Abstract
West Nile virus (WNV) is a neurotropic flavivirus that has emerged globally as a significant cause of viral encephalitis in humans, especially in immunocompromised individuals. Previous studies have shown essential protective roles for antiviral cytokines (e.g., alpha interferon [IFN-α] and IFN-γ) against WNV in mice. However, studies using cell culture offer conflicting answers regarding whether tumor necrosis factor alpha (TNF-α) has an anti-WNV function. To test the biological significance of TNF-α against WNV in vivo, experiments were performed with TNF receptor-1 (TNF-R1)-deficient and TNF-α-depleted C57BL/6 mice. TNF-R1−/− mice had enhanced mortality and decreased survival time after WNV infection compared to congenic wild-type mice. Consistent with this, administration of a neutralizing anti-TNF-α monoclonal antibody also decreased survival after WNV infection. Relatively small differences in viral burdens in peripheral tissues of TNF-R1−/− mice were observed, and this occurrence correlated with a modest antiviral effect of TNF-α on primary macrophages but not dendritic cells. In contrast, the viral titers detected in the central nervous systems of TNF-R1−/− mice were significantly increased compared to those of wild-type mice, although TNF-α did not have a direct antiviral effect in primary neuron cultures. Whereas no defect in priming of adaptive B- and T-cell responses in TNF-R1−/− mice was observed, there were significant reductions in accumulations of CD8+ T cells and macrophages in the brain. Our data are most consistent with a model in which interaction of TNF-α with TNF-R1 protects against WNV infection by regulating migration of protective inflammatory cells into the brain during acute infection.
West Nile virus (WNV) is a mosquito-borne, neurotropic flavivirus that has emerged globally as a significant cause of epidemic viral encephalitis, especially in elderly and immunocompromised individuals. In humans, WNV infection is usually associated with a mild febrile illness, with a small subset of cases progressing to meningitis, encephalitis, or an acute flaccid paralysis syndrome (42, 43). At the present, treatment for WNV infection is supportive and no vaccine is approved for human use (14, 21). Rodent pathogenesis models suggest that innate and adaptive immune responses cooperatively orchestrate control of pathogenic strains of WNV (reviewed in reference 38). Alpha/beta interferon (IFN-α/β), IFN-γ, γδ T cells, and early immunoglobulin M (IgM) responses initially restrict WNV infection, whereas antigen-specific CD4+ and CD8+ T cells and neutralizing antibodies clear WNV from peripheral nervous system and central nervous system (CNS) tissues.
Tumor necrosis factor alpha (TNF-α) is a proinflammatory cytokine that is produced by activated macrophages, natural killer cells, and CD4+ and CD8+ T cells (6). TNF-α limits viral infections by several independent mechanisms (reviewed in reference 3), including a direct antiviral effect, enhanced class I and II major histocompatibility complex expression and antigen presentation, activation of phagocytic myeloid cells, and polarization of helper-T-cell responses. TNF-α also modulates leukocyte trafficking by altering the chemokine expression patterns in different tissues (35, 41). TNF-α functions by binding to one of two cell surface ligands, TNF receptor types 1 (TNF-R1/p55/CD120a) and 2 (TNF-R2/p75/CD120b), which are expressed on diverse cell types (22, 33, 37). Experiments using receptor-specific antibodies and TNF-R1−/− or TNF-R2−/− mice (13, 22, 52) suggest that interaction of TNF-α with TNF-R1 induces the proinflammatory signaling response whereas binding to TNF-R2 suppresses TNF-mediated inflammation (33).
As a recent report suggested that pharmacological administration of anti-TNF-α antibodies contributed to a severe neuroinvasive case of WNV infection in a human patient (9), we evaluated the role of TNF-α on WNV disease pathogenesis by using a well-established mouse model of disease. Here, using TNF-R1−/− and TNF-α-depleted C57BL/6 mice, we dissect the mechanism by which TNF-TNF-R1 signaling modulates WNV infection. We found that a TNF-R1 deficiency is associated with an increased viral burden in the CNS and enhanced mortality despite normal priming of adaptive B- and T-cell immune responses. However, disruption of TNF-α signaling reduced the accumulation of CD8+ T cells and activated macrophages into the brain. Our data are most consistent with a model in which protective CD8+ T cells and/or macrophages require TNF-α-dependent signals to migrate into the CNS parenchyma and control WNV infection.
MATERIALS AND METHODS
Viruses and cells.
The lineage I WNV strain isolated in New York in 2000 (3000.0259 [WNV-NY]) was propagated once in Vero cells and used as a stock virus (4 × 107 PFU/ml) for in vivo experiments (16). The lineage II WNV strain isolated in Madagascar in 1978 (DakAnMg798 [WNV-Mad]) was amplified once in Vero cells (4 × 107 PFU/ml) and used for intracranial (IC) survival studies as described previously (26). BHK21-15 cells were cultivated in Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum and were used for titration of the viral burdens of infected mouse tissues (16).
Mouse experiments and tissue preparation.
C57BL/6J strain (H-2b) inbred wild-type mice were obtained from a commercial vendor (Jackson Laboratories, Bar Harbor, ME). The congenic backcrossed TNF-R1−/− mice were a gift from J. Russell (Washington University School of Medicine, St. Louis, MO). All mice were genotyped and bred under pathogen-free conditions in the animal facilities of Washington University Animal Studies Committee. Eight- to 10-week-old age-matched wild-type and TNF-R1−/− mice were inoculated with 102 PFU of WNV-NY via a footpad route or 101 PFU of WNV-Mad via an IC route. In some studies, wild-type mice were treated with a single 500-μg dose of a rat/mouse chimeric anti-TNF (CNTO 2213) or isotype control (CNTO 1322) monoclonal antibody (MAb) (IgG2a, generous gift of D. Shealy, Centocor, Horsham, PA) one day prior to infection via a footpad route.
Histopathology.
Nine-week-old wild-type or TNF-R1−/− mice were inoculated with 102 PFU of WNV by footpad injection. Brain tissues were harvested at day 10 after extensive perfusion with phosphate-buffered saline (PBS) and 4% paraformaldehyde. Tissues were incubated in PBS-paraformaldehyde for two hours at 4°C, embedded in paraffin, sectioned, stained with hematoxylin and eosin (48), and examined for pathological changes by light microscopy with a Olympus BX51 microscope (Center Valley, PA).
Quantitation of viral burden in mouse tissues.
To analyze the kinetics of virus replication in different tissues, groups of wild-type and TNF-R1−/− mice were infected with 102 PFU of WNV and euthanized on days 2, 4, 6, 8, and 10 after infection. Blood was collected by cardiac puncture, and serum was isolated and stored in aliquots at −80°C. After extensive tissue perfusion with PBS, organs were removed and homogenized by using a bead beater apparatus, and titers for BHK21-15 cells were determined as described previously (16). Viremia was measured both by plaque assay with Vero cells and by fluorogenic quantitative reverse transcription-PCR (46) using an ABI 7000 sequence detection system (Applied Biosystems, Foster City, CA).
TNF-α treatment of primary cells and virus infection.
To assay the antiviral function of TNF-α in primary cells, bone marrow-derived macrophages and dendritic cells, as well as cortical neurons, were generated from C57BL/6 mice as described previously (12, 40, 49). Macrophages, dendritic cells, and cortical neurons were pretreated with 100, 10, or 1 ng/ml of recombinant TNF-α (PeproTech Inc., Rocky Hill, NJ) one day prior to infection. Subsequently, macrophages, dendritic cells, and cortical neurons were infected with WNV at a multiplicity of infection of 0.1 for 1 h, followed by extensive washing to remove free unbound virus. Supernatants were harvested 24 and 48 h after infection, and the production of infectious virus was measured by a plaque assay with Vero cells as described previously (39).
Measurement of WNV-specific antibodies.
The levels of WNV-specific IgM and IgG were detected by enzyme-linked immunosorbent assay using purified WNV E protein as described previously (16). Titers representing the serum dilution yielding an optical density at 450 nm equivalent to three times the background level of the assay were determined. The titer of neutralizing antibody was determined by using a plaque reduction neutralization assay with BHK21 cells (16). Plaques were counted and plotted, and the plaque reduction neutralization titer for 50% inhibition was calculated.
Intracellular IFN-γ staining of T cells.
Splenocytes from wild-type and TNF-R1−/− mice were harvested at day 7 after infection and stimulated ex vivo with an immunodominant Db-restricted NS4B peptide of WNV (SSVWNATTAI) (7, 34) or with a nonspecific agonist, phorbol ester and ionomycin. Four hours after stimulation in the presence of brefeldin A, splenocytes were cooled to 4°C and immunostained for cell surface markers and intracellular IFN-γ as described previously (34). The percentage of CD8+ T cells that expressed IFN-γ was determined by flow cytometry using CellQuest software (BD Biosciences).
Leukocyte isolation from CNS.
Leukocytes were isolated and quantified from the CNS as described previously (27). Briefly, wild-type and TNF-R1−/− mouse brains were harvested on day 9 after infection, dispersed into single-cell suspension, and digested with 0.05% collagenase D, 0.1 μg/ml trypsin inhibitor TLCK (Nα-p-tosyl-l-lysine chloromethyl ketone), 10 μg/ml DNase I (all from Sigma Chemical), and 10 mM of HEPES (Life Technologies) in Hanks balanced salt solution for 1 h. Viable cells were separated by discontinuous Percoll gradient (70%/37%/30%) centrifugation for 30 min (850 × g at 4°C). After being washed and counted, cells were stained for CD4, CD8, CD45, and CD11b with directly conjugated antibodies (BD Pharmingen) for 30 min at 4°C and then fixed with 1% paraformaldehyde. Data collection and analysis were performed with a FACSCalibur flow cytometer and CellQuest Software.
Statistical analysis.
All data were analyzed using Prism software (GraphPad Prism, San Diego, CA). Kaplan-Meier survival curves were analyzed by the log rank test. For antibody titers, a two-tailed unpaired t test was used to determine statistical significance. For viral burden analysis, differences in log titers were analyzed by the Mann-Whitney test.
RESULTS
Treatment with neutralizing TNF-α antibodies increases susceptibility to WNV infection.
TNF-α is a proinflammatory cytokine that can inhibit virus infection through direct antiviral or immunomodulatory effects (22). Several studies have demonstrated that TNF-α accumulates in serum and tissues of mice infected with WNV (19, 20, 27, 50, 53). To determine the significance of TNF-α in the pathogenesis of WNV infection, we passively administered a single dose of neutralizing rat anti-mouse TNF-α MAb or isotype control MAb by an intraperitoneal route to 9-week-old wild-type C57BL/6 mice. One day after MAb administration, mice were infected subcutaneously with 102 PFU of a North American pathogenic WNV isolate and monitored for survival. Mice that were treated with the anti-TNF-α MAb had a decreased survival rate (50%) compared to that of mice treated with the isotype control MAb (78%) (P = 0.04) (Fig. 1A), suggesting that TNF-α has a protective function against WNV infection.
FIG. 1.
Effect of TNF-α on WNV infection. (A) Effect of neutralizing TNF-α MAbs on survival. Nine-week-old wild-type mice were treated with 500 μg of anti-TNF-α or isotype control IgG2a MAb one day before infection with 102 PFU of WNV by a subcutaneous route. The survival differences between anti TNF-α antibody and isotype control antibody mice were statistically significant (P = 0.04; >22 mice per group). (B) Survival analysis of TNF-R1−/− mice. Wild-type (n = 40) and TNF-R1−/− (n = 34) mice were infected subcutaneously with 102 PFU of WNV and monitored for mortality for 28 days. Survival differences were statistically significant (P < 0.0001). WNV burdens in wild-type and TNF-R1−/− mouse sera (C), spleens (D), brains (E), and spinal cords (F) were analyzed by using a viral plaque assay after tissues were harvested at the indicated time points. Data were derived from 10 to 12 mice per time point between days 2 and 10. For the graphs showing the results of viral burden experiments, the dotted line represents the limit of sensitivity for viral detection. An asterisk indicates a P value of <0.05 and two asterisks indicate a P value of <0.005 in comparison to values for wild-type mice.
Lack of TNF-R1 increases susceptibility to WNV.
Although TNF-α signals through two receptors (TNF-R1 and TNF-R2), TNF-α-TNF-R1 interactions are responsible for the majority of its proinflammatory functions in adult mice (22). To independently confirm a protective role of TNF-α against WNV infection, we compared mortalities of wild-type and TNF-R1−/− mice after infection with 102 PFU of the same strain of WNV isolated from New York. Because of their immune system developmental defects, which include a lack of splenic primary B-cell follicles and germinal centers (31, 32), congenic TNF−/− mice were not used. The rate of survival after WNV infection for TNF-R1−/− mice was noticeably lower than that for wild-type mice (33% compared to 75%; P < 0.0001) (Fig. 1B). Mortality also occurred earlier in the TNF-R1−/− mice, as the mean time to death was shorter than that for wild-type mice (11.7 ± 3 days compared to 13.9 ± 2 days, respectively; P = 0.02). Thus, an absence of TNF-R1 caused more-severe WNV infection with increased mortality after subcutaneous inoculation.
Increased WNV burden in TNF-R1−/− mice.
To better understand how an absence of TNF-TNF-R1 interaction affected the susceptibility of mice to WNV infection, viral burdens in peripheral nervous system and CNS tissues were analyzed. Wild-type and TNF-R1−/− mice were infected with 102 PFU of WNV, and viral burdens were measured by plaque assay at days 2, 4, 6, 8, and 10 after infection in sera, spleens, brains, and spinal cords.
Viremia was measured by plaque assay and by fluorogenic reverse transcription-PCR. The kinetics and magnitude of viremia for wild-type and TNF-R1−/− mice were virtually identical. Infectious WNV or viral RNA was detected from day 2 to day 4 after inoculation but was cleared from circulation by day 6 (Fig. 1C and data not shown).
In contrast to the case for sera, the levels of WNV detected in the spleens of TNF-R1−/− mice were eightfold higher than the levels detected in wild-type mouse spleens at day 4 after infection (P = 0.02) (Fig. 1D). However, by day 6, no significant difference between the two groups was observed (P > 0.4). Thus, a lack of TNF-R1 did not alter the clearance phase of WNV infection in the spleen, despite the presence of a modestly higher viral burden in the TNF-R1−/− mice during the initial stages.
No statistically significant difference in the kinetics of the WNV spread to the brain was observed in TNF-R1−/− mice (P = 0.08) (Fig. 1E). However, by days 8 and 10 after infection, levels of WNV detected in the brains of TNF-R1−/− mice were significantly higher (18- and 68-fold, respectively; P < 0.04) than those detected in the brains of wild-type mice. In the spinal cord, WNV was detected earlier for more TNF-R1−/− mice than wild-type mice (at day 6, WNV was detected in a subset [5 of 8] of the TNF-R1−/− mice but was detected in only one wild-type mouse [1 of 8]), although this difference did not reach statistical significance (Fig. 1F). By day 10, WNV titers in the spinal cords of TNF-R1−/− mice were 85-fold higher than those in the spinal cords of wild-type mice (P < 0.01). Collectively, these data show that TNF-α-TNF-R1 interactions restrict WNV replication in the CNS, and the phenotype becomes more evident late during the course of infection.
Antiviral effect of TNF-α on WNV infection of primary cells.
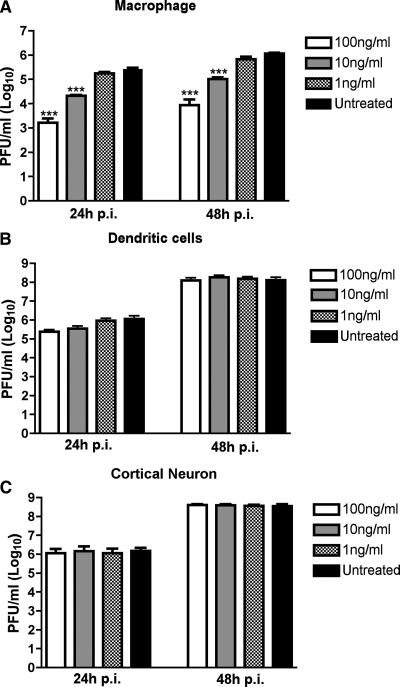
The in vivo studies suggested that interaction of TNF-α with TNF-R1 modestly limited WNV replication in the spleen and to a greater degree inhibited infection in the CNS. Based on these data, we speculated that TNF-α has a direct antiviral role and restricts infection in a cell-specific manner. To address this, three WNV-permissive primary cells (macrophages, myeloid dendritic cells, and cortical neurons) were pretreated with increasing doses of TNF-α one day prior to infection. Importantly, no differences in cell viability were observed after a 24-h preincubation with TNF-α (data not shown). Supernatants were analyzed for the production of WNV 24 and 48 h after infection by plaque assay with Vero cells. Notably, TNF-α pretreatment of dendritic cells and cortical neurons did not affect WNV production (P > 0.5) (Fig. 2B and C). In contrast, WNV accumulation in macrophage supernatants was significantly reduced after treatment with 10 and 100 ng/ml of TNF-α (Fig. 2A). At 48 h after infection, WNV titers were reduced in macrophage supernatants approximately 12- to 160-fold (P < 0.0001) after incubation with 10 and 100 ng/ml of TNF-α, respectively, in comparison to the titers in untreated macrophage supernatants.
FIG. 2.
Antiviral effect of TNF-α in primary cells. Macrophages (A), dendritic cells (B), and cortical neurons (C) derived from C57BL/6 mice were pretreated with the indicated doses of recombinant TNF-α one day prior to infection with WNV at a multiplicity of infection of 0.1. Supernatants were harvested at 24 and 48 h postinfection (p.i.) for viral plaque assays with Vero cells. The data are averages for three independent experiments performed in duplicate. The error bars indicate standard errors of the means, and the asterisks indicate statistically significant (P < 0.0001) differences in comparison to values for the condition without treatment.
Normal WNV-specific antibody responses in TNF-R1−/− mice.
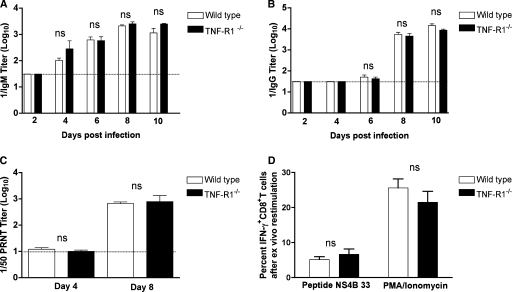
As defects in TNF-α signaling can impair B-cell priming (4, 5, 32) and antibody responses are essential to controlling WNV infection (16, 17), we evaluated whether the enhanced viral burden in TNF-R1−/− mice was associated with blunted WNV-specific antibody responses. Notably, levels of WNV-specific IgM (Fig. 3A) and IgG (Fig. 3B) in wild-type and TNF-R1−/− mice were observed to be similar throughout the time course. Correspondingly, no difference in neutralizing activity was detected on day 4 or 8 after infection (P > 0.7) (Fig. 3C). Thus, an absence of TNF-R1 did not affect the magnitude or quality of early antibody responses after WNV infection.
FIG. 3.
WNV-specific antibody and T-cell responses in wild-type and TNF-R1−/− mice. The development of WNV-specific IgM (A) or IgG (B) antibodies was determined from serum at the indicated time points by enzyme-linked immunosorbent assay using purified WNV E protein. The data are the averages of 5 mice per time point. (C) Neutralizing antibody titers. Neutralizing antibody titers were determined using a plaque reduction neutralization titer assay on sera from days 4 and 8 after infection. The data are expressed as antibody titers that reduce the number of plaques by 50% (1/50 PRNT titer) and are the averages for five mice per time point. (D) IFN-γ production by WNV-primed CD8+ T cells from wild-type and TNF-R1−/− mice. Uninfected or WNV-infected splenocytes from wild-type or TNF-R1−/− mice on day 7 were stimulated ex vivo with an immunodominant Db-restricted NS4B peptide or phorbol ester (phorbol myristate acetate [PMA]) and ionomycin. Cells were costained for CD8 and IFN-γ and analyzed by flow cytometry. The percentage of CD8+ T cells that expressed IFN-γ after restimulation was calculated. The data are averages for five mice per group. No statistically significant differences were observed between the wild-type and TNF-R1−/− mice in any of the antibody or T-cell response assays (P > 0.1). ns, not significant.
Normal CD8+-T-cell priming in TNF-R1−/− mice.
Based on the increased mortality, modest direct antiviral effects of TNF-α, and elevated viral burden in the CNS late in the time course, we speculated that there might be a deficit in WNV-specific CD8+-T-cell responses in TNF-R1−/− mice. Previous studies had suggested that TNF signaling could modulate CD8+-T-cell priming (8, 24) and that CD8+ T cells clear WNV from infected neurons in the CNS (7, 46, 47, 49, 54). To determine whether a deficiency in TNF signaling altered WNV-specific CD8+-T-cell responses, we analyzed intracellular IFN-γ production ex vivo at day 7 postinfection after antigen-specific restimulation with a Db-restricted immunodominant NS4B peptide (34) or a nonspecific agonist, phorbol ester and ionomycin. Notably, no significant difference was observed between the percentages or numbers of IFN-γ-positive splenic CD8+ T cells from wild-type and TNF-R1−/− mice after restimulation with NS4B peptides (P > 0.4) or phorbol myristate acetate-ionomycin (P > 0.3) (Fig. 3D). Thus, a deficiency in TNF-TNF-R1 interactions did not impair priming of WNV-specific CD8+ T cells in the spleen.
Effect of TNF-R1 on survival after IC WNV infection.
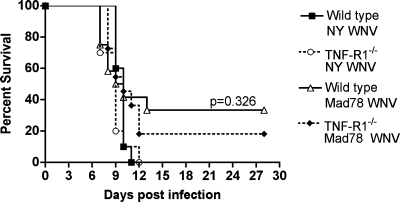
Our virologic data for primary neuron cultures suggested that TNF-α may act indirectly to control WNV replication in the CNS. To confirm this, we performed IC infections with wild-type and TNF-R1−/− mice and virulent (WNV-NY) and attenuated (WNV-Mad) viral strains (Fig. 4): because of the rapid kinetic of WNV replication, inoculation into the brain allows a more direct evaluation of the effect of TNF-α in the CNS without its potentially confounding effects on the peripheral adaptive immune response, which takes 5 to 7 days to develop. IC infection with WNV-NY caused complete lethality (100%) for both wild-type and TNF-R1−/− mice, with no difference between average survival times (P > 0.1). Although both wild-type (17%) and TNF-R1−/− (33%) mice showed incomplete mortality after IC infection with WNV-Mad, the differences were not statistically significant (P > 0.3). As an absence of TNF-R1 expression failed to promote more-severe disease with virulent or attenuated WNV strains after direct inoculation into the brain, TNF-α does not appear to have a dominant direct antiviral effect in the CNS.
FIG. 4.
Survival analysis after WNV infection by an IC route. Wild-type and TNF-R1−/− mice were infected IC with 101 PFU of virulent WNV-NY or attenuated WNV-Mad (Mad78 WNV) and monitored for 28 days. The survival curves were constructed with data from two independent experiments (10 to 12 mice per group). The survival differences between wild-type and TNF-R1−/− mice were not statistically significant (P > 0.3).
TNF-R1−/− mice show a defect in accumulation of inflammatory cells in the CNS.
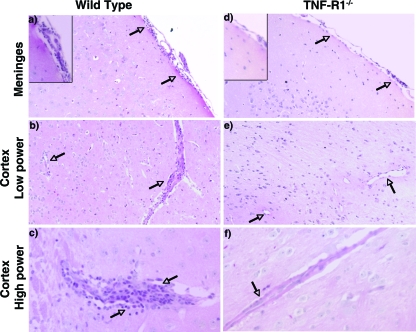
Although T-cell priming in the spleen was normal in TNF-R1−/− mice, because viral burden accumulated at a late stage in pathogenesis, we speculated there might be a defect of leukocyte trafficking into the CNS. Inefficient migration of leukocytes into the CNS results in increased WNV burden in the brain and vulnerability to lethal infection in CXCL10−/−, CXCR3−/−, CD40−/−, and CCR5−/− mice (20, 27, 51, 57). To address this, histopathological analysis was performed on brain sections at day 10 after infection (Fig. 5). Hematoxylin and eosin staining of brain sections from wild-type mice showed noticeable inflammation, as evidenced by perivascular cuffing and the accumulation of leukocytes within the meninges. In contrast, for TNF-R1−/− mice, despite the higher viral burden (Fig. 1E), fewer leukocytes were apparent in the meninges or near blood vessels.
FIG. 5.
Hematoxylin and eosin staining of brain sections after WNV infection. Wild-type and TNF-R1−/− mice were infected subcutaneously with WNV and brains were harvested at day 10, sectioned, and stained with hematoxylin and eosin. Representative sections from the cortex and meninges are shown at low and high powers after thorough review of five independent wild-type or TNF-R1−/− mouse brains. Arrows indicate areas of leukocyte infiltration.
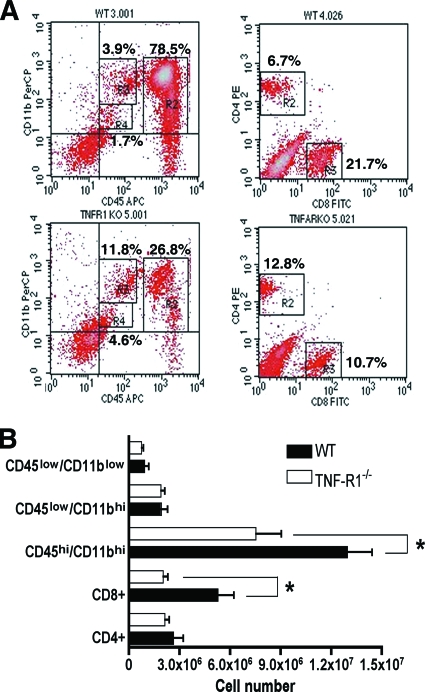
To better understand the basis for the decreased number of mononuclear leukocytes in the CNS in the TNF-R1−/− mice after WNV infection, leukocytes were recovered from brains at day 9 after extensive perfusion and analyzed by flow cytometry (Fig. 6A). Although no difference between the percentages or numbers of CD4+ T cells in the brain was identified (P > 0.2), a ∼50% reduction (P = 0.004) in CD8+ T cells was observed in the brains of TNF-R1−/− mice (Fig. 6B), despite the higher viral burden. Additionally, the number of CD11bhi/CD45hi macrophages detected in the brains of TNF-R1−/− mice were lower than that detected in wild-type mice (1.2 × 107 and 7.5 × 106 cells in wild-type and TNF-R1−/− mice, respectively; P < 0.01). In contrast, no difference was observed between the numbers of resting CD11blo/CD45lo (P > 0.2) or activated CD11bhi/CD45lo (P > 0.4) microglia (Fig. 6B) in TNF-R1−/− mice. Taken together, the histopathologic and flow cytometric analyses suggest that an absence of TNF-TNF-R1 interactions blunts leukocyte trafficking into and accumulation in the brain, resulting in a failure to clear WNV infection in a timely manner.
FIG. 6.
Trafficking of inflammatory cells into the CNS after WNV infection. Wild-type (WT) and TNF-R1−/− mice were infected with 102 PFU of WNV, and brains were harvested after extensive perfusion at day 9 after infection. CNS leukocytes were isolated afterwards by Percoll centrifugation and analyzed by flow cytometry. (A) Representative flow cytometry profiles of inflammatory cells (CD4+ T cells, CD8+ T cells, CD45+ cells, and CD11b+ cells) that were present in the brain. PerCP, peridinin chlorophyll protein; PE, phycoerythrin; APC, antigen-presenting cell; FITC, fluorescein isothiocyanate; KO, knockout; TNFARKO, TNF-RI−/−. (B) The absolute numbers of specific inflammatory cells in brains of wild-type and TNF-R1−/− mice after WNV infection were calculated and reflect averages for 6 to 10 mice per group from two independent experiments (*, P < 0.005).
DISCUSSION
Antiviral cytokines, such as type I (IFN-α/β) or II (IFN-γ) IFN, have essential roles in the immediate defense against WNV, as they restrict infection in both peripheral nervous system and CNS tissues. However, the role of TNF-α, which has both antiviral and immunomodulatory functions (22), in WNV pathogenesis remains uncertain. Here, we showed that an absence of TNF-α-TNF-R1 interactions in TNF-R1−/− and TNF-α-depleted mice causes enhanced mortality compared to that of congenic wild-type mice. In TNF-R1−/− mice, the decreased survival rate correlated with an increase in viral burden in the CNS at later stages of infection and was associated with reduced accumulation of CD8+ T cells and macrophages in the CNS despite normal priming of adaptive immune responses after WNV infection. Moreover, pretreatment of primary neuron cultures with TNF-α did not reduce WNV titers, and correspondingly, no difference in survival was observed between TNF-R1−/− mice and wild-type mice after direct IC infection. These results suggest that TNF-α likely enhances clearance of WNV infection by facilitating migration or accumulation of protective leukocytes in the CNS.
TNF-α accumulates in serum and tissues after WNV infection (19, 20, 27, 50, 53). In one study, accumulation of TNF-α at day 3 in wild-type C57BL/6 mice was associated with enhanced blood-brain barrier permeability that resulted in early entry of WNV into the CNS and increased mortality (53). In contrast, here, we observed a distinct phenotype consisting of antibody neutralization of TNF-α or a genetic deficiency of TNF-R1 increasing susceptibility to WNV infection. Although the reasons remain unclear, several experimental differences could account for the disparity in results: these include differences in the route of infection (footpad versus intraperitoneal infection), infectious dose (102 versus 104 PFU), or viral strain (WNV-NY versus CT2471). Consistent with our findings, the administration of neutralizing anti-TNF-α MAbs to a human patient with rheumatoid arthritis was associated with a severe case of WNV-induced encephalitis (9).
The survival phenotype in animals given a neutralizing TNF-α antibody appeared to be slightly weaker than that of WNV-infected TNF-R1−/− mice. This could be due to incomplete antibody neutralization of TNF-α in specific tissue compartments (e.g., the CNS) or to an independent pathological effect of TNF-R2 signaling in the absence of TNF-R1. Indeed, experiments with mice suggest that TNF-R2 may have a counterregulatory function to inhibit inflammation and cytokine responses (33). Future studies with TNF-R2−/− mice may be required to address this directly.
In other animal models of flavivirus infection that induce vascular permeability, TNF-α appears to contribute to pathogenesis. During dengue virus (DENV) infection, TNF-α mediates damage to the endothelium and promotes virus-induced vascular leakage and hemorrhage (10), and treatment with anti-TNF-α antibodies improves the outcome for mice (2, 45). Correspondingly, human DENV-infected patients with a single-nucleotide polymorphism of a TNF-α allele have elevated TNF-α levels in serum and an increased risk of severe hemorrhagic fever and shock syndrome (18). Our examinations of the role of TNF-α during infection with an encephalitic strain of the flavivirus WNV are more consistent with results from studies with other nonflavivirus models of encephalitis. For example, neutralization of TNF-α significantly increased the rate and severity of encephalitis due to herpes simplex virus in mice (28), and pretreatment of mice with TNF-α prolonged survival (36). Moreover, TNF-R1−/− mice showed impaired clearance of lymphocytic choriomeningitis virus (52).
By combining virological and immunological analyses for TNF-R1−/− mice, we have begun to define the primary mechanism by which TNF-α-TNF-R1 interactions protect against WNV infection. A deficiency in TNF-TNF-R1 signaling resulted in increased CNS viral burden and lethality without a noticeable impairment in adaptive immune system priming. Indeed, the integrity of the early B- and T-cell responses in TNF-R1−/− mice correlated with efficient control of viremia and rapid clearance of infection in the spleen, which occur at stages in WNV disease pathogenesis that require efficient antibody and CD8+-T-cell priming (17, 47). These results contrast with other models in which TNF-α has a more dominant role in priming CD8+-T-cell and CD8+-B-cell responses (8, 24, 28).
TNF-α could act locally as a direct antiviral cytokine, as it is rapidly induced in peripheral nervous system and CNS tissues after WNV infection (19, 20, 27, 50, 53). Indeed, one group has shown that WNV replicates to higher levels in TNF−/− murine embryonic fibroblasts, although in that study, treatment of cells with exogenous recombinant TNF-α did not reduce WNV infection (11). Analogously, pretreatment of human hepatoma cells or monocyte-derived macrophages with TNF-α did not reduce infection with DENV (15, 55). Our primary culture data are interesting, as they may explain apparent conflicts in the literature; we observed cell-specific inhibitory effects of TNF-α, with dose-dependent reductions of WNV propagation in bone marrow-derived macrophages yet no effect on primary dendritic cells or neurons. Consistent with the lack of antiviral effect in neurons, no difference in survival was observed when a virulent or attenuated WNV strain was directly inoculated into the brain.
The combination of elevated WNV burden at day 10 after infection with blunted levels of CD8+ T cells and activated macrophages in the brains of TNF-R1−/− mice is most consistent with a model in which TNF-α protects against WNV infection by regulating accumulation of protective inflammatory cells in the brain during acute infection. In support of this, histopathologic analysis showed depressed meningeal and perivascular inflammation in TNF-R1−/− mice, even though their viral loads were ∼70-fold higher than those of wild-type mice. TNF-α regulates leukocyte trafficking (41) by modulating the expression of cell adhesion molecules and leukocyte-specific chemokines (23, 30, 44). Analogously, TNF-α increases the expression of leukocyte adhesion molecules on endothelial cells lining the blood-brain barrier to facilitate leukocyte trafficking into the CNS parenchyma during experimental autoimmune encephalomyelitis (25, 56). Although TNF-TNF-R1 interactions modulate the trafficking of CD8+ T cells and activated macrophages into the CNS after WNV infection, the precise mechanism remains uncertain. As preliminary studies show significantly reduced expression of T-cell- and macrophage-specific chemokines (e.g., CCL4, CCL5, and CXCL10) in the spleens of WNV-infected TNF-R1−/− mice (B. Zhang and R. Klein, unpublished data), a combination of decreased adhesion and chemotaxis may explain the blunted number of leukocytes in the CNS of TNF-R1−/− mice after WNV infection.
In summary, our experiments using a peripheral-inoculation model demonstrate a protective role of TNF-α during WNV infection. TNF-α appears to function by regulating steps that allow accumulation of CD8+ T cells and activated macrophages in the CNS, which ultimately leads to a decrease in viral burden and improvement in survival. Patients who require TNF-α antagonist therapy to control autoimmune disease thus may be appropriate initial candidates to receive WNV vaccines (21, 29) or prophylaxis (1) as it becomes available.
Acknowledgments
We thank members of our laboratories for constructive advice on experiments and D. Shealy (Centocor, Inc.) for the generous gift of the anti-TNF and control antibodies. We also thank Dawn Koch and Michelle Noll for technical assistance.
The study was supported by grants from the National Institutes of Health (R01NS052632 to R.S.K. and M.S.D.) and the Midwest Regional Center for Excellence in Emerging Infectious Diseases (5U54 AI057160).
Footnotes
Published ahead of print on 16 July 2008.
REFERENCES
- 1.Agrawal, A. G., and L. R. Petersen. 2003. Human immunoglobulin as a treatment for West Nile virus infection. J. Infect. Dis. 1881-4. [DOI] [PubMed] [Google Scholar]
- 2.Atrasheuskaya, A., P. Petzelbauer, T. M. Fredeking, and G. Ignatyev. 2003. Anti-TNF antibody treatment reduces mortality in experimental dengue virus infection. FEMS Immunol. Med. Microbiol. 3533-42. [DOI] [PubMed] [Google Scholar]
- 3.Benedict, C. A. 2003. Viruses and the TNF-related cytokines, an evolving battle. Cytokine Growth Factor Rev. 14349-357. [DOI] [PubMed] [Google Scholar]
- 4.Benihoud, K., S. Esselin, D. Descamps, B. Jullienne, B. Salone, P. Bobe, D. Bonardelle, E. Connault, P. Opolon, I. Saggio, and M. Perricaudet. 2007. Respective roles of TNF-alpha and IL-6 in the immune response-elicited by adenovirus-mediated gene transfer in mice. Gene Ther. 14533-544. [DOI] [PubMed] [Google Scholar]
- 5.Borrow, P., S. Hou, S. Gloster, M. Ashton, and L. Hyland. 2005. Virus infection-associated bone marrow B cell depletion and impairment of humoral immunity to heterologous infection mediated by TNF-alpha/LTalpha. Eur. J. Immunol. 35524-532. [DOI] [PubMed] [Google Scholar]
- 6.Bradley, J. R. 2008. TNF-mediated inflammatory disease. J. Pathol. 214149-160. [DOI] [PubMed] [Google Scholar]
- 7.Brien, J. D., J. L. Uhrlaub, and J. Nikolich-Zugich. 2007. Protective capacity and epitope specificity of CD8(+) T cells responding to lethal West Nile virus infection. Eur. J. Immunol. 371855-1863. [DOI] [PubMed] [Google Scholar]
- 8.Bromberg, J. S., K. D. Chavin, and S. L. Kunkel. 1992. Anti-tumor necrosis factor antibodies suppress cell-mediated immunity in vivo. J. Immunol. 1483412-3417. [PubMed] [Google Scholar]
- 9.Chan-Tack, K. M., and G. Forrest. 2006. West Nile virus meningoencephalitis and acute flaccid paralysis after infliximab treatment. J. Rheumatol. 33191-192. [PubMed] [Google Scholar]
- 10.Chen, H. C., F. M. Hofman, J. T. Kung, Y. D. Lin, and B. A. Wu-Hsieh. 2007. Both virus and tumor necrosis factor alpha are critical for endothelium damage in a mouse model of dengue virus-induced hemorrhage. J. Virol. 815518-5526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng, Y., N. J. King, and A. M. Kesson. 2004. The role of tumor necrosis factor in modulating responses of murine embryo fibroblasts by flavivirus, West Nile. Virology 329361-370. [DOI] [PubMed] [Google Scholar]
- 12.Daffis, S., M. A. Samuel, B. C. Keller, M. Gale, Jr., and M. S. Diamond. 2007. Cell-specific IRF-3 responses protect against West Nile virus infection by interferon-dependent and independent mechanisms. PLoS Pathog. 3e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deckert-Schlüter, M., H. Bluethmann, A. Rang, H. Hof, and D. Schluter. 1998. Crucial role of TNF receptor type 1 (p55), but not of TNF receptor type 2 (p75), in murine toxoplasmosis. J. Immunol. 1603427-3436. [PubMed] [Google Scholar]
- 14.Diamond, M. S. 2005. Development of effective therapies against West Nile virus infection. Expert Rev. Anti-Infect. Ther. 3931-944. [DOI] [PubMed] [Google Scholar]
- 15.Diamond, M. S., T. Roberts, D. Edgil, B. Lu, J. Ernst, and E. Harris. 2000. Modulation of dengue virus infection in human cells by alpha, beta, and gamma interferons. J. Virol. 744957-4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diamond, M. S., B. Shrestha, A. Marri, D. Mahan, and M. Engle. 2003. B cells and antibody play critical roles in the immediate defense of disseminated infection by West Nile encephalitis virus. J. Virol. 772578-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diamond, M. S., E. Sitati, L. Friend, B. Shrestha, S. Higgs, and M. Engle. 2003. A critical role for induced IgM in the protection against West Nile virus infection. J. Exp. Med. 1981853-1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernández-Mestre, M. T., K. Gendzekhadze, P. Rivas-Vetencourt, and Z. Layrisse. 2004. TNF-alpha-308A allele, a possible severity risk factor of hemorrhagic manifestation in dengue fever patients. Tissue Antigens 64469-472. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Tapia, D., D. E. Hassett, W. J. Mitchell, Jr., G. C. Johnson, and S. B. Kleiboeker. 2007. West Nile virus encephalitis: sequential histopathological and immunological events in a murine model of infection. J. Neurovirol. 13130-138. [DOI] [PubMed] [Google Scholar]
- 20.Glass, W. G., J. K. Lim, R. Cholera, A. G. Pletnev, J. L. Gao, and P. M. Murphy. 2005. Chemokine receptor CCR5 promotes leukocyte trafficking to the brain and survival in West Nile virus infection. J. Exp. Med. 2021087-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall, R. A., and A. A. Khromykh. 2004. West Nile virus vaccines. Expert Opin. Biol. Ther. 41295-1305. [DOI] [PubMed] [Google Scholar]
- 22.Herbein, G., and W. A. O'Brien. 2000. Tumor necrosis factor (TNF)-alpha and TNF receptors in viral pathogenesis. Proc. Soc. Exp. Biol. Med. 223241-257. [DOI] [PubMed] [Google Scholar]
- 23.Hickey, M. J., P. H. Reinhardt, L. Ostrovsky, W. M. Jones, M. A. Jutila, D. Payne, J. Elliott, and P. Kubes. 1997. Tumor necrosis factor-alpha induces leukocyte recruitment by different mechanisms in vivo and in vitro. J. Immunol. 1583391-3400. [PubMed] [Google Scholar]
- 24.Hu, H. M., H. Winter, J. Ma, M. Croft, W. J. Urba, and B. A. Fox. 2002. CD28, TNF receptor, and IL-12 are critical for CD4-independent cross-priming of therapeutic antitumor CD8+ T cells. J. Immunol. 1694897-4904. [DOI] [PubMed] [Google Scholar]
- 25.Kahn, M. A., J. M. Dopp, S. Liva, A. J. MacKenzie-Graham, R. Chang, A. Huang, R. Nazarian, P. Dell'Albani, D. Condorelli, R. R. Voskuhl, and J. de Vellis. 1999. Temporal kinetics and cellular phenotype of TNF p55/p75 receptors in experimental allergic encephalomyelitis. J. Neuroimmunol. 9519-34. [DOI] [PubMed] [Google Scholar]
- 26.Keller, B. C., B. L. Fredericksen, M. A. Samuel, R. E. Mock, P. W. Mason, M. S. Diamond, and M. Gale, Jr. 2006. Resistance to alpha/beta interferon is a determinant of West Nile virus replication fitness and virulence. J. Virol. 809424-9434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klein, R. S., E. Lin, B. Zhang, A. D. Luster, J. Tollett, M. A. Samuel, M. Engle, and M. S. Diamond. 2005. Neuronal CXCL10 directs CD8+ T cell recruitment and control of West Nile virus encephalitis. J. Virol. 7911457-11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lundberg, P., P. V. Welander, C. K. Edwards III, N. van Rooijen, and E. Cantin. 2007. Tumor necrosis factor (TNF) protects resistant C57BL/6 mice against herpes simplex virus-induced encephalitis independently of signaling via TNF receptor 1 or 2. J. Virol. 811451-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monath, T. P., J. Liu, N. Kanesa-Thasan, G. A. Myers, R. Nichols, A. Deary, K. McCarthy, C. Johnson, T. Ermak, S. Shin, J. Arroyo, F. Guirakhoo, J. S. Kennedy, F. A. Ennis, S. Green, and P. Bedford. 2006. A live, attenuated recombinant West Nile virus vaccine. Proc. Natl. Acad. Sci. USA 1036694-6699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Norman, M. U., K. J. Lister, Y. H. Yang, A. Issekutz, and M. J. Hickey. 2005. TNF regulates leukocyte-endothelial cell interactions and microvascular dysfunction during immune complex-mediated inflammation. Br. J. Pharmacol. 144265-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pasparakis, M., L. Alexopoulou, V. Episkopou, and G. Kollias. 1996. Immune and inflammatory responses in TNF alpha-deficient mice: a critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J. Exp. Med. 1841397-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pasparakis, M., L. Alexopoulou, M. Grell, K. Pfizenmaier, H. Bluethmann, and G. Kollias. 1997. Peyer's patch organogenesis is intact yet formation of B lymphocyte follicles is defective in peripheral lymphoid organs of mice deficient for tumor necrosis factor and its 55-kDa receptor. Proc. Natl. Acad. Sci. USA 946319-6323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peschon, J. J., D. S. Torrance, K. L. Stocking, M. B. Glaccum, C. Otten, C. R. Willis, K. Charrier, P. J. Morrissey, C. B. Ware, and K. M. Mohler. 1998. TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J. Immunol. 160943-952. [PubMed] [Google Scholar]
- 34.Purtha, W. E., N. Myers, V. Mitaksov, E. Sitati, J. Connolly, D. H. Fremont, T. H. Hansen, and M. S. Diamond. 2007. Antigen-specific cytotoxic T lymphocytes protect against lethal West Nile virus encephalitis. Eur. J. Immunol. 371845-1854. [DOI] [PubMed] [Google Scholar]
- 35.Roach, D. R., A. G. Bean, C. Demangel, M. P. France, H. Briscoe, and W. J. Britton. 2002. TNF regulates chemokine induction essential for cell recruitment, granuloma formation, and clearance of mycobacterial infection. J. Immunol. 1684620-4627. [DOI] [PubMed] [Google Scholar]
- 36.Rossol-Voth, R., S. Rossol, K. H. Schutt, S. Corridori, W. de Cian, and D. Falke. 1991. In vivo protective effect of tumour necrosis factor alpha against experimental infection with herpes simplex virus type 1. J. Gen. Virol. 72143-147. [DOI] [PubMed] [Google Scholar]
- 37.Ruby, J., H. Bluethmann, and J. J. Peschon. 1997. Antiviral activity of tumor necrosis factor (TNF) is mediated via p55 and p75 TNF receptors. J. Exp. Med. 1861591-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samuel, M. A., and M. S. Diamond. 2006. Pathogenesis of West Nile virus infection: a balance between virulence, innate and adaptive immunity, and viral evasion. J. Virol. 809349-9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Samuel, M. A., and M. S. Diamond. 2005. Type I IFN protects against lethal West Nile virus infection by restricting cellular tropism and enhancing neuronal survival. J. Virol. 7913350-13361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Samuel, M. A., K. Whitby, B. C. Keller, A. Marri, W. Barchet, B. R. G. Williams, R. H. Silverman, M. Gale, and M. S. Diamond. 2006. PKR and RNase L contribute to protection against lethal West Nile virus infection by controlling early viral spread in the periphery and replication in neurons. J. Virol. 807009-7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sedgwick, J. D., D. S. Riminton, J. G. Cyster, and H. Korner. 2000. Tumor necrosis factor: a master-regulator of leukocyte movement. Immunol. Today 21110-113. [DOI] [PubMed] [Google Scholar]
- 42.Sejvar, J. J., A. V. Bode, A. A. Marfin, G. L. Campbell, J. Pape, B. J. Biggerstaff, and L. R. Petersen. 2006. West Nile virus-associated flaccid paralysis outcome. Emerg. Infect. Dis. 12514-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sejvar, J. J., M. B. Haddad, B. C. Tierney, G. L. Campbell, A. A. Marfin, J. A. Van Gerpen, A. Fleischauer, A. A. Leis, D. S. Stokic, and L. R. Petersen. 2003. Neurologic manifestations and outcome of West Nile virus infection. JAMA 290511-515. [DOI] [PubMed] [Google Scholar]
- 44.Shen, J., S. S. T-To, L. Schrieber, and N. J. King. 1997. Early E-selectin, VCAM-1, ICAM-1, and late major histocompatibility complex antigen induction on human endothelial cells by flavivirus and comodulation of adhesion molecule expression by immune cytokines. J. Virol. 719323-9332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shresta, S., K. L. Sharar, D. M. Prigozhin, P. R. Beatty, and E. Harris. 2006. Murine model for dengue virus-induced lethal disease with increased vascular permeability. J. Virol. 8010208-10217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shrestha, B., and M. S. Diamond. 2007. Fas ligand interactions contribute to CD8+ T-cell-mediated control of West Nile virus infection in the central nervous system. J. Virol. 8111749-11757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shrestha, B., and M. S. Diamond. 2004. The role of CD8+ T cells in the control of West Nile virus infection. J. Virol. 788312-8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shrestha, B., D. I. Gottlieb, and M. S. Diamond. 2003. Infection and injury of neurons by West Nile encephalitis virus. J. Virol. 7713203-13213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shrestha, B., M. A. Samuel, and M. S. Diamond. 2006. CD8+ T cells require perforin to clear West Nile virus from infected neurons. J. Virol. 80119-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shrestha, B., T. Wang, M. A. Samuel, K. Whitby, J. Craft, E. Fikrig, and M. S. Diamond. 2006. Gamma interferon plays a crucial early antiviral role in protection against West Nile virus infection. J. Virol. 805338-5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sitati, E., E. E. McCandless, R. S. Klein, and M. S. Diamond. 2007. CD40-CD40 ligand interactions promote trafficking of CD8+ T cells into the brain and protection against West Nile virus encephalitis. J. Virol. 819801-9811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suresh, M., X. Gao, C. Fischer, N. E. Miller, and K. Tewari. 2004. Dissection of antiviral and immune regulatory functions of tumor necrosis factor receptors in a chronic lymphocytic choriomeningitis virus infection. J. Virol. 783906-3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang, T., T. Town, L. Alexopoulou, J. F. Anderson, E. Fikrig, and R. A. Flavell. 2004. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat. Med. 101366-1373. [DOI] [PubMed] [Google Scholar]
- 54.Wang, Y., M. Lobigs, E. Lee, and A. Mullbacher. 2003. CD8+ T cells mediate recovery and immunopathology in West Nile virus encephalitis. J. Virol. 7713323-13334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wati, S., P. Li, C. J. Burrell, and J. M. Carr. 2007. Dengue virus (DV) replication in monocyte-derived macrophages is not affected by tumor necrosis factor alpha (TNF-alpha), and DV infection induces altered responsiveness to TNF-alpha stimulation. J. Virol. 8110161-10171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Willenborg, D. O., R. D. Simmons, T. Tamatani, and M. Miyasaka. 1993. ICAM-1-dependent pathway is not critically involved in the inflammatory process of autoimmune encephalomyelitis or in cytokine-induced inflammation of the central nervous system. J. Neuroimmunol. 45147-154. [DOI] [PubMed] [Google Scholar]
- 57.Zhang, B., Y. K. Chan, B. Lu, M. S. Diamond, and R. S. Klein. 2008. CXCR3 mediates region-specific antiviral T cell trafficking within the central nervous system during West Nile virus encephalitis. J. Immunol. 1802641-2649. [DOI] [PubMed] [Google Scholar]