Abstract
Assembly of adenovirus particles is thought to be similar to that of bacteriophages, in which the double-stranded DNA genome is inserted into a preformed empty capsid. Previous studies from our and other laboratories have implicated the viral IVa2 protein as a key component of the encapsidation process. IVa2 binds to the packaging sequence on the viral chromosome in a sequence-specific manner, alone and in conjunction with the viral L4 22K protein. In addition, it interacts with the viral L1 52/55-kDa protein, which is required for DNA packaging. Finally, a mutant virus that does not produce IVa2 is unable to produce any capsids. Therefore, it has been proposed that IVa2 nucleates capsid assembly. A prediction of such a model is that the IVa2 protein would be found at a unique vertex of the mature virion. In this study, the location of IVa2 in the virion has been analyzed using immunogold staining and electron microscopy, and the copy number of IVa2 in virions was determined using three independent methods, quantitative mass spectrometry, metabolic labeling, and Western blotting. The results indicate that it resides at a unique vertex and that there are approximately six to eight IVa2 molecules in each particle. These findings support the hypothesis that the IVa2 protein plays multiple roles in the viral assembly process.
Human adenoviruses are the causative agents of respiratory, ocular, and gastrointestinal infections that are generally self-limiting in otherwise healthy individuals. In immunocompromised hosts, especially pediatric patients who received a transplant, the virus can cause severe disease that is often fatal, and effective antiviral treatments are not available (21, 38). While much is understood about viral gene expression and genome replication, less is known about the later steps in infection, including capsid assembly and DNA packaging. The growing concern about adenovirus as a pathogen and ongoing efforts to develop it as a gene delivery vehicle underscore the importance of dissecting the complete life cycle of the virus.
The adenovirus virion is comprised of a nonenveloped icosahedral capsid surrounding a double-stranded, linear DNA genome (8, 32, 33). Virus assembly is thought to occur by formation of the capsid, followed by insertion of the genome and its associated core proteins (5). Hexons are assembled into trimers in the cytoplasm with the assistance of the viral chaperone, the L4 100,000-molecular-weight (100K) protein, and are transported into the nucleus where they associate with the penton base, fiber, and other minor structural proteins to form an empty capsid. DNA packaging has been studied using both genetic and biochemical approaches, and a number of viral factors have been found to play a role in the process. The viral packaging sequence is a cis-acting element that is found between the inverted terminal repeat at the left end of the genome and the E1A transcription unit (19). In adenovirus type 5 (Ad5), this sequence encompasses approximately 200 bp and contains seven functional elements known as A repeats. On the basis of the characterization of viruses containing mutations engineered into the packaging sequence, Hearing and colleagues have proposed the consensus sequence TTTGN8CG as being critical for packaging to occur (11, 12, 28, 34).
Biochemical studies of the viral IVa2 protein have confirmed the importance of this consensus sequence. The IVa2 protein was first implicated in packaging when it was found to form a complex with another viral protein, the L1 52/55-kDa protein, during infection (16). Viruses containing mutations in the 52/55-kDa gene are either partially or fully defective for encapsidation of the genome, although they assemble capsids in a manner similar to that of the wild-type virus (15, 18). On the basis of this interaction and knowing that the IVa2 protein is a sequence-specific DNA binding protein in its role as an activator of the viral major late promoter (24), we asked whether IVa2 binds to the packaging sequence in vitro and determined that it does indeed bind to the A-repeat consensus sequence (40) and forms multimeric structures on the entire packaging sequence (35). Chromatin immunoprecipitation experiments have demonstrated interactions of the IVa2 and 52/55-kDa proteins with the packaging sequence in infected cells (29, 31). Most recently, another viral protein, the L4 22K protein, has been implicated in packaging (27). This protein acts together with the IVa2 protein to form higher-order complexes on the A repeats and the entire packaging sequence (7, 27). Our current understanding of the DNA binding properties of the IVa2 and 22K proteins is that the IVa2 protein recognizes the CG motif in the A-repeat consensus sequence and binding of the 22K protein requires the TTTG motif. IVa2 can bind to DNA either as what appears to be a homodimer or as a complex with the 22K protein (7, 31). A related product of the L4 region, the 33K protein, shares its amino-terminal 105 amino acids with the 22K protein and may also contribute to DNA binding complexes at the packaging sequence (1). Interestingly, unlike mutant viruses with mutations in the 52/55-kDa protein, which assemble capsids, a mutant virus that does not express the IVa2 protein is completely assembly defective, implicating the IVa2 protein in both capsid assembly and genome encapsidation (41).
The dual requirement of the IVa2 protein for capsid assembly and DNA packaging suggests that it would occupy a structure in the virus that might facilitate these roles. For example, viral portals comprise structures that are situated at a single vertex of the icosahedron, can nucleate capsid assembly, and act as the site of entry of the genome into the capsid by their association with enzymatic functions that translocate the DNA. We therefore wished to address whether the IVa2 protein is associated with a single vertex. One prediction of such a model is that IVa2 would be detectable at a unique vertex on each viral particle using immunological reagents, similar to studies of the herpes simplex virus (HSV) UL6 and bacteriophage PRD1 P6 and P20 proteins, all of which are located at a single vertex (10, 26, 39). A second prediction is that the copy number of the IVa2 protein in each virion would be small. In the present study, we have examined the location of the IVa2 protein by immunogold labeling and electron microscopy, and we found that it is present at a single vertex. In addition, using three independent quantitative methods, we demonstrate that there are approximately six to eight IVa2 molecules per viral particle. These data are consistent with a model in which the IVa2 protein acts to nucleate capsid assembly and subsequently facilitates insertion of the viral genome into the capsid.
MATERIALS AND METHODS
Cells and virus.
HeLa and 293 cells (14) were maintained in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum, 100 U of penicillin per ml, and 100 μg of streptomycin per ml. The cells were grown in a 5% CO2, humidified environment. Wild-type Ad5 (American Type Culture Collection) was propagated on 293 cells and purified by CsCl gradient centrifugation as previously described (13, 15). To prepare 35S-labeled virions, Ad5 was used to infect monolayers of HeLa cells in 15-cm tissue culture dishes at 5 PFU per cell. At 20 and 27 h postinfection, 500 μCi of [35S]methionine/cysteine was added per dish (Promix [14.3 μCi/μl]; GE Healthcare) in 15 ml labeling medium consisting of 9 parts of DMEM (methionine and cysteine free) (Invitrogen) and 1 part of complete DMEM plus 10% fetal bovine serum. At 42 h postinfection, the labeled virus was collected and purified on CsCl gradients. After centrifugation, the band of mature virions was collected from the side of the tube using an 18-gauge needle and dialyzed (Pierce Slide-A-Lyzer, 10,000-kDa molecular-mass cutoff) against TNE (20 mM Tris Cl [pH 7.5], 500 mM NaCl, 1 mM EDTA, 10% glycerol) for 4 h at 4°C with two changes of buffer.
Immunogold labeling.
Ad5 was purified by banding twice on CsCl gradients. The mature virus band was collected from the side of the centrifuge tube using an 18-gauge needle, allowing the virus to flow by gravity. The collected virus was then dialyzed overnight at 4°C against TNE. Dialyzed particles were diluted 1/10 in 0.5× TNE, and 10-μl aliquots were applied to glow-discharged 400-mesh Formvar-carbon-coated nickel electron microscopy grids (Electron Microscopy Sciences) for 5 min at room temperature. Unattached capsids were washed away with 0.5× TNE. Grids were blocked for 2 h at room temperature by floating the grid face down on drops of blocking solution (5% normal serum, 5% bovine serum albumin, and 0.1% cold water fish skin gelatin in 0.5× TNE). Primary antibody incubations were performed for 1 h at room temperature in blocking solution; rabbit anticapsid serum (Abcam) was used at a 1/50 dilution, and serum against goat glutathione S-transferase (GST) fused to IVa2 (goat anti-GST-IVa2 serum) (31) was used at a 1/20 dilution. The grids were then washed with blocking solution followed by secondary antibody incubation for 45 min at room temperature with either goat anti-rabbit immunoglobulin (Ig) (1/20) or rabbit anti-goat Ig (1/10) conjugated to 15-nm gold particles (E Y Labs). Grids were washed with 0.5× TNE and fixed for 5 min with 10 μl of 2.5% glutaraldehyde in 0.1 M Sorensen's buffer (pH 7.4) and then negatively stained with 10 μl of 1% uranyl acetate. Dried grids were examined on a Phillips CM100 electron microscope. Images were captured with a high-resolution (2K pixels by 2K pixels) digital camera driven by AMT software.
Determination of IVa2 copy number from Western blots.
Capsids and purified IVa2 (35) were electrophoresed on 5 to 8% sodium dodecyl sulfate (SDS)-polyacrylamide gels, using the buffer conditions described by Laemmli (23). The number of virions was calculated from the absorbance at 260 nm using the formula 1 OD260 (optical density at 260 nm) unit = 1 × 1012 particles/ml (25). For Western blotting, the proteins were transferred from the gels to nitrocellulose on a Bio-Rad semidry transfer apparatus for 1 h at 25 V, blocked 1 h at room temperature in 5% nonfat dry milk in phosphate-buffered saline (PBS) plus 0.1% Tween 20 (PBS-T). The filters were then probed overnight at 4°C with either goat anti-GST-IVa2 or rabbit anti-IVa2 N-terminal peptide (35) antibodies. After incubation in primary antibody solutions, filters were washed three times in PBS-T and probed for 1 h at room temperature with the appropriate horseradish peroxidase (HRP)-conjugated secondary antibody. The signal was detected using ECL Plus (GE Healthcare). The band intensities of the known amounts of purified IVa2 in multiple immunoblots were compared with the band intensities of IVa2 in known numbers of virions after quantification with an AlphaImager gel documentation system.
Analysis of metabolically labeled virions.
Duplicate lanes of 1.4 × 1011 35S-labeled viral particles were electrophoresed to allow simultaneous analysis of separated virion proteins by immunoblotting and liquid scintillation counting. Fifty-three horizontal 2-mm slices were cut from adjacent lanes using a single-edge razor blade. Gel slices from one lane were placed in liquid scintillation vials, and 500 μl of 50% H2O2 was added to each vial; the tubes were then placed at 50°C for 2 days with rocking. Scintillation cocktail (BioSafeII; RPI) (4.5 ml) was then added to each vial, and the radioactivity in each vial was counted with a Beckman scintillation counter for 10 min using the wide window. Gel slices from the adjacent lane were used for Western blotting. Each gel slice was placed in a grid on a rectangle of nitrocellulose, and the proteins were transferred using a Bio-Rad semidry transfer apparatus for 0.5 h at 25 V in a buffer containing 48 mM Tris, 39 mM glycine, 0.04% SDS, and 20% methanol. The nitrocellulose membranes were blocked in 5% nonfat dry milk in PBS-T for 1 h at room temperature. Primary antibodies were added to blocking solution and incubated with the membranes either for 1 h at room temperature or overnight at 4°C. The antibodies used were goat anti-GST-IVa2, rabbit anti-Ad5 capsids (Abcam), mouse monoclonal anti-penton base (gift of David Ornelles), mouse anti-adenovirus protein V (gift of Jane Flint), and mouse monoclonal antifiber antibody (S. A. Byrd and M. J. Imperiale, unpublished result). Following incubation with primary antibodies, filters were washed in PBS-T and then incubated with the appropriate HRP-conjugated secondary antibody for 1 h at room temperature. The HRP-conjugated secondary antibodies used were donkey anti-goat IgG (Santa Cruz), donkey anti-rabbit IgG (GE Healthcare), and sheep anti-mouse IgG (GE Healthcare). After 1 h in secondary antibody, filters were washed in PBS-T and developed in Luminol (Millipore) according to the manufacturer's instructions.
Quantitative mass spectrometry (MS). (i) Digestion.
Viral particles (100 fmol) were denatured in 50 mM triethylammonium bicarbonate (TEAB) (pH 8.5) containing 8 M urea. Protein was digested with 3 μg of Achromobacter lyticus lysyl endopeptidase (LysC; Waco) for 2 h at room temperature. The LysC digestion product was diluted with 50 mM TEAB (2 M urea [final concentration]), and a second digestion was performed with trypsin (1.25 μg modified porcine; Promega) for an additional 3 to 22 h at 37°C. Digestion was terminated with 1% trifluoroacetic acid (TFA). Two other digestion protocols were also used. Virus particles were suspended in 50 mM TEAB and 0.1% SDS and digested with trypsin for 16 h at 37°C, or particles were incubated with 70% formic acid overnight at room temperature, dried, suspended in 50 mM TEAB and 0.1% SDS, and digested with trypsin.
(ii) Reversed-phase HPLC.
Peptides were separated by C18 nano-liquid chromatography (nano-LC) using a 1100 series nano high-performance liquid chromatograph (HPLC) equipped with μWPS autosampler, 2/10 microvalve, MWD UV detector (214 nm), and micro-FC fraction collector/spotter (Agilent). The viral protein digestion product (40 μl) was injected onto a C18 enrichment cartridge (Zorbax300SB [5 μm, 0.3 by 5 mm]; Agilent) and desalted with solvent C (CH3CN-H2O-TFA, 5:95:0.1) at 20 μl/min for 9 min, and the effluent was directed to waste. The enrichment cartridge was placed ahead of a C18 column (Zorbax300SB [3.5 μm, 0.075 by 15 mm]; Agilent) equilibrated with solvent A (CH3CN-H2O-TFA, 6.5:93.5:0.1). Peptides were eluted with a gradient of solvent B (CH3CN-H2O-TFA, 90:10:0.1) from 6.5% solvent B to 50% solvent B over 90 min at a flow rate of 0.4 μl/minute. Column effluent was mixed (micro Tee; Agilent) with matrix (2.6 mg of α-cyano-4-hydroxycinnamic acid per ml in CH3OH-isopropanol-CH3CN-H2O-acetic acid [14:30:22:33:0.7] containing 10 mM ammonium phosphate) delivered with a PHD200 infusion pump (Harvard Apparatus) at 0.9 μl/min. Fractions were spotted at 25-s intervals onto a stainless steel matrix-assisted laser desorption ionization (MALDI) target plate (192 wells/LC separation) (Applied Biosystems).
(iii) Mass spectrometry.
Mass spectra were acquired on a Sciex model 4800 MALDI TOF/TOF analyzer (AB/MDS Sciex) using 4000 series Explorer software (v. 3.0). Mass spectra from m/z 800 to 3,500 were acquired for each fraction using 750 laser shots. Extracted ion chromatograms were generated for each mass of interest to identify MALDI wells in which each native/labeled peptide pair elute. Tandem mass spectra (MS-MS) confirmed the identity of each peptide in all fractions in which its mass was observed. For MS-MS, the atmosphere was used as the collision gas with a pressure of ∼6 × 10−7 torrs and a collision energy of 2 kV. For peak detection, seven-point Gaussian smoothing and a signal-to-noise setting of 30 were applied using cluster area signal-to-noise optimization. MS-MS for native/labeled peptide pairs were submitted to a database search to confirm the identities of the peptides. For quantification, 10 spectra were acquired for each peptide in each fraction. The laser power was independently adjusted to optimize the intensity and shape of the peaks of interest. Acceptance criteria were applied to the acquisition of subspectra so that the most intense peak in a given mass range was required to be between 500 and 50,000 ion counts for the subspectrum to be accumulated into the final spectrum (1,500 to 2,000 subspectra) to prevent detector saturation. For each peptide mass pair, the window used for acquisition of spectra for quantification was set from 2 Da below the m/z of the native peptide to 5 Da above the m/z of the isotopically labeled form of the peptide. Subsequent to acquisition of MS for quantification, MS-MS were acquired for the 12 most intense peaks above a signal-to-noise setting of 60 in each fraction. A job-wide interpretation method was used so that the MS-MS was acquired from only the most intense instance of a mass if it was observed in adjacent fractions. Acceptance criteria were six fragment ion peaks with a signal-to-noise setting of 60 and a minimum of 1,000 and maximum of 4,000 laser shots.
Database searches were performed using Mascot (v. 2.2; Matrix Science) and Protein Pilot (v. 2.0.1, Applied Biosystems). Mascot searches were performed using NCBI (8 August 2007; 5,359,649 sequences), Swiss-Prot (9 August 2007; 276,256 sequences), and a database containing only proteins from Ad5 (NCBI). For searches against NCBI and Swiss-Prot, no species filters were used. Deamidation, N-terminal pyro-Glu, and oxidized methionine were set as variable modifications. Mass tolerances for the peptides and fragments were set at 0.5 and 0.3 Da, respectively. Searches were performed using both trypsin and semi-trypsin for enzyme specificity, and up to two missed cleavages were allowed. Protein Pilot searches were performed using NCBI and the Ad5 database. Instrument type was set at 4800. Digestion and cysteine alkylation were set at none. Mass tolerances, missed cleavages, and modifications are not user selectable in a Protein Pilot database search.
(iv) Quantification of penton and IVa2.
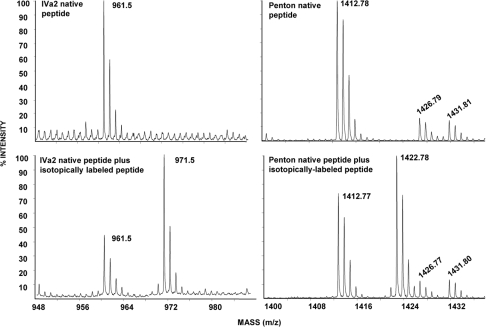
Stable isotopically labeled peptides were used as internal standards by the method of Gerber et al. (9) to determine the relative molar ratios of selected tryptic peptides derived from penton, which has a known copy number of 60 per virion, and from IVa2. Mature virions were digested with trypsin in the presence of 0.1% SDS, and tryptic peptides were separated by reversed-phase HPLC. MALDI-MS-MS identified candidates for synthesis of stable isotope peptides. Nine unique tryptic peptides (22% sequence coverage) were identified for penton, and six peptides (17% coverage) were identified for IVa2 (Table 1). Only two peptides from each protein satisfied criteria for selection as isotope-labeled standards: penton-1412, penton-1808, IVa2-961, and IVa2-1633. The peptides were eluted in three to five fractions, and the MS of each showed that the mass region 10 Da greater was free of other peptides as shown for penton-1412 and IVa2-961 (Fig. 1). Similar results were obtained for penton-1808 and IVa2-1633 (data not shown). Figure 1 also shows the relative signal intensities of the native viral peptide and its isotope-labeled partner for penton-1412 and IVa2-961, which permits determination of the relative molar amounts of these peptides in the digest. Tandem spectra of penton-1412 and its isotope-labeled counterpart penton-1422 demonstrated an almost complete y-ion series that can be assigned to the sequence YSELAPLFDTTR, confirming the peptide identity. Several ions (y*1, y*3, y*7, y*9, and y*10-H2O) derived from the penton-1422 peptide are also seen in the penton-1412 MS-MS due to bleed-through in the timed-ion selector; however all other major fragment ions observed can be assigned to penton-1412, suggesting the absence of other virally derived peptide ions at the selected precursor ion mass m/z 1,412.6. Similar results were observed for IVa2-1633 and IVa2-961 (data not shown).
TABLE 1.
Selection of peptides for synthesis of isotopically labeled peptide internal standards
| Protein | Peptide designation | Sequencea |
m/z
|
Δm | Mascot ion score | εpeptide (M−1 cm−1) | |
|---|---|---|---|---|---|---|---|
| Observed | Theoretical | ||||||
| Penton | Penton-1412 | YSELAPLFDTTR | 1,412.717 | 1,412.706 | 0.011 | 79 | |
| Penton-1808 | SYNLISNDSTFTQYR | 1,808.821 | 1,808.845 | −0.024 | 91 | ||
| pQFTSLTHVFNR | 1,332.703 | 1,332.670 | 0.033 | 64 | |||
| QFTSLTHVFNR | 1,349.717 | 1,349.700 | 0.017 | 77 | |||
| SWYLAYNYGDPQTGIR | 1,903.990 | 1,903.900 | 0.090 | 70 | |||
| RAEAEAAAEAAAPAAQPEVEKPQK | 2,433.265 | 2,433.237 | 0.028 | 91 | |||
| pQPFQEGFR | 991.483 | 991.463 | 0.020 | 48 | |||
| QPFQEGFR | 1,008.480 | 1,008.490 | −0.010 | 57 | |||
| pQNGVLESDIGVK | 1,242.631 | 1,242.621 | 0.010 | 58 | |||
| KPVIKPLTEDSK | 1,354.860 | 1,354.794 | 0.066 | 38 | |||
| TCmmtsPYVYKALGIVSPR | 1,712.937 | 1,712.886 | 0.051 | 31 | |||
| IVa2 | IVa2-961 | NIFAQAAAR | 961.471 | 961.521 | −0.050 | 46 | |
| IVa2-1633 | NFASLQELLSLGGER | 1,633.858 | 1,633.854 | 0.004 | 79 | ||
| GLPLAISLLLK | 1,137.720 | 1,137.760 | −0.040 | 49 | |||
| DMLNEVAPLLR | 1,270.690 | 1,270.682 | 0.008 | 48 | |||
| RPAALQHQQDQPQAHPGQR | 2,163.156 | 2,163.092 | 0.064 | 42 | |||
| SAPLHRDPDYADEDPAPVER | 2,250.085 | 2,250.042 | 0.043 | 36 | |||
| Synthetic labeled | AQUA-1422 | YSELAPLFDTTR* | 1,422.729 | 1,422.714 | 0.015 | 86 | 23,879 |
| peptides | AQUA-1818 | SYNLISNDSTFTQYR* | 1,818.831 | 1,818.853 | −0.022 | 110 | 29,720 |
| AQUA-971 | NIFAQAAAR* | 971.563 | 971.530 | 0.033 | 34 | 13,137 | |
| AQUA-1643 | NFASLQELLSLGGER* | 1,643.865 | 1,643.863 | 0.002 | 129 | 18,980 | |
pQ, pyro-Glu; DP, acid-sensitive AspPro; R and K, missed cleavages; Cmmts, thiomethylCys; R*, 13C615N4-arginine.
FIG. 1.
Mass spectra of IVa2 and penton peptides used for the stable heavy isotope-labeled experiments. Mass spectra of tryptic peptides IVa2-961 (NFASLQELLSLGGER) and penton-1412 (YSELAPLFDTTR) alone (top panels) and spiked with the corresponding isotopically labeled peptides IVa2-971 and penton-1422 (bottom panels).
Heavy isotope-labeled peptides were obtained from Sigma-Aldrich. Stock solutions (ca. 1 nmol/ml) were prepared, and their concentrations were determined on the basis of absorbance at 214 nm. Peptide molar extinction coefficients were calculated as described by Kuipers and Gruppen (22). Following reversed-phase LC, integrated isotope-labeled peptide peak areas at 214 nm were used to determine molar concentrations relative to the concentration of penton-1412, which was chosen as 1 nmol/ml (i.e., assuming 100% solubility of vial contents as indicated by the manufacturer).
For quantification of IVa2 relative to penton, known amounts of isotopically labeled peptide standards were added to LysC digests of viral particles prior to tryptic digestion. Integrated peak cluster areas were obtained for each native and isotopically labeled peptide pair in their appropriate chromatographic fractions. The ratio of ion cluster areas of the native peptide to the isotopically labeled peptide was determined for a minimum of 10 spectra for each fraction to obtain a molar ratio of native to isotopically labeled peptide. The ratio of areas was consistent across the elution profile, indicating that each native peptide coeluted with its isotopically labeled equivalent. The weighted mean and standard error of each peptide pair (femtomole of IVa2 peptide/femtomole of peptide) was used to calculate the molar amount of native peptide in the digest from the known amount of isotopically labeled peptide added. The weighted mean molar ratio r was calculated using the equation:
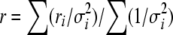 |
where ri is the average molar ratio of a native peptide/isotopically labeled peptide pair for 10 measurements in LC fraction i, having standard deviation σi. The ratio ri was measured in three to five consecutive LC fractions. The error estimate σ is given by:
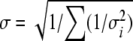 |
RESULTS
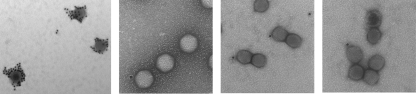
If adenovirus were similar to double-stranded DNA bacteriophages and herpesviruses in how it inserts its genome into a preformed capsid, one would expect to find proteins involved in encapsidation at a unique vertex. To determine the location of IVa2 in capsids, we examined freshly prepared, purified mature viruses using immunogold labeling and electron microscopy. Aliquots of CsCl-purified mature virions were applied to glow-discharged Formvar-coated nickel electron microscope grids. The adsorbed capsids were then stained with goat polyclonal antiserum against purified GST-IVa2 and an anti-goat secondary antibody conjugated to 15-nm gold particles. Control experiments were performed with an anticapsid polyclonal antiserum (positive control) and two negative controls, an anti-GST-L1 52/55-kDa protein antiserum (L1 52/55-kDa protein is not present in mature virions [15, 17]) and gold-conjugated secondary antibody alone. The ability of the anti-GST-IVa2 to recognize IVa2 using immunogold staining was confirmed on grids coated with purified IVa2 protein: an even distribution of gold particles was seen on these grids only when the IVa2-specific primary antibody was used (data not shown). Virus-coated immunogold-stained grids were subsequently examined for specific staining with an electron microscope after fixation and a brief treatment with uranyl acetate (Fig. 2). Data were accumulated from the staining of four preparations of virus, used in independent immunogold experiments. The total number of capsids counted at a given magnification was consolidated from all experiments to increase the accuracy of the results (Table 2). On average, after staining with the IVa2-specific antibody, 19% of the capsids were labeled with gold beads. Where this staining was seen, it was predominantly with a single gold bead and at a single vertex. Labeling was seen at more than one vertex in only 1% of cases, and then it was never more than two vertices. In contrast, use of an anticapsid antibody routinely resulted in the staining of 100% of the virions, with an average of 25 gold beads distributed over the surface of the capsid (Fig. 2). No signal was seen on control grids incubated with an anti-GST-L1 antibody, which addresses both the contribution from the GST portion of the GST-IVa2 antibody and the specificity for IVa2 itself, or when primary antibody was omitted. These results strongly indicate that the IVa2 protein is present at a single vertex.
FIG. 2.
Immunogold labeling of Ad5 particles. Mature virions were applied to grids and stained as described in the text. Anticapsid (leftmost panel) (magnification, 130,000) and anti-IVa2 (other panels) (magnification, 180,000) antibodies were used.
TABLE 2.
Immunogold labeling of IVa2 in mature virions
| Expt | No. of virions counted | % of particles labeled at:
|
|
|---|---|---|---|
| One vertex | Multiple vertices | ||
| 1 | 633 | 21 | 2 |
| 2 | 211 | 28 | 2 |
| 3 | 356 | 10 | 0 |
| 4 | 385 | 19 | 0 |
| Total | 1,585 | 19 | 1 |
We next wished to determine how many molecules of IVa2 protein were present in a single virus particle. As an initial approach, we used immunoblots to compare known numbers of viral particles with known amounts of purified IVa2 protein. Dilutions of virions, whose concentrations in particles per milliliter were determined from their absorbance at 260 nm, were separated by SDS-polyacrylamide gel electrophoresis along with serial dilutions of purified IVa2 protein. Following immunodetection with anti-IVa2 antibodies, band densities were measured using an AlphaImager gel documentation system to generate a standard curve. A representative immunoblot is shown in Fig. 3. In this experiment, 1.2 × 1011 viral particles contained an estimated 75 ng of IVa2, equivalent to 8.9 × 1011 molecules of IVa2, or 7.3 copies of IVa2 per virion; 3.0 × 1010 viral particles contained an estimated 22.5 ng of IVa2, or 2.7 × 1011 molecules, which represents 9.0 copies of IVa2 per virion.
FIG. 3.
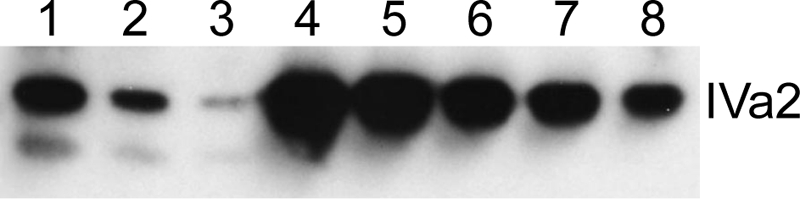
Semiquantitative Western blot. Dilutions of purified mature virions or purified IVa2 protein were electrophoresed, blotted, and probed with anti-IVa2 antiserum as described in the text. Lanes: 1, 1.2 × 1011 virus particles; 2, 6.0 × 1010 particles; 3, 3.0 × 1010 particles; 4, 200 ng of IVa2; 5, 100 ng of IVa2; 6, 50 ng of IVa2; 7, 25 ng of IVa2; 8, 12.5 ng of IVa2. The band below the IVa2 in lanes 1 to 3 is a truncated product that has been previously reported (30) and was not included in the copy number calculation.
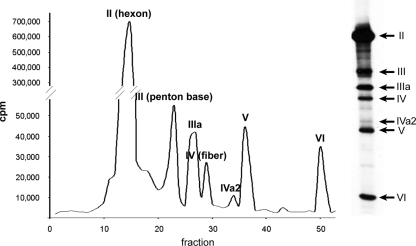
To obtain a more accurate measurement of the IVa2 copy number, we used the metabolic labeling approach previously used by van Oostrum and Burnett to determine the copy numbers of viral structural proteins in 1985 (37). At that time, the IVa2 protein had not been discovered and therefore was not quantified. Mature virions that had been metabolically labeled with [35S]cysteine and [35S]methionine were prepared and purified by CsCl gradient sedimentation. The viral proteins were then separated by SDS-polyacrylamide gel electrophoresis (Fig. 4). Duplicate lanes were subsequently fractionated; fractions from one lane were solubilized, and the 35S present was measured by liquid scintillation counting; the duplicate fractions from the adjacent lane were transferred to nitrocellulose for Western blot analysis (data not shown) of the proteins present. In this way, it was possible to ascertain unambiguously how many counts were derived from IVa2. Using hexon as a reference for copy number (720 molecules/virion), we calculated a copy number for IVa2 (Table 3). The results presented are from three independent labeling and fractionation experiments. The copy numbers arrived at for the internal control virion proteins were in close agreement with published values: the average value for fiber was identical, 36; and the value for penton base was within 10% of the published value, at 66 rather than 60. The average for IVa2 in these experiments was 6.3 ± 1.5 copies per mature virion.
FIG. 4.
Analysis of metabolically labeled virion components. Ad5-infected cells were labeled with [35S]methionine and cysteine, and virions were purified by gradient centrifugation and SDS-polyacrylamide gel electrophoresis. The graph on the left shows the counts incorporated into the various proteins as determined by scintillation counting of slices. The radiogram on the right shows the viral proteins as they appeared on an adjacent lane. In this experiment, penton base was present in fraction 23, fiber in fraction 29, IVa2 in fraction 34, and V in fractions 36 and 37, as analyzed by Western blotting (data not shown). Intervening fractions were negative for all four proteins.
TABLE 3.
IVa2 copy number in mature virions
| Protein | Calculated copy no. (normalized to hexon)
|
Mean calculated copy no.a | Published copy no. | ||
|---|---|---|---|---|---|
| Expt 1 | Expt 2 | Expt 3 | |||
| Hexon | 720 | ||||
| Penton | 62 | 68 | 67 | 66 | 60 |
| Fiber | 40 | 37 | 30 | 36 | 36 |
| IVa2 | 8 | 6 | 5 | 6 | |
Mean of the calculated copy numbers for experiments 1 to 3.
We also verified these results using a quantitative mass spectrometry technique employing stable isotope internal standard peptides (9). Cesium chloride gradient-purified mature virions were subjected to trypsin digestion followed by MALDI-TOF MS-MS. Data from preliminary MS-MS analyses allowed the selection of only two tryptic peptides each from both IVa2 (IVa2-961 and IVa2-1633) and an internal control of penton base (penton-1412 and penton-1808) for production of synthetic isotope-labeled peptides. These synthetic peptides were introduced during trypsin digestion of virions that were then subjected to MS-MS to allow relative quantification of these peptides. The copy number of IVa2 was determined with reference to penton base, whose copy number is 60, using multiple measures of peak intensities in adjacent wells. An experiment measuring the time course of digestion revealed that IVa2 was fully digested after 3 hours at which time the yield of peptides IVa2-961 and IVa2-1633 peaked compared to penton-1412 (Table 4). The yield of penton-1808 did not peak even after 22 h of digestion and thus was not used to determine IVa2 copy number (data not shown). Three additional experiments were performed, utilizing two independent viral preparations and overnight digestion with trypsin. Similar IVa2 copy numbers were obtained (Table 4). Averaging the results yielded 6.6 ± 0.2 and 8.2 ± 0.5 copies of IVa2 based on the IVa2-961 and IVa2-1633 peptides, respectively.
TABLE 4.
IVa2 copy number in mature virions normalized to penton-1412
| Expt | Digestion time (h) | Calculated copy no. of IVa2 based on peptide:
|
|
|---|---|---|---|
| IVa2-961 | IVa2-1633 | ||
| 1 | 3 | 6.4 | 8.3 |
| 4 | 6.7 | 8.4 | |
| 5 | 6.7 | 8.6 | |
| 22 | 6.8 | 8.4 | |
| 2 | O/Na | 6.3 | NDb |
| 3 | O/N | 6.8 | ND |
| 4 | O/N | 6.8 | 7.4 |
| Mean ± SEM | 6.6 ± 0.2 | 8.2 ± 0.5 | |
O/N, overnight digestion.
ND, not determined.
DISCUSSION
Biochemical and genetic analyses of the adenovirus IVa2 protein have implicated it in capsid assembly and DNA packaging. The protein binds to specific motifs in the packaging sequence that are required for encapsidation of the genome (19, 40), and a mutant virus that does not express the IVa2 protein is unable to produce capsids (41). On the basis of these data, we hypothesized that the IVa2 protein might be associated with a structure at a unique vertex of the capsid where it performs two functions, nucleating capsid assembly and assisting with the packaging of the viral chromosome. The data in this report are consistent with this model. Using immunogold labeling of purified mature virions, we found that the IVa2 protein is present at a single vertex. Quantification of the number of IVa2 molecules in the virion supports its presence at one vertex. Semiquantitative Western blotting, analysis of radiolabeled viral particles, and quantitative mass spectrometry all indicate that there are approximately six to eight copies of IVa2 per mature virion. If IVa2 were present at more than one vertex, it would be unevenly distributed among the 12 vertices of the icosahedral viral particle.
Immunogold labeling has been used previously to locate portal proteins in bacteriophage T4 and herpes simplex virus (6, 26, 39), which by definition are present at a single vertex, as well as the phage PRD1 P6 and P20 proteins, which are also located at a unique vertex (10). When HSV virions were examined by immunogold labeling for the presence of the UL6 portal protein, 16 to 41% of the capsids stained at a single vertex (26, 39). These numbers are in agreement with our experiments, in which we obtained labeling of virions for IVa2 at a single vertex in approximately 19% of the particles. Very few particles (1%) were stained at more than one vertex. In the HSV studies, it was proposed that 100% staining of a unique vertex could not be obtained due to the position of the unique vertex relative to the electron microscope grid or steric blocking of antibody access on a proportion of the capsids. A similar argument would apply to our experiments with adenovirus. The bacteriophage PRD1, which shares many structural similarities with adenovirus, has a unique vertex that contains two proteins, P6 and P20. These proteins were detected using immunogold labeling in 19.2% and 7.8% of virions, respectively (10). Thus, our results with IVa2 are similar to those for proteins located at a unique vertex in other prokaryotic and eukaryotic viruses.
The number of IVa2 molecules in each mature virion is consistent with its presence at a unique vertex. Using three independent measures, semiquantitative Western blotting, quantification of 35S-labeled protein, and isotopically labeled peptides, we found the copy number to be between six and eight molecules per virion. We believe that the 35S-labeling approach is the most accurate of the three for the following reasons. First, quantifying by Western blotting is prone to errors introduced during quantification of the purified virions and IVa2 protein. Second, the isotopically labeled technique relies on complete digestion of particles and release of each peptide being analyzed during spectrometry: if not all the penton protein was digested, for example, it would artificially inflate the ratio of IVa2 to penton. Similarly, if the IVa2 peptides were not completely released, the estimate would be low. Nonetheless, the values obtained using this method are consistent with the other two methods. In the metabolic labeling study, the analysis of each slice of the gel is unbiased, and the amount of label incorporated into each protein is determined in situ. Therefore, there is little chance of loss of signal. Moreover, the metabolic labeling experiment contains internal standards, penton and fiber, the numbers for which were in agreement with published data (37). We conclude, then, that there are most likely six to eight molecules of IVa2 present in the mature virion.
Variations in copy numbers for IVa2 depending on which IVa2 isotopically labeled peptide is used (6.6 copies using IVa2-961 versus 8.2 copies using IVa2-1633) underscores the need for accurate measures of the peptide concentration. The heavy isotope-labeled peptide must be completely solubilized and must be stable in solution, and its concentration should not change within the time frame of the experiment. Underestimation of the amount of labeled peptide present will result in an overestimate of the copy number. Synthetic peptides are routinely certified with an accuracy of ca. 10% and are supplied as a dried film which may not be at the bottom of the vial. Peptide solubility is difficult to assess and is a major source of variation (36). In this study, the ability to calculate a molar extinction coefficient at 214 nm for specific peptides (22) allows correction for variation. The peptide concentration can be monitored over time both in solution and under various storage conditions. We found that the concentration of one of the labeled peptides, penton-1643, decreased over a 1-week period (data not shown). It is the most hydrophobic of the labeled peptides used in this study, and peptide could have been lost by its aggregation or adsorption to surfaces during pipetting. This would lead to an overestimate of IVa2-1633.
It is not known at this time whether adenovirus encodes a portal structure through which DNA is inserted into the empty capsid. All known portal structures in viruses containing double-stranded DNA genomes are dodecamers (4). If IVa2 were part of such a structure, there would need to be six copies of another protein that completes the dodecameric structure. Alternatively, the six copies of IVa2 may bridge dimers of a dodecamer of another protein. It has recently been shown that the IVa2 protein can form complexes on the packaging sequence with another viral protein, the L4 22K protein (7, 27). It is possible, then that there are also six copies of the L4 22K protein per virion. Experiments to determine the number of L4 22K molecules in the adenovirus particle are in progress. If the portal were a hexamer of IVa2-22K dimers, one would expect that a virus containing a nonfunctional L4 22K gene would exhibit the same phenotype as an IVa2-null virus and be unable to build capsids. While a mutant virus with mutant L4 22K protein is nonviable, whether it makes capsids has not been examined (27). Alternatively, the adenovirus portal may be comprised of a different protein(s) that has not yet been identified.
It is more likely that the IVa2 protein is not part of the portal structure itself but is a component of the packaging machinery that is associated with the portal. Although there is no direct biochemical evidence, it is likely that the IVa2 protein is an ATPase. It contains Walker A and B motifs, which are characteristic of ATPases in the ABC and AAA+ families. A recent analysis of protein sequences and predicted secondary structures indicates that IVa2 is very closely related to a subset of ABC ATPases (3). While most of the members of this family are enzymes that transport molecules across membranes, others are involved in transactions such as repair and recombination with DNA (2, 20). The IVa2 Walker motifs are extremely highly conserved in all adenoviruses whose genomes have been sequenced, including those that infect avian species, while other parts of the IVa2 protein are not as well conserved. This conservation suggests that the function associated with the Walker motifs is essential. The Walker A motif in ABC and AAA+ ATPases contains a conserved lysine residue that is involved in substrate nucleotide binding. Pardo-Mateos and Young mutated this lysine to alanine or arginine and reported that these mutant viruses are nonviable (30). It is logical that adenovirus would require an ATPase to provide the energy necessary to insert its genome into a preformed capsid. The behavior of the Walker A motif mutants, the ability of the IVa2 protein to bind the packaging sequence, and the requirement for energy in order to insert DNA into a preformed capsid strongly suggest that the IVa2 protein provides that function. We therefore suggest that the IVa2 protein is an ATPase, or a subunit of one, that is located at a unique vertex where it facilitates encapsidation. The data from the IVa2-null mutant virus, which does not assemble capsids (41), also indicate that the presence of IVa2 at a unique vertex may nucleate capsid assembly.
As we learn more about adenovirus assembly and DNA encapsidation, it is becoming clear that while the virus shares some features with bacteriophage and other eukaryotic DNA viruses, such as HSV, it also has unique properties. Understanding the role of the IVa2 protein in the adenovirus life cycle will allow a more thorough dissection of how adenovirus is similar to, and different from, these other viruses.
Acknowledgments
We thank the members of the Imperiale lab for their thoughtful comments and insights into this work, Ryan Tyler for purified IVa2 protein, David Ornelles and Jane Flint for antisera, Bill Newcomb for advice on immunogold staining, Chris Edwards and Dorothy Sorenson for help with electron microscopy, and Kathy Spindler for helpful comments during the course of this work and on the manuscript.
This work was supported by NIH grant AI052150 to M.J.I. and NRPP grant 5P41RR018627-05 to P.C.A.
Footnotes
Published ahead of print on 9 July 2008.
REFERENCES
- 1.Ali, H., G. LeRoy, G. Bridge, and S. J. Flint. 2007. The adenovirus L4 33-kilodalton protein binds to intragenic sequences of the major late promoter required for late phase-specific stimulation of transcription. J. Virol. 811327-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aravind, L., D. R. Walker, and E. V. Koonin. 1999. Conserved domains in DNA repair proteins and evolution of repair systems. Nucleic Acids Res. 271223-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burroughs, A. M., L. M. Iyer, and L. Aravind. 2007. Comparative genomics and evolutionary trajectories of viral ATP dependent DNA-packaging systems. Genome Dyn. 348-65. [DOI] [PubMed] [Google Scholar]
- 4.Catalano, C. E. 2005. Viral genome packaging machines: genetics, structure, and mechanism. Kluwer Academic/Plenum, New York, NY.
- 5.D'Halluin, J. C. 1995. Virus assembly. Curr. Top. Microbiol. Immunol. 19947-66. [PubMed] [Google Scholar]
- 6.Driedonks, R. A., and J. Caldentey. 1983. Gene 20 product of bacteriophage T4. II. Its structural organization in prehead and bacteriophage. J. Mol. Biol. 166341-360. [DOI] [PubMed] [Google Scholar]
- 7.Ewing, S. G., S. A. Byrd, J. B. Christensen, R. E. Tyler, and M. J. Imperiale. 2007. Ternary complex formation on the adenovirus packaging sequence by the IVa2 and L4 22-kilodalton proteins. J. Virol. 8112450-12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fabry, C. M., M. Rosa-Calatrava, J. F. Conway, C. Zubieta, S. Cusack, R. W. Ruigrok, and G. Schoehn. 2005. A quasi-atomic model of human adenovirus type 5 capsid. EMBO J. 241645-1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerber, S. A., J. Rush, O. Stemman, M. W. Kirschner, and S. P. Gygi. 2003. Absolute quantification of proteins and phosphoproteins from cell lysates by tandem MS. Proc. Natl. Acad. Sci. USA 1006940-6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gowen, B., J. K. Bamford, D. H. Bamford, and S. D. Fuller. 2003. The tailless icosahedral membrane virus PRD1 localizes the proteins involved in genome packaging and injection at a unique vertex. J. Virol. 777863-7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gräble, M., and P. Hearing. 1990. Adenovirus type 5 packaging domain is composed of a repeated element that is functionally redundant. J. Virol. 642047-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gräble, M., and P. Hearing. 1992. cis and trans requirements for the selective packaging of adenovirus type 5 DNA. J. Virol. 66723-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graham, F. L., and L. Prevec. 1991. Manipulation of adenovirus vectors. Methods Mol. Biol. 7109-128. [DOI] [PubMed] [Google Scholar]
- 14.Graham, F. L., J. Smiley, W. C. Russell, and R. Nairn. 1977. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 3659-74. [DOI] [PubMed] [Google Scholar]
- 15.Gustin, K. E., and M. J. Imperiale. 1998. Encapsidation of viral DNA requires the adenovirus L1 52/55-kilodalton protein. J. Virol. 727860-7870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gustin, K. E., P. Lutz, and M. J. Imperiale. 1996. Interaction of the adenovirus L1 52/55-kilodalton protein with the IVa2 gene product during infection. J. Virol. 706463-6467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasson, T. B., D. A. Ornelles, and T. Shenk. 1992. Adenovirus L1 52- and 55-kilodalton proteins are present within assembling virions and colocalize with nuclear structures distinct from replication centers. J. Virol. 666133-6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasson, T. B., P. D. Soloway, D. A. Ornelles, W. Doerfler, and T. Shenk. 1989. Adenovirus L1 52- and 55-kilodalton proteins are required for assembly of virions. J. Virol. 633612-3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hearing, P., R. J. Samulski, W. L. Wishart, and T. Shenk. 1987. Identification of a repeated sequence element required for efficient encapsidation of the adenovirus type 5 chromosome. J. Virol. 612555-2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hopfner, K. P., and J. A. Tainer. 2003. Rad50/SMC proteins and ABC transporters: unifying concepts from high-resolution structures. Curr. Opin. Struct. Biol. 13249-255. [DOI] [PubMed] [Google Scholar]
- 21.Kojaoghlanian, T., P. Flomenberg, and M. S. Horwitz. 2003. The impact of adenovirus infection on the immunocompromised host. Rev. Med. Virol. 13155-171. [DOI] [PubMed] [Google Scholar]
- 22.Kuipers, B. J., and H. Gruppen. 2007. Prediction of molar extinction coefficients of proteins and peptides using UV absorption of the constituent amino acids at 214 nm to enable quantitative reverse phase high-performance liquid chromatography-mass spectrometry analysis. J. Agric. Food Chem. 555445-5451. [DOI] [PubMed] [Google Scholar]
- 23.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227680-685. [DOI] [PubMed] [Google Scholar]
- 24.Lutz, P., and C. Kedinger. 1996. Properties of the adenovirus IVa2 gene product, an effector of late-phase-dependent activation of the major late promoter. J. Virol. 701396-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maizel, J. V., Jr., D. O. White, and M. D. Scharff. 1968. The polypeptides of adenovirus. I. Evidence for multiple protein components in the virion and a comparison of types 2, 7A, and 12. Virology 36115-125. [DOI] [PubMed] [Google Scholar]
- 26.Newcomb, W. W., R. M. Juhas, D. R. Thomsen, F. L. Homa, A. D. Burch, S. K. Weller, and J. C. Brown. 2001. The UL6 gene product forms the portal for entry of DNA into the herpes simplex virus capsid. J. Virol. 7510923-10932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ostapchuk, P., M. E. Anderson, S. Chandrasekhar, and P. Hearing. 2006. The L4 22-kilodalton protein plays a role in packaging of the adenovirus genome. J. Virol. 806973-6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ostapchuk, P., and P. Hearing. 2003. Minimal cis-acting elements required for adenovirus genome packaging. J. Virol. 775127-5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ostapchuk, P., J. Yang, E. Auffarth, and P. Hearing. 2005. Functional interaction of the adenovirus IVa2 protein with adenovirus type 5 packaging sequences. J. Virol. 792831-2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pardo-Mateos, A., and C. S. Young. 2004. A 40 kDa isoform of the type 5 adenovirus IVa2 protein is sufficient for virus viability. Virology 324151-164. [DOI] [PubMed] [Google Scholar]
- 31.Perez-Romero, P., R. E. Tyler, J. R. Abend, M. Dus, and M. J. Imperiale. 2005. Analysis of the interaction of the adenovirus L1 52/55-kilodalton and IVa2 proteins with the packaging sequence in vivo and in vitro. J. Virol. 792366-2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Philipson, L. 1984. Structure and assembly of adenoviruses. Curr. Top. Microbiol. Immunol. 1091-52. [DOI] [PubMed] [Google Scholar]
- 33.Saban, S. D., M. Silvestry, G. R. Nemerow, and P. L. Stewart. 2006. Visualization of alpha-helices in a 6-angstrom resolution cryoelectron microscopy structure of adenovirus allows refinement of capsid protein assignments. J. Virol. 8012049-12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmid, S. I., and P. Hearing. 1997. Bipartite structure and functional independence of adenovirus type 5 packaging elements. J. Virol. 713375-3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tyler, R. E., S. G. Ewing, and M. J. Imperiale. 2007. Formation of a multiple protein complex on the adenovirus packaging sequence by the IVa2 protein. J. Virol. 813447-3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Midwoud, P. M., L. Rieux, R. Bischoff, E. Verpoorte, and H. A. Niederlander. 2007. Improvement of recovery and repeatability in liquid chromatography-mass spectrometry analysis of peptides. J. Proteome Res. 6781-791. [DOI] [PubMed] [Google Scholar]
- 37.van Oostrum, J., and R. M. Burnett. 1985. Molecular composition of the adenovirus type 2 virion. J. Virol. 56439-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walls, T., A. G. Shankar, and D. Shingadia. 2003. Adenovirus: an increasingly important pathogen in paediatric bone marrow transplant patients. Lancet Infect. Dis. 379-86. [DOI] [PubMed] [Google Scholar]
- 39.Wills, E., L. Scholtes, and J. D. Baines. 2006. Herpes simplex virus 1 DNA packaging proteins encoded by UL6, UL15, UL17, UL28, and UL33 are located on the external surface of the viral capsid. J. Virol. 8010894-10899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang, W., and M. J. Imperiale. 2000. Interaction of the adenovirus IVa2 protein with viral packaging sequences. J. Virol. 742687-2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang, W., and M. J. Imperiale. 2003. Requirement of the adenovirus IVa2 protein for virus assembly. J. Virol. 773586-3594. [DOI] [PMC free article] [PubMed] [Google Scholar]