. 4e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lawrence, M. B. 1999. Selectin-carbohydrate interactions in shear flow. Curr. Opin. Chem. Biol. 3659-664. [DOI] [PubMed] [Google Scholar]
- 48.Lee, K. H., M. S. Yoon, and W. H. Chun. 1997. The effects of monoclonal antibodies against iC3b receptors in mice with experimentally induced disseminated candidiasis. Immunology 92104-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lloyd, K. L., and P. Kubes. 2006. GPI-linked endothelial CD14 contributes to the detection of LPS. Am. J. Physiol. Heart Circ. Physiol. 291H473-H481. [DOI] [PubMed] [Google Scholar]
- 50.Lo, H. J., J. R. Kohler, B. DiDomenico, D. Loebenberg, A. Cacciapuoti, and G. R. Fink. 1997. Nonfilamentous C. albicans mutants are avirulent. Cell 1997939-949. [DOI] [PubMed] [Google Scholar]
- 51.Lossinsky, A. S., A. Jong, M. Fiala, M. Mukhtar, K. F. Buttle, and M. Ingram. 2006. The histopathology of Candida albicans invasion in neonatal rat tissues and in the human blood-brain barrier in culture revealed by light, scanning, transmission and immunoelectron microscopy. Histol. Histopathol. 211029-1041. [DOI] [PubMed] [Google Scholar]
- 52.MacCallum, D. M., and F. C. Odds. 2005. Temporal events in the intravenous challenge model for experimental Candida albicans infections in female mice. Mycoses 48151-161. [DOI] [PubMed] [Google Scholar]
- 53.Meri, T., A. M. Blom, A. Hartmann, D. Lenk, S. Meri, and P. F. Zipfel. 2004. The hyphal and yeast forms of Candida albicans bind the complement regulator C4b-binding protein. Infect. Immun. 726633-6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Millsap, K. W., R. Bos, H. J. Busscher, and H. C. van der Mei. 1999. Surface aggregation of Candida albicans on glass in the absence and presence of adhering Streptococcus gordonii in a parallel-plate flow chamber: a surface thermodynamical analysis based on acid-base interactions. J. Colloid Interface Sci. 212495-502. [DOI] [PubMed] [Google Scholar]
- 55.Mouy, R., A. Fischer, E. Vilmer, R. Seger, and C. Griscelli. 1989. Incidence, severity and prevention of infections in chronic granulomatous disease. J. Pediatr. 114555-560. [DOI] [PubMed] [Google Scholar]
- 56.Nadir, E., and M. Kaufshtein. 2005. Images in clinical medicine. Candida albicans in a peripheral-blood smear. N. Engl. J. Med. 353e9. [DOI] [PubMed] [Google Scholar]
- 57.Netea, M. G., G. D. Brown, B. J. Kullberg, and N. A. Gow. 2008. An integrated model of the recognition of Candida albicans by the innate immune system. Nat. Rev. Microbiol. 667-78. [DOI] [PubMed] [Google Scholar]
- 58.Netea, M. G., N. A. Gow, C. A. Munro, S. Bates, C. Collins, G. Ferwerda, R. P. Hobson, G. Bertram, H. B. Hughes, T. Jansen, L. Jacobs, E. T. Buurman, K. Gijzen, D. L. Williams, R. Torensma, A. McKinnon, D. M. MacCallum, F. C. Odds, J. W. M. Van der Meer, A. J. P. Brown, and B. J. Kullberg. 2006. Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. J. Clin. Investig. 1161642-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Netea, M. G., C. A. Van Der Graaf, J. W. M. Van der Meer, and B. J. Kullberg. 2004. Toll-like receptors and the host defense against microbial pathogens: bringing specificity to the innate-immune system. J. Leukoc. Biol. 75749-755. [DOI] [PubMed] [Google Scholar]
- 60.Netea, M. G., C. A. Van Der Graaf, A. G. Vonk, I. Verschueren, J. W. M. Van Der Meer, and B. J. Kullberg. 2002. The role of Toll-like receptor (TLR) 2 and TLR4 in the host defense against disseminated candidiasis. J. Infect. Dis. 1851483-1489. [DOI] [PubMed] [Google Scholar]
- 61.Opitz, B., S. Hippenstiel, J. Eitel, and N. Suttorp. 2007. Extra- and intracellular innate immune recognition in endothelial cells. Thromb. Haemost. 98319-326. [PubMed] [Google Scholar]
- 62.Parker, J. C. J., J. J. McCloskey, and K. A. Knauer. 1976. Pathobiologic features of human candidiasis. A common deep mycosis of the brain, heart and kidney in the altered host. Am. J. Clin. Pathol. 65991-1000. [DOI] [PubMed] [Google Scholar]
- 63.Pfaller, M. A. 1996. Nosocomial candidiasis: emerging species, reservoirs and modes of transmission. Clin. Infect. Dis. 22S89-S94. [DOI] [PubMed] [Google Scholar]
- 64.Phan, Q. T., P. H. Belanger, and S. G. Filler. 2000. Role of hyphal formation in interactions of Candida albicans with endothelial cells. Infect. Immun. 683485-3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Phan, Q. T., C. L. Myers, Y. Fu, D. C. Sheppard, M. R. Yeaman, W. H. Welch, A. S. Ibrahim, J. E. J. Edwards, and S. G. Filler. 2007. Als3 is a Candida albicans invasin that binds to cadherins and induces endocytosis by host cells. PLoS Biol. 5e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rotrosen, D., J. E. J. Edwards, T. R. Gibson, J. C. Moore, A. H. Cohen, and I. Green. 1985. Adherence of Candida to cultured vascular endothelial cells: mechanisms of attachment and endothelial cell penetration. J. Infect. Dis. 1521264-1274. [DOI] [PubMed] [Google Scholar]
- 67.Sanchez, A. A., D. A. Johnston, C. Myers, J. E. J. Edwards, A. P. Mitchell, and S. G. Filler. 2004. Relationship between Candida albicans virulence during experimental hematogenously disseminated infection and endothelial cell damage in vitro. Infect. Immun. 72598-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sanglard, D., and F. C. Odds. 2002. Resistance of Candida species to antifungal agents: molecular mechanisms and clinical consequences. Lancet Infect. Dis. 273-85. [DOI] [PubMed] [Google Scholar]
- 69.Santoni, G., R. Lucciarini, C. Amantini, J. Jacobelli, E. Spreghini, P. Ballarini, M. Piccoli, and A. Gismondi. 2002. Candida albicans expresses a focal adhesion kinase-like protein that undergoes increased tyrosine phosphorylation upon yeast cell adhesion to vitronectin and the EA.hy 926 human endothelial cell line. Infect. Immun. 703804-3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Santoni, G., E. Spreghini, R. Lucciarini, C. Amantini, and M. Piccoli. 2001. Involvement of alpha(v)beta3 integrin-like receptor and glycosaminoglycans in Candida albicans germ tube adhesion to vitronectin and to a human endothelial cell line. Microb. Pathog. 31159-172. [DOI] [PubMed] [Google Scholar]
- 71.Saville, S. P., A. L. Lazzell, C. Monteagudo, and J. L. Lopez-Ribot. 2003. Engineered control of cell morphology in vivo reveals distinct roles for yeast and filamentous forms of Candida albicans during infection. Eukaryot. Cell 21053-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sheppard, D. C., M. R. Yeaman, W. H. Welch, Q. T. Phan, Y. Fu, A. S. Ibrahim, S. G. Filler, M. Zhang, A. J. Waring, and J. E. J. Edwards. 2004. Functional and structural diversity in the Als protein family of Candida albicans. J. Biol. Chem. 27930480-30489. [DOI] [PubMed] [Google Scholar]
- 73.Spreghini, E., A. Gismondi, M. Piccoli, and G. Santoni. 1999. Evidence for alphavbeta3 and alphavbeta5 integrin-like vitronectin (VN) receptors in Candida albicans and their involvement in yeast cell adhesion to VN. J. Infect. Dis. 180156-166. [DOI] [PubMed] [Google Scholar]
- 74.Tada, H., E. Nemoto, H. Shimauchi, T. Watanabe, T. Mikami, T. Matsumoto, N. Ohno, H. Tamura, K. Shibata, S. Akashi, K. Miyake, S. Sugawara, and H. Takada. 2002. Saccharomyces cerevisiae and Candida albicans-derived mannan induced production of tumor necrosis factor alpha by human monocytes in a CD14- and Toll-like receptor 4-dependent manner. Microbiol. Immunol. 46503-512. [DOI] [PubMed] [Google Scholar]
- 75.Thijssen, V. L., F. Poirier, L. G. Baum, and A. W. Griffioen. 2007. Galectins in the tumor endothelium: opportunities for combined cancer therapy. Blood 1102819-2827. [DOI] [PubMed] [Google Scholar]
- 76.Todeschini, G. T. 1997. Treatment of candidiasis: a perspective on recent advances and future challenges. Int. J. Infect. Dis. 1S37-S41. [Google Scholar]
- 77.van Der Graaf, C. A., M. G. Netea, I. Verschueren, J. W. M. van der Meer, and B. J. Kullberg. 2005. Differential cytokine production and Toll-like receptor signaling pathways by Candida albicans blastoconidia and hyphae. Infect. Immun. 737458-7464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Villamon, E., D. Gozalbo, P. Roig, C. Murciano, J. E. O'Connor, D. Fradelizi, and M. L. Gil. 2004. Myeloid differentiation factor 88 (MyD88) is required for murine resistance to Candida albicans and is critically involved in Candida-induced production of cytokines. Eur. Cytokine Netw. 15263-271. [PubMed] [Google Scholar]
- 79.Wilson, L. S., C. M. Reyes, M. Stolpman, J. Speckman, K. Allen, and J. Beney. 2002. The direct cost and incidence of systemic fungal infections. Value Health 526-34. [DOI] [PubMed] [Google Scholar]
- 80.Wurzner, R., M. Langgartner, L. Spotl, A. Eder, H. Bujdakova, K. Schroppel, and M. P. Dierich. 1996. Temperature-dependent surface expression of the beta-2-integrin analogue of Candida albicans and its role in adhesion to the human endothelium. Exp. Clin. Immunogenet. 13161-172. [PubMed] [Google Scholar]
- 81.Yamamoto, Y., T. W. Klein, and H. Friedman. 1997. Involvement of mannose receptor in cytokine interleukin-1β (IL-1β), IL-6, and granulocyte-macrophage colony-stimulating factor responses, but not in chemokine macrophage inflammatory protein 1β (MIP-1β), MIP-2, and KC responses, caused by attachment of Candida albicans to macrophages. Infect. Immun. 651077-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yokomura, I., Y. Iwasaki, K. Nagata, M. Nakanishi, A. Natsuhara, H. Harada, Y. Kubota, and M. Ueda. 2001. Role of intercellular adhesion molecule 1 in acute lung injury induced by candidemia. Exp. Lung Res. 27417-431. [DOI] [PubMed] [Google Scholar]
- 83.Zhao, X., S. H. Oh, G. Cheng, C. B. Green, J. A. Nuessen, K. Yeater, R. P. Leng, A. J. Brown, and L. L. Hoyer. 2004. ALS3 and ALS8 represent a single locus that encodes a Candida albicans adhesin; functional comparisons between Als3p and Als1p. Microbiology 1502415-2428. [DOI] [PubMed] [Google Scholar]
- 84.Zhao, X., S. H. Oh, and L. L. Hoyer. 2007. Deletion of ALS5, ALS6 or ALS7 increases adhesion of Candida albicans to human vascular endothelial and buccal epithelial cells. Med. Mycol. 45429-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhao, X., S. H. Oh, and L. L. Hoyer. 2007. Unequal contribution of ALS9 alleles to adhesion between Candida albicans and human vascular endothelial cells. Microbiology 1532342-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhao, X., S. H. Oh, K. Yeater, and L. L. Hoyer. 2005. Analysis of the Candida albicans Als2p and Als4p adhesins suggests the potential for compensatory function within the Als family. Microbiology 1511619-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]