Abstract
Summary: To better understand the underlying mechanisms of aerovirology, accurate sampling of airborne viruses is fundamental. The sampling instruments commonly used in aerobiology have also been used to recover viruses suspended in the air. We reviewed over 100 papers to evaluate the methods currently used for viral aerosol sampling. Differentiating infections caused by direct contact from those caused by airborne dissemination can be a very demanding task given the wide variety of sources of viral aerosols. While epidemiological data can help to determine the source of the contamination, direct data obtained from air samples can provide very useful information for risk assessment purposes. Many types of samplers have been used over the years, including liquid impingers, solid impactors, filters, electrostatic precipitators, and many others. The efficiencies of these samplers depend on a variety of environmental and methodological factors that can affect the integrity of the virus structure. The aerodynamic size distribution of the aerosol also has a direct effect on sampler efficiency. Viral aerosols can be studied under controlled laboratory conditions, using biological or nonbiological tracers and surrogate viruses, which are also discussed in this review. Lastly, general recommendations are made regarding future studies on the sampling of airborne viruses.
INTRODUCTION
Any microorganism, including viruses, can become airborne. Contaminated material can be aerosolized in many different ways, ranging from wind to human and animal activities such as sneezing, mechanical processes, etc. If the aerodynamic size of an infectious particle is appropriate, it can remain airborne, come into contact with humans or animals, and potentially cause an infection. The probability of an airborne microorganism-laden particle causing an infection depends on its infectious potential and its ability to resist the stress of aerosolization.
Airborne microorganisms can represent major health and economic risks to human and animal populations. Appropriate preventive actions can be taken if the threat posed by such microorganisms is better understood. Authorities need to be aware of the nature, concentration, and pathogenicity of airborne microorganisms to better control them. This information can be obtained by using various air sampling methods, each of which has its particular advantages and disadvantages. Many types of samplers and analytical methods have been used over the years (Fig. 1). The purpose of this review is to present the principles underlying viral aerosol sampling methods, with their advantages and pitfalls.
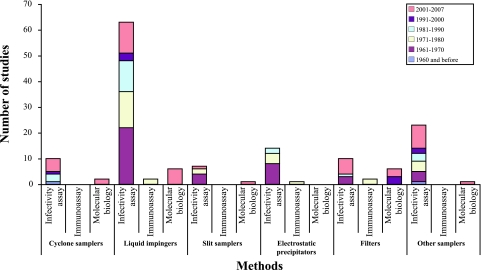
FIG. 1.
Airborne virus sampling studies, according to date and analysis method.
CONTACT VERSUS AEROSOLS
The route of transmission of infections is not always easily determined in an environment with undefined parameters. Infection by direct contact can occur when infected hosts are in close proximity with a susceptible population. On the other hand, infected hosts can transmit the disease without direct contact. Moreover, many microorganisms, including viruses (110), can remain infectious outside their hosts for prolonged periods of time, and this can lead to infections by indirect contact. For example, a surface can become contaminated by deposited infectious droplets and eventually cause the infection of susceptible hosts coming into contact with it. The probability of airborne transmission of an infectious disease can be determined by conducting epidemiological studies (145) and/or by analyzing the microbiological content of air samples.
EPIDEMIOLOGICAL EVIDENCE OF AIRBORNE SPREAD OF VIRUSES
Studies on the aerobiology of infectious diseases, including viral diseases, have been rather limited (115). This is due mainly to the difficulty in collecting and analyzing airborne biological contaminants, which is an even greater problem for viruses. This technical challenge has made epidemiological studies particularly useful. While data inferred from epidemiological studies using computer-based analytical methods are more equivocal than those from air sampling coupled with microbial analyses, epidemiological studies can provide very valuable information.
Many epidemiological studies have proposed that viruses can spread from one host to another by using air for transport. The capacity of the foot-and-mouth disease (FMD) virus to spread by air has been studied and reviewed (36) over the years and is now being investigated using computer models. One of these models predicted that in a “worst-case scenario” of an FMD outbreak, cattle could be infected as far as 20 to 300 kilometers away from an infectious source (37). Dispersion models based on meteorological data and information on the spread of FMD at the beginning of the 1967-1968 epidemic in the United Kingdom strongly suggested that the infection may have spread by the airborne route over a distance of 60 km (59). Airborne transmission of FMD was also reported to have occurred during the 1982-1983 epidemic in Denmark. In the latter case, an analysis of epidemiological dynamics using molecular methods coupled with meteorological data concluded that the infection had spread by air over a distance of 70 km (27). Similarly, the results of a Canadian study on an FMD epidemic reported that airborne viruses may have traveled 20 km downwind from the contaminated source (29). Nevertheless, a recent study on the O/UKG/2001 strain of FMD virus indicated that it does not spread efficiently between sheep by the airborne route. However, other strains may behave differently (134).
In 2001, a Norwalk-like virus outbreak in a school in the United Kingdom was believed to have been caused by airborne transmission (89). A similar occurrence has also been reported for a hotel restaurant (88). A retrospective cohort study conducted after a severe acute respiratory syndrome (SARS) epidemic in Hong Kong in 2003 suggested that airborne spread may have played an important role in the transmission of the disease (146). The same mode of transmission was also hypothesized in other studies of SARS (87, 104, 145). Aerosols may also be responsible for the transmission of other viral diseases (63, 83, 113).
COMMON SOURCES OF AIRBORNE VIRUSES
A virus can multiply only within a host cell. Infected cells can spread viruses directly into the surrounding air (primary aerosolization) or to fluids and surfaces, which can become sources for airborne transmission (secondary aerosolization). Secondary aerosolization can occur for any virus, predominantly when air displacements or movements around contaminated surfaces or fluids disperse the viruses into the air. It can also occur by liquid splashes, which can aerosolize viruses in liquids or on surfaces. In fact, almost any kind of disturbance of infected organisms or materials, even the bursting of bubbles in seawater (9), can produce airborne, virus-laden particles.
The most important aerosol source representing a risk for human health is humans themselves. Since the interspecies barrier is not a factor in the transmission of infections from human to human, aerosol-mediated infections from human sources can occur in everyday situations. Human infections through viral aerosol sources have been studied in various environments, including office buildings (102), hospitals (3, 10, 11, 13, 19, 41, 92, 95, 117, 126), restaurants (88), and schools (89). The mechanisms of dispersion of infectious aerosols originating from humans are described in detail in a recent review (98). It is important to recognize that viruses can be spread by airborne particles released by humans but also by other means. Simply flushing a toilet containing infectious particles can aerosolize significant concentrations of airborne viruses (14, 136). Wastewater treatment plants (24, 51) and sewage sprinklers (97, 125) can also produce viral aerosols.
Farm animals have also been studied for their emission of airborne viruses. The FMD virus, which is one of the most widely studied airborne animal viruses, has been detected in air contaminated by infected pigs and ruminants (7, 8, 38, 39, 56, 60, 121) in both laboratory settings and farm environments. This single-stranded RNA (ssRNA) virus of the Picornaviridae family is excreted in all body fluids of infected animals (100) and can become airborne directly from the animals or from the secondary aerosolization of deposited viruses or virus-laden particles. Other suspected sources of airborne viruses, such as burning carcasses of infected animals (26), have not yet been identified formally as true sources because additional investigations are needed.
Poultry farms are also potential producers of virus-laden airborne particles. The exotic Newcastle disease virus (Paramyxoviridae family) was probably the first virus isolated from a naturally contaminated environment (35) of poultry houses sheltering infected birds. This 150-nm-diameter ssRNA virus was detected in air samples from two farms during an outbreak in Southern California in 2002-2003 (74). Air samples in and around broiler poultry houses have also been studied for the presence of viruses such as Escherichia coli bacteriophages, which are a fecal contamination tracer (50). Other animals, such as bats (rabies virus) (144), rabbits (rabbit poxvirus) (128, 141), and mice (polyomavirus) (94), have been studied as sources of bioaerosols. These viruses can be released into the air directly from animals by their breathing, coughing, and sneezing or by secondary aerosolization. It should be noted that the means of aerosolization has a critical impact on the aerodynamic size and, thus, on the behavior of the airborne particles.
SIZE DISTRIBUTION OF VIRUS-LADEN PARTICLES
For humans, most particles larger than 10 μm will not pass the upper airways; while smaller particles will travel more easily toward the lungs, the particles will be trapped at different proportions in the head airways and the tracheobronchial and alveolar regions (75). The particle size determines whether or not it can be inhaled and retained in the respiratory tract.
Given that virus-laden particles are a complex mixture of various components (salts, proteins, and other organic and inorganic matter, including virus particles), it is essential to realize that the size of the viral particle itself does not rule the airborne particle size. The influence of viruses alone on the granulometric distribution of aerosols is likely negligible compared to that of the remainder of the aerosol. To support this, it was demonstrated that the particle size distribution of artificially produced submicrometer and ultrafine aerosols of culture media is not affected by the presence of bacteriophages (76).
Infectious bioaerosols spontaneously released by sick animals are composed of variously sized particles. The smaller size limit of a viral aerosol is limited to the virus diameter itself, which can be as small as 20 to 30 nm, while the larger limit depends on the size of the particle with which it is associated. Size also dictates the capacity of a particle to remain airborne. A study investigating the natural excretion of the FMD virus (25 to 30 nm in diameter) into the air by infected pigs, using a multistage liquid impinger sampler, showed that 65% to 71% of the virus-laden particles were over 6 μm in diameter, 19% to 24% ranged from 3 to 6 μm in diameter, and 10% to 11% were under 3 μm in diameter (121). Similar results were also obtained with infected sheep (56). The same type of bioaerosol sampler was also used to establish a link between the concentration and the size of infectious particles, using artificially and naturally produced aerosols of FMD virus. This study reported that over 85% of the particles in the artificially produced aerosols were less than 3 μm in diameter, whereas the size distributions of the natural aerosols were similar in all three stages of the sampler (39). Another study investigating pigs infected with Aujeszky's disease virus (Herpesviridae family; approximately 150 nm in diameter; double-stranded DNA [dsDNA] virus) found that the infectivity of the aerosols collected in each stage of the three-stage impinger varied over time. The investigators reported that the size distributions of the aerosols in the three stages were comparable on day 2 of the infection but that there was an increase in infectivity associated with larger particles on days 3 and 4 (40). Nevertheless, no clear association has been made between aerosol infectivity and a particular size range (60).
While single virus particles exist in the air (76), they tend to aggregate rapidly. Aggregation speed depends on the size distribution of the airborne particles, the concentration of the aerosol, and the thermodynamic conditions (142). Many factors influence the size distribution of both naturally and artificially produced viral aerosols. Artificially produced aerosols are normally used in controlled environments where there are no other aerosols to which the nebulized particles can bind. They are thus influenced only by the size of the original droplet created by the nebulizer and by the solute concentration in the droplet. When a droplet evaporates (Fig. 2), its final size (the droplet nucleus) depends on the relative humidity (RH) in the chamber. To some extent, this phenomenon can also be observed with natural aerosols. For example, infectious droplets exhaled by animals shrink rapidly with the lower humidity outside the respiratory airway, creating smaller aerosols. However, the size distribution of such naturally generated bioaerosols depends on the sizes of the particles to which the microorganisms bind. This binding may occur by diffusion, impaction, interception, or electrostatic attraction (98). If mostly large particles are encountered in the air of a given environment, then the particles making up the infectious aerosol will also tend to be large. Interestingly, larger particles may be relatively less hazardous than smaller ones. It has been shown on pig farms that a visually clean environment may be more contaminated by bioaerosols than a visually dirty one (43). This may be due to the fact that larger particles tend to settle faster than smaller particles do; the settling velocity of 0.001-μm particles is 6.75E−09 m/s, while 10-μm particles settle at 3.06E−03 m/s and 100-μm particles settle at 2.49E−01 m/s (75). Airborne particles in a “clean” environment are more likely to remain small and inhalable by animals and humans than are particles in a dirty environment, which tend to grow larger by sticking to other airborne particles.
FIG. 2.
Evaporation of a liquid droplet (left) to a droplet nucleus (right). As the liquid evaporates, the nonevaporative content concentrates until a droplet nucleus is obtained.
FACTORS AFFECTING THE RECOVERY OF AIRBORNE VIRUSES BY SAMPLING
Organic and inorganic materials in viral aerosols can affect the size of the aerosolized particles and their infectious potential. Many factors, such as RH, temperature, radiation, aerosolization medium, exposure period, chemical composition of the air, and sampling methods, can affect the infectivity of airborne viruses. Each virus reacts in its own particular way to each factor or combination of factors, depending on the structural composition of the virus and its interactions with other aerosol components. However, the structural composition of airborne viruses alone cannot be used to predict survival under different environmental conditions (78).
RH is the most widely studied of the factors that affect airborne virus infectivity (Table 1). Depending on the virus, optimal preservation of infectivity may require a low RH (under 30%), an intermediate RH (30% to 70%), or a high RH (over 70%). Influenza virus (65, 118), Semliki Forest virus (17), Japanese B encephalitis virus (86), porcine reproductive and respiratory syndrome virus (72), Newcastle disease virus, and vesicular stomatitis virus (122), all of which are enveloped, are most stable at low RH, while rhinovirus (79, 84), poliovirus (65, 79, 81), T3 coliphage (45, 122), rhinotracheitis virus (122), picornavirus (5), and viruses of the Columbia SK group (4), which are nonenveloped (with the exception of the rhinotracheitis virus), are most stable at high RH. Human coronavirus 229E (79), pseudorabies virus (119), and rotavirus (81, 82, 116) are most stable at intermediate RH. The first two are enveloped, while mature rotaviruses are usually nonenveloped. RH has no effect on the stability of airborne St. Louis encephalitis virus under the conditions tested (112).
TABLE 1.
Effects of RH on infectivity of a selection of airborne viruses
| Virus | Optimal RH for maximum infectivity | Family | Genetic material | Size (nm) | Envelope |
|---|---|---|---|---|---|
| Influenza virus | Low | Orthomyxoviridae | ssRNA (−) | 80-120 | Yes |
| Newcastle disease virus | Low | Paramyxoviridae | ssRNA (−) | 150 | Yes |
| Vesicular stomatitis virus | Low | Rhabdoviridae | ssRNA (−) | 60 × 200 | Yes |
| Japanese encephalitis virus | Low | Flaviviridae | ssRNA (+) | 40-60 | Yes |
| Porcine reproductive and respiratory syndrome virus | Low | Arteriviridae | ssRNA (+) | 45-60 | Yes |
| Semliki Forest virus | Low | Togaviridae | ssRNA (+) | 70 | Yes |
| Human coronavirus 229E | Mid-range | Coronaviridae | ssRNA (+) | 120-160 | Yes |
| Rotavirus | Mid-range | Reoviridae | dsRNA | 100 | No |
| Pseudorabies virus | Mid-range | Herpesviridae | dsDNA | 200 | Yes |
| Rhinovirus | High | Picornaviridae | ssRNA (+) | 25-30 | No |
| Poliovirus | High | Picornaviridae | ssRNA (+) | 25-30 | No |
| Picornavirus | High | Picornaviridae | ssRNA (+) | 25-30 | No |
| Columbia SK group | High | Picornaviridae | ssRNA (+) | 25-30 | No |
| T3 coliphage | High | Podoviridae | dsDNA | 60 (capsid) | No |
| Rhinotracheitis virus | High | Herpesviridae | dsDNA | 200 | Yes |
| St. Louis encephalitis virus | All | Flaviviridae | ssRNA (+) | 40-60 | Yes |
Seasonal variations in indoor RH have also been correlated with fluctuations in the morbidity of influenza (low RH) and poliomyelitis (high RH) viruses, with the highest morbidity occurring at the optimal RH for each virus (68, 69). Seasonal variations have also been observed with measles virus (34) and respiratory syncytial virus (147). An intriguing study comparing the effect of RH on the stability of an airborne picornavirus to that on its genomic RNA (5) indicated that the inactivation of airborne picornaviruses by low RH levels is not due to the instability of the RNA but, rather, to structural damage to the virion (5). The findings of these studies indicate that there is no absolute correlation between RH and the preservation of viral infectivity in aerosols and that the impact of RH should be determined for each virus. However, it appears that low RH tends to preserve the infectivity of enveloped viruses, while the stability of nonenveloped viruses is best preserved at high RH.
Temperature can also have an impact on the infectivity of airborne viruses. For example, the stability of certain infectious airborne viruses (47, 65, 72, 79, 119, 122) is improved at low temperatures but does not depend on RH. UV radiation is another factor that influences survivability. UV germicidal lamps, for instance, can be used to inactivate airborne microorganisms, including viruses, in indoor settings (53). However, in certain cases, RH must be taken into consideration. For example, vaccinia virus is more susceptible to UV radiation at low RH than at high RH (93).
Interestingly, aerosolization can inactivate some viruses to a certain extent, depending upon the nature of the spray fluid, the temperature, and the RH (80). This was reported for the recovery of bovine parainfluenza virus (47) and infectious bovine rhinotracheitis virus, where various combinations of these factors generated different results (48). Certain chemicals also have diverse effects on the stability of airborne viruses. For example, adding salt to a spray suspension reduces the recovery of airborne infectious Semliki Forest virus at high RH in a controlled chamber (17). On the other hand, polyhydroxy compounds (17, 118) and peptones (69) are protective. Similarly, adding dextrose to the spray fluid significantly enhances the recovery of coliphage T3 at mid-range RH, but spermine, spermine-phosphate, thiourea, galacturonic acid, and glucosaminic acid have no effect on virus recovery (45). Mid-range RH and fecal matter as a spray fluid have been shown to enhance the recovery of a strain of human rotavirus (82). Organic matter and chemical compounds probably exert their protective effect by reducing desiccation and other environmental stresses.
Lastly, the gas composition of the air can also have an influence on viruses, as ozone has been shown to inactivate airborne viruses (96). In fact, virus susceptibility to ozone is much higher than those of bacterial and fungal bioaerosols (133). However, the ozone efficacy will vary from virus to virus. For example, phage φX174 is more susceptible to ozone than are phages MS2 and T7 (133). Ions in the air can also reduce the recovery rate of certain viruses, such as aerosolized T1 bacteriophage, with positive ions having the most detrimental effect (64).
AIR SAMPLING METHODOLOGIES
Most air sampling technologies depend on the aerodynamic diameter of the airborne particles, the adhesion properties of airborne particles, Brownian motion, thermal gradients, and the inertia of the particles. Aerosolized particles attach to any surface with which they come into contact (75). Adhesive forces such as van der Waals forces, electrostatic forces, and surface tension partly explain this adhesion. Most of the sampling methodologies presented in this review are based on this principle.
Airborne particles with aerodynamic diameters on the order of 100 nm or less are prone to a particular way of moving, mainly due to the billions of collisions they encounter with gas molecules. This is called Brownian motion, and the smaller the particle, the greater the movement and the more likely that the particle will diffuse, come into contact with a surface, and adhere to it. When this happens, the other suspended particles occupy the space left vacant by the particle that has adhered to the surface. This phenomenon is the basis for the efficient removal of very small particles by filtration, particularly when the distance between two surfaces of the filter is sufficient for the particles to pass through.
Larger particles with aerodynamic diameters on the order of a micrometer or more are less influenced by Brownian motion but have greater inertia. Gravitational attraction has a significant impact on these particles and causes them to settle on surfaces. These particles are also more easily diverted from a gas streamline, leading to impaction on surfaces, especially at high velocity and when the angle of the airflow is drastically altered. Very small particles have less inertia and will more likely follow the streamline.
AIR SAMPLING FOR VIRUS RECOVERY
Various sampling devices can be used to recover airborne viruses, and some are illustrated in Fig. 3. The most common are liquid and solid impactors as well as filters. Electrostatic precipitators have also been tested. Table 2 presents an extensive compilation of studies on the recovery of viral particles. The history of use of air samplers for viral aerosols is summarized in Fig. 1.
FIG. 3.
Diagrams of six different bioaerosol samplers. Red lines and arrows represent the airflow into the sampler. Blue arrows represent airflow out of the sampler. These drawings are simplified representations.
TABLE 2.
Summary of virus aerosol sampling studies
| Sampler type | Air sampling rate or capacity | Sampling environment | Virus and/or tracer | Particle size (μm) | Analytical method | Comments | Aerosol source | Reference | Yr of publication |
|---|---|---|---|---|---|---|---|---|---|
| Wells air centrifuges | 200-ft3 chamber | Influenza virus strain Puerto Rico 8 | Inoculation of ferrets | All ferrets inoculated with material collected from the air contracted influenza when the sampling was done within an hour after the material was suspended | Atomizer | 140 | 1936 | ||
| Sampling atomizers | 540 and 1,080 liters | Poultry houses | Pneumoencephalitis virus | Inoculation of chick embryos | Air samples contained sufficient viruses to infect chick embryos | Poultry | 35 | 1948 | |
| Impingers | 11 liters/min for 15 to 120 s | Modified Henderson apparatus and rotating stainless steel drum | Vaccinia virus, influenza A virus strain PR8, Venezuelan equine encephalomyelitis (VEE) virus, poliomyelitis virus type I (Brunhilde), formalin-killed Pasteurella tularensis cells labeled with 32P for VEE virus and virus suspension labeled with 32P for the other three viruses | Inoculation of eggs or mice, culture | Airborne viruses were recovered up to 23 h after aerosolization at different RH and temperatures | Collison atomizer | 65 | 1961 | |
| Slit sampler (solid gelatin medium) | 1 ft3/min for 1 h | 1,500-liter Plexiglas chamber | Bacteriophage T3 | 0.5-1 (96% of particles) | Culture | Slit sampler with 12% gelatin medium recovered ∼75% of phages compared to AGI-30 sampler | Vaponefrin nebulizer | 30 | 1961 |
| AGI-30 | 12.8 liters/min for 15 min | 1,500-liter Plexiglas chamber | Bacteriophage T3 | 0.5-1 (96% of particles) | Culture | Slit sampler with a 12% gelatin medium recovered ∼75% of phages compared to AGI-30 sampler | Vaponefrin nebulizer | 30 | 1961 |
| Slit sampler | 1 ft3/min for 4 and 15 min | Modified Henderson apparatus | VEE virus | Inoculation of mice, titration with embryonated hen's eggs, and culture | Results with the slit sampler and the AGI-30 sampler were comparable | Collison generator | 85 | 1961 | |
| AGI-30 | 12.5 liters/min for 4 and 15 min | Modified Henderson apparatus | VEE virus | Inoculation of mice, titration with embryonated hen's eggs, and culture | Results with the slit sampler and the AGI-30 sampler were comparable | Collison generator | 85 | 1961 | |
| Porton impinger | 10 to 20 liters/min for 0.5 and 1 min | 14-ft3 aerosol chamber with fan | Bacteriophage T3 | Culture | The electrostatic precipitator was very efficient; ozone can be produced at high RH with the electrostatic precipitator; a 0.1% peptone solution can protect phage T3 from ozone | Collison spray | 99 | 1961 | |
| Electrostatic precipitator | 5 to 40 liters/min for 5 min | 14-ft3 aerosol chamber with fan | Bacteriophage T3 | Culture | The electrostatic precipitator was very efficient; ozone can be produced at high RH with the electrostatic precipitator; a 0.1% peptone solution can protect phage T3 from ozone | Collison spray | 99 | 1961 | |
| Glass sampler containing tightly packed dry cotton | 20 to 18,000 liters | Hospital | Variola virus | Inoculation of chick embryos | Viruses were recovered in 1 of 38 samples | Humans | 95 | 1961 | |
| Modified capillary impingers | 11 liters/min for 10 s | 4-m3 conditioned room | Bacteriophage T5, influenza virus, poliomyelitis virus | 5-6 (mean size 5 cm from the outlet) | Culture | Recovery depended on the aerosolization medium and the RH, but the effect of RH did not depend on the composition of the medium; the survival curves at different RH were not the same for influenza and poliomyelitis viruses | All-glass indirect-type spray | 69 | 1962 |
| AGI-30 | 12.5 liters/min for 1 min | 1,600-liter Plexiglas chamber with fan | Bacteriophage T3 | 0.5-5.0 (peak at 2.0 μm) | Culture | The best recovery was at high RH | Hartman atomizer | 45 | 1964 |
| Short-stem AGI | 12.0 liters/min for 5 to 20 min | Hospital rooms of infected patients | Viruses associated with respiratory diseases | Culture | Viruses were recovered in 1 of 23 trials | Humans | 10 | 1964 | |
| Liquid impingers | |||||||||
| AGI-4 | 12.5 liters/min for 5 min | Aerosol chamber | Bacteriophage T1, 32P | Mass median diameter, 0.2 | Culture | Recovered the most viable phages, but major sample loss (30 to 40%) | Dautreband D301 aerosol generator | 66 | 1965 |
| Capillary impinger | 2.5 L/min for 5 min | Aerosol chamber | Bacteriophage T1, 32P | Mass median diameter, 0.2 | Culture | Recovered the most viable phages, but major sample loss (30 to 40%) | Dautreband D301 aerosol generator | 66 | 1965 |
| Filters | |||||||||
| Chemical Corps type 6 | 1 liter/min (8 cm/s) for 5 min | Aerosol chamber | Bacteriophage T1, 32P | Mass median diameter, 0.2 | Culture | Very destructive but most efficient collection of submicrometer airborne particles | Dautreband D301 aerosol generator | 66 | 1965 |
| Glass filter paper (MSA 1106BH) | 1 liter/min (8 cm/s) for 5 min | Aerosol chamber | Bacteriophage T1, 32P | Mass median diameter, 0.2 | Culture | Very destructive but most efficient collection of submicrometer airborne particles | Dautreband D301 aerosol generator | 66 | 1965 |
| Fritted bubbler | 1 liter/min for 5 min | Aerosol chamber | Bacteriophage T1, 32P | Mass median diameter, 0.2 | Culture | Important sample loss (>80%) | Dautreband D301 aerosol generator | 66 | 1965 |
| Porton impinger | 10 liters/min for 5 to 10 min | Hospital rooms | Smallpox virus | Inoculation of chick embryos | Large particles from patients' bed clothes seemed to be mostly responsible for contamination of the air in the vicinity of patients | Humans | 41 | 1965 | |
| Petri dish (settling plates) | 5 to 10 min | Hospital rooms | Smallpox virus | Inoculation of chick embryos | Large particles from patients' bed clothes seemed to be mostly responsible for contamination of the air in the vicinity of patients | Humans | 41 | 1965 | |
| Top part of an Andersen sampler | 10 liters/min for 5 to 10 min | Hospital rooms | Smallpox virus | Inoculation of chick embryos | Large particles from patients' bed clothes seemed to be mostly responsible for contamination of the air in the vicinity of patients | Humans | 41 | 1965 | |
| Capillary impingers | 4-m3 conditioned room | Measles virus | 5 (average diameter) | Culture | Recovery depended on RH | 34 | 1965 | ||
| LVS | 1,785 ft3 for 5 min | 1,440-ft3 Army hospital room | Adenovirus type 4 | Culture | Viable viruses were recovered at very low concentrations | Humans | 11 | 1966 | |
| LVS | 10,000 liters/min for 3.5 min | 32,800-liter room with atomized virus suspension | Coxsackie virus A type 21, sodium fluorescein | 1-15 | Culture | LVS consistently recovered more fluorescein than the AGI-30 did | University of Chicago Toxicity Laboratories atomizer | 54 | 1966 |
| AGI-30 | 12.5 liters/min for 1 min | 32,800-liter room with atomized virus suspension | Coxsackie virus A type 21, sodium fluorescein | 1-15 | Culture | LVS consistently recovered more fluorescein than the AGI-30 did | University of Chicago Toxicity Laboratories atomizer | 54 | 1966 |
| Electrostatic precipitator | Rooms containing infected rabbits | Rabbit pox virus strain Utrecht and Rockefeller Institute strain | Culture | Low concentrations were recovered with the electrostatic precipitator, and none was recovered with the impinger | Rabbits | 141 | 1966 | ||
| Raised glass impinger | Rooms containing infected rabbits | Rabbit pox virus strain Utrecht and Rockefeller Institute strain | Culture | Low concentrations were recovered with the electrostatic precipitator, and none was recovered with the impinger | Rabbits | 141 | 1966 | ||
| AGI-4 | 12.5 liters/min for 5 min | Aerosol chamber | Bacteriophage T1 | <1 | Culture | The AGI-4 sampler recovered more airborne viruses than type 6 filter paper did; ions affected the stability of submicrometer T1 phage | Dautrebande aerosol generator | 64 | 1966 |
| Chemical Corps type 6 filter paper | 5 min at 1.0 liter/min | Aerosol chamber | Bacteriophage T1 | <1 | Culture | The AGI-4 sampler recovered more airborne viruses than type 6 filter paper did; ions affected the stability of submicrometer T1 phage | Dautrebande aerosol generator | 64 | 1966 |
| AGI-30 | 12.5 liters/min for 5 min | Rotating drum | Columbia SK group viruses | Culture | Inactivation of the airborne viruses depended on RH | Modified Wells refluxing atomizer | 4 | 1966 | |
| AGI | 12 liters/min (3-liter samples) | 140-liter aluminum drum | Newcastle disease virus, infectious bovine rhinotracheitis virus, vesicular stomatitis virus, T3 phage, rhodamine B | Culture | Best recovery at low RH for Newcastle disease virus and vesicular stomatitis virus and at high RH for bovine rhinotracheitis virus and T3 | De Vilbiss no. 40 nebulizer | 122 | 1967 | |
| LVS | 10,000 liters/min for 6 or 7 min | 1,440-ft3 Army hospital room | Adenovirus | Culture | Recovery of one viral unit per 204 to 1,970 ft3 of air in 10 of 14 samples | Humans | 13 | 1967 | |
| AGI-4 | 10 liters/min for 10 min | Frio Cave, TX | Rabies virus | Inoculation of animals | Rabies virus was isolated from four of eight samples collected with the LVS L and from none of the five AGI-4 samples | Bats | 144 | 1968 | |
| LVS model L | 10,000 liters/min for 10 to 30 min | Frio Cave, TX | Rabies virus | Inoculation of animals | Rabies virus was isolated from four of eight samples collected with the LVS L and from none of the five AGI-4 samples | Bats | 144 | 1968 | |
| Impinger | 2 min | 500-liter rotating toroid drum | Mengovirus 37A | Culture | Inactivation of the virus was due to damage to the virion structure | Modified Wells refluxing atomizer | 5 | 1968 | |
| LVS with added preimpactor | 357 ft3 for 1 min or full room for 3 min | 986-ft3 room | Adenovirus type 4 | Depended on individual subjects | Culture | Recovery of one viral unit per 86 to 448 ft3 of air in 3 of 11 samples; infectious particles were present in both small- and large-particle aerosols | Humans | 12 | 1968 |
| LVS model M | 1,000 liters/min for 1 h | 3.65- by 3.35- by 3.05-m loose boxes | Four strains of FMD virus (01 Lombardy, 01 Swiss 1/66, 01 BFS 1860, and 02 Brescia) | Culture and inoculation of unweaned mice | The amt of virus recovered by the impinger was similar to that recovered by the LVS | Cattle, sheep, and pigs | 121 | 1969 | |
| Multistage liquid impinger | 55 L/min for 45 min | 3.65 × 3.35 × 3.05 m loose boxes | Four strains of FMD virus (01 Lombardy, 01 Swiss 1/66, 01 BFS 1860, and 02 Brescia) | Culture and inoculation of unweaned mice | The amt of virus recovered by the impinger was similar to that recovered by the LVS | Cattle, sheep, and pigs | 121 | 1969 | |
| AGI-30 | Dual-aerosol transport apparatus | T3 and S13 coliphages, mengovirus-37A, and vesicular stomatitis virus | Culture | While the recovery of some viruses from aerosols was increased by prehumidification, no generalization could be drawn | Modified Wells reflux atomizer | 137 | 1969 | ||
| AGI-30 with a humidifier bulb | Dual-aerosol transport apparatus | T3 and S13 coliphages, mengovirus-37A, and vesicular stomatitis virus | Culture | While the recovery of some viruses from aerosols was increased by prehumidification, no generalization could be drawn | Modified Wells reflux atomizer | 137 | 1969 | ||
| AGI-30 | 12.5 liters/min | Two 500-liter rotating drums | Bacteriophage T3 | Culture | Virus recovery was best at higher RH | Atomizer designed by the lab (similar to the Wells atomizer) | 138 | 1969 | |
| Raised impingers | Semliki Forest virus, washed Bacillus subtilis spores | Culture | Best recovery at low RH, with good protective effect of polyhydroxy compounds at low RH | Collison spray | 17 | 1969 | |||
| AGI-30, with or without a humidifier bulb | Two 500-liter rotating drums | Bacteriophage T3 and Pasteurella pestis bacteriophage | 1-5 | Culture | Prehumidification of the air samples significantly enhanced the recovery of airborne viruses | Atomizer designed by the lab (similar to the Wells atomizer) | 67 | 1969 | |
| AGI-30 | 1 min | 500-liter rotating toroid drum | Bacteriophages S13 and MS2 | Culture | RH and aerosol composition had a major impact on viral recovery | Modified Wells reflux atomizer | 42 | 1970 | |
| AGI-30 with humidifier bulb | 1 min | 500-liter rotating toroid drum | Bacteriophages S13 and MS2 | Culture | RH and aerosol composition had a major impact on viral recovery | Modified Wells reflux atomizer | 42 | 1970 | |
| Slit sampler with adhesive surface petri dishes | Room containing infected rabbits (4,500 ft3) | Rabbit poxvirus strain Utrecht | Culture | More viruses were recovered in the top stages of the Andersen sampler than in the lower stages; both the slit samplers and the Andersen sampler successfully recovered airborne viruses | Rabbits | 128 | 1970 | ||
| Automated slit sampler with adhesive surface petri dishes | 1 h (60 ft3) | Room containing infected rabbits (4,500 ft3) | Rabbit poxvirus strain Utrecht | Culture | More viruses were recovered in the top stages of the Andersen sampler than in the lower stages; both the slit samplers and the Andersen sampler successfully recovered airborne viruses | Rabbits | 128 | 1970 | |
| Andersen sampler with adhesive surface petri dishes | 1 h (60 to 120 ft3) | Room containing infected rabbits (4,500 ft3) | Rabbit poxvirus strain Utrecht | Culture | More viruses were recovered in the top stages of the Andersen sampler than in the lower stages; both the slit samplers and the Andersen sampler successfully recovered airborne viruses | Rabbits | 128 | 1970 | |
| Modified Andersen sampler | 1 ft3/min | Aerosol apparatus (Henderson) | Vaccinia virus, poliovirus | Culture | The modified Andersen sampler gave the best percentage recovery, followed by the impinger and the slit sampler; the adhesive surface sampling method indicated the number of virus-bearing particles | Spray | 127 | 1970 | |
| Slit sampler with sucrose, glycerol, and bovine serum albumin (SGB) medium | 1 ft3/min for 0.5 to 10 min | Aerosol apparatus (Henderson) | Vaccinia virus, poliovirus | Culture | The modified Andersen sampler gave the best percentage recovery, followed by the impinger and the slit sampler; the adhesive surface sampling method indicated the number of virus-bearing particles | Spray | 127 | 1970 | |
| Porton impinger | 11.5 liters/min | Aerosol apparatus (Henderson) | Vaccinia virus, poliovirus | Culture | The modified Andersen sampler gave the best percentage recovery, followed by the impinger and the slit sampler; the adhesive surface sampling method indicated the number of virus-bearing particles | Spray | 127 | 1970 | |
| AGI | 6 liters/min | 650-liter aerosol chamber | VEE virus | 1.5 (median diameter) | Culture | Sodium fluorescein affected the recovery of VEE virus in the presence (or not) of simulated solar radiation, depending on RH | FK-8 gun | 18 | 1971 |
| Porton raised impingers | 120-liter rotating drum | Semliki Forest virus, Langat virus, poliovirus Sabin type I strain, T7 coliphage, 32P-labeled T7 coliphage or radioactive sodium phosphate | Culture | Salts in the aerosolization medium influenced the recovery of some viruses at different RH; prehumidification enhanced the recovery of T7 coliphage and poliovirus | Three-jet Collison spray | 16 | 1971 | ||
| May's subsonic impinger | 120-liter rotating drum | Semliki Forest virus, Langat virus, poliovirus Sabin type I strain, T7 coliphage, 32P-labeled T7 coliphage or radioactive sodium phosphate | Culture | Salts in the aerosolization medium influenced the recovery of some viruses at different RH; prehumidification enhanced the recovery of T7 coliphage and poliovirus | Three-jet Collison spray | 16 | 1971 | ||
| AGI | 12.9 liters/min | Chamber (aerosol inoculator) | Type A influenza virus strain PR8, sodium fluorescein | 50% Egg infective dose | No significant difference was noted in the physical tracer or viral recovery of the two samplers | Collison atomizer | 55 | 1971 | |
| Shipe impinger | 10 liters/min | Chamber (aerosol inoculator) | Type A influenza virus strain PR8, sodium fluorescein | 50% Egg infective dose | No significant difference was noted in the physical tracer or viral recovery of the two samplers | Collison atomizer | 55 | 1971 | |
| Raised impingers | 11 liters/min | 10-ft by 10-ft by 10-ft rooms, poultry houses with infected chickens, and a modified Henderson apparatus with a 500-liter rotating stainless drum | Three strains of Newcastle disease virus (Herts '33/56, Eastwood '67, and Essex '70), Bacillus globigii spores | Inoculation of eggs | Viruses were recovered with all the samplers, but under different sampling conditions | Poultry or Collison atomizer | 77 | 1973 | |
| LVAS | 1,000 liters/min | 10-ft by 10-ft by 10-ft rooms, poultry houses with infected chickens, and a modified Henderson apparatus with a 500-liter rotating stainless drum | Three strains of Newcastle disease virus (Herts '33/56, Eastwood '67, and Essex '70), Bacillus globigii spores | Inoculation of eggs | Viruses were recovered with all the samplers, but under different sampling conditions | Poultry or Collison atomizer | 77 | 1973 | |
| Cascade impactor | 17 liters/min | 10-ft by 10-ft by 10-ft rooms, poultry houses with infected chickens, and a modified Henderson apparatus with a 500-liter rotating stainless drum | Three strains of Newcastle disease virus (Herts '33/56, Eastwood '67, and Essex '70), Bacillus globigii spores | Inoculation of eggs | Viruses were recovered with all the samplers, but under different sampling conditions | Poultry or Collison atomizer | 77 | 1973 | |
| Multistage liquid impinger | 55 liters/min | 10-ft by 10-ft by 10-ft rooms, poultry houses with infected chickens, and a modified Henderson apparatus with a 500-liter rotating stainless drum | Three strains of Newcastle disease virus (Herts '33/56, Eastwood '67, and Essex '70), Bacillus globigii spores | Inoculation of eggs | Viruses were recovered with all the samplers, but under different sampling conditions | Poultry or Collison atomizer | 77 | 1973 | |
| AGI-30 | 1 min | 500-liter rotating drum | Simian virus 40 | 2 (mean diameter) | Culture | The viruses were stable at all RH tested at 21°C but were inactivated at mid-range RH at 32°C | Collison three-jet atomizer | 6 | 1973 |
| Raised Porton impinger | 11.5 liters/min for 1 min | 2,000-liter double-walled static system with fan | Bacteriophage MS2 | 2 (before evaporation) | Culture | The composition of the aerosolization medium can affect virus recovery | Direct spray apparatus (FK8) | 130 | 1973 |
| Lower stage of a multistage liquid impinger | 275 liters in 5 min | 2,000-liter static air cabinet with fan | Poliovirus type I strain LSc2ab, fluorescein | Culture | The infectivity of the virus depended on the RH, but the infectivity of the RNA remained unchanged | FK8 direct-type nebulizer | 32 | 1973 | |
| Multistage impinger | 30 min | Loose boxes | Swine vesicular disease virus | Culture | Viruses were recovered in the top, middle, and bottom stages of the multistage impinger | Pigs | 120 | 1974 | |
| May's multistage liquid impinger | 275 liters in 5 min | 2,000-liter double-walled stainless steel tank | Encephalomyocarditis virus | Culture with intact viruses or infectious RNA, hemagglutination activity, and antibody-blocking activity | Viruses were recovered from the air; the infectivity of the virus decreased, but the viral RNA was unaffected | FK-8 direct-type spray gun | 33 | 1974 | |
| Porton impinger | 2,000-liter static system | Bacteriophage T3 | Culture | Viruses were recovered with and without prehumidification of the aerosols | FK-8 spray gun | 131 | 1974 | ||
| Slit sampler with SGB medium | 1 ft3/min for 30 to 60 min | Wards of smallpox isolation hospital | Variola virus | Inoculation of hen's eggs, culture | The slit sampler and sedimentation plates gave positive results; the impinger samples were all negative | Humans | 126 | 1974 | |
| Porton impinger | 11.5 liters/min for 15 min | Wards of smallpox isolation hospital | Variola virus | Inoculation of hen's eggs, culture | The slit sampler and sedimentation plates gave positive results; the impinger samples were all negative | Humans | 126 | 1974 | |
| Sedimentation plates with SGB medium | Many hours at a time | Wards of smallpox isolation hospital | Variola virus | Inoculation of hen's eggs, culture | The slit sampler and sedimentation plates gave positive results; the impinger samples were all negative | Humans | 126 | 1974 | |
| LVS | 1,000 liters/min for 30 to 120 min | Field in proximity to wastewater treatment plants | Bacteriophages of E. coli strains C3000 and K-12 HfrD | Culture (most-probable-number and plaque counts) | Both the multislit impinger and the LVS successfully recovered airborne bacteriophages | Wastewater treatment facilities | 51 | 1976 | |
| Multislit impinger | 1,000 liters/min for 35 min | Field in proximity to wastewater treatment plants | Bacteriophages of E. coli strains C3000 and K-12 HfrD | Culture (most-probable-number and plaque counts) | Both the multislit impinger and the LVS successfully recovered airborne bacteriophages | Wastewater treatment facilities | 51 | 1976 | |
| Membrane filter (0.45-μm pore size) | 14 liters/min for 3 to 30 min in the chamber and 23 to 25 liters/min for 30 to 45 min in the hemodialysis center | Aerosol chamber and a 20-bed hemodialysis center | Hepatitis B virus surface antigen (HBsAg), Bacillus subtilis var. niger (in chamber) | Radioimmunoassay | HBsAg was detected in chamber aerosols but not in the samples from the hemodialysis center | Airflow directed on dust reservoir, nebulizer, or humans | 107 | 1976 | |
| AGI-30 | 12.5 liters in 1 min | Two 208-liter chambers | Influenza A virus strain WSNH | Inoculation of embryonated eggs, culture | Differences were noted in percentages of viral recovery depending on RH | Wells refluxing atomizer | 118 | 1976 | |
| Raised Porton impinger | 1 or 5 min | Chamber (50 or 2,000 liters) | Bacteriophage φX174, Bacillus globigii spores, or no tracer | Culture | Bacteriophage infectivity decreased in the presence of ozone | Three-jet Collison nebulizer or spray gun (FK-8 type) | 96 | 1977 | |
| LVS | 1,100 liters/min | 18-m3 animal laboratory housing infected mice | Polyomavirus | Mouse antibody production tests and culture | Airborne viruses were detected with the LVS in four of six samples; no airborne viruses were recovered with the AGI-4 sampler | Infected mice | 94 | 1978 | |
| AGI-4 | 12.3 liters/min | 18-m3 animal laboratory housing infected mice | Polyomavirus | Mouse antibody production tests and culture | Airborne viruses were detected with the LVS in four of six samples; no airborne viruses were recovered with the AGI-4 sampler | Infected mice | 94 | 1978 | |
| Large-volume aerojet-general liquid scrubber | 15 to 20 min at 600 liters/min | In the vicinity of an effluent-irrigated field | Enteric viruses | Culture | Four of 12 samples taken 40 m downwind from the aerosol source were positive for echovirus 7 | Sewage sprinklers | 125 | 1978 | |
| AGI-30 | 18 s | 200-liter stainless steel rotating drum | Parainfluenza virus type 3, bovine adenovirus type 3, rhodamine | Culture | Viruses were recovered with different efficiencies that depended on nebulization medium, RH, and ambient temperature | Devilbiss 40 nebulizer | 47 | 1979 | |
| LVS | 1,000 liters/min for 30 min (6 to 8 samples from each sampler were pooled; eight samples were operated simultaneously) | Field in proximity to a source of spray irrigation of wastewater | Enteric viruses | Culture | Low concentrations of coliphages, poliovirus, and Coxsackie virus were recovered | Sewage sprinklers | 97 | 1979 | |
| AGI-30 | 18 s | 200-liter stainless steel rotating drum | Infectious bovine rhinotracheitis virus strain Cooper, rhodamine B | Under 5 (diameter) (over 88% of the particles) | Culture | The decay rate depended on RH and the aerosolization medium | Devilbiss 40 nebulizer | 48 | 1979 |
| Membrane filter (0.45-μm pore size) | 15 liters/min for the duration of the treatment (3 to 13 min) | Dental unit, while treating infected patients | HBsAg | Radioimmunoassay | None of the 40 samples was positive for HBsAg | Humans | 106 | 1979 | |
| AGI-30 | 12.5 liters/min for 1 min | 6,200-liter static aerosol chamber | Japanese B encephalitis virus | 4.0 (median diameter) | Culture | Viral recovery was inversely related to RH | FK-8 atomizer | 86 | 1980 |
| AGI | 2 to 5 min | 1,000-liter stainless steel dynamic aerosol toroid with mixing chamber | Reovirus, Bacillus subtilis var. niger spores | Mean diameters of 2 for Collison nebulizer and 5 for Chicago atomizer | Culture | Reovirus particles were relatively stable when airborne; they were least stable at mid-range RH | Collison three-jet nebulizer and Chicago atomizer | 1 | 1982 |
| Andersen viable-type stacked sieve | 28.3 liters/min | 930-liter Plexiglas chamber | Coliphage f2 | 2.0 or 3.9 (median aerodynamic particle size) | Culture | The Andersen sampler had an efficiency of 28.2% compared to the AGI samplers | All-glass two-fluid nebulizer for 2.0-μm median aerodynamic particle diameter; spinning-disk aerosol generator for 3.9-μm particles | 15 | 1982 |
| AGI-30 | 12.5 liters/min | 930-liter Plexiglas chamber | Coliphage f2 | 2.0 or 3.9 (median aerodynamic particle size) | Culture | The Andersen sampler had an efficiency of 28.2% compared to the AGI samplers | All-glass two-fluid nebulizer for 2.0-μm median aerodynamic particle diameter; spinning-disk aerosol generator for 3.9-μm particles | 15 | 1982 |
| LVS model M | (i) 1,000 liters/min for 30 min | (i) 3.6-m by 3.3-m by 3.0-m loose boxes with confined infected pigs; (ii) 610-liter chamber, one pig at a time | FMD virus type C strain Noville | Culture | The LVS had a higher viral recovery rate, but the cyclone sampler was much easier to use | Pigs | 38 | 1982 | |
| All-glass cyclone separator | 700 liters/min for (i) 30 min or (ii) 15 min | (i) 3.6-m by 3.3-m by 3.0-m loose boxes with confined infected pigs; (ii) 610-liter chamber, one pig at a time | FMD virus type C strain Noville | Culture | The LVS had a higher viral recovery rate, but the cyclone sampler was much easier to use | Pigs | 38 | 1982 | |
| LVS | 1,000 liters/min for 30 min | 3.65- by 3.35- by 3.05-m loose boxes for groups of pigs and 610-liter chamber for individual pigs | Four strains of Aujeszky's disease virus (UK AD 74/33, Northern Ireland NIA-2, U 298/81, and UK AD 82/196) | Culture | The cyclone sampler had a slightly lower recovery rate than the LVS; the settling plates were successful in recovering viruses; the three-stage impinger showed daily variations in the size distributions of virus-containing particles | Pigs | 40 | 1983 | |
| All-glass cyclone sampler | 1,000 liters/min for 10 and 30 min | 3.65- by 3.35- by 3.05-m loose boxes for groups of pigs and 610-liter chamber for individual pigs | Four strains of Aujeszky's disease virus (UK AD 74/33, Northern Ireland NIA-2, U 298/81, and UK AD 82/196) | Culture | The cyclone sampler had a slightly lower recovery rate than the LVS; the settling plates were successful in recovering viruses; the three-stage impinger showed daily variations in the size distributions of virus-containing particles | Pigs | 40 | 1983 | |
| Square settling plates with 20 ml of collection fluid | 30 min | 3.65- by 3.35- by 3.05-m loose boxes for groups of pigs and 610-liter chamber for individual pigs | Four strains of Aujeszky's disease virus (UK AD 74/33, Northern Ireland NIA-2, U 298/81, and UK AD 82/196) | Culture | The cyclone sampler had a slightly lower recovery rate than the LVS; the settling plates were successful in recovering viruses; the three-stage impinger showed daily variations in the size distributions of virus-containing particles | Pigs | 40 | 1983 | |
| Three-stage liquid impinger | 55 liters/min for 15 min | 3.65- by 3.35- by 3.05-m loose boxes for groups of pigs and 610-liter chamber for individual pigs | Four strains of Aujeszky's disease virus (UK AD 74/33, Northern Ireland NIA-2, U 298/81, and UK AD 82/196) | Culture | The cyclone sampler had a slightly lower recovery rate than the LVS; the settling plates were successful in recovering viruses; the three-stage impinger showed daily variations in the size distributions of virus-containing particles | Pigs | 40 | 1983 | |
| AGI | 5.6 liters in 1 min | 300-liter stainless steel rotating drum | Rotavirus SA11, rhodamine | Culture | Best survival of the virus at medium (50% ± 5%) RH; high (80% ± 5%) humidity was the least favorable | Six-jet Collison nebulizer | 116 | 1984 | |
| AGI | 5.6 liters/min for 2 min | 300-liter stainless steel rotating drum | Human rotavirus strain Wa, uranine | Culture | Best recovery at 50% ± 5% RH and 6 ± 1°C; recovery was enhanced when feces were used in the aerosolization medium | Six-jet Collison nebulizer | 82 | 1985 | |
| AGI | 5.6 liters/min for 2 min | 300-liter stainless steel rotating drum | Calf rotavirus strain C-486, poliovirus type 1 (Sabin), uranine | Culture | The best survival rates were at 50% ± 5% RH for the rotavirus and 80% ± 5% RH for the poliovirus | Six-jet Collison nebulizer | 81 | 1985 | |
| Impinger | 5.6 liters/min for 2 min | 300-liter stainless steel rotating drum | Human coronavirus 229E, poliovirus type 1, uranine | Culture | The half-life of aerosolized coronavirus was determined under different temperature and RH conditions | Six-jet Collison nebulizer | 78 | 1985 | |
| AGI | 300-liter rotating drum | Rhinovirus type 14, uranine | <5 in diameter (theoretically; > 90% of particles) | Culture | Best viral recovery at high (80% ± 5%) RH | Six-jet Collison nebulizer | 84 | 1985 | |
| Aerosol collection device with Filterite filters (pore size, 0.4 μm) moistened with glycine buffer | 100 liters/min for 10 to 15 s before and 2 min after flushing | Sampling device placed over a toilet bowl seeded with poliovirus | Poliovirus | Culture | Recovery of viruses from the air was possible, but virus adsorption to the filter was hampered if the filter became dry; large volumes of dry air would be problematic | Toilet flush | 136 | 1985 | |
| Three-stage liquid impinger | 55 liters/min for 10 min | 610-liter chamber | FMD virus type O1 strain BFS 1860 | Culture | The three-stage liquid impinger recovered smaller amounts of virus than the Porton impingers did | Pigs | 56 | 1986 | |
| Porton raised AGI | 11 to 13 liters/min for 10 min | 610-liter chamber | FMD virus type O1 strain BFS 1860 | Culture | The three-stage liquid impinger recovered smaller amounts of virus than the Porton impingers did | Pigs | 56 | 1986 | |
| Three-stage liquid impinger | 55 liters/min for 2 to 5 min | 610-liter chamber | FMD virus strains O1 and SAT 2 | <3 with spinning-top aerosol generator | Culture | Artificial aerosols were found mostly in the lower stage of the three-stage impinger; natural aerosols were found in equal amounts in all three stages; the minimal infectious dose of the airborne virus was determined with the Porton impinger; no viruses were recovered from the cyclone sampler | Modified May spinning-top aerosol generator or pigs | 39 | 1987 |
| Porton raised AGI | 11 to 13 liters/min for 1 min 47 s to 2 min 40 s | 610-liter chamber | FMD virus strains O1 and SAT 2 | <3 with spinning-top aerosol generator | Culture | Artificial aerosols were found mostly in the lower stage of the three-stage impinger; natural aerosols were found in equal amounts in all three stages; the minimal infectious dose of the airborne virus was determined with the Porton impinger; no viruses were recovered from the cyclone sampler | Modified May spinning-top aerosol generator or pigs | 39 | 1987 |
| Glass cyclone sampler | 700 liters/min | Corridor | FMD virus strains O1 and SAT 2 | <3 with spinning-top aerosol generator | Culture | Artificial aerosols were found mostly in the lower stage of the three-stage impinger; natural aerosols were found in equal amounts in all three stages; the minimal infectious dose of the airborne virus was determined with the Porton impinger; no viruses were recovered from the cyclone sampler | Modified May spinning-top aerosol generator or pigs | 39 | 1987 |
| AGI | 5.6 liters/min for 1 min | 300-liter stainless steel rotating drum | Simian rotavirus SA-11 strain H-96, human rotavirus subgroup 2 strain Wa, bovine rotavirus C-486, mouse rotavirus, bovine rotavirus Campton UK isolate, poliovirus type I Sabin strain, human coronavirus strain 229E, rhinovirus type 14/75Se (for radiolabeling), rhodamine B, or uranine | 1.0-3.3 (over 87% of the infectious viruses were collected in the last three stages of the Andersen sampler) | Culture | The Andersen sampler was used to determine the size distribution of the aerosolized particles; both samplers successfully recovered viruses; uranine is safer as a tracer than radiolabeling and, unlike rhodamine B, does not affect viral infectivity | Six-jet Collison nebulizer | 79 | 1987 |
| Andersen sampler with 3% gelatin medium | 28 liters/min for 1 min | 300-liter stainless steel rotating drum | Simian rotavirus SA-11 strain H-96, human rotavirus subgroup 2 strain Wa, bovine rotavirus C-486, mouse rotavirus, bovine rotavirus Campton UK isolate, poliovirus type I Sabin strain, human coronavirus strain 229E, rhinovirus type 14/75Se (for radiolabeling), rhodamine B, or uranine | 1.0-3.3 (over 87% of the infectious viruses were collected in the last three stages of the Andersen sampler) | Culture | The Andersen sampler was used to determine the size distribution of the aerosolized particles; both samplers successfully recovered viruses; uranine is safer as a tracer than radiolabeling and, unlike rhodamine B, does not affect viral infectivity | Six-jet Collison nebulizer | 79 | 1987 |
| AGI | 24 liters in 2 min | 500-liter stainless steel rotating drum | Pseudorabies virus, Bacillus subtilis spores | Culture | Recovery was best at 55% RH and 4°C | Nebulizer | 119 | 1990 | |
| Stainless steel cyclone sampler | 300 liters/min for 15 min | 60-m3 isolated units (six units with eight pigs each) | Aujeszky's disease virus strain 75V19 | Culture | Air sampling was less sensitive than nasal swab sampling; however, virus concentrations in the air were closely related to those in the nasal cavity | Pigs | 20 | 1992 | |
| AGI | 300-liter rotating drum | Bovine rotavirus UK isolate, murine rotavirus, uranine | Culture | Both viruses were recovered from the air | Collison nebulizer | 80 | 1994 | ||
| Cellulose filter (0.45-μm pore size) | 2.5 to 9.4 liters/min for 15 min to 24 h | Hospital rooms of patients with active varicella-zoster virus (VZV) infections | VZV | PCR | VZV DNA was detected in 64 of 78 air samples | Humans | 117 | 1994 | |
| Surface air system | 0.9 m3 of air | Near aeration tank of an activated sludge treatment plant | Coliphages and enteroviruses | Culture | Coliphages and enteroviruses were recovered from air samples, but no relationship was found between the two | Aeration tank of an activated sludge treatment plant | 24 | 1995 | |
| Polycarbonate membrane filter (0.1-μm pore size) | 1.9 liters/min for 6 h | Rooms of patients with active and latent cytomegalovirus (CMV) infection | Human CMV | PCR | CMV DNA was detected in the rooms of all three patients | Humans | 92 | 1996 | |
| AGI-30 | 15 min at 12.5 liters/min | Exposure room | Aujeszky's disease virus | Culture | A virus-containing aerosol was recovered from the breath of only one pig; viruses were recovered more easily from the nebulized aerosol; the sampler inactivated the virus, making the method less sensitive | Pigs or a DeVilbiss ultrasonic nebulizer (model 99) | 57 | 1996 | |
| AGI-30 | 1 or 2 liters/min | 80-liter aluminum chamber | St. Louis encephalitis virus strain MS1-7, Bacillus subtilis var. niger | Culture | Collison spray | 109 | 1997 | ||
| Cellulose filters (0.45-μm pore size) | 2.0 liters/min for <6 h, >18 h, or 24 h | Hospital rooms | Respiratory syncytial virus (RSV) | PCR-based detection methods | RSV DNA was detected in 17 of the 27 rooms housing infected patients and in 32 of the 143 samples | Humans | 3 | 1998 | |
| Surface air system agarized terrain impactor | 1,800 liters indoors and 3,000 liters outdoors | Urban sewage plants | Reovirus and enterovirus | Culture | Both viruses were detected in some samples | Urban sewage treatment plants | 25 | 2000 | |
| AGI-30 | 28-m3 isolation rooms | Aujeszky's disease virus | Culture | No virus was detected | Pigs | 58 | 2000 | ||
| AGI-30 | 12.5 liters/min for 10 min | Exhaust air from an infected barn | Porcine reproductive and respiratory syndrome virus (PRRSV) | PCR and culture | No virus was detected in the air samples by PCR or by culture | Pigs | 105 | 2002 | |
| All-glass cyclone sampler | 170 liters/min for 20 min | Animal rooms | FMD virus O UKG 34/2001 | Culture | Viruses were recovered with both samplers | Sheep and heifers | 8 | 2002 | |
| Three-stage liquid impinger | 55 liters/min for 5 min | 610-liter cabinet | FMD virus O UKG 34/2001 | Culture | Viruses were recovered with both samplers | Sheep and heifers | 8 | 2002 | |
| All-glass cyclone sampler | 170 liters/min for 2 min | Chamber | FMD virus strain O1 Lausanne Sw/65 | Culture | FMD virus was recovered from the air samples, but the efficiency of the samplers was not compared | Pigs | 7 | 2002 | |
| Porton AGI | 10 to 13 liters/min for 2 or 5 min | Chamber | FMD virus strain O1 Lausanne Sw/65 | Culture | FMD virus was recovered from the air samples, but the efficiency of the samplers was not compared | Pigs | 7 | 2002 | |
| Three-stage liquid impinger | 55 liters/min for 5 min | Chamber | FMD virus strain O1 Lausanne Sw/65 | Culture | FMD virus was recovered from the air samples, but the efficiency of the samplers was not compared | Pigs | 7 | 2002 | |
| Modified SAS-100 | 100 liters/min for 2.5 min | Within and around poultry broiler houses | Male-specific coliphages | Culture | Coliphages were recovered from air samples when a premoistened cellulose ester filter collection medium was used for sampling by impaction | Poultry | 50 | 2002 | |
| PTFE filters (2.0-μm pore size) | 10 min | Chamber with UV light | Rhinovirus 16 strain 11757 from ATCC, uranine | 2 (mass-median diameter of droplets) | Seminested RT-PCR | The detection limit was 1.3 50% tissue culture infective doses/filter for aerosolized virus | Six-jet Collison nebulizer (CN-38) | 101 | 2003 |
| MD8 air sampler with sterile gelatin membrane filter (3-μm pore size) | 100 liters in 1 or 2 min | Dairy factory, close proximity to a running whey separator | Lactococcus lactis bacteriophages | Culture | The MD8 and AirPort MD8 results were very similar; the phage recovery rates for the MAS-100 (impaction) sampler were 1 to 5% that for the MD8 (filtration) sampler | Whey separator in a dairy factory | 103 | 2003 | |
| AirPort MD8 sampler with sterile gelatin membrane filters (3-μm pore size) | 100 liters in 2 min | Dairy factory, close proximity to a running whey separator | Lactococcus lactis bacteriophages | Culture | The MD8 and AirPort MD8 results were very similar; the phage recovery rates for the MAS-100 (impaction) sampler were 1 to 5% that for the MD8 (filtration) sampler | Whey separator in a dairy factory | 103 | 2003 | |
| MAS-100 device (with five different setups) | 100 liters in 1 min | Dairy factory, close proximity to a running whey separator | Lactococcus lactis bacteriophages | Culture | The MD8 and AirPort MD8 results were very similar; the phage recovery rates for the MAS-100 (impaction) sampler were 1 to 5% that for the MD8 (filtration) sampler | Whey separator in a dairy factory | 103 | 2003 | |
| AGI-30 | 12.5 liters/min for 10 min | Exhaust air from an infected barn | PRRSV | PCR, culture, and pig bioassay | All 168 air samples were negative by PCR, culture, and pig bioassay | Pigs | 129 | 2004 | |
| PTFE filters (2.0-μm pore size) | Average of 47 h, from 9 a.m. to 5 p.m. at 4 liters/min | Office buildings | Picornaviruses (rhinovirus and enteroviruses) | Nested RT-PCR and sequencing | Fifty-eight (32%) of 181 filters were positive for picornavirus | Humans | 102 | 2004 | |
| Bubbling sampler | 4 liters/min for 5 min | 400-liter aerosol chamber | Influenza virus A/Aichi/2/68 (H3N2), vaccinia virus strain LIVP (C0355 K0602), uranine | 0.5-2.2 (majority of particles) | Culture and titration on chicken embryos | The average recovery rate was 20% for the influenza virus and 89% for the vaccinia virus | Collison nebulizer | 2 | 2005 |
| Portable, single-sieve, MicroBio MB1 impactor | 100 to 600 liters | Domestic toilet in a 2.6-m3 room | Bacteriophage MS2 | Culture | Bacteriophages were recovered with both sampling methods; the impactor sampler recovered phages from the air 60 min after toilet flushing | Toilet flush | 14 | 2005 | |
| Settling plates | 30 min | Domestic toilet in a 2.6-m3 room | Bacteriophage MS2 | Culture | Bacteriophages were recovered with both sampling methods; the impactor sampler recovered phages from the air 60 min after toilet flushing | Toilet flush | 14 | 2005 | |
| High-resolution slit sampler system | 30 liters/min for 18 min (10 sampling heads for a total of 180 min) | Hospital rooms of patients with SARS | SARS coronavirus | RT-PCR, quantitative PCR, culture, and DNA sequencing | Two of 10 samples from the room of a recovering SARS patient collected using the slit sampler were PCR positive but culture negative; the PTFE membrane filters used in the other rooms were all PCR and culture negative; low concentrations (or absence) of airborne viruses may explain the negative results | Humans | 19 | 2005 | |
| PTFE membrane filters (0.3-μm pore size) | 2 liters/min for 10.5 to 13 h | Hospital rooms of patients with SARS | SARS coronavirus | RT-PCR, quantitative PCR, culture, and DNA sequencing | Two of 10 samples from the room of a recovering SARS patient collected using the slit sampler were PCR positive but culture negative; the PTFE membrane filters used in the other rooms were all PCR and culture negative; low concentrations (or absence) of airborne viruses may explain the negative results | Humans | 19 | 2005 | |
| Portable air sampler | 450 liters/min | 10.16-cm-diameter polyvinyl chloride pipe of 3 to 150 m long attached to a blower | PRRSV strain MN 30-100 | Quantitative PCR and culture | Viruses were recovered from a distance of up to 150 m | Cooking oil spritzer | 31 | 2005 | |
| Cyclone samplers | 700 (± 50) liters/min for 5 min | Chamber | Bacteriophage MS2 | Culture | Viruses were recovered | One or two three-jet or six-jet Collison nebulizers | 62 | 2005 | |
| Wetted-wall cyclone-style air sampler | 265 liters/min for 8 h | Commercial poultry flocks | Exotic Newcastle disease virus | Real-time RT-PCR (RRT-PCR), inoculation of eggs, culture, and sequencing | Low concentrations of virus particles were detected by RRT-PCR; culture was apparently more sensitive than RRT-PCR for detecting viruses | Poultry | 74 | 2005 | |
| AGI-30 | Set 2 experiments, 12.5 liters/min | Closed system | Bacteriophages MS2 and T3 | Particle size was influenced mainly by the properties of the liquid medium and the method of aerosolization, not by the physical size of the viruses; the particles studied were under 300 nm in diameter | Set 4 experiments, culture | The capture efficiency for particles in the 30- to 100-nm size range was 10% or lower; the efficiency increased for particles smaller than 30 nm and larger than 100 nm; all three samplers exhibited low capture efficiencies for ultrafine particles that varied over time, as well as a potential loss of virus viability during sampling | Constant output atomizer | 76 | 2005 |
| SKC BioSampler | Set 2 experiments, 12.5 liters/min | Closed system | Bacteriophages MS2 and T3 | Particle size was influenced mainly by the properties of the liquid medium and the method of aerosolization, not by the physical size of the viruses; the particles studied were under 300 nm in diameter | Set 4 experiments, culture | The capture efficiency for particles in the 30- to 100-nm size range was 10% or lower; the efficiency increased for particles smaller than 30 nm and larger than 100 nm; all three samplers exhibited low capture efficiencies for ultrafine particles that varied over time, as well as a potential loss of virus viability during sampling | Constant output atomizer | 76 | 2005 |
| Frit bubbler | Set 2 experiments, 12.5 liters/min | Closed system | Bacteriophages MS2 and T3 | Particle size was influenced mainly by the properties of the liquid medium and the method of aerosolization, not by the physical size of the viruses; the particles studied were under 300 nm in diameter | Set 4 experiments, culture | The capture efficiency for particles in the 30- to 100-nm size range was 10% or lower; the efficiency increased for particles smaller than 30 nm and larger than 100 nm; all three samplers exhibited low capture efficiencies for ultrafine particles that varied over time, as well as a potential loss of virus viability during sampling | Constant output atomizer | 76 | 2005 |
| SKC BioSampler | 12.5 liters/min for 20 min | Field downwind from a biosolid spray or seeded groundwater | Bacteriophage MS2 | Culture | Bacteriophages were recovered downwind from the seeded groundwater spray but not from the biosolid spray | Spray tanker | 124 | 2005 | |
| Andersen one-stage impactor; a six-stage impactor was also used for size distribution | 28.3 liters/min | 29-cm-diameter, 32-cm-high chamber | Bacteriophages φX174, MS2, T7, and φ6 | 1.23-1.25 (mean aerodynamic diameter; >95% of PFU recovered with the six-stage Andersen sampler were <2.1 μm in diameter) | Culture | The capture efficiency for infectious viruses was highly dependent on the properties of the viruses and the RH; viral recovery was very low with the Nuclepore filter | Three-jet Collison nebulizer | 132 | 2005 |
| AGI-30 | 12.5 liters/min for 5 min | 29-cm-diameter, 32-cm-high chamber | Bacteriophages φX174, MS2, T7, and φ6 | 1.23-1.25 (mean aerodynamic diameter; >95% of PFU recovered with the six-stage Andersen sampler were <2.1 μm in diameter) | Culture | The capture efficiency for infectious viruses was highly dependent on the properties of the viruses and the RH; viral recovery was very low with the Nuclepore filter | Three-jet Collison nebulizer | 132 | 2005 |
| Gelatin filter (3.0-μm pore size) | 30 liters/min for 5 min | 29-cm-diameter, 32-cm-high chamber | Bacteriophages φX174, MS2, T7, and φ6 | 1.23-1.25 (mean aerodynamic diameter; >95% of PFU recovered with the six-stage Andersen sampler were <2.1 μm in diameter) | Culture | The capture efficiency for infectious viruses was highly dependent on the properties of the viruses and the RH; viral recovery was very low with the Nuclepore filter | Three-jet Collison nebulizer | 132 | 2005 |
| Nuclepore filter (polycarbonate membrane; 0.4-μm pore size) | 2 liters/min for 20 min | 29-cm-diameter, 32-cm-high chamber | Bacteriophages φX174, MS2, T7, and φ6 | 1.23-1.25 (mean aerodynamic diameter; >95% of PFU recovered with the six-stage Andersen sampler were <2.1 μm in diameter) | Culture | The capture efficiency for infectious viruses was highly dependent on the properties of the viruses and the RH; viral recovery was very low with the Nuclepore filter | Three-jet Collison nebulizer | 132 | 2005 |
| AGI-30 | 12.5 liters/min | Glass chamber | PRRSV (North American prototype) and swine influenza virus strain A/Swine/Iowa/73 (H1N1) | Culture and quantitative RT-PCR | The BioSampler and the AGI-30 sampler collected more viruses than the AGI-4 sampler at 10, 15, and 20 min; the BioSampler collected more viruses than the AGI-30 and AGI-4 samplers at 15 and 20 min | 24-Jet Collison nebulizer | 73 | 2006 | |
| AGI-4 | 12.5 liters/min | Glass chamber | PRRSV (North American prototype) and swine influenza virus strain A/Swine/Iowa/73 (H1N1) | Culture and quantitative RT-PCR | The BioSampler and the AGI-30 sampler collected more viruses than the AGI-4 sampler at 10, 15, and 20 min; the BioSampler collected more viruses than the AGI-30 and AGI-4 samplers at 15 and 20 min | 24-Jet Collison nebulizer | 73 | 2006 | |
| SKC BioSampler | 6 liters/min | Glass chamber | PRRSV (North American prototype) and swine influenza virus strain A/Swine/Iowa/73 (H1N1) | Culture and quantitative RT-PCR | The BioSampler and the AGI-30 sampler collected more viruses than the AGI-4 sampler at 10, 15, and 20 min; the BioSampler collected more viruses than the AGI-30 and AGI-4 samplers at 15 and 20 min | 24-Jet Collison nebulizer | 73 | 2006 | |
| Andersen six-stage sampler (for size distribution) | 23-liter exposure chamber | Bacteriophages MS2, φX174, φ6, and T7 | 0.5-3.0 (>95% of virus-containing particles were <2.1 μm) | Culture | The surviving fraction of airborne viruses decreased exponentially as the ozone concentration increased; viruses with more complex capsid architectures were less susceptible to ozone inactivation | Three-jet Collison nebulizer | 133 | 2006 | |
| Andersen one-stage sampler | 28.3 liters/min for 0.5 to 5 min | 23-liter exposure chamber | Bacteriophages MS2, φX174, φ6, and T7 | 0.5-3.0 (>95% of virus-containing particles were <2.1 μm) | Culture | The surviving fraction of airborne viruses decreased exponentially as the ozone concentration increased; viruses with more complex capsid architectures were less susceptible to ozone inactivation | Three-jet Collison nebulizer | 133 | 2006 |
| AGI-30 | 12.5 liters/min for 10 min or less | Stainless steel closed-loop wind tunnel | Transmissible gastroenteritis virus, avian pneumovirus, and fowlpox virus | Culture | Viruses were detected upstream but not downstream from the filter | 500 ELSD nebulizer | 52 | 2006 | |
| Porton sampler | 55 liters in 5 min | 610-liter air sampling cabinet (for each pig) and a loose box (for six pigs) (approximately 4 by 4 by 3 m) | FMD virus O UKG FMD 34/2001 | 0.015-20 (no clear association of viable virus with a particular size range) | Culture and RRT-PCR | The number of infectious FMD virus and RNA copies was independent of the sampling method | Pigs | 60 | 2007 |
| May sampler | 165 liters in 5 min | 610-liter air sampling cabinet (for each pig) and a loose box (for six pigs) (approximately 4 by 4 by 3 m) | FMD virus O UKG FMD 34/2001 | 0.015-20 (no clear association of viable virus with a particular size range) | Culture and RRT-PCR | The number of infectious FMD virus and RNA copies was independent of the sampling method | Pigs | 60 | 2007 |
| Cyclone sampler | 3,900 liters in 5 min | 610-liter air sampling cabinet (for each pig) and a loose box (for six pigs) (approximately 4 by 4 by 3 m) | FMD virus O UKG FMD 34/2001 | 0.015-20 (no clear association of viable virus with a particular size range) | Culture and RRT-PCR | The number of infectious FMD virus and RNA copies was independent of the sampling method | Pigs | 60 | 2007 |
| 25-mm filters with an SKC Button inhalable aerosol sampler | |||||||||
| Gelatin filter (3-μm pore size) | 4 liters/min | Chamber | Bacteriophage MS2 | 10-80 nm | Particle counters (airborne particle concentrations were measured downstream and upstream from the filters) | Physical efficiency was over 96% | Six-jet Collison-type air-jet nebulizer | 23 | 2007 |
| PC filter (0.4-μm pore size) | 4 liters/min | Chamber | Bacteriophage MS2 | 10-80 nm | Particle counters (airborne particle concentrations were measured downstream and upstream from the filters) | Left aside due to a high pressure drop | Six-jet Collison-type air-jet nebulizer | 23 | 2007 |
| PC filter (1-μm pore size) | 4 liters/min | Chamber | Bacteriophage MS2 | 10-80 nm | Particle counters (airborne particle concentrations were measured downstream and upstream from the filters) | Physical collection efficiency was 68% | Six-jet Collison-type air-jet nebulizer | 23 | 2007 |
| PC filter (3-μm pore size) | 4 liters/min | Chamber | Bacteriophage MS2 | 10-80 nm | Particle counters (airborne particle concentrations were measured downstream and upstream from the filters) | Physical collection efficiency was 27% | Six-jet Collison-type air-jet nebulizer | 23 | 2007 |
| PTFE filter (0.5-μm pore size) | 4 liters/min | Chamber | Bacteriophage MS2 | 10-80 nm | Particle counters (airborne particle concentrations were measured downstream and upstream from the filters) | More than 96% physical collection efficiency | Six-jet Collison-type air-jet nebulizer | 23 | 2007 |
| PTFE filter (1-μm pore size) | 4 liters/min | Chamber | Bacteriophage MS2 | 10-80 nm | Particle counters (airborne particle concentrations were measured downstream and upstream from the filters) | More than 96% physical collection efficiency | Six-jet Collison-type air-jet nebulizer | 23 | 2007 |
| PTFE filter (3-μm pore size) | 4 liters/min | Chamber | Bacteriophage MS2 | 10-80 nm | Particle counters (airborne particle concentrations were measured downstream and upstream from the filters) | More than 96% physical collection efficiency | Six-jet Collison-type air-jet nebulizer | 23 | 2007 |
| Preloaded in three-part 37-mm plastic cassettes | |||||||||
| PTFE filter (0.3-μm pore size) | 2 liters/min | Chamber | Bacteriophage MS2 | 10-80 nm | Particle counters (airborne particle concentrations were measured downstream and upstream from the filters) | More than 96% physical collection efficiency | Six-jet Collison-type air-jet nebulizer | 23 | 2007 |
| SKC BioSampler impingers | 12.5 liters/min for 1 min | 133-liter stainless steel dynamic aerosol toroid | PRRSV, rhodamine B | Microinfectivity assays and quantitative RT-PCR | Temperature had a greater effect than RH on the stability of PRRSV | 24-Jet Collison nebulizer | 72 | 2007 | |
| Bioaerosol personal sampler | 4 liters/min for 5 min | Aerosol chamber | Influenza virus A strain | Culture and RRT-PCR | Viruses were detected in aerosol samples | Three-jet Collison nebulizer | 111 | 2007 | |
| 37-mm gelatin filter | 28.3 liters/min for 5 or 10 min | 3.0-m by 4.6-m by 3.0-m chamber with upper-room UV germicidal irradiation | Vaccinia virus (WR strain) | 2.5 (median diameter) | Culture | Concentrations of infective vaccinia virus can be diminished by upper-room UV germicidal irradiation | Six-jet Collison nebulizer | 53 | 2007 |
| 37-mm gelatin filter in a polystyrene air sampling cassette | Aerosol chamber with UV light | Vaccinia virus (WR strain) | Culture | Inactivation of viruses by UVC decreased at high RH | Six-jet Collison nebulizer | 93 | 2007 | ||
| Andersen sampler with gelatin filters | Aerosol chamber with UV light | Vaccinia virus (WR strain) | Culture | Inactivation of viruses by UVC decreased at high RH | Six-jet Collison nebulizer | 93 | 2007 |
Solid Impactors
Solid impactors, such as Andersen samplers, slit samplers, and cyclone samplers, are usually more efficient at capturing large particles. Andersen and slit samplers accelerate the particles through narrow holes or slits. The streamline moves toward a solid surface and abruptly changes direction. The inertia of the particles deviates them from the airflow and impacts them on the surface, which usually holds a petri dish with a culture medium. The medium is either washed to collect the particles or used directly for plaque assays. Andersen samplers contain a number of stages, each of which traps particles of a specific aerodynamic size range, and is often used to determine the sizes of virus-laden particles (54, 79, 132, 133). The multistage configuration is designed to accelerate the incoming particles. The first stage induces moderate acceleration so that only the largest particles deviate from their trajectory. The second stage accelerates the smaller particles a little more, and so on. A six-stage Andersen sampler recovers particles ranging from 0.65 μm in diameter on the lowest stage to 7.5 μm and over on the top stage. Single-stage Andersen samplers can also be used to capture particles. The lower recovery limit of these samplers is a function of the diameter of the holes through which the particles are accelerated.
Slit samplers are used mostly to determine aerosol concentrations of bacteria as a function of time. The accelerated particles are impacted onto a rotating petri dish containing a culture medium. This makes it possible to determine the time when each particle was sampled. Time-dependent results can be obtained only if the samples are grown directly on the solid culture medium. Obviously, the use of a liquid medium or buffer on top of a solid medium compromises this function. Nevertheless, slit samplers have been used with a liquid layer on the culture medium to recover viruses. The particles that impact the solid surface are immediately resuspended in a liquid medium to maximize the recovery of both infective viruses and viral nucleic acids. This method was used successfully in Toronto, Canada, during the 2003 SARS outbreak (19). In fact, two air sampling methods were used during this outbreak, namely, a modified high-resolution slit sampler system and polytetrafluoroethylene (PTFE) membrane filters with 0.3-μm pores. The samples were tested for viruses by reverse transcriptase PCR (RT-PCR) and culture assays. The cultures were all negative, and only 2 of the 10 slit samples were PCR positive. These results might be explained by the absence or a very low concentration of airborne viruses.
Centrifugal forces have also been used to sample artificially generated airborne influenza viruses. The samples collected by centrifugation within an hour after aerosolization caused influenza in inoculated ferrets (140). The centrifugal sampler recovered close to 100% of 2.3-μm-diameter particles and 50% of 0.77-μm-diameter particles at 4,500 rpm, which corresponds to an airflow of 1.44 to 1.90 cubic feet/min (40 to 54 liters/min) (108). Errington and Powell developed small and large cyclone separators. The small cyclone separator has a flow rate of 75 liters/min, and the large one has a flow rate of 350 liters/min. Both cyclones accelerate the air by using a centrifugal vortex, pushing the airborne particles into contact with a solid surface by using the inertia of the particles. A scrubbing liquid is constantly injected into the cyclone and collected in a bottle at its base. The concentration of the aerosol in the liquid depends on the air sampling and liquid injection rates. The smaller sampler can, for example, concentrate 100 liters of air in 1 ml of liquid (49). This first generation of cyclone separators inspired the development of similar apparatuses, which can sample the air at various rates.
A 170-liters/min flow rate (2- and 20-min samples) has been used to sample air contaminated with FMD virus released by infected pigs (7) as well as by sheep and heifers (8). In another study, a 300-liters/min flow rate (15-min sample) was successfully used to sample the air of isolated units housing pigs infected with Aujeszky's disease virus. However, the sampler was unable to detect low levels of airborne virus (20). Cyclone samplers have also been used at high flow rates, ranging from 700 to 1,000 liters/min (5- to 30-min samples), to recover airborne viruses (38-40, 60, 62). A recirculating liquid cyclone-style air sampler operating at 265 liters/min (8-h sample), combined with culture and RT-PCR, has been used to detect exotic Newcastle disease virus in naturally contaminated commercial poultry flocks (74). The capacity of cyclones to concentrate aerosols in large volumes of air over long, uninterrupted periods is one of the major advantages of this type of sampler. However, some investigators have reported that cyclone samplers are much less efficient than other samplers at recovering low concentrations of airborne viruses (20). This may be due to the physical stress caused by cyclone samplers, which may cause structural damage to the viruses and thus decrease their infectivity (20).
Liquid Impactors
All-glass impingers (AGIs) (Fig. 3), also called Porton impingers, and AGI-like samplers are the most often used samplers for the capture of airborne viruses (Fig. 1; Table 2). The liquid impinger, which was first described by May and Harper (91), works by accelerating airborne particles through a narrow orifice placed at a fixed distance from the bottom of a flask containing a liquid. A pressure drop is created in the flask and forces the air to enter through the inlet of the impinger. The air enters horizontally through a glass tube, which curves to a vertical position, forcing the air to change direction and flow downward. The diameter of the tubing abruptly narrows and acts as a critical flow orifice, accelerating the air passing through it to sonic velocity. The flow remains constant as long as there is at least half an atmosphere of suction in the impinger. The curve in the tube is intended to trap the larger particles by inertial impaction and mimics the airway of the human nose. The largest particles entering the flask through the critical flow orifice are impacted onto the liquid. The formation of small bubbles in the liquid of the impinger can also help to sample very small particles by diffusion. However, the reaerosolization of particles due to the scavenging properties of the air bubbles can be a problem, especially for hydrophobic particles. The liquid prevents desiccation and facilitates the extraction of genetic material for subsequent analysis. The AGI-4 sampler (the number refers to the distance, in millimeters, separating the tip of the critical orifice from the bottom of the flask) and the AGI-30 sampler, also called a raised impinger, are often used as standard reference samplers (71). Multistage liquid impingers are also available. Particles impinge into liquids in successive stages as a function of their aerodynamic size. This type of sampler, like the Andersen sampler, is used mainly to determine the size distribution of infectious particles (39, 40).
According to Harstad (66), who compared the sampling efficiencies of two types of liquid impingers, two types of filters, and a fritted bubbler, using submicrometer aerosols of a suspension of bacteriophage T1 (a tailed bacterial virus) with a radioactive tracer, liquid impingers are the least destructive samplers, with a relative efficiency, as determined by culture, superior by 18% to that of the next best sampler, although 30% to 48% of the sample was physically lost. Harstad also reported that filters are very destructive for this bacterial virus but are the most efficient at collecting submicrometer particles and that the fritted bubbler is the least efficient sampler, with a physical loss of over 80% of the sample (66). These differences in the recovery rates of AGI samplers and filters were confirmed in a later study (64). The gentler sampling process leading to better recovery of infective viruses seems to be the main reason for the wide use of AGI samplers in aerovirology. Many studies involving airborne virus sampling have been conducted using the AGI-30 or AGI-4 sampler as the main sampling device (4, 10, 17, 42, 45-48, 57, 58, 65, 67, 78, 81, 82, 84, 86, 105, 116, 118, 119, 122, 129-131, 138). Most were done to determine the effects of various factors on the recovery rates of airborne viruses. Although some studies indicate that the AGI has a lower recovery potential than other samplers, such as the large-volume sampler (LVS) (94, 144), the Andersen sampler (127), and the slit sampler (126), other studies suggest that the AGI recovers concentrations of viruses that are equivalent to those with the LVS (121), greater than those with the Andersen sampler (15), and greater than (30) or equal to (85) those with the slit sampler.
A recently developed impinger model, the “swirling aerosol collector” (Fig. 3) (143), commercialized as the BioSampler, has also been used to study viral aerosols in the same way as AGIs (22, 72, 124). This newer impinger works much like the AGI, with a curved inlet tube and a vacuum in the flask to force the air through the sampler. The major difference is the number and positions of nozzles. Instead of forcing air at sonic speed through a single nozzle directed toward the base of the flask, as with the AGI, the BioSampler has three tangential sonic nozzles. The collection liquid in the flask moves in a swirling motion during sampling. The sampling procedure is less violent and less destructive than that with the AGI-30 sampler. Hermann et al. (73) studied the BioSampler and reported that it, as well as the AGI-30 sampler, collects significantly more aerosolized porcine reproductive and respiratory syndrome viruses than the AGI-4 sampler does. They also reported that the collection efficiency of the BioSampler is significantly greater than that of the AGI-30 sampler after 15 and 20 min of sampling (73). However, both the AGI-30 and the BioSampler (as well as the frit bubbler) are surprisingly inefficient at recovering submicrometer and ultrafine virus aerosols, with collection efficiencies of <10% for all three samplers for the 30- to 100-nm particle size range (76).
Prehumidifying aerosols by using a humidifier bulb in combination with an AGI-30 sampler can have both positive and negative effects on the recovery of infectious viruses from airborne material, depending on the virus. The AGI-30 with humidifier bulb has been shown to increase the recovery of airborne coliphages T3 (67, 137), T2, and T7 (16), as well as Pasteurella pestis bacteriophages (67). Recovery increases fivefold at high RH and up to 1,000-fold at low RH when a peptone solution or saliva is used as the spray medium. However, adding NaCl to the spray medium has no effect on the recovery of T3 (131). Prehumidification has no effect on the recovery of mengovirus 37A (Picornaviridae family; ssRNA virus) or vesicular stomatitis virus (Rhabdoviridae family; ssRNA virus) but increases the recovery of bacteriophage S13 (Microviridae family; ssDNA virus) at mid-range RH (137). One possible explanation for the beneficial effect of prehumidification may be that the median size of the particles is increased at high RH by the condensation of the water vapor on the airborne particles. The condensation may also have a negative effect by dissolving the particle nuclei, exposing the viruses to high concentrations of solutes, which may structurally damage the virus, leading to a loss of infectivity. No definitive explanation has yet been proposed to explain the effect of prehumidification on viral infectivity.
Filters
Since most samplers cannot efficiently trap particles with an aerodynamic size of <500 nm, filters are frequently used to sample airborne viruses. Filter efficiency is based on the following five basic mechanisms: (i) interception, (ii) inertial impaction, (iii) diffusion, (iv) gravitational settling, and (v) electrostatic attraction (75). While each mechanism depends on the aerodynamic diameter of the particle, interception also depends on particle radius. Interception occurs when a particle follows the streamline going around an obstacle but, because of its size, comes into contact with and is intercepted by the obstacle. This is the only mechanism that does not depend on particles being diverted from the streamline. Inertial impaction occurs when a particle impacts an obstacle when the streamline changes direction. The inertia of the particle forces it to divert from the streamline and to impact a surface. As mentioned previously, only very small particles are affected by the diffusion mechanism based on Brownian motion. Gravitational settling affects mostly particles of much larger aerodynamic diameter by pushing them downward due to gravity. The importance of this force depends on the other forces affecting the particle in various directions. Lastly, electrostatic forces also influence the trajectory of particles. This mechanism depends on the size and charge of the particle and the charge difference with the filter.
Given that small particles are highly governed by the diffusion phenomenon and that larger particles have a tendency to impact and to be intercepted, it was shown that both types of behaviors are at their lowest at 0.3 μm (75). Thus, filters are least efficient at removing 0.3-μm particles. Filtration efficiency improves with increasing and decreasing particle size. This is why filter efficiency is based on the 0.3-μm benchmark.
Many different types of filters have been used to sample airborne viruses. They differ mainly in composition, pore size, and thickness. To our knowledge, the first filters used to sample airborne viruses were made out of tightly packed cotton and were used to sample variola virus (Poxviridae family; dsDNA virus) in a hospital (95). Cellulose filters (0.45-μm pore size) have also been used to sample hospital air; PCR analysis of these samples permitted the detection of naturally produced aerosols of varicella-zoster virus (Herpesviridae family; dsDNA virus; 200 nm) (117) and respiratory syncytial virus (Paramyxoviridae family; ssRNA virus; 150 nm) (3). PTFE filters (2.0-μm pore size) have been used to collect artificial rhinovirus (Picornaviridae family; ssRNA virus; 25 to 30 nm) aerosols in a small aerosol chamber (101) and naturally produced rhinovirus aerosols in office buildings (102). In both cases, PCR was used to detect the viruses. While polycarbonate filters are much less efficient than gelatin or PTFE filters (23), 0.1-μm polycarbonate filters have been used in combination with PCR to detect human cytomegalovirus (Herpesviridae family; dsDNA virus; 200 nm) in samples of naturally produced aerosols (92). The low filtration efficiency of polycarbonate filters may be due to the structure of the filter. The contact area of filters with uniform cylindrical pores, such as polycarbonate filters, is much smaller than that of filters with a complex structure, such as PTFE filters, where the probability of adherence is greater because airborne particles are exposed to a greater surface area.
However, filters are not commonly used to sample airborne viruses because they can cause structural damage. In addition, the desiccation of the samples that occurs during sampling can interfere with culture analysis of the samples. While modern molecular biology tools do not require infectious particles to detect viruses, studies investigating the effects of environmental factors on viral infectivity, for example, require the collection of infectious viruses. Gelatin filters can be used because they do not appear to significantly affect viral infectivity. For example, MD8 air samplers equipped with 80-mm gelatin membrane filters with a pore size of 3 μm in combination with culture techniques have been used successfully to detect Lactococcus lactis tailed bacteriophages in a cheese factory (103). Gelatin filters, as well as the Andersen sampler and the AGI-30 impinger, are 10 times more efficient than polycarbonate filters at collecting active bacteriophages (132). The physical collection efficiencies of both gelatin and PTFE filters, calculated by placing particle counters before and after the filters, exceed 96% (23). While gelatin filters can be very useful for sampling functional viruses, their use can be limited by environmental conditions. Low humidity can cause them to dry out and break, while high humidity or water droplets can cause them to dissolve. On the positive side, this property can be used to recover viruses or virus-laden particles by dissolving the filters in water. Nevertheless, 0.3-μm PTFE filters appear to be the best option for long-term sampling of 10- to 900-nm-diameter virus-laden particles (23).
Filter materials mounted on three-piece cassettes all have the same limitation. These cassettes are hollow cylinders made out of plastic or metal, with an inlet or outlet hole at the center of the base of each cylinder. A filter is deposited on a porous support pad (cellulose, plastic, or metal) on part one, the base. The second part, the cylinder, is placed on the base to seal the edge of the filter and ensure that the air pumped from the outlet of the cassette passes through the filter. If only the first two parts are used, it is considered an open cassette. The third part is placed on top to close the cassette. The pressure used to seal the cassettes can influence the outcome of an experiment. If the cassette is not properly sealed, aerosol slippage can occur. The airflow goes around the filter and into the pump, preventing most of the airborne particles from being captured by the filter and significantly contaminating the pump in the process. On the other hand, if the cassette is sealed too tightly, the filter can be damaged or weakened, especially with fragile filters such as gelatin membranes, and aerosol slippage can also occur (135). If premounted cassettes are available with the desired filters, their efficiency should be compared to that of laboratory-prepared cassettes.
Standardized filters are still available but are often left aside because of the damage they can cause to viruses. In comparison studies, filters often provide the best physical recovery of nanoscale particles, but the filtering process can damage viruses and complicate the analysis, especially if culture is used to assess virus levels. Comparative studies using culture as an analytical tool have shown that filters are less efficient than other, less destructive methods, such as liquid impingers, for recovering airborne infectious viruses (64, 66, 132).
Electrostatic Precipitators
LVS use electrostatic precipitation to sample air. One example is the LVS designed for the U.S. army in the 1960s (54). This device can draw up to 10,000 liters of air per minute through a high-voltage corona, where the particles are charged before being precipitated onto a grounded rotating disc. A recirculating fluid is used to wash off and concentrate the precipitated particles. LVS have been used to recover airborne adenoviruses (Adenoviridae family; nonenveloped dsDNA virus; 70 to 90 nm in diameter) in a military hospital, where 50,545 liters of air were sampled in 5 minutes, with the particulate content collected in 180 ml of fluid (11). LVS have also been used in other circumstances to recover low concentrations of airborne adenoviruses and picornaviruses (12, 13, 38, 40, 51, 77, 94, 97, 121, 144). These samplers are commonly used with a preimpactor attached to the air inlet to capture particles of over 15 μm in diameter. Smaller particles are subsequently collected by electrostatic precipitation (12). Although LVS recover slightly more infective viruses than does the cyclone separator described by Errington and Powell (49), LVS are more complicated to operate (38). In addition, the production of ozone at high RH in the presence of the intense electric field may damage viruses (28).
Other Sampling and Detection Methods
Other methods can be used to detect airborne viruses without any actual air sampling. Swabbing the surfaces of air purifier filters (44, 123) can be used to assess the viral composition of air. Settling dishes (14, 40, 41, 126) can also be used, but this method is more suitable for larger droplets that settle by gravity on a petri dish. While cumbersome, sentinel animals can be used to detect the presence of viruses (21, 57, 105, 129). Susceptible host animals can be used as air samplers and culture support systems (90) by exposing the animals to potentially contaminated air and then noting symptoms or performing laboratory tests. Sufficient concentrations of airborne virus and appropriate conditions are required for this approach to detect viruses.
ASSESSING THE EFFICIENCY OF AIRBORNE VIRUS SAMPLERS BY USE OF TRACERS
In order to study the efficiency of samplers for airborne virus sampling, a variety of strategies have been used. It is important to note the difference between the capture efficiency of a sampler and its efficiency for viral recovery. The capture efficiency (or total physical efficiency) is based on the rate of recovery of different particle sizes and is measured with methods independent of the integrity of the viruses, while the efficiency of viral recovery is, in most studies, an indicator of the remaining integrity and infectivity of the sampled viruses.
Tracers can be used to measure the total capture efficiency of samplers. P-32 (16, 65, 66), uranine (fluorescein sodium salt) (2, 32, 54, 55, 78, 81, 82, 84, 101), rhodamine B (46-48, 72, 116, 122), and Bacillus spores (17, 77, 119) have all been used as tracers to detect airborne viruses. Uranine remains the most popular tracer because it is safer than radiolabeling for aerosol studies. In addition, unlike rhodamine B, it does not affect viral infectivity (79). New molecular methods have led to alternative tracers for determining sampler efficiency. For example, viral genetic material can be used to estimate the total number of viruses sampled. Genomic tracers do not depend on viral infectivity or, to a certain degree, viral integrity. However, the lack of information on the degradation of viral genetic material in aerosols makes the replacement of physical tracers by quantitative PCR premature (72). The major weakness of the quantitative PCR method is the detection limit. Lastly, particle counters can be installed upstream and downstream from the sampler to determine the total number of particles trapped by the sampler. However, this method cannot be used to determine the proportion of captured particles that can be extracted for further analysis.
LABORATORY STUDIES OF AIRBORNE VIRUSES
To our knowledge, the oldest study on the sampling of airborne viruses was performed with a laboratory setup using a chamber and an artificially produced aerosol of influenza virus (140). Since then, many chamber setups have been used to study artificially produced infectious aerosols (61, 70, 139) and airborne viruses. Rotating drum or dynamic aerosol toroids (61) have been used to study the biological decay rates of airborne viruses under different temperature and/or RH conditions (4, 5, 16, 42, 46-48, 65, 67, 72, 78, 80-82, 84, 116, 119, 122, 138). These devices make it possible to study aerosols under controlled atmospheric conditions for extended periods, with little loss of airborne particles to gravitational settling. A variety of chambers and other types of closed and/or controlled systems, some inspired by previous technologies, have been used to study artificially and naturally produced aerosols (2, 7, 8, 15, 20, 23, 30-33, 38, 39, 45, 54-58, 60, 62, 64, 66, 73, 76, 86, 99, 101, 107, 109, 118, 120, 121, 130-133, 137). Most setups for viral aerosol studies are handmade for specific purposes. The aerosol source or generator, temperature, RH, radiation, time of exposure, aerosolization medium, sampling method, viruses, tracers, and analytical methods are rarely the same. As such, even controlled studies are difficult to compare.
Aerosol generators are most often used to study the behavior of airborne viruses. For the generation of submicrometer aerosols, neutralizers are placed between the generator and the chamber to prevent uncommon aerosol behavior by removing charges on particles created during the nebulization process. Desiccators are also often used to shrink particles by evaporation. The median size of aerosol particles is controlled by the intensity of the aerosolization process and by preimpactors to stop larger particles. Since the concentration of the nonevaporative solutes in the nebulization medium determines the size of the droplet nuclei, low solute concentrations can be used to produce small particles and high solute concentrations can be used for large particles. The concentration of the aerosol in the chamber can be modified by adding clean dilution air. The size of the droplet nuclei can be calculated using equations that take into account the initial droplet diameter and the volume fraction of solid material (75).
Many types of devices can be used to determine the concentration of particles in the air. Spectrometers equipped with various technologies can count and size airborne particles in real time or near real time. However, they are often limited to measuring particles of >300 nm to 500 nm in diameter. Scanning mobility particle sizers can be used to count and measure smaller particles. These devices neutralize airborne particles before separating them based on electrical mobility (charge and size). The particles are then passed through a condenser to increase their size so they can be detected by photometry.
SURROGATE VIRUSES
While aerobiological studies using hazardous viruses can provide valuable information, they can also expose personnel to unnecessary risks. Surrogate viruses, such as bacteriophages of E. coli and other bacteria, can be used to mimic the behavior of pathogenic viruses. Bacteriophages of the order Caudovirales, such as T7-like (16, 30, 42, 45, 67, 76, 99, 122, 131-133, 137, 138), T1-like (64, 66), and T5-like (69) bacteriophages, were the first surrogates used. The genomic material of these bacteriophages consists of dsDNA, the capsid is nonenveloped, and the presence of a tail for host recognition renders the viruses susceptible to physical damage. They are thus unreliable for infectivity assays. In addition, the morphological characteristics of these bacteriophages do not resemble those of any mammalian viruses. As such, they are used more rarely nowadays and have largely been replaced by other surrogate viruses. Bacteriophage MS2 (Leviviridae family) has been used as a surrogate virus in many studies (14, 23, 42, 62, 76, 130, 132, 133). It is a nonenveloped ssRNA coliphage with a very small (25 to 27 nm) icosahedral capsid. It has no tail and is morphologically similar to members of the Picornaviridae family, which includes many pathogenic viruses, such as poliovirus, rhinovirus, and FMD virus. Another surrogate virus is bacteriophage φX174 (Microviridae family), which possesses a ssDNA genome and a morphology similar to that of MS2. Bacteriophage φX174 has been compared to the most resistant human-pathogenic viruses, such as polioviruses and parvoviruses (114), and has been used in aerobiology (96, 132, 133) along with another microvirus, S13 (42, 137). Bacteriophage φ6 (Cystoviridae family; dsRNA virus; 85 nm) can be used as a surrogate for small enveloped viruses (132, 133).
Since every virus has a unique response to environmental factors, no surrogate is perfect. Nonetheless, nonpathogenic models can greatly simplify virus studies, especially when aerosols are used.
CONCLUSIONS
Sampling techniques have been improved greatly over the years, and we are definitely better equipped today to tackle the important health issue of airborne viruses. However, the lack of standardization has to be addressed, as it limits the development of general recommendations for sampling of airborne viruses. Given the wide range of aerodynamic properties of airborne viruses, which can be nanometer- to micrometer-sized particles, the issue of standardization is of the utmost importance. The detection of viruses in air samples depends on the type of aerosol and the sampling and analytical methodologies. Studies to date have rarely included quantitative analyses of total viral load. While culture is often used to determine virus concentrations, most sampling methods affect viral infectivity, making culture inadequate for calculating the true concentrations of infectious airborne viruses. Technologies such as PCR can be used to detect viruses in air samples even when they are no longer infectious. While filters cause more damage to viruses than other methods do, they are more efficient for determining viral loads in aerosols. Lastly, viruses are an astonishingly diverse group of microorganisms, so this diversity has to be taken into consideration to select the most appropriate sampling devices. Whatever techniques or recommendations are proposed for sampling virus aerosols, it is important to keep in mind that a representative sample should contain nanoparticles together with larger airborne particles. Future studies will contribute to better predicting the potential risk of infection by airborne viruses.
Acknowledgments
This study was funded by a concerted grant from FQRNT-NOVALAIT-MAPAQ and by Agriculture and Agri-Food Canada. S.M. and C.D. also received funding from the Natural Sciences and Engineering Research Council of Canada (NSERC). C.D. is the recipient of an FRSQ Junior 2 scholarship.
REFERENCES
- 1.Adams, D. J., J. C. Spendlove, R. S. Spendlove, and B. B. Barnett. 1982. Aerosol stability of infectious and potentially infectious reovirus particles. Appl. Environ. Microbiol. 44903-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agranovski, I. E., A. S. Safatov, A. I. Borodulin, O. V. Pyankov, V. A. Petrishchenko, A. N. Sergeev, A. A. Sergeev, V. Agranovski, and S. A. Grinshpun. 2005. New personal sampler for viable airborne viruses: feasibility study. J. Aerosol Sci. 36609-617. [Google Scholar]
- 3.Aintablian, N., P. Walpita, and M. H. Sawyer. 1998. Detection of Bordetella pertussis and respiratory syncytial virus in air samples from hospital rooms. Infect. Control Hosp. Epidemiol. 19918-923. [DOI] [PubMed] [Google Scholar]
- 4.Akers, T. G., S. Bond, and L. J. Goldberg. 1966. Effect of temperature and relative humidity on survival of airborne Columbia SK group viruses. Appl. Microbiol. 14361-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akers, T. G., and M. T. Hatch. 1968. Survival of a picornavirus and its infectious ribonucleic acid after aerosolization. Appl. Microbiol. 161811-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akers, T. G., C. M. Prato, and E. J. Dubovi. 1973. Airborne stability of simian virus 40. Appl. Microbiol. 26146-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexandersen, S., I. Brotherhood, and A. I. Donaldson. 2002. Natural aerosol transmission of foot-and-mouth disease virus to pigs: minimal infectious dose for strain O1 Lausanne. Epidemiol. Infect. 128301-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alexandersen, S., Z. Zhang, S. M. Reid, G. H. Hutchings, and A. I. Donaldson. 2002. Quantities of infectious virus and viral RNA recovered from sheep and cattle experimentally infected with foot-and-mouth disease virus O UK 2001. J. Gen. Virol. 831915-1923. [DOI] [PubMed] [Google Scholar]
- 9.Aller, J. Y., M. R. Kuznetsova, C. J. Jahns, and P. F. Kemp. 2005. The sea surface microlayer as a source of viral and bacterial enrichment in marine aerosols. J. Aerosol Sci. 36801-812. [Google Scholar]
- 10.Artenstein, M. S., and F. C. Cadigan, Jr. 1964. Air sampling in viral respiratory disease. Arch. Environ. Health 3958-60. [DOI] [PubMed] [Google Scholar]
- 11.Artenstein, M. S., and W. S. Miller. 1966. Air sampling for respiratory disease agents in army recruits. Bacteriol. Rev. 30571-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Artenstein, M. S., W. S. Miller, T. H. Lamson, and B. L. Brandt. 1968. Large-volume air sampling for meningococci and adenoviruses. Am. J. Epidemiol. 87567-577. [DOI] [PubMed] [Google Scholar]
- 13.Artenstein, M. S., W. S. Miller, J. H. Rust, Jr., and T. H. Lamson. 1967. Large-volume air sampling of human respiratory disease pathogens. Am. J. Epidemiol. 85479-485. [DOI] [PubMed] [Google Scholar]
- 14.Barker, J., and M. V. Jones. 2005. The potential spread of infection caused by aerosol contamination of surfaces after flushing a domestic toilet. J. Appl. Microbiol. 99339-347. [DOI] [PubMed] [Google Scholar]
- 15.Bausum, H. T., S. A. Schaub, K. F. Kenyon, and M. J. Small. 1982. Comparison of coliphage and bacterial aerosols at a wastewater spray irrigation site. Appl. Environ. Microbiol. 4328-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benbough, J. E. 1971. Some factors affecting the survival of airborne viruses. J. Gen. Virol. 10209-220. [DOI] [PubMed] [Google Scholar]
- 17.Benbough, J. E. 1969. The effect of relative humidity on the survival of airborne Semliki Forest virus. J. Gen. Virol. 4473-477. [DOI] [PubMed] [Google Scholar]
- 18.Berendt, R. F., and E. L. Dorsey. 1971. Effect of simulated solar radiation and sodium fluorescein on the recovery of Venezuelan equine encephalomyelitis virus from aerosols. Appl. Microbiol. 21447-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Booth, T. F., B. Kournikakis, N. Bastien, J. Ho, D. Kobasa, L. Stadnyk, Y. Li, M. Spence, S. Paton, B. Henry, B. Mederski, D. White, D. E. Low, A. McGeer, A. Simor, M. Vearncombe, J. Downey, F. B. Jamieson, P. Tang, and F. Plummer. 2005. Detection of airborne severe acute respiratory syndrome (SARS) coronavirus and environmental contamination in SARS outbreak units. J. Infect. Dis. 1911472-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bourgueil, E., E. Hutet, R. Cariolet, and P. Vannier. 1992. Air sampling procedure for evaluation of viral excretion level by vaccinated pigs infected with Aujeszky's disease (pseudorabies) virus. Res. Vet. Sci. 52182-186. [DOI] [PubMed] [Google Scholar]
- 21.Brielmeier, M., E. Mahabir, J. R. Needham, C. Lengger, P. Wilhelm, and J. Schmidt. 2006. Microbiological monitoring of laboratory mice and biocontainment in individually ventilated cages: a field study. Lab. Anim. 40247-260. [DOI] [PubMed] [Google Scholar]
- 22.Brooks, J. P., B. D. Tanner, C. P. Gerba, C. N. Haas, and I. L. Pepper. 2005. Estimation of bioaerosol risk of infection to residents adjacent to a land applied biosolids site using an empirically derived transport model. J. Appl. Microbiol. 98397-405. [DOI] [PubMed] [Google Scholar]
- 23.Burton, N. C., S. A. Grinshpun, and T. Reponen. 2007. Physical collection efficiency of filter materials for bacteria and viruses. Ann. Occup. Hyg. 51143-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carducci, A., S. Arrighi, and A. Ruschi. 1995. Detection of coliphages and enteroviruses in sewage and aerosol from an activated sludge wastewater treatment plant. Lett. Appl. Microbiol. 21207-209. [DOI] [PubMed] [Google Scholar]
- 25.Carducci, A., E. Tozzi, E. Rubulotta, B. Casini, L. Cantiani, E. Rovini, M. Muscillo, and R. Pacini. 2000. Assessing airborne biological hazard from urban wastewater treatment. Water Res. 341173-1178. [Google Scholar]
- 26.Champion, H. J., J. Gloster, I. S. Mason, R. J. Brown, A. I. Donaldson, D. B. Ryall, and A. J. Garland. 2002. Investigation of the possible spread of foot-and-mouth disease virus by the burning of animal carcasses on open pyres. Vet. Rec. 151593-600. [DOI] [PubMed] [Google Scholar]
- 27.Christensen, L. S., P. Normann, S. Thykier-Nielsen, J. H. Sorensen, K. de Stricker, and S. Rosenorn. 2005. Analysis of the epidemiological dynamics during the 1982-1983 epidemic of foot-and-mouth disease in Denmark based on molecular high-resolution strain identification. J. Gen. Virol. 862577-2584. [DOI] [PubMed] [Google Scholar]
- 28.Cox, C. S. 1987. The aerobiological pathway of microorganisms. John Wiley & Sons, Chichester, United Kingdom.
- 29.Daggupaty, S. M., and R. F. Sellers. 1990. Airborne spread of foot-and-mouth disease in Saskatchewan, Canada, 1951-1952. Can. J. Vet. Res. 54465-468. [PMC free article] [PubMed] [Google Scholar]
- 30.Dahlgren, C. M., H. M. Decker, and J. B. Harstad. 1961. A slit sampler for collecting T3 bacteriophage and Venezuelan equine encephalomyelitis virus. I. Studies with T3 bacteriophage. Appl. Microbiol. 9103-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dee, S. A., J. Deen, L. Jacobson, K. D. Rossow, C. Mahlum, and C. Pijoan. 2005. Laboratory model to evaluate the role of aerosols in the transport of porcine reproductive and respiratory syndrome virus. Vet. Rec. 156501-504. [DOI] [PubMed] [Google Scholar]
- 32.de Jong, J. C., M. Harmsen, and T. Trouwborst. 1973. The infectivity of the nucleic acid of aerosol-inactivated poliovirus. J. Gen. Virol. 1883-86. [DOI] [PubMed] [Google Scholar]
- 33.de Jong, J. C., M. Harmsen, T. Trouwborst, and K. C. Winkler. 1974. Inactivation of encephalomyocarditis virus in aerosols: fate of virus protein and ribonucleic acid. Appl. Microbiol. 2759-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Jong, J. G. 1965. The survival of measles virus in air, in relation to the epidemiology of measles. Arch. Gesamte Virusforsch. 1697-102. [DOI] [PubMed] [Google Scholar]
- 35.DeLay, P. D., K. B. DeOme, and R. A. Bankowski. 1948. Recovery of pneumoencephalitis (Newcastle) virus from the air of poultry houses containing infected birds. Science 107474-475. [DOI] [PubMed] [Google Scholar]
- 36.Donaldson, A. I. 1983. Quantitative data on airborne foot-and-mouth-disease virus—its production, carriage and deposition. Philos. Trans. R. Soc. Lond. B 302529-534. [Google Scholar]
- 37.Donaldson, A. I., and S. Alexandersen. 2002. Predicting the spread of foot and mouth disease by airborne virus. Rev. Sci. Technol. 21569-575. [DOI] [PubMed] [Google Scholar]
- 38.Donaldson, A. I., N. P. Ferris, and J. Gloster. 1982. Air sampling of pigs infected with foot-and-mouth disease virus: comparison of Litton and cyclone samplers. Res. Vet. Sci. 33384-385. [PubMed] [Google Scholar]
- 39.Donaldson, A. I., C. F. Gibson, R. Oliver, C. Hamblin, and R. P. Kitching. 1987. Infection of cattle by airborne foot-and-mouth disease virus: minimal doses with O1 and SAT 2 strains. Res. Vet. Sci. 43339-346. [PubMed] [Google Scholar]
- 40.Donaldson, A. I., R. C. Wardley, S. Martin, and N. P. Ferris. 1983. Experimental Aujeszky's disease in pigs: excretion, survival and transmission of the virus. Vet. Rec. 113490-494. [DOI] [PubMed] [Google Scholar]
- 41.Downie, A. W., M. Meiklejohn, L. St. Vincent, A. R. Rao, B. V. Sundara Babu, and C. H. Kempe. 1965. The recovery of smallpox virus from patients and their environment in a smallpox hospital. Bull. W. H. O. 33615-622. [PMC free article] [PubMed] [Google Scholar]
- 42.Dubovi, E. J., and T. G. Akers. 1970. Airborne stability of tailless bacterial viruses S13 and MS2. Appl. Microbiol. 19624-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duchaine, C., Y. Grimard, and Y. Cormier. 2000. Influence of building maintenance, environmental factors, and seasons on airborne contaminants of swine confinement buildings. AIHAJ 6156-63. [PubMed] [Google Scholar]
- 44.Echavarria, M., S. A. Kolavic, S. Cersovsky, F. Mitchell, J. L. Sanchez, C. Polyak, B. L. Innis, and L. N. Binn. 2000. Detection of adenoviruses (AdV) in culture-negative environmental samples by PCR during an AdV-associated respiratory disease outbreak. J. Clin. Microbiol. 382982-2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ehrlich, R., S. Miller, and L. S. Idoine. 1964. Effects of environmental factors on the survival of airborne T3 coliphage. Appl. Microbiol. 12479-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elazhary, M. A., and J. B. Derbyshire. 1979. Aerosol stability of bovine adenovirus type 3. Can. J. Comp. Med. 43305-312. [PMC free article] [PubMed] [Google Scholar]
- 47.Elazhary, M. A., and J. B. Derbyshire. 1979. Aerosol stability of bovine parainfluenza type 3 virus. Can. J. Comp. Med. 43295-304. [PMC free article] [PubMed] [Google Scholar]
- 48.Elazhary, M. A., and J. B. Derbyshire. 1979. Effect of temperature, relative humidity and medium on the aerosol stability of infectious bovine rhinotracheitis virus. Can. J. Comp. Med. 43158-167. [PMC free article] [PubMed] [Google Scholar]
- 49.Errington, F. P., and E. O. Powell. 1969. A cyclone separator for aerosol sampling in the field. J. Hyg. (London) 67387-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Espinosa, I. Y., and S. D. Pillai. 2002. Impaction-based sampler for detecting male-specific coliphages in bioaerosols. J. Rapid Methods Autom. Microbiol. 10117-127. [Google Scholar]
- 51.Fannin, K. F., J. C. Spendlove, K. W. Cochran, and J. J. Gannon. 1976. Airborne coliphages from wastewater treatment facilities. Appl. Environ. Microbiol. 31705-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Farnsworth, J. E., S. M. Goyal, S. W. Kim, T. H. Kuehn, P. C. Raynor, M. A. Ramakrishnan, S. Anantharaman, and W. Tang. 2006. Development of a method for bacteria and virus recovery from heating, ventilation, and air conditioning (HVAC) filters. J. Environ. Monit. 81006-1013. [DOI] [PubMed] [Google Scholar]
- 53.First, M., S. N. Rudnick, K. F. Banahan, R. L. Vincent, and P. W. Brickner. 2007. Fundamental factors affecting upper-room ultraviolet germicidal irradiation. I. Experimental. J. Occup. Environ. Hyg. 4321-331. [DOI] [PubMed] [Google Scholar]
- 54.Gerone, P. J., R. B. Couch, G. V. Keefer, R. G. Douglas, E. B. Derrenbacher, and V. Knight. 1966. Assessment of experimental and natural viral aerosols. Bacteriol. Rev. 30576-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gerone, P. J., R. B. Couch, and V. Knight. 1971. Aerosol inoculator for exposure of human volunteers. Appl. Microbiol. 22899-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gibson, C. F., and A. I. Donaldson. 1986. Exposure of sheep to natural aerosols of foot-and-mouth disease virus. Res. Vet. Sci. 4145-49. [PubMed] [Google Scholar]
- 57.Gillespie, R. R., M. A. Hill, and C. L. Kanitz. 1996. Infection of pigs by aerosols of Aujeszky's disease virus and their shedding of the virus. Res. Vet. Sci. 60228-233. [DOI] [PubMed] [Google Scholar]
- 58.Gillespie, R. R., M. A. Hill, C. L. Kanitz, K. E. Knox, L. K. Clark, and J. P. Robinson. 2000. Infection of pigs by Aujeszky's disease virus via the breath of intranasally inoculated pigs. Res. Vet. Sci. 68217-222. [DOI] [PubMed] [Google Scholar]
- 59.Gloster, J., A. Freshwater, R. F. Sellers, and S. Alexandersen. 2005. Re-assessing the likelihood of airborne spread of foot-and-mouth disease at the start of the 1967-1968 UK foot-and-mouth disease epidemic. Epidemiol. Infect. 133767-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gloster, J., P. Williams, C. Doel, I. Esteves, H. Coe, and J. F. Valarcher. 2007. Foot-and-mouth disease—quantification and size distribution of airborne particles emitted by healthy and infected pigs. Vet. J. 17442-53. [DOI] [PubMed] [Google Scholar]
- 61.Goldberg, L. J., H. M. Watkins, E. E. Boerke, and M. A. Chatigny. 1958. The use of a rotating drum for the study of aerosols over extended periods of time. Am. J. Hyg. 6885-93. [DOI] [PubMed] [Google Scholar]
- 62.Griffiths, W. D., A. Bennett, S. Speight, and S. Parks. 2005. Determining the performance of a commercial air purification system for reducing airborne contamination using model microorganisms: a new test methodology. J. Hosp. Infect. 61242-247. [DOI] [PubMed] [Google Scholar]
- 63.Hammond, G. W., R. L. Raddatz, and D. E. Gelskey. 1989. Impact of atmospheric dispersion and transport of viral aerosols on the epidemiology of influenza. Rev. Infect. Dis. 11494-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Happ, J. W., J. B. Harstad, and L. M. Buchanan. 1966. Effect of air ions on submicron T1 bacteriophage aerosols. Appl. Microbiol. 14888-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Harper, G. J. 1961. Airborne microorganisms: survival tests with four viruses. J. Hyg. (London) 59479-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harstad, J. B. 1965. Sampling submicron T1 bacteriophage aerosols. Appl. Microbiol. 13899-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hatch, M. T., and J. C. Warren. 1969. Enhanced recovery of airborne T3 coliphage and Pasteurella pestis bacteriophage by means of a presampling humidification technique. Appl. Microbiol. 17685-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hemmes, J. H., K. C. Winkler, and S. M. Kool. 1960. Virus survival as a seasonal factor in influenza and poliomyelitis. Nature 188430-431. [DOI] [PubMed] [Google Scholar]
- 69.Hemmes, J. H., K. C. Winkler, and S. M. Kool. 1962. Virus survival as a seasonal factor in influenza and poliomyelitis. Antonie van Leeuwenhoek 28221-233. [DOI] [PubMed] [Google Scholar]
- 70.Henderson, D. W. 1952. An apparatus for the study of airborne infection. J. Hyg. (London) 5053-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Henningson, E. W., and M. S. Ahlberg. 1994. Evaluation of microbiological aerosol samplers—a review. J. Aerosol Sci. 251459-1492. [Google Scholar]
- 72.Hermann, J., S. Hoff, C. Munoz-Zanzi, K. J. Yoon, M. Roof, A. Burkhardt, and J. Zimmerman. 2007. Effect of temperature and relative humidity on the stability of infectious porcine reproductive and respiratory syndrome virus in aerosols. Vet. Res. 3881-93. [DOI] [PubMed] [Google Scholar]
- 73.Hermann, J. R., S. J. Hoff, K. J. Yoon, A. C. Burkhardt, R. B. Evans, and J. J. Zimmerman. 2006. Optimization of a sampling system for recovery and detection of airborne porcine reproductive and respiratory syndrome virus and swine influenza virus. Appl. Environ. Microbiol. 724811-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hietala, S. K., P. J. Hullinger, B. M. Crossley, H. Kinde, and A. A. Ardans. 2005. Environmental air sampling to detect exotic Newcastle disease virus in two California commercial poultry flocks. J. Vet. Diagn. Investig. 17198-200. [DOI] [PubMed] [Google Scholar]
- 75.Hinds, W. C. 1999. Aerosol technology, 2nd ed. Wiley-Interscience, New York, NY.
- 76.Hogan, C. J., Jr., E. M. Kettleson, M. H. Lee, B. Ramaswami, L. T. Angenent, and P. Biswas. 2005. Sampling methodologies and dosage assessment techniques for submicrometre and ultrafine virus aerosol particles. J. Appl. Microbiol. 991422-1434. [DOI] [PubMed] [Google Scholar]
- 77.Hugh-Jones, M., W. H. Allan, F. A. Dark, and G. J. Harper. 1973. The evidence for the airborne spread of Newcastle disease. J. Hyg. (London) 71325-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ijaz, M. K., A. H. Brunner, S. A. Sattar, R. C. Nair, and C. M. Johnson-Lussenburg. 1985. Survival characteristics of airborne human coronavirus 229E. J. Gen. Virol. 662743-2748. [DOI] [PubMed] [Google Scholar]
- 79.Ijaz, M. K., Y. G. Karim, S. A. Sattar, and C. M. Johnson-Lussenburg. 1987. Development of methods to study the survival of airborne viruses. J. Virol. Methods 1887-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ijaz, M. K., S. A. Sattar, T. Alkarmi, F. K. Dar, A. R. Bhatti, and K. M. Elhag. 1994. Studies on the survival of aerosolized bovine rotavirus (UK) and a murine rotavirus. Comp. Immunol. Microbiol. Infect. Dis. 1791-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ijaz, M. K., S. A. Sattar, C. M. Johnson-Lussenburg, and V. S. Springthorpe. 1985. Comparison of the airborne survival of calf rotavirus and poliovirus type 1 (Sabin) aerosolized as a mixture. Appl. Environ. Microbiol. 49289-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ijaz, M. K., S. A. Sattar, C. M. Johnson-Lussenburg, V. S. Springthorpe, and R. C. Nair. 1985. Effect of relative humidity, atmospheric temperature, and suspending medium on the airborne survival of human rotavirus. Can. J. Microbiol. 31681-685. [DOI] [PubMed] [Google Scholar]
- 83.Johnson, N., R. Phillpotts, and A. R. Fooks. 2006. Airborne transmission of lyssaviruses. J. Med. Microbiol. 55785-790. [DOI] [PubMed] [Google Scholar]
- 84.Karim, Y. G., M. K. Ijaz, S. A. Sattar, and C. M. Johnson-Lussenburg. 1985. Effect of relative humidity on the airborne survival of rhinovirus-14. Can. J. Microbiol. 311058-1061. [DOI] [PubMed] [Google Scholar]
- 85.Kuehne, R. W., and W. S. Gochenour, Jr. 1961. A slit sampler for collecting T3 bacteriophage and Venezuelan equine encephalomyelitis virus. II. Studies with Venezuelan equine encephalomyelitis virus. Appl. Microbiol. 9106-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Larson, E. W., J. W. Dominik, and T. W. Slone. 1980. Aerosol stability and respiratory infectivity of Japanese B encephalitis virus. Infect. Immun. 30397-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li, Y., X. Huang, I. T. Yu, T. W. Wong, and H. Qian. 2005. Role of air distribution in SARS transmission during the largest nosocomial outbreak in Hong Kong. Indoor Air 1583-95. [DOI] [PubMed] [Google Scholar]
- 88.Marks, P. J., I. B. Vipond, D. Carlisle, D. Deakin, R. E. Fey, and E. O. Caul. 2000. Evidence for airborne transmission of Norwalk-like virus (NLV) in a hotel restaurant. Epidemiol. Infect. 124481-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Marks, P. J., I. B. Vipond, F. M. Regan, K. Wedgwood, R. E. Fey, and E. O. Caul. 2003. A school outbreak of Norwalk-like virus: evidence for airborne transmission. Epidemiol. Infect. 131727-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mars, M. H., C. J. Bruschke, and J. T. van Oirschot. 1999. Airborne transmission of BHV1, BRSV, and BVDV among cattle is possible under experimental conditions. Vet. Microbiol. 66197-207. [DOI] [PubMed] [Google Scholar]
- 91.May, K. R., and G. J. Harper. 1957. The efficiency of various liquid impinger samplers in bacterial aerosols. Br. J. Ind. Med. 14287-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McCluskey, R., R. Sandin, and J. Greene. 1996. Detection of airborne cytomegalovirus in hospital rooms of immunocompromised patients. J. Virol. Methods 56115-118. [DOI] [PubMed] [Google Scholar]
- 93.McDevitt, J. J., K. M. Lai, S. N. Rudnick, E. A. Houseman, M. W. First, and D. K. Milton. 2007. Characterization of UVC light sensitivity of vaccinia virus. Appl. Environ. Microbiol. 735760-5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McGarrity, G. J., and A. S. Dion. 1978. Detection of airborne polyoma virus. J. Hyg. (London) 819-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Meiklejohn, G., C. H. Kempe, A. W. Downie, T. O. Berge, L. St. Vincent, and A. R. Rao. 1961. Air sampling to recover variola virus in the environment of a smallpox hospital. Bull. W. H. O. 2563-67. [PMC free article] [PubMed] [Google Scholar]
- 96.Mik, G., I. de Groot, and J. L. Gerbrandy. 1977. Survival of aerosolized bacteriophage phiX174 in air containing ozone-olefin mixtures. J. Hyg. (London) 78189-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Moore, B. E., B. P. Sagik, and C. A. Sorber. 1979. Procedure for the recovery of airborne human enteric viruses during spray irrigation of treated wastewater. Appl. Environ. Microbiol. 38688-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Morawska, L. 2006. Droplet fate in indoor environments, or can we prevent the spread of infection? Indoor Air 16335-347. [DOI] [PubMed] [Google Scholar]
- 99.Morris, E. J., H. M. Darlow, J. F. Peel, and W. C. Wright. 1961. The quantitative assay of mono-dispersed aerosols of bacteria and bacteriophage by electrostatic precipitation. J. Hyg. (London) 59487-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Musser, J. M. 2004. A practitioner's primer on foot-and-mouth disease. J. Am. Vet. Med. Assoc. 2241261-1268. [DOI] [PubMed] [Google Scholar]
- 101.Myatt, T. A., S. L. Johnston, S. Rudnick, and D. K. Milton. 2003. Airborne rhinovirus detection and effect of ultraviolet irradiation on detection by a semi-nested RT-PCR assay. BMC Public Health 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Myatt, T. A., S. L. Johnston, Z. Zuo, M. Wand, T. Kebadze, S. Rudnick, and D. K. Milton. 2004. Detection of airborne rhinovirus and its relation to outdoor air supply in office environments. Am. J. Respir. Crit. Care Med. 1691187-1190. [DOI] [PubMed] [Google Scholar]
- 103.Neve, H., A. Laborius, and K. J. Heller. 2003. Testing of the applicability of battery-powered portable microbial air samplers for detection and enumeration of airborne Lactococcus lactis dairy bacteriophages. Kieler Milchw. Forsch. 55301-315. [Google Scholar]
- 104.Olsen, S. J., H. L. Chang, T. Y. Cheung, A. F. Tang, T. L. Fisk, S. P. Ooi, H. W. Kuo, D. D. Jiang, K. T. Chen, J. Lando, K. H. Hsu, T. J. Chen, and S. F. Dowell. 2003. Transmission of the severe acute respiratory syndrome on aircraft. N. Engl. J. Med. 3492416-2422. [DOI] [PubMed] [Google Scholar]
- 105.Otake, S., S. A. Dee, L. Jacobson, M. Torremorell, and C. Pijoan. 2002. Evaluation of aerosol transmission of porcine reproductive and respiratory syndrome virus under controlled field conditions. Vet. Rec. 150804-808. [DOI] [PubMed] [Google Scholar]
- 106.Petersen, N. J., W. W. Bond, and M. S. Favero. 1979. Air sampling for hepatitis B surface antigen in a dental operatory. J. Am. Dent. Assoc. 99465-467. [DOI] [PubMed] [Google Scholar]
- 107.Petersen, N. J., W. W. Bond, J. H. Marshall, M. S. Favero, and L. Raij. 1976. An air sampling technique for hepatitis B surface antigen. Health Lab. Sci. 13233-237. [PubMed] [Google Scholar]
- 108.Phelps, E. B., and L. Buchbinder. 1941. Studies on microorganisms in simulated room environments. I. A study of the performance of the Wells air centrifuge and of the settling rates of bacteria through the air. J. Bacteriol. 42321-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Phillpotts, R. J., T. J. Brooks, and C. S. Cox. 1997. A simple device for the exposure of animals to infectious microorganisms by the airborne route. Epidemiol. Infect. 11871-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pirtle, E. C., and G. W. Beran. 1991. Virus survival in the environment. Rev. Sci. Technol. 10733-748. [DOI] [PubMed] [Google Scholar]
- 111.Pyankov, O. V., I. E. Agranovski, O. Pyankova, E. Mokhonova, V. Mokhonov, A. S. Safatov, and A. A. Khromykh. 2007. Using a bioaerosol personal sampler in combination with real-time PCR analysis for rapid detection of airborne viruses. Environ. Microbiol. 9992-1000. [DOI] [PubMed] [Google Scholar]
- 112.Rabey, F., R. J. Janssen, and L. M. Kelley. 1969. Stability of St. Louis encephalitis virus in the airborne state. Appl. Microbiol. 18880-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Remington, P. L., W. N. Hall, I. H. Davis, A. Herald, and R. A. Gunn. 1985. Airborne transmission of measles in a physician's office. JAMA 2531574-1577. [PubMed] [Google Scholar]
- 114.Rheinbaben, F., S. Schunemann, T. Gross, and M. H. Wolff. 2000. Transmission of viruses via contact in a household setting: experiments using bacteriophage straight phiX174 as a model virus. J. Hosp. Infect. 4661-66. [DOI] [PubMed] [Google Scholar]
- 115.Roy, C. J., and D. K. Milton. 2004. Airborne transmission of communicable infection—the elusive pathway. N. Engl. J. Med. 3501710-1712. [DOI] [PubMed] [Google Scholar]
- 116.Sattar, S. A., M. K. Ijaz, C. M. Johnson-Lussenburg, and V. S. Springthorpe. 1984. Effect of relative humidity on the airborne survival of rotavirus SA11. Appl. Environ. Microbiol. 47879-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sawyer, M. H., C. J. Chamberlin, Y. N. Wu, N. Aintablian, and M. R. Wallace. 1994. Detection of varicella-zoster virus DNA in air samples from hospital rooms. J. Infect. Dis. 16991-94. [DOI] [PubMed] [Google Scholar]
- 118.Schaffer, F. L., M. E. Soergel, and D. C. Straube. 1976. Survival of airborne influenza virus: effects of propagating host, relative humidity, and composition of spray fluids. Arch. Virol. 51263-273. [DOI] [PubMed] [Google Scholar]
- 119.Schoenbaum, M. A., J. J. Zimmerman, G. W. Beran, and D. P. Murphy. 1990. Survival of pseudorabies virus in aerosol. Am. J. Vet. Res. 51331-333. [PubMed] [Google Scholar]
- 120.Sellers, R. F., and K. A. Herniman. 1974. The airborne excretion by pigs of swine vesicular disease virus. J. Hyg. (London) 7261-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sellers, R. F., and J. Parker. 1969. Airborne excretion of foot-and-mouth disease virus. J. Hyg. (London) 67671-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Songer, J. R. 1967. Influence of relative humidity on the survival of some airborne viruses. Appl. Microbiol. 1535-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Suzuki, K., T. Yoshikawa, A. Tomitaka, K. Matsunaga, and Y. Asano. 2004. Detection of aerosolized varicella-zoster virus DNA in patients with localized herpes zoster. J. Infect. Dis. 1891009-1012. [DOI] [PubMed] [Google Scholar]
- 124.Tanner, B. D., J. P. Brooks, C. N. Haas, C. P. Gerba, and I. L. Pepper. 2005. Bioaerosol emission rate and plume characteristics during land application of liquid class B biosolids. Environ. Sci. Technol. 391584-1590. [DOI] [PubMed] [Google Scholar]
- 125.Teltsch, B., and E. Katzenelson. 1978. Airborne enteric bacteria and viruses from spray irrigation with wastewater. Appl. Environ. Microbiol. 35290-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Thomas, G. 1974. Air sampling of smallpox virus. J. Hyg. (London) 731-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Thomas, G. 1970. An adhesive surface sampling technique for airborne viruses. J. Hyg. (London) 68273-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Thomas, G. 1970. Sampling rabbit pox aerosols of natural origin. J. Hyg. (London) 68511-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Trincado, C., S. Dee, L. Jacobson, S. Otake, K. Rossow, and C. Pijoan. 2004. Attempts to transmit porcine reproductive and respiratory syndrome virus by aerosols under controlled field conditions. Vet. Rec. 154294-297. [DOI] [PubMed] [Google Scholar]
- 130.Trouwborst, T., and J. C. de Jong. 1973. Interaction of some factors in the mechanism of inactivation of bacteriophage MS2 in aerosols. Appl. Microbiol. 26252-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Trouwborst, T., and S. Kuyper. 1974. Inactivation of bacteriophage T3 in aerosols: effect of prehumidification on survival after spraying from solutions of salt, peptone, and saliva. Appl. Microbiol. 27834-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tseng, C. C., and C. S. Li. 2005. Collection efficiencies of aerosol samplers for virus-containing aerosols. J. Aerosol Sci. 36593-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tseng, C. C., and C. S. Li. 2006. Ozone for inactivation of aerosolized bacteriophages. Aerosol Sci. Technol. 40683-689. [Google Scholar]
- 134.Valarcher, J. F., J. Gloster, C. A. Doel, B. Bankowski, and D. Gibson. 2007. Foot-and-mouth disease virus (O/UKG/2001) is poorly transmitted between sheep by the airborne route. Vet. J. doi: 10.1016/j.tvjl.2007.05.023. [DOI] [PubMed]
- 135.Verreault, D., G. Marchand, Y. Cloutier, J. Barbeau, S. Moineau, and C. Duchaine. 2007. Experimental viral aerosol sampling: sampler comparison and challenges of the method, abstr. A71. Abstr. CSM 57th Annu. Conf., Quebec, Canada.
- 136.Wallis, C., J. L. Melnick, V. C. Rao, and T. E. Sox. 1985. Method for detecting viruses in aerosols. Appl. Environ. Microbiol. 501181-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Warren, J. C., T. G. Akers, and E. J. Dubovi. 1969. Effect of prehumidification on sampling of selected airborne viruses. Appl. Microbiol. 18893-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Warren, J. C., and M. T. Hatch. 1969. Survival of T3 coliphage in varied extracellular environments. I. Viability of the coliphage during storage and in aerosols. Appl. Microbiol. 17256-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wells, W. F. 1940. An apparatus for the study of experimental air-borne disease. Science 91172-174. [DOI] [PubMed] [Google Scholar]
- 140.Wells, W. K., and H. W. Brown. 1936. Recovery of influenza virus suspended in air. Science 8468-69. [DOI] [PubMed] [Google Scholar]
- 141.Westwood, J. C., E. A. Boulter, E. T. Bowen, and H. B. Maber. 1966. Experimental respiratory infection with poxviruses. I. Clinical virological and epidemiological studies. Br. J. Exp. Pathol. 47453-465. [PMC free article] [PubMed] [Google Scholar]
- 142.Wichmann, H. E., C. Spix, T. Tuch, G. Wolke, A. Peters, J. Heinrich, W. G. Kreyling, and J. Heyder. 2000. Daily mortality and fine and ultrafine particles in Erfurt, Germany. I. Role of particle number and particle mass. Res. Rep. Health Eff. Inst. 20005-86. [PubMed] [Google Scholar]
- 143.Willeke, K., X. J. Lin, and S. A. Grinshpun. 1998. Improved aerosol collection by combined impaction and centrifugal motion. Aerosol Sci. Technol. 28439-456. [Google Scholar]
- 144.Winkler, W. G. 1968. Airborne rabies virus isolation. Wildl. Dis. 437-40. [DOI] [PubMed] [Google Scholar]
- 145.Yu, I. T., Y. Li, T. W. Wong, W. Tam, A. T. Chan, J. H. Lee, D. Y. Leung, and T. Ho. 2004. Evidence of airborne transmission of the severe acute respiratory syndrome virus. N. Engl. J. Med. 3501731-1739. [DOI] [PubMed] [Google Scholar]
- 146.Yu, I. T., T. W. Wong, Y. L. Chiu, N. Lee, and Y. Li. 2005. Temporal-spatial analysis of severe acute respiratory syndrome among hospital inpatients. Clin. Infect. Dis. 401237-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yusuf, S., G. Piedimonte, A. Auais, G. Demmler, S. Krishnan, P. Van Caeseele, R. Singleton, S. Broor, S. Parveen, L. Avendano, J. Parra, S. Chavez-Bueno, T. Murguía De Sierra, E. A. Simoes, S. Shaha, and R. Welliver. 2007. The relationship of meteorological conditions to the epidemic activity of respiratory syncytial virus. Epidemiol. Infect. 1351077-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]