Abstract
Syndecan-1 is a developmentally regulated cell surface heparan sulfate proteoglycan (HSPG). It functions as a coreceptor for a variety of soluble and insoluble ligands and is implicated in several biological processes, including differentiation, cell migration, morphogenesis, and recently feeding behavior. The extracellular domain of syndecan-1 is proteolytically cleaved at a juxtamembrane site by tissue inhibitor of metalloprotease-3 (TIMP-3)-sensitive metalloproteinases in response to a variety of physiological stimulators and stress in a process known as shedding. Shedding converts syndecan-1 from a membrane-bound coreceptor into a soluble effector capable of binding the same ligands. We found that replacing the syndecan-1 juxtamembrane amino acid residues A243SQSL247 with human CD4 amino acid residues can completely block PMA-induced syndecan-1 ectodomain shedding. Furthermore, using Liquid Chromatography Electrospray Ionization Mass Spectrometry (LC-ESI-MS), we identified the proteolytic cleavage site of syndecan-1 to amino acids A243 and S244, generated by constitutive and PMA-induced shedding from murine NMuMG cells. Finally, we show that basal cleavage of syndecan-1 utilizes the same in vivo site as the in vitro site. Indeed, as predicted, transgenic mice expressing the syndecan-1/CD4 cDNA do not shed the syndecan-1 ectodomain in vivo. These results suggest that the same cleavage site is utilized for basal syndecan-1 ectodomain shedding both in vitro from NMuMG and CHO cells as well as in vivo.
Keywords: syndecan, shedding, cleavage site
Syndecan-1 is one of four members of the syndecan family of heparan sulfate proteoglycans (1, 2) Most cells express at least one type of syndecan, and their expression pattern is highly regulated (1, 3). Syndecans are abundant on the surface of all adherent mammalian cells. About 1% of membrane-anchored proteins undergo regulated proteolytic cleavage near the plasma membrane, resulting in release of their ectodomains in a process known as shedding (4, 5). The syndecan ectodomain is shed constitutively by cultured cells, though the release can be accelerated by growth factor receptor activation, e.g. by thrombin and epidermal growth factor (6, 7). In vivo, stress or skin injury/wounding can lead to increased levels of soluble syndecan-1 ectodomain in biological fluids (7, 8). The process of ectodomain shedding not only reduces the number of surface receptors, an effective way to down-regulate signal transduction through these receptors, it also converts the membrane-bound cell surface receptors into soluble effectors that can effectively compete for the same ligand as dominant negative modulators as well as act as paracrine effectors at a remote location. The significance of this has been shown in vivo in syndecan-1-deficient and syndecan-1 overexpressing mice, which lack or contain excessive amounts of soluble ectodomains in their tissues, respectively. Syndecan-1 deficient mice are resistant to Pseudomonas aeruginosa lung infections, due to the absence of soluble syndecan-1 (9). Soluble syndecan-1, complexed to chemokines, is apparently also required for the formation of chemotactic gradients in a model of lung inflammation (10), which may also be the underlying cause for increased leukocyte-endothelial interactions and angiogenesis in these mice (11-13). In contrast, syndecan-1 overexpressing mice accumulate excessive amounts of shed syndecan-1 in skin wound fluids, which leads to a delay in wound repair concomitant with enhanced elastolytic activity, reduced cell proliferation rates and abnormal blood vessel morphology (8). Finally, shedding of syndecan ectodomains appears to modulate feeding behaviour and weight in rodents (14).
The precise mechanism of syndecan-1 ectodomain shedding has not been elucidated, yet there is accumulating evidence which suggests that diverse signal transduction pathways, such as the protein kinase C (PKC), protein tyrosine kinase (PTK) and MAP kinase pathways, are involved (6, 15, 16). Inhibitors of PKC, PTK or MAP kinase can selectively inhibit the syndecan-1 ectodomains shedding (6). Previous work suggested that syndecan-1 ectodomain shedding appears to involve several metalloproteinases, since both a peptide hydroxamate metalloproteinases inhibitor and tissue inhibitor of metalloprotease-3 (TIMP-3) can specifically inhibit syndecan-1 ectodomain shedding (6). Peptide hydroxamates inhibit both constitutive and PMA-accelerated syndecan-1 ectodomain shedding, though TIMP-3 appears to inhibit only the PMA-accelerated shedding. This result implies that constitutive and PMA-stimulated shedding of syndecan-1 is mediated by different metalloproteinases. This finding appears to be corroborated in an in vivo model demonstrating matrilysin (MMP-7)-mediated murine syndecan-1 ectodomain shedding (10). Furthermore, MT1-MMP and MT3-MMP appear to be involved in human syndecan-1 ectodomain shedding as demonstrated in an in vitro study (17).
The syndecan-1 ectodomain shedding cleavage site is thought to be the dibasic peptide near the plasma membrane (16, 18), though current evidence does not support this hypothesis. In the case of human syndecan-1, a point mutant, G245L, was shed at 50% reduced extend in MT-1-MMP overexpressing 293T cells compared to mock-transfected cells (17). Thus, we sought to identify the cleavage site of murine syndecan-1, both in vitro and in vivo. Our study found that the syndecan-1 ectodomain generated by constitutive and PMA-accelerated shedding are cleaved at the same site, about 9 amino acids from the plasma membrane. This same cleavage site used in vivo for constitutively shed syndecan-1. Our results demonstrate that under basal or phorbol ester (PMA) stimulated conditions, syndecan-1 is cleaved at a site 9 amino acids from the membrane. These results are based on a molecular and biophysical analysis of the syndecan-1 obtained from both in vitro and in vivo sources. These results lay the foundation for the identification of the metalloprotease responsible for murine syndecan-1 shedding.
Materials and Methods
Materials
PMA, phorbol myristate acetate, PMSF, phenylmethylsulfonyl fluoride were purchased from Calbiochem-Novbiochem (La Jolla, CA). Lipofectamine was purchased from Invitrogen (Carlsbad, CA). The rat monoclonal anti-mouse syndecan-1 ectodomain antibody (281−2) was purchased from Pharmingen (San Diego, CA). All other reagents were from Sigma (St. Louis, MO).
Production of full-length syndecan-1 fusion constructs with mutated juxtamembrane domains
Expression vectors for the synthesis of full-length and mutated juxtamembrane domains were used for transient transfection assays. The construction of syn1-WTJM and syn1-CD4JM has been described previously (6). All of the syndecan-1 mutant constructs were generated by the same procedure with the following primers sets: Syn-1/D 5’ primer GGA CGA AGG AGC CAC AGG TAC ATG TGTC CAC CCC G and 3’ primer GTA CCT GTG GCT CCT TCG TCC ACC GGG GGC TG; syn-1/E 5’ primer GCC CGC TTC TCA GAG CCT TGT GCA GCC AAT GGC and 3’ primer GCA CAA GGC TCT GAG AAG CGG GCA GAA CCT TGA C; syn-1/F 5’ primer TGG ACA GGA AGG AAG TGC TGG GAG GTG TC and 3’ primer TCC TTC CTG TCC AAC GGG GTG GAC CAT GTG. The constitutive shed construct (CS) was generated by converting the amino acid residue 245 glutamine into a stop codon by point mutation, CAG to TAG, with the 5’ primer TAG AGC CTT TTG GAC AGG AAG GAA GTG CTG GG and the 3’ primer CTA AGAAGC ACC TGT GGC TCC TTC GTC CAC CGG GGG CT. The expression of CS results in a truncated syndecan-1 ectodomain. In brief, the 5’ primer was amplified with primer OR40 5'-CACAAGCTTCCCGCCGCCGGTCTG-3' and 3’ primer was amplified with primer OR39 5'-GTGCAGCCAATGGCCGTGCTGGGAGGTGTC-3'. Then the PCR products were gel purified and combined to be reamplified with the 5’ primer OR40 5'-CACAAGCTTCCCGCCGCCGGTCTG-3' and the 3’ primer OR39 5'-GTGCAGCCAATGGCCGTGCTGGGAGGTGTC-3' to generate full-length cDNA with the desired mutation. These PCR products were subcloned into a PCR3 vector (Invitrogen Corp) and sequenced to confirm the presence of the mutations. The full-length cDNA with mutation was excised with the restriction endonucleases BamH I and Hind III and subcloned into the BamH I and Hind III sites of pcDNA3.
Cell lines and culture conditions
Normal murine mammary gland (NMuMG), Chinese Hamster Ovary, and COS-7 cells were from our culture collection and cultured exactly as previously described (19).
Shedding assay
To determine whether the putative uncleavable mutant ectodomain is shed constitutively by cultured cells, wild type plasmids (syn1-WTJM) and plasmids containing the syn1-CD4JM mutant were linearized using the restriction enzyme DraIII (NEB, Beverly, MA) and transfected into CHO cells using Lipofectamine (Invitrogen, Carlsbad, CA). Stable transfectants were selected by G418. Cells were trypsinized and replated in 6-well plates for shedding assays. For transient transfection, 10 μg of syndecan-1 298 and 10 μg of syndecan-1 mutants were transfected into CHO cells with Lipofectamine according to the manufacturers’ instructions. Cells were trypsinized 24 hours after transfection and re-plated in 6-well plates for shedding assay at 36 hours after transfection. Cells were washed with serum free medium 2 times and then the cells were subjected to 1 uM of PMA stimulation in serum free medium for 15 minutes. An equal volume of DMSO was used as a control. Conditioned media were collected and briefly centrifuged at 2000 × g to remove any remaining cells. The conditioned media were stored at −20°C or used for dot blot assays immediately. Syndecan-1 expression in the total cell lysate was determined from cells that were washed twice with PBS to remove any soluble syndecan-1 ectodomain. Cells were extracted in RIPS (50 mM Tris pH 8.0, 150 mM NaCl, 0.5% Triton X-100, and 0.5 mM EDTA) and analyzed in dot blot assays.
Dot immunoassay
Quantitation and specificity of the dot immunoassay has been described previously (16). Detection was performed by incubating the immobilon N (Millipore) membrane with 125I labeled 281−2 rat anti-mouse syndecan-1 monoclonal antibody overnight at 4°C with gentle shaking. After several washes, the filter was exposed to X-ray film and dots were cut out for quantification. The radioactivity of each dot was determined in a gamma counter (Beckman, Palo Alto).
Purification of syndecan-1 ectodomain and LC-MS analysis
Syndecan-1 ectodomain from NMuMG mouse mammary epithelial cell conditioned medium was purified using QAE-Sephadex A-25 (Pharmacia, Uppsala, Sweden), cesium chloride density gradient separation, and followed by affinity purification with a 281−2 monoclonal antibody column as described previously by Rapraeger and Bernfield (20). Syndecan-1 ectodomain was also purified from conditioned media of PMA-stimulated NMuMG cells. Briefly, cells were washed 2 times with serum-free DME containing glucose at 4.5 g/L (Mediatech, Herndon, VA) followed by incubation with 1μM of PMA in DME containing glucose at 4.5 g/L for 15 minutes. The soluble syndecan-1 ectodomain from PMA-stimulated NMuMG cell conditioned medium was purified as described above. Mouse plasma from transgenic mice overexpressing syndecan-1 was collected, pooled from over 50 mice, followed by the same purification procedure as described above. All three purified soluble syndecan-1 ectodomain proteins were analyzed by LC-ESI-MS analysis at the Harvard Biopolymer Lab (Boston, MA).
Generation of transgenic mice expressing syndecan-1 and syndecan-1/CD4JM
A full-length mouse syndecan-1 cDNA and syndecan-1/CD4JM were inserted into pcDNA3 (Invitrogen, Carlsbad, CA) as described previously (17). The restriction endonucleases Mlu I and Dra III (NEB, Beverly, MA) were used to isolate a 2.5 kB fragment for oocyte injection. The injection fragment contains 660 bp of the CMV promoter, the syndecan-1 cDNA including 203 bp of its 3’ UTR, and the 3’ polyadenylation signal of bovine growth hormone. The fragments were injected into fertilized FVB oocytes and implanted into recipient pseodopregnant mice. The transgenic founders were screened by Southern blot analysis using CMV promoter probes. For routine genotyping, a pair of PCR primers, oligo 464 GCTTTGCCAGATCATTTGTCACGGC and oligo 465 GTGTTCTCCCCAGATGTTTCAAAGG, were designed which amplify the endogenous syndecan-1 as a 933 bp DNA fragment and the syndecan-1 transgene as a 511 bp DNA fragment. Amplified fragments were separated on 1% agarose TBE gels and visualized by eithidium bromide staining.
Analysis of soluble sydecan-1 ectodomain in plasma
Blood was collected from ad libitum fed animals by puncture of the retro-orbital sinus with heparinized capillary tubes and collected in SARSTEDT centrifuge tubes with K-EDTA (Sarstedt, Newton, NC) to obtain plasma. An equal volume of plasma was used in a dot Immunoassay to determine the presence of soluble syndecan-1 ectodomain as described above. All samples were loaded in triplicate.
Results
Proteolytic cleavage of syndecan-1 from basal and PMA stimulated CHO cells occurs at an extracellular site located between amino acid residues A243SQSL247
We previously reported that the construct 388 syn-1/CD4JM is not shed in response to PMA stimulation. The 388 construct contains a domain swap between the syndecan-1 juxtamembrane 15 amino acid residues E238GATGASQSLLDTKE252 and the human CD4 receptor amino acid residues V238KVLPTWSTRVQPMA252 (6). Cells transfected with this construct have reduced basal shedding of syndecan-1 and shedding of the syndecan-1 is not induced by phorbol ester. To further localize the cleavage site, we performed additional domain-swapping experiments within this 15 amino acid region. Indeed, as reported previously, the 388 syn-1CD4 construct does not respond to PMA accelerated shedding (Fig.1). Constructs syn-1D and syn-1F encoding domain swaps between the syndecan-1 juxtamembrane amino acid residues A243SQSLLDTKE252 and E238GATGASQSL247 were replaced with human CD4 amino acid residues T238WSTRVQPMA252 and V238KVLPTWSTR247, respectively, failed to respond to PMA-stimulated shedding (Fig. 1). The basal shedding rates of the 388 syn-1/CD4, Syn-1D, and Syn-1F mutants were reduced by 60, 80, and 80%, respectively (Fig. 2), whereas the basal and PMA stimulated shedding rates for the wild-type cDNA (mSyn-1) and for Syn-1E were identical (Fig.1 and 2). These observations indicate that the syndecan-1 cleavage site is not located within amino acid residues E238GATG242 or L248DTKE252. In contrast, construct syn-1E containing a domain swap that retains the sequence A243SQSL247 flanked by human CD4 juxtamembrane sequences VKVLP and VQPMA was shed in response to PMA-stimulation (Fig.1). These observations suggest that A243SQSL247 may contain the metalloproteinase recognition site but more importantly contain the cleavage site.
Figure.1.
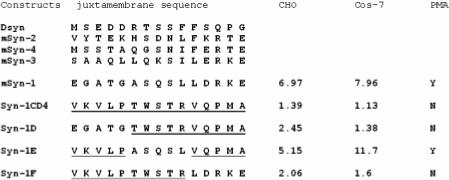
PMA-accelerated shedding of syndecan-1 ectodomains results from cleavage at a juxtamembrane site in the core protein within amino acid residues A243SQSL247. CHO cells or COS-7 cells were transfected with wild type syndecan-1 (mSyn-1), Syn-1CD4, Syn-1D, Syn-1E, and Syn-1F cDNAs. PMA-accelerated shedding was analyzed by incubating the transfected cells in 6 well plates with or without PMA (1μM) in serum-free medium for 15 minutes. The soluble syndecan-1 ectodomain in the conditioned media was analyzed by a dot blot assay. The fold induction is presented as the ratio of the soluble syndecan-1 ectodomains in conditioned media from PMA stimulated cells compared to DMSO treated controls. The human CD4 amino acid residues are underlined.
Figure 2.
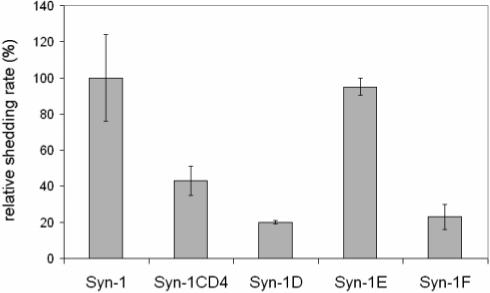
Basal shedding of syndecan-1 ectodomain is reduced in mutants with domain swap containing amino acid residues A243SQSL247. CHO cells were transfected with wild type syndecan-1cDNA, syndecan-1CD cDNA or syndecan-1 mutant cDNAs, syn-1D, syn-1E, and syn-1F (cf Figure 1). Basal shedding was analyzed by incubating the transfected cells in 6 well plates in serum-free medium for 15 minutes. The soluble syndecan-1 ectodomain in the conditioned media was analyzed by a dot blot assay and normalized to the syndecan-1 content in total cell lysates. Expression of syndecan-1 in the total cell lysate was extracted was determined to be similar among all the cDNA constructs (data not shown). The relative shedding rate is expressed as the percentage of control (wild-type Syn-1) value. (Error bars = SD, n=3).
The cleavage site of PMA-stimulated or constitutively shed syndecan-1 is localized to amino acids A243 and S244
The location of the cleavage site within the juxtamembrane region of the syndecan-1 core protein was determined using liquid chromatography electron spray ionization mass spectrometry (LC-ESI-MS). Soluble syndecan-1 ectodomain was purified from conditioned media of NMuMG cells that were stimulated with PMA or constitutively shed as described in the Materials and Methods section. The affinity purified soluble syndecan-1 ectodomain was subjected to trypsin digestion followed by online LC-ESI-MS. We reasoned that the potential shedding cleavage site is located within the amino acid residues of A243SQSL247, thus the potential C-terminal tryptic peptide with a single charge should have a m/z value of 1155 to 1954. As shown in Figure 3a, a prominent peak of singly charged [M+H]+ with m/z = 1155.64 was obtained. This m/z value corresponds to the peptide sequence N232QPPVDEGATGA243. Since this peptide is the last potential tryptic fragment and the final amino acid residue is within the potential region of cleavage site, these data suggests that the cleavage site is located between A243 and S244. To confirm the identity of the peptide, the mass fragmentation products of the m/z=1155.64 peptide were analyzed (Figure 3b). The peptide fragments with one (B11), two (B10), three (B9), or four (B8) amino acid residues breaking away from the C-terminus can be easily identified (Table 1). The peptide fragments with two (Y10) or three (Y9) amino acid residues breaking away from the N-terminus can also be identified (Table 1). These results further confirmed that the m/z value of the 1155.64 fragment is indeed the peptide N232QPPVDEGATGA243 suggesting the cleavage site is located between A243 and S244.
Figure 3.

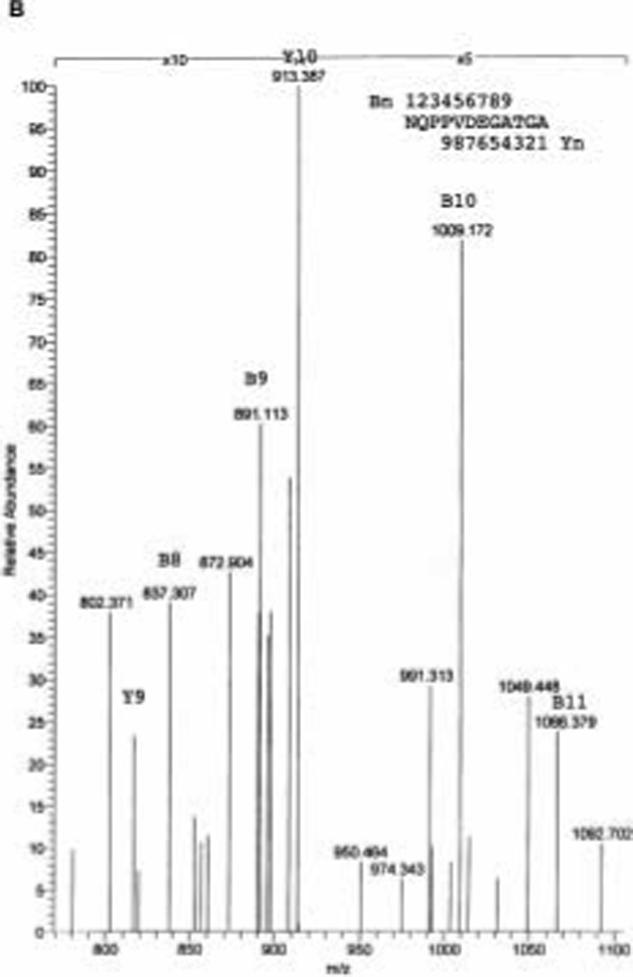
On-line LC-ESI-MS analysis of tryptic digests of PMA-stimulated soluble syndecan-1 ectodomain. A. Total ion current trace showing the distribution of tryptic peptides of PMA-stimulated syndecan-1 ectodomain. B. Collision induced decomposition spectrum of the singly charged ion of m/z = 1155.64 from PMA-stimulated syndecan-1 ectodomain.
Table 1.
Predicted and obtained mass of fragments derived from the juxtamembrane syndecan-1 peptide.
| Peptide | Sequence | Predicted Mass | Obtained Mass |
|---|---|---|---|
| Parent | NQPPVDEGATGA | 1155.19 | 1155.64 |
| Y11 | QPPVDEGATGA | 1041.08 | 1041.31 |
| Y10 | PPVDEGATGA | 912.95 | 913.31 |
| B11* | NQPPVDEGATG | 1067.11 | 1066.38 |
| B10* | NQPPVDEGAT | 1010.06 | 1009.12 |
| B9* | NQPPVDEGA | 908.95 | 909.00 |
| B8* | NQPPVDEG | 837.87 | 837.31 |
Fragmentation from the carboxy terminal leads to loss of hydroxyl group so predicted mass of the peptide has been corrected.
As we reported previously, tissue inhibitor of metalloprotease-3 (TIMP-3) can specifically inhibit the PMA-accelerated syndecan-1 ectodomain shedding in a variety of cell types, while the TIMP-3 does not inhibit constitutive syndecan-1 ectodomain shedding. To determine whether constitutive shedding of the syndecan-1 ectodomain also occurs at the same cleavage site, the soluble syndecan-1 ectodomain was subjected to tryptic digestion and LC-ESI-MS. As shown in Figure 4a, a prominent single charged peptide fragment with a m/z value of 1155.55 is identified. The mass fragmentation analysis indicates this peptide has the same fragmentation pattern and readily identifiable mass fragments such as B11, B10, B9, Y11 and Y10, suggesting that the constitutively shed syndecan-1 ectodomains are cleaved at the same juxtamembrane site as in the case of PMA-accelerated shedding (Fig.4b; Table 1).
Figure 4.
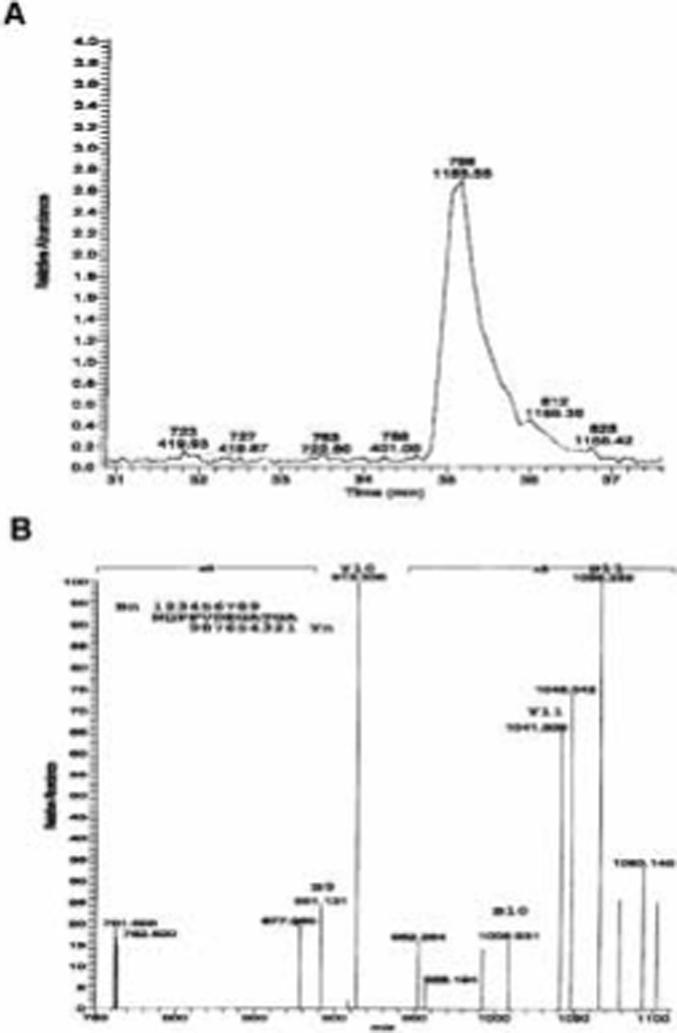
A. On-line LC-ESI-MS analysis of tryptic digests of basal constitutively shed syndecan-1 ectodomain. A. Total ion current trace showing the distribution of tryptic peptides of basal constitutively shed syndecan-1 ectodomain. B. Collision induced decomposition spectrum of the singly charged ion of m/z = 1155.55 from basal constitutively shed syndecan-1 ectodomain.
Transgenic mice expressing the syndecan-1/CD4JM do not shed the soluble syndecan-1 ectodomain
We next determined whether replacing the 15-juxtamembrane amino acid residues with human CD4 amino acid residues is sufficient to block syndecan-1 ectodomain shedding in vivo. Three transgenic lines were generated in FVB mice, one expressing the full-length syndecan-1 cDNA, the second expressing Syn-1CD4 cDNA, and the third expressing a constitutively shed syndecan-1 (Fig. 5a). The level of syndecan-1 ectodomain in plasma was analyzed in these transgenic mice as shown in Figure 5b. The levels of soluble syndecan-1 ectodomain in wild type littermates are at or below the limit of detection. The transgenic mice expressing the Syn-1CD4 cDNA shed syndecan-1 at a level two times above background, while the transgenic mice expressing the wild type syndecan-1 cDNA contain 20 times higher levels of soluble syndecan-1 ectodomains than both transgenic mice expressing the Syn1 CD4 or wild type mice. These results suggest that replacing the 15-juxtamembrane amino acid residues of syndecan-1 with human CD4 amino acid residues can effectively block syndecan-1 ectodomain shedding in vivo.
Figure 5.
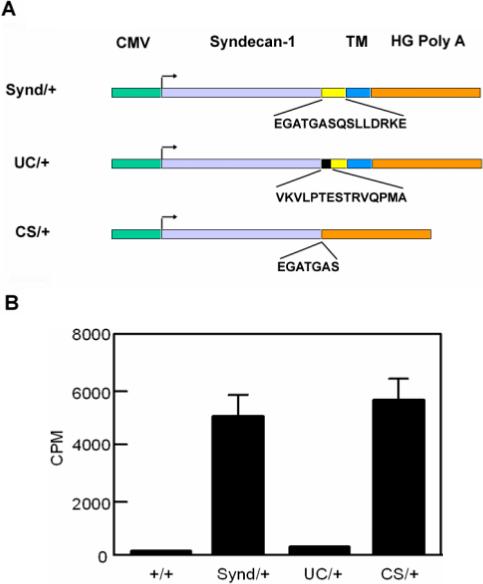
Generation of transgenic mouse lines carrying mutations in the juxtamembrane region of syndecan-1. a) DNA constructs used for the generation of transgenic mice. Synd/+ = mice overexpressing wild-type syndecan-1, UC/+ mice overexpressing an uncleavable syndecan-1 construct; CS/+ = mice overexpressing a syndecan-1 construct lacking the transmembrane domain (‘constitutively shed’); CMV= cytomegalovirus promoter, TM = transmembrane domain, HG-Poly A = Poly A tail. b) Transgenic mice expressing Syn-1CD4 cDNA do not shed syndecan-1 ectodomain into blood. The relative levels of circulating soluble syndecan-1 ectodomains in 10 μl of plasma were analyzed by a dot blot assay using a 125I labeled 281−2 monoclonal antibody against murine syndecan-1 ectodomain. cf Fig 5 for genotype symbols. (Data are shown as mean ± SD).
Shed syndecan-1 ectodomains are cleaved at the same juxtamembrane site in vivo and in vitro
The location of the cleavage site from in vivo shed syndecan-1 was evaluated from the plasma of syndecan-1 overexpressing mice. The soluble syndecan-1 ectodomain was affinity purified from the pooled plasma and subjected to tryptic digestion and LC-ESI-MS (Fig. 6). The same single charged peptide with an m/z value of 1155.56 was detected. Analysis of the peptide fragmentation indicated the presence of the peptide fragment Y10 (Fig 6). These data indicate that the same juxtamembrane cleavage site is used by proteases in vivo.
Figure 6.
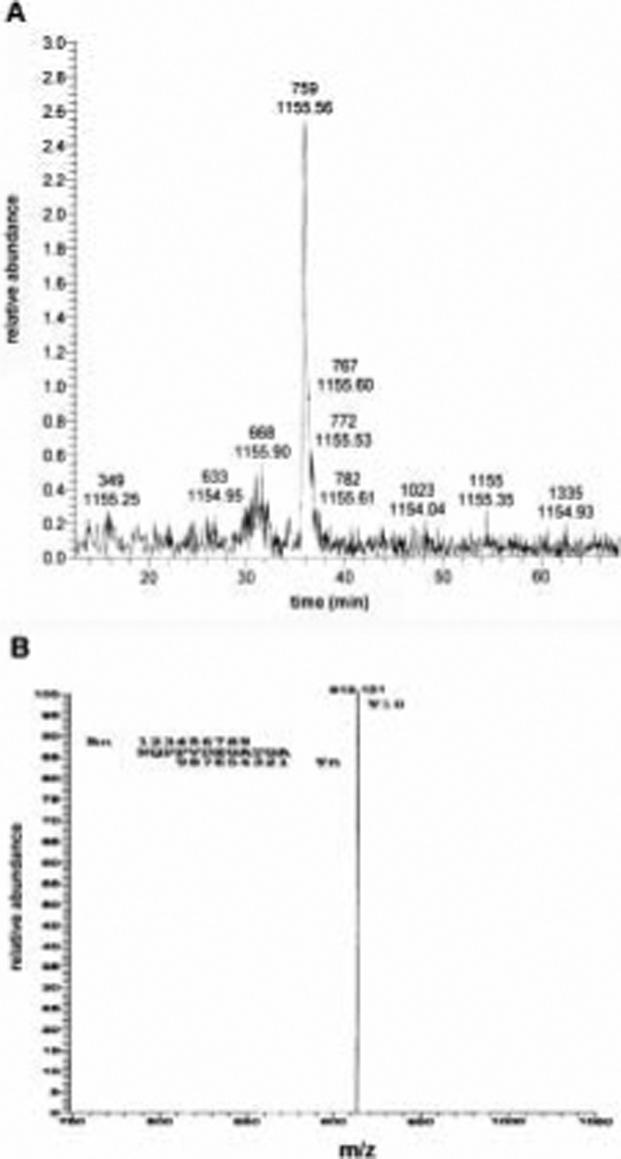
On-line LC-ESI-MS analysis of tryptic digest of syndecan-1 ectodomain purified from transgenic mice expressing wild type syndecan-1 cDNA. Plasma from transgenic mice expressing the syndecan-1 cDNA was collected and pooled. Affinity purified soluble syndecan-1 ectodomain was subjected to tryptic digestion and LC-MS. A. Total ion current trace showing the distribution of tryptic peptides of syndecan-1 ectodomain purified from the plasma of mice. B. Collision induced decomposition spectrum of the singly charged ion of m/z = 1155.56 of syndecan-1 ectodomain purified from murine plasma.
Discussion
Shedding is a physiological process that releases the extracellular domain of cell surface proteins, thus modulating their signaling activities. All four mammalian syndecans and the single drosphila syndecan are shed in vitro and in vivo (1, 21). The shedding of syndecans occurs at a juxtamembrane site adjacent to the membrane. We localized a cleavage site that is important for basal and PMA stimulated shedding to amino acids A243 and S244; a site 9 amino acids from the plasma membrane. Our data is based on molecular domain swapping experiments as well as biochemical mapping using LC-ESI-MS. The cleavage site is apparently conserved, since it was identified in the syndecan-1 ectodomain purified from conditioned media under basal and PMA-stimulated conditions as well as ectodomain constitutively shed in vivo. In addition, mice expressing the Syn-1CD4 cDNA were found to have substantially lower levels of circulating soluble syndecan-1 ectodomain than mice expressing the syndecan-1 wild type cDNA. This result further supports our finding that the same shedding cleavage site is used in vivo. Although the constitutive shedding of syndecan-1 ectodomain by NMuMG cells is not inhibited by TIMP-3, it is likely that the same group of metalloproteinases may be responsible for syndecan-1 ectodomain shedding events both in vitro and in vivo. Our finding that syndecan-1 appears to be cleaved at the same site in from basal and PMA stimulated conditions suggests that a defined length of the shed ectodomain may be a structural requirement in order for syndecan-1 to be converted into soluble form to fulfill its physiological functions.
All four mammalian syndecans contain a dibasic peptide adjacent to the plasma membrane suggesting that this may be the shedding cleavage site. However, the single Drosophila syndecan, which does not contain the dibasic peptide, is also constitutively shed into the conditioned media of cultured insect cells and suggests that the dibasic peptide is not the cleavage site (21). This view is supported by studies in transfected human fibrosarcoma cells that have demonstrated that a substitution of the glycine residue at position 245 of syndecan-1 with a leucine reduces MT1-MMP-mediated shedding by about 50 % (17). Our data indicates that the syndecan-1 shedding cleavage site is not localized to the dibasic sequence, rather a site between the Ala and Ser residues 9 amino acids from the plasma membrane. Our previous results also suggested that the potential cleavage site may located within the 15 amino acid juxtamembrane region near the plasma membrane and confirmed that the shedding is a membrane surface event (6). Our current results from domain swapping pinpoint the cleavage site to the five amino acid residues A243SQSL247, five to ten amino acid away from putative transmembrane domain of syndecan-1. Transfection studies in human HEK293 cell line have demonstrated that TIMP-2, but not TIMP-1, is able to inhibit syndecan-1-shedding induced by MT-MMP-1 overexpression (17). Importantly, the cDNA construct Syn-1E, in which the A243SQSL247 five amino acid residues was used to replace the corresponding CD4 amino acid residues T243WSTR247, can convert syndecan-1/CD4JM to respond to PMA-accelerated shedding in transient transfection assay. LC-ESI-MS results further confirm our observation and identify the proteolytic cleavage site of the syndecan-1 ectodomain between A243 and S244. Importantly, we do not know how this data extends to shedding of other syndecans or orthologs of syndecan-1 in human cells. Based on primary amino acid sequence comparisons it is clear that other murine syndecans do not share the same juxtamembrane sequence. Furthermore, studies by Park and colleagues (22) suggest that syndecan-1 and -4 shedding occurs via independent mechanisms.
The current study does not address what metalloprotease is responsible for the cleavage of murine syndecan-1 in NMuMg or CHO cells. It is unlikely that a single metalloprotease is involved in syndecan-1 shedding. Indeed, different proteases may be involved under different shedding conditions, e.g. MMP-7 cleaves syndecan-1 in bleomycin-induced lung fibrosis (10), yet MMP-7 null mice syndecan-1 shedding still occurs under allergen induced lung inflammation (23). It is important to note that the specific cleavage site of murine syndecan-1 lies in the same region as a point mutation in human syndecan-1 (G245L) recently reported to result in a 50% reduction in MT1-MMP-induced shedding of syndecan-1 in transfected HT1080 fibrosarcoma cells (17). Furthermore, the recombinant human syndecan-1 fusion proteins are cleaved by MT1-MMP and MT3-MMP preferentially at the Gly245-Leu246 bond. However, this specific bond does not exist in murine syndecan-1, where a serine residue is located in place of the glycine (mouse: GASQSLLDR, human: GASQGLLDR). The sequence equivalent of the murine cleavage site is present in human syndecan-1. These studies lead us to speculate that murine and human syndecan-1 MMPs utilize different cleavage sites. Alternatively, cleavage at the A243-S244 in human syndecan-1 may be utilized to some extent, since the point mutation studies show incomplete blockade of shedding in the human G245L syndecan-1 mutant (17). In fact, the lack of complete blockade in the human syndecan-1 G245L mutant suggests that the cleavage site may indeed localize to A243-S244. It is possible that the G245L mutant does not show complete blockade due to a reduction in cleavage efficiency at the A243-S244 cleavage site.
The shedding of mammalian syndecan-1 and -4 ectodomains is accelerated by direct proteolytic cleavage (thrombin or plasmin), cell stress, and by interfering with several intracellular signaling pathways (PMA, pervanadate, or stress) (6, 15, 16). Shedding of the the murine syndecan-1 and-4 ectodomain is inhibited by peptide hydrooxamate and TIMP-3, but not by TIMP-1 or -2 (6). These findings suggest the proteinase responsible for syndecan-1 and-4 ectodomains shedding belongs to the ADAM (A Disintegrin And Metalloproteinase) family of metalloproteinases. The shedding of other syndecans has not been fully explored. Of particular interest will be the future analysis of syndecan-3 an HSPG that appears to modulate feeding behavior through its regulation of the melanocortin signaling pathway (14). Shedding appears to play an integral role in the regulation of syndecan-3 modulated feeding behavior. The identification of the cleavage sites in different members of the syndecan family represents an important step towards the development of specific shedding inhibitors with potential of being used in a variety of clinical contexts, including obesity, wound repair and inflammation.
ACKNOWLEDGEMENT
This study was financially supported by IMF (Innovative Medizinische Forschung, Muenster University Hospital) GÖ 1 2 04 15 (M.G.), Deutsche Forschungsgemeinschaft GO 1392/1-1 (M.G.) and National Cancer Institute Grant RO1-CA-28735 (M.B.).
The abbreviations used are
- CHO
Chinese hamster ovary
- PMA
phorbol myristate acetate
- PMSF
phenylmethylsulfonyl fluoride
- TIMP-3
tissue inhibitor of metalloprotease-3
- LC-ESI-MS
Liquid Chromatography Electrospray Ionization Mass Spectrometry
- NMuMG
Normal murine mammary gland
- HSPG
surface heparan sulfate proteoglycan
- PKC
protein kinase C
- PTK
protein tyrosine kinase
- ADAM
A Disintegrin And Metalloproteinase
- UC
uncleavable
- CS
constitutive shed
References
- 1.Bernfield M, Gotte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- 2.Gotte M, Joussen AM, Klein C, Andre P, Wagner DD, Hinkes MT, Kirchhof B, Adamis AP, Bernfield M. Role of syndecan-1 in leukocyte-endothelial interactions in the ocular vasculature. Invest Ophthalmol Vis Sci. 2002;43:1135. [PubMed] [Google Scholar]
- 3.Bernfield M, Kokenyesi R, Kato M, Hinkes MT, Spring J, Gallo RL, Lose EJ. Biology of the syndecans: a family of transmembrane heparan sulfate proteoglycans. Annu Rev Cell Biol. 1992;8:365. doi: 10.1146/annurev.cb.08.110192.002053. [DOI] [PubMed] [Google Scholar]
- 4.Turner AJ, Hooper NM. Role for ADAM-family proteinases as membrane protein secretases. Biochem Soc Trans. 1999;27:255. doi: 10.1042/bst0270255. [DOI] [PubMed] [Google Scholar]
- 5.Werb Z, Yan Y. A cellular striptease act. Science. 1998;282:1279. doi: 10.1126/science.282.5392.1279. [DOI] [PubMed] [Google Scholar]
- 6.Fitzgerald ML, Wang Z, Park PW, Murphy G, Bernfield M. Shedding of syndecan-1 and -4 ectodomains is regulated by multiple signaling pathways and mediated by a TIMP-3-sensitive metalloproteinase. J Cell Biol. 2000;148:811. doi: 10.1083/jcb.148.4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kainulainen V, Wang H, Schick C, Bernfield M. Syndecans, heparan sulfate proteoglycans, maintain the proteolytic balance of acute wound fluids. J Biol Chem. 1998;273:11563. doi: 10.1074/jbc.273.19.11563. [DOI] [PubMed] [Google Scholar]
- 8.Elenius V, Gotte M, Reizes O, Elenius K, Bernfield M. Inhibition by the soluble syndecan-1 ectodomains delays wound repair in mice overexpressing syndecan-1. J Biol Chem. 2004;279:41928. doi: 10.1074/jbc.M404506200. [DOI] [PubMed] [Google Scholar]
- 9.Park PW, Pier GB, Hinkes MT, Bernfield M. Exploitation of syndecan-1 shedding by Pseudomonas aeruginosa enhances virulence. Nature. 2001;411:98. doi: 10.1038/35075100. [DOI] [PubMed] [Google Scholar]
- 10.Li Q, Park PW, Wilson CL, Parks WC. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell. 2002;111:635. doi: 10.1016/s0092-8674(02)01079-6. [DOI] [PubMed] [Google Scholar]
- 11.Gotte M. Syndecans in inflammation. Faseb J. 2003;17:575. doi: 10.1096/fj.02-0739rev. [DOI] [PubMed] [Google Scholar]
- 12.Gotte M, Echtermeyer F. Syndecan-1 as a regulator of chemokine function. ScientificWorldJournal. 2003;3:1327. doi: 10.1100/tsw.2003.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mulivor AW, Lipowsky HH. Inflammation- and ischemia-induced shedding of venular glycocalyx. Am J Physiol Heart Circ Physiol. 2004;286:H1672. doi: 10.1152/ajpheart.00832.2003. [DOI] [PubMed] [Google Scholar]
- 14.Reizes O, Benoit SC, Strader AD, Clegg DJ, Akunuru S, Seeley RJ. Syndecan-3 modulates food intake by interacting with the melanocortin/AgRP pathway. Ann N Y Acad Sci. 2003;994:66. doi: 10.1111/j.1749-6632.2003.tb03163.x. [DOI] [PubMed] [Google Scholar]
- 15.Reiland J, Ott VL, Lebakken CS, Yeaman C, McCarthy J, Rapraeger AC. Pervanadate activation of intracellular kinases leads to tyrosine phosphorylation and shedding of syndecan-1. Biochem J. 1996;319:39. doi: 10.1042/bj3190039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Subramanian SV, Fitzgerald ML, Bernfield M. Regulated shedding of syndecan-1 and -4 ectodomains by thrombin and growth factor receptor activation. J Biol Chem. 1997;272:14713. doi: 10.1074/jbc.272.23.14713. [DOI] [PubMed] [Google Scholar]
- 17.Endo K, Takino T, Miyamori H, Kinsen H, Yoshizaki T, Furukawa M, Sato H. Cleavage of syndecan-1 by membrane type matrix metalloproteinase-1 stimulates cell migration. J Biol Chem. 2003;278:40764. doi: 10.1074/jbc.M306736200. [DOI] [PubMed] [Google Scholar]
- 18.Jalkanen M, Rapraeger A, Saunders S, Bernfield M. Cell surface proteoglycan of mouse mammary epithelial cells is shed by cleavage of its matrix-binding ectodomain from its membrane-associated domain. J Cell Biol. 1987;105:3087. doi: 10.1083/jcb.105.6.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim CW, Goldberger OA, Gallo RL, Bernfield M. Members of the syndecan family of heparan sulfate proteoglycans are expressed in distinct cell-, tissue-, and development-specific patterns. Mol Biol Cell. 1994;5:797. doi: 10.1091/mbc.5.7.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rapraeger AC, Bernfield M. Heparan sulfate proteoglycans from mouse mammary epithelial cells. A putative membrane proteoglycan associates quantitatively with lipid vesicles. J Biol Chem. 1983;258:3632. [PubMed] [Google Scholar]
- 21.Spring J, Paine-Saunders SE, Hynes RO, Bernfield M. Drosophila syndecan: conservation of a cell-surface heparan sulfate proteoglycan. Proc Natl Acad Sci U S A. 1994;91:3334. doi: 10.1073/pnas.91.8.3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park PW, Foster TJ, Nishi E, Duncan SJ, Klagsbrun M, Chen Y. Activation of syndecan-1 ectodomain shedding by Staphylococcus aureus alpha-toxin and beta-toxin. J Biol Chem. 2004;279:251. doi: 10.1074/jbc.M308537200. [DOI] [PubMed] [Google Scholar]
- 23.Xu J, Park PW, Kheradmand F, Corry DB. Endogenous Attenuation of Allergic Lung Inflammation by Syndecan-1. J Immunol. 2005;174:5758. doi: 10.4049/jimmunol.174.9.5758. [DOI] [PubMed] [Google Scholar]


